Play all audios:
ABSTRACT The aim of this study was two-fold: (1) identify suitable bio-indicators to assess elemental status in elephants using captive elephant samples, and (2) understand how geochemistry
influences mineral intake. Tail hair, toenail, faeces, plasma and urine were collected quarterly from 21 elephants at five UK zoos. All elephant food, soil from enclosure(s), and drinking
water were also sampled. Elemental analysis was conducted on all samples, using inductively coupled plasma mass spectrometry, focusing on biologically functional minerals (Ca, Cu, Fe, K, Mg,
Mn, Na, P, Se and Zn) and trace metals (As, Cd, Pb, U and V). Linear mixed modelling was used to identify how keeper-fed diet, water and soil were reflected in sample bio-indicators. No
sample matrix reflected the status of all assessed elements. Toenail was the best bio-indicator of intake for the most elements reviewed in this study, with keeper-fed diet being the
strongest predictor. Calcium status was reflected in faeces, (p 0.019, R2 between elephant within zoo - 0.608). In this study urine was of no value in determining mineral status here and
plasma was of limited value. Results aimed to define the most suitable bio-indicators to assess captive animal health and encourage onward application to wildlife management. SIMILAR CONTENT
BEING VIEWED BY OTHERS STABLE ISOTOPES OF C AND N DIFFER IN THEIR ABILITY TO RECONSTRUCT DIETS OF CATTLE FED C3–C4 FORAGE DIETS Article Open access 13 October 2022 EVALUATION OF FACTORS
INDUCING VARIABILITY OF FAECAL NUTRIENTS IN CAPTIVE RED DEER UNDER VARIABLE DEMANDS Article Open access 27 January 2021 STABLE ISOTOPE ANALYSES OF CARBON AND NITROGEN IN HAIR KERATIN OF
SUSPECTED MAN-EATING WOLVES FROM 1880S Article Open access 28 February 2024 INTRODUCTION Formulation of an appropriate zoo diet requires husbandry skills and applied nutritional science1.
Although there is limited agreement in the literature, the use of appropriate bio-indicators to assess elemental status was suggested by Combs _et al_.2 to support evidence-based zoo diet
assessment. Zoos in the United Kingdom have a responsibility to provide appropriate nutrition to all animals within their care3 to prevent nutritional-related disease, compromised welfare
and potential reproductive failure. Limited information exists for estimated mineral requirements of elephants4, with cases of specific mineral deficiency documented. Due to elephants’ low
growth rate and large size, clinical signs of nutrient deficiency may go unnoticed for long periods of time5, making nutritional evaluation challenging. Jansman and Pas (2015)6 defined
mineral status as the balance between dietary intake of a nutrient and its requirement in the body. Twenty-eight “essential” mineral elements have known metabolic roles in the mammalian
system, for which dietary deficiency will lead to clinical deficiency. These include calcium (Ca), phosphorus (P), magnesium (Mg), selenium (Se) and zinc (Zn)7,8. Minerals are utilized
within the body in various forms or individual compartments, with a central reserve or interchange compartment, usually blood and one or more storage compartments, usually bone or liver.
Element and animal species affects the speed of mobilisation of the mineral between compartment(s)7,8. Mineral status can also be altered by interactions between dietary components; for
example an increase in dietary P causes a decrease in serum Ca9,10, and variations in individuals’ metabolism, circadian patterns and pathological state. ANALYSING ELEMENTAL STATUS IN
ELEPHANTS No single sample matrix exists for analysing elemental status in elephants or for other mammalian species. Circulating blood fraction concentrations, and/or liver tissues have
provided the standards for assessment criteria, with limited evidence as to the accuracy of reflection of elemental status in an animal11. Having a non-invasive method to assess elephant
elemental status would enable diet evaluation, regular assessment of mineral status within the animal and the development of more accurate reference ranges for the species. The practically
available sample matrices for this include plasma, toenail and tail hair, urine and faeces. Blood samples have been significantly correlated to nutritional status in livestock for trace
minerals such as copper (Cu), cobalt (Co) and Zn, as determined by health status6,12. However, mineral concentrations in the body are under homeostatic control, even when intake is
insufficient13. Fluctuations in dietary intake may affect plasma mineral levels too slowly or too rapidly to demonstrate true nutritional status in the animal12,14,15. Blood sample
collection requires elephant and keeper training16 and sample storage outside the laboratory may be problematic. Urine samples indicate excesses of certain minerals that have been absorbed,
potentially metabolised, and then excreted. They can be useful in determining Ca, iodine (I) and arsenic (As) status, however, a single sample from an individual is insufficient to reflect
elemental status, due to homeostatic controls within the mammalian system and variability in hydration status affecting solute concentrations14,17,18,19. Collection from elephants can be
problematic as samples must be collected mid-flow without substrate contamination. Toenail and tail hair samples reflect longer term patterns of dietary intake, over weeks or months20,21.
Human toenails have been shown to reflect dietary Se levels, and to correlate with blood plasma levels22. Additionally, both sample matrices have been used to identify exposure to toxic
trace elements such as As23,24,25. Sample collection is less invasive than for blood samples and storage in the field, handling and health and safety is easier. Faecal samples reflect
unabsorbed dietary minerals, as well as those re-excreted into the intestines as excess26. Separating these relative component contributions in faecal mineral contents is challenging.
Elephants consume diets of mixed digestibility, thus less digestible components, including minerals, could be over-represented in samples21. However, faecal samples are inexpensive and
non-invasive to collect, in both captive and wild elephants. From existing evidence, there is no recognised sample matrix for assessing mineral status in elephants, other than by using
samples of various sample matrices that will differ in suitability for different elements. The approach proposed in this study attempted to model the different sample matrices in terms of
measured intake, focussing on minerals essential for health, including Ca, Cu, iron (Fe), potassium (K), Mg, manganese (Mn), sodium (Na), P, Se and Zn and trace metals including As, cadmium
(Cd), lead (Pb), uranium (U) and vanadium (V). The overarching aim of this study was to identify the most suitable bio-indictor to reflect elemental intake, and thus elemental status in
elephants. MATERIALS AND METHODS STATEMENT OF ETHICAL APPROVAL * (i) Ethical approval was obtained from the University of Nottingham School of Veterinary Medicine and Science Clinical
Ethical Review Panel (Reference: 1499,150622) prior to commencing the study. * (ii) All experiments were performed in accordance with relevant UK guidelines and regulations and appropriate
permission obtained from each participating zoo. SITE AND ELEPHANT SELECTION The study was conducted between March 2016 and July 2017, using 21 elephants at five UK zoos, as detailed in
Table 1. Each zoo was visited four times over one year throughout the study period to account for potential seasonal variation within the keeper-fed diet and in pasture grazing. Zoos were
selected to provide a geographical spread across the UK so as to have regional geochemical variation in soils. Approximately equal numbers of each elephant species were included in the
study, with only adult elephants selected (over 10 years old). Animals included in the study represented approximately 40% of the total UK zoo elephant population, sampled from 55% of zoos
holding more than one elephant27. SAMPLE COLLECTION Samples of all offered food items, soil and water available to the elephants, elephant tail hair, toenail, plasma, faeces and urine were
collected from each study zoo as summarised in Supplementary Information, Table 1. SAMPLE PREPARATION Soil samples were air-dried, crushed and sieved to ≤2 mm and further milled to ≤40 µm in
an agate ball mill (Retsch, Germany). Water samples were filtered with a hydrophilic 25 mm Minisart filter in the field and acidified with 1% HNO3 and 0.5% HCl. Elephant food samples and
faecal samples were freeze dried, and passed through a food blender as described by Watts _et al_.28. Elephant tail hair and toenail samples were cleaned as described in Middleton _et
al_.25. Although this method was developed for human hair and toenails, due to the similarities in the sample composition, it was applicable to use with elephant hair and toenails. Blood
samples were collected as described in Bourne (2005)16, using Vacutainer heparin 10 ml collection tubes. Blood samples were mixed by inversion of the sample 10 times immediately after
collection; plasma was immediately separated by centrifugation at 1500 g for 10 minutes and transferred into trace element-free tubes (up to 5 × 1 ml aliquots for each sample) at each
individual zoo. Samples were transported on ice and frozen at −20 °C upon return to the laboratory. SAMPLE DIGESTION FOR ICP-MS ANALYSIS Soil samples and elephant faecal samples (0.25 g)
were digested in a mixed acid solution (HF: 2.5 ml/HNO3:2 ml/HClO4:1 ml/H2O2:2.5 ml) on a programmable hot block; 0.5 g of elephant food samples were digested in HNO3:10 ml/H2O2:1 ml mixed
solution in a closed vessel microwave heating system (CEM MARS Xpress, USA) as described in Watts _et al_.28. In summary, food samples were heated to 100 °C over 5 minutes with 10 ml HNO3,
held for 1 minute and then heated to 200 °C over 5 minutes and held for 15 minutes. The vessels were then cooled and 1 ml of H2O2 added to the solution, allowed to settle for 30 mins before
repeating the heating cycle, but with the latter stage held at 200 °C for 25 minutes. Sample digests were then diluted to an appropriate acid matric content for ICP-MS analysis. Elephant
tail hair samples and elephant toenail samples (0.1 g sample) were digested also using the microwave heating system, but with HNO3:4 ml/H2O2:1 ml and with only one heating cycle, heated to
100 °C over 5 minutes, held for 1 minute and then heated to 200 °C over 5 minutes and held for 30 minutes as described in Middleton _et al_.25. Urine samples were diluted 1-in-10 with 2%
HNO3 prior to analysis as described in Middleton _et al_.17. Plasma samples were diluted 1-in-20 with 0.5% HNO3 prior to analysis as described in, Phiri _et al_.29. ELEMENTAL ANALYSIS
Elemental analysis was conducted on all prepared samples by inductively coupled plasma mass spectrometry (ICP-QQQ; Agilent 8900×, USA) using collision reaction cell mode (reactive gas modes:
H2 for Se at 7.0 ml/min, O2 for As at 30%, He for all remaining elements at 5.1 ml/min) for a suite of 55 elements using internal standards (Sc, Ge, Rh, In and Ir) for drift correction.
ICP-MS operating parameters were: Rf power 1550 W; plasma gas flow 15 L/min; carrier gas flow rate 1.0 ml/min. Fifteen biologically functional elements that are routinely used for health
assessment were selected for this study, Ca, Cu, Fe, K, Mg, Mn, Na, P, Se, Zn, As, Cd, Pb, U and V. Internal and external analytical quality controls were used including appropriate
certified reference materials, selected based upon the sample matrix. Sample blanks were run to determine practical Limit of Detection (LOD, 3*STDEV). Additionally, soil pH was measured
using 10 g soil and 25 ml CaCl2 and organic matter content was estimated for soil and faecal samples using loss on ignition (LOI) at 450 °C for 1 g of sample, as described in Watts _et
al_.28. For normalisation across urinary samples, urinary creatinine was determined using the JAFFE method30. ANALYTICAL QUALITY CONTROL The accuracy of the elemental analysis was verified
by analysing the following Certified Reference Materials (CRM): * Human Hair (GBW09101, China) * Spinach leaves (SRM1570A, NIST, USA) * Tomato leaves (SRM1573A, NIST, USA) * Seronorm Trace
Elements Urine L-1 (Sero AS, Norway) * Seronorm Trace Elements Plasma L-1 (Sero AS, Norway) * Basalt rock (BCR-2 United States Geological Survey, USA) * Soil (SRM2711a, NIST USA) * Soil (BGS
102, British Geological Survey, UK) * In house toenail (BAPS 2014) The concentrations of all reference materials were found to be accurate within an acceptable percentage of the certified
values for all elements studied here (average % recovery = 97% ± 20, see Supplementary Information Table 2 for keeper-fed diet CRM data and Supplementary Information Table 3 for all other
CRM data). INPUT SAMPLING AND ANALYSIS Quantities of all food items which were presented to each elephant in their keeper-fed-diets on the day of sample collection were recorded, and water
consumption was estimated at 200 litres per elephant per day based on the literature11,31,32 details of keeper-fed diets are in Supplementary Information Tables 4–8. These data were entered
into Zootrition software33 along with elemental analysis data (nutritional breakdown of feed items in Supplementary Information Table 2 keeper-fed diet analysis) to give an estimation of
elemental intake in keeper-fed diet for each individual at each sampling point in time. Elemental intake was estimated from diet, grass and water consumption for each individual elephant to
give a combined input value for each element. Grass consumption from pasture grazing for each season was approximately estimated from total dry matter (DM) intake per elephant, based on
individual body weight for that season34, as shown in Supplementary Information Table 9. Soil consumption could not be estimated and thus was not included in the combined input value.
Therefore, intake values may be considered a conservative underestimate. STATISTICAL ANALYSIS The objective of the analysis was to assess the evidence that particular measures of intake
(e.g. soil, water, keeper-fed diet) for each element were predictive of measured status in a particular sample matrix (e.g. toenail, tail hair, blood, faeces, urine). This was done using a
linear mixed model8 using R software, version 3.5.035; the outcome of interest was the elemental level in elephant sample matrices (as predicted by inputs). In all models, species and sex
were included as fixed effects. Zoo, individual and season were treated as nested random effects, accounting for correlations between individuals in the same zoo, and between repeated
observations on the same individual. The linear mixed model (lmm) was fitted with the lme procedure from the nlme library for the R platform36,37. The evidence that combined input was
predictive of element status was assessed by fitting a lmm, with species, sex and combined input as the fixed effects. The models were fitted by residual maximum likelihood. The anova.lme
command was applied to test the null hypotheses that the fixed effects on the model were zero. The sequential (default) option was used so that the test on the last-named fixed effect
(combined input here) is a test of the null hypothesis given that the other fixed effects were already included in the model. Next, the separate measures of intake were considered as
potential predictors of status by a sequential modelling process. First, and without any reference to data, the measures were ordered from the one regarded as most likely to be predictive to
the one least likely. The order of predictors was keeper-fed diet, grass, water and lastly, soil. The first predictor in this order was then added to sex and species as fixed effects in an
alternative model to the null, with sex and species only. The evidence for an effect of the added predictor was assessed by anova.lme command as described above. If the null hypothesis of no
effect of the predictor was rejected with _P_ < 0.05 then the predictor was retained, and a new model was estimated with the second predictor added to the fixed effects as last-named in
the sequence. If the null hypothesis for the first predictor was accepted, then it was dropped from the fixed effects, and a new model was fitted with the second predictor added to sex and
species as fixed effects. This procedure was iterated until all the predictors had been considered. The objective of this model fitting was to identify measures of intake that may be
predictive of elemental status. The inference in the procedure above from p-values is properly done to test single hypotheses, and the procedure here is multiple hypothesis testing in which
our question was ‘are any of the measures of intake predictive?’. It is well known that multiple hypothesis testing in which each hypothesis is evaluated on the basis of its own _p-valu_e is
likely to be anticonservative, in the sense that the final set of selected predictors is likely to contain some, which result from the rejection of null hypotheses, which should have been
accepted. Here we used the control of marginal discovery rate to control the error, based on the approach of Benjamini and Hochberg (1995)38. The false discovery rate is the probability that
a rejected null hypothesis in some family of tests should have been accepted. We controlled marginal false discovery rate (mFDR) at <0.05, following the method of alpha-investment
proposed by Foster & Stine (2008)39. Under Foster and Stine’s (2008)39 procedure, the p-values for a set of tests conducted in some order are compared against a set of threshold values.
The threshold value for the _i_th test is not fixed in advance but depends on a quantity called the alpha-wealth, which is depleted (by acceptance of null hypotheses at positions 1 to
(_i-_1) in the sequence) or augmented by rejection of these hypotheses. Foster and Stine (2008)39 present rules for the development of the alpha wealth, which ensure that the false discovery
rate is controlled below the specified value. These mean that judicious ordering of the hypotheses, so that the least plausible nulls are tested early, (i.e. the predictors most likely to
be informative) will increase the power of the procedure to detect real effects (although this is not valid if the ordering is based on prior examination of the data). More detail of the
theory of this procedure is presented by Foster and Stine (2008)39 and Lark (2017)40 gives an example of its application. It has been used in various studies to improve the efficiency with
which large data bases are interrogated to identify effects of interest while controlling false discovery rate41. The p-values from the successive tests of null hypotheses for each element
and sample matrix were evaluated against threshold values determined by the method of Foster and Stine (2008)39, specifying that the false discovery rate be kept below 0.05. The process
above results in a predictive model for a mineral in a particular sample matrix, with selected predictors related to possible sources of intake, or no predictors are selected. In the former
case we compute a measure of the extent to which the predictive model succeeds in accounting for variation in the observed concentrations in the sample matrix of interest. This is called the
approximate adjusted R2 and it is computed for each random effect in the model: the between-zoo, between-elephant within zoo and between observations within elephant random effects (the
latter including measurement error). If the estimated between-zoo variance component for the null model, with sex and species the only fixed effects, is _s_2Z,0, and the corresponding
variance component for the model with additional selected predictors is _s_2Z,1, then the approximate adjusted R2 at between-zoo level may be computed as
$${R}_{Z}^{2}=1-{s}_{Z,1}^{2}/{s}_{Z,0}^{2}.$$ (1) We may think of this quantity as the approximate proportion of variance (at the between-zoo level) accounted for by adding the extra
predictors to sex and species. It should be noted that, unlike the R2 values customarily computed with statistical software, this one is based on likelihood-based estimates of variance
components rather than those obtained by partition of a sum of squared residuals. It is therefore possible that an estimated variance component could be negative, due to estimation error42,
and so the value computed in Eq. [1] might not be bounded by [0,1]. Negative values simply imply that the effect of adding the predictor, at this level, is smaller than the estimation error,
and a value of 1 implies that the variance component in the model with predictors is very small and has been estimated as zero as (or less than zero). A value of _R_2 Z < 0 should
therefore be interpreted as evidence that the predictor has negligible effect in terms of accounting for variation in the observations at the between-zoo level, and a value of 1 that it
accounts for most of the variation at this level. RESULTS OVERALL RESULTS Tables 2 and 3 summarise results of all intakes sampled (keeper-fed diet, grass, soil and water) at the five study
zoos. All individual data points are shown in Supplementary Information Tables 2 and 4 to 8. ELEMENTAL DATA FOR INTAKES (DIET, GRASS, WATER AND SOIL) Based on observations of the elephants,
and keeper feedback, on all four visits to each of the five zoos, it was clear that the elephants consumed all the food presented to them in their keeper-fed diets, over a 24-hour period. At
all five zoos, hay made up the largest contribution to the diet by weight, on average 52 kg per elephant per day (+/− 15 kg) out of an average keeper-fed diet of 81 kg per elephant per day
(over 60% of intake), other than possible grazing for which accurate quantification was not possible. Seasonally, there was little variation in mineral provision from the hay and amounts fed
were consistent throughout the year in all but one zoo (Zoo C), as shown in Supplementary Information Tables 2–8. Commercial pellets were the main source of dietary minerals. Across the
five zoos, 10 different commercial pellets were fed with four out of the five zoos feeding a pellet that was manufactured specifically for the species. One pellet had a Zn supplement milled
into it (fed at Zoo C) and was fed to specific individuals that were suspected to be Zn deficient from previous in house assessment. All but one zoo fed wheat bran to their elephants in
minimal quantities as a medicant carrier. Five additional nutraceutical or vitamin/mineral powdered supplements were added to the keeper- fed diets across three zoos (Zoos A, C and D):
Bladder-rite (Gold Label, UK), Newmarket Joint Supplement (Newmarket, UK), multivitamin supplement (Farm and Stables Supplies, UK), multivitamin with additional vitamin E and Se supplement
(Farm and Stables Supplies, UK), and calcium carbonate (CaCO3) (Farm and Stables Supplies, UK). Two zoos provided no additional supplements (Zoos B and E). Grass from pasture grazing was not
available to all elephants throughout the year, as access depended on the weather conditions; however, all zoos offered grazing to all animals for at least part of the year (generally in
spring, summer or autumn). Mineral content in grass from pasture grazing, and from browse consumption, varied considerably across seasons and between zoos. Generally, less browse was
presented to elephants during winter months due to the challenges of sourcing palatable material in the UK climate. Fruit and vegetables comprised a very small proportion of all elephant
diets (by weight) despite the wide variety of items fed (18 varieties of vegetables and 9 varieties of fruit over the five zoos), thus contributed minimally to the mineral provision in the
diet due to the very small quantities fed per day and their high water content. For certain minerals, provision from water contributed substantially to the overall mineral intake for the
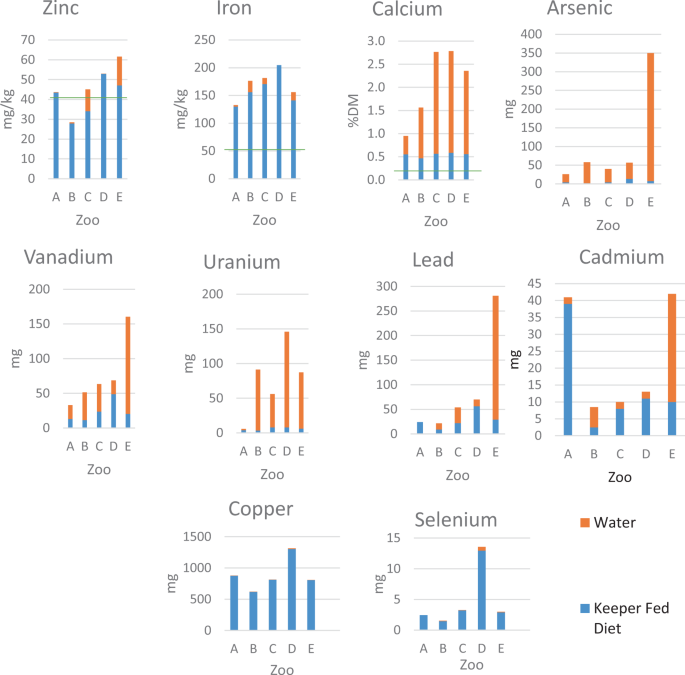
elephants, with considerable variation between zoos noted, as shown in Fig. 1. Specific elements of interest provided through drinking water included Zn where provision in zoos C and E were
noteworthy, Ca in all the study zoos, and As and U in which provision from water contributed to intake more than keeper-fed diets. Additionally, at Zoo E, levels of Cd, Pb and V in the water
contributed substantially to overall intake of these elements at this zoo. All water samples fell within the safe water limits issued for humans by the World Health Organization (WHO
2018)43. It was not possible to quantify elemental provision from soil, although anecdotally, keepers reported that elephants were seen to consume soil in very small quantities on occasion.
Table 3 indicates that there is considerable variation in elemental provision from soil at the various zoos. This is likely to be due to geographical location of each zoo and geological
makeup of the associated soil44. DIETARY PROVISION COMPARED TO PUBLISHED RECOMMENDATIONS Calcium, Fe and Zn all have published recommended dietary intakes for elephants4. Figure 1 shows a
comparison of mineral levels in keeper-fed diets from each zoo to captive recommendations produced by Ullrey _et al_.34. In all zoos, Ca and Fe concentrations in provisioned diets were well
in excess compared with intake recommendations, whereas Zn was below the recommended levels in keeper-fed diets in two of the five zoos. However, when Zn provision from water was also
considered (based on assumed consumption of 200 litres per elephant per day11,31,32), the Zn level in Zoo C rose to within the recommended levels and only intake in Zoo B remained below the
recommended level of 40 mg/kg (DM basis). ELEMENTAL REFLECTION IN ELEPHANT SAMPLE MATRICES Tables 4 and 5 and Supplementary Information Table 10, summarise the results of all outputs from
elephants at the five study zoos (toenail, tail hair, faeces, urine and plasma), divided into fluids and solids. Urine elemental analysis was corrected for hydration status using creatinine
normalization30. Elephant tail hair is estimated to grow at approximately 2–3.5 cm per month, with two studies of 50 and 32 hairs, respectively, documenting this growth rate and reported
that tail hair of males grew up to 1.5 cm more slowly per month than those of females20,45. These data reported for zoo elephants are therefore assumed to represent the median elemental
analysis for approximately the most recent 6 months of growth: 3 × 3 cm sections for males and 5 × 3 cm sections for females, given that growth rate of males is documented to be slower than
females. IDENTIFYING BEST SAMPLE MATRICES FOR EACH ELEMENT Table 6 summarises results from the linear mixed model, and the significance of correlation with combined inputs, including the
keeper-fed diet, estimated grass consumption and estimated water consumption. Toenail was found to best reflect Na and K intakes. Iron intake was best reflected in both tail hair and toenail
samples. Phosphorus and Ca levels in faeces correlated with combined inputs and Se intake correlated with toenail and faecal levels. Finally, Cd and U intake were reflected in plasma
samples. Elemental levels in urine were found to have no significant correlation with any combined inputs. Table 7 shows summary results from within the linear mixed model to identify the
best sample matrices for reflecting bio-indicators of intake and therefore a good proxy of elemental status. The R2 value (in Tables 6 and 7) considers other factors which may influence the
significant predictor; R2 E = between elephant within zoo and R2 O = between observations within elephant random effects (the latter including measurement of error). This value increases
confidence towards the predictor. Supplementary Information Table 11 details all p-values resulting from this model. Inputs were considered sequentially, with diet as the first predictor
followed by grass, water and finally soil. Toenail was found to reflect the greatest number of elements, followed by faeces and blood. Tail hair and urine reflected the least number of
elements. Diet was the most significant predictor for the greatest number of elements (8) followed by grass, soil and water. Due to the reduced sample size for urine, there is inadequate
balanced replication to estimate the full model, so sex and species effects were removed as random effects. No significant predictors were found for Cd, Na, Pb and V. DISCUSSION The aim of
this study was to identify appropriate sample matrices to use as bio-indicators of elemental status in elephants. The variability in dietary provision by zoos presented a challenge, and the
complexity of estimating the ‘inputs’, i.e. keeper-fed diet including browse, grass provision from grazing, and intake of water and soil, demonstrates the need to be able to simply obtain
non-invasive samples for assessing elemental status in the species. A strong bio-indicator must respond well to variation in intake, so we examined relationships between elemental
composition of sampled sample matrices and known inputs as shown in Tables 6 and 7. Tables 6 and 7 demonstrate that non-invasive sampling of tail hairs and toenails can potentially provide
useful indications of As, Fe, K, Mg, Na, P, Se, and Zn status in elephants. Results suggest that the current clinical practice of elemental measurement using plasma is of limited value, as
such measures are rarely responsive to dietary variation. There were no useful biomarkers of intake for Cd, Na, Pb and V when considered sequentially and for As, Cu, Mg, Mn, Pb, V and Zn
when considered on a combined input basis. Additionally, faeces reflected As, Ca, Cu and Se intake on a sequential basis and Ca, Se and P on an estimated combined input basis. Manganese and
U intakes were reflected in blood sequentially. Where there is no elephant-derived sample that can indicate intake of an element, zoos may find it useful to be aware of alternative measures
(e.g. haemoglobin status for Fe) and consider the elemental composition of their water sources. Toenails were found to best reflect elemental intake for the largest number of elements in
elephants: As, Fe, K, Mg, Na, P and Se as shown in Tables 6 and 7. Unfortunately, it was not possible to estimate the growth rate of the toenails or the time during which the material
analysed was laid down by the elephant. A number of factors including substrate, exercise, weather, foot care, nutritional and health status of the animal, as well as behaviour will affect
toenail growth rates46. Tail hair proved to be a bio-indicator of total intake of key minerals, including As and Fe. Tail hair growth rate, as with other mammals, is also likely to be
affected by multiple factors including overall nutritional plane, weather, health of the elephant and age of the elephant47. Therefore, estimated growth rate was used to calculate the
analysed length20,45, which is an estimation of the previous 6 months of growth, although in practice this may not always be accurate due to variability in growth rate. Faecal samples were
indicative of estimated elemental intake in the elephant for As, Ca, Cu, Se and P, as shown in Tables 6 and 7. Due to historic concern around insufficient vitamin E and other antioxidants in
zoo elephant diets, Se is likely being fed in excess as an additive within the manufactured elephant pellets48. This could indicate why high concentrations were excreted and therefore
detected within faecal samples. Macro-mineral concentrations within blood plasma were not found to be reflective of intake. This is unsurprising, as mammals homeostatically control levels of
these minerals within the blood and store excess as needed12,14,15. Urine was not found to indicate estimated intake of any element. It was only possible to obtain 32 samples from 9 animals
at two zoos. Therefore, there was inadequate balanced replication to estimate the full model, thus sex and species effects were removed. Generally, UK zoo elephants are not mineral
deficient, and often for several minerals, keeper-fed diets contain excess provision. For specific elements such as Pb, inputs were very low (in keeper-fed diet, water, soil or grass) and
thus, Pb was not present in sufficient quantities in any inputs, to be reflected in any elephant samples. A linear mixed model with the application of alpha wealth was appropriate to
identify significant relationships between inputs and sample matrices (outputs) both on a combined and sequential method. The use of alpha wealth to reduce the false discovery rate within
the linear mixed model provided greater confidence in the findings; nine previously significant relationships were eliminated through this correction. The application of R2 allows for
consideration to be given to the weighting of factors other than the one being investigated that may have affected relationships, including between-elephant within zoo and between
observations within elephant random effects (the latter including measurement error). Elemental combined input based on keeper-fed diet, estimated water consumption (200 litres/day) and
estimated grass intake from grazing (based on %DM consumption), resulted in less significant relationships with sample matrices than when considered sequentially. This is likely because
estimations were made of inputs, especially of grass intake, to form the combined input figure. Keeper-fed diet was the most likely predictor of elemental values measured in any sample
matrix and was the major source of minerals, as shown in Table 7. From this source, the pellet ration within the diet contributed most to elemental provision, even though the weight of
pellet fed in the overall keeper-fed diet was comparatively low, on average 9% of the diet as fed (an average 7 ± 9 kg per elephant per day). However, only Zoo B and C fed less than the
recommended 3 kg of pellet per day49. Variation in elemental provision from hay throughout the year was minimal, mineral or trace metal degradation would not be expected with storage. Hay
and browse used within each zoo were produced within close proximity (within approx. 20 km) of each zoo. The variation in elemental analysis in hay and browse between zoos, is likely to
reflect the differing geology of the five areas surrounding the zoos. Water mineral concentrations varied widely both among zoos and seasonally, often depending on source / availability,
e.g. borehole, rainwater harvesting or mains water supply. Elemental provision from water is rarely considered as significant when evaluating human or animal diets, except when investigating
exposure to trace metals. However, Fig. 1 shows that water contributed substantially to the intake of specific minerals (As, Ca, Cd, Pb, U, V and Zn, Cd) and requires further study. At zoo
C, Zn provision from keeper-fed diet was below the recommended levels. With the addition of Zn from the water provided (assuming average levels of water intake from the literature, 200
litres /day), apparent levels of Zn became sufficient. Beal (2017)50 demonstrated the significance of Ca provision in human drinking water, where Ca from water contributed up to 11% of
national Ca supplied, based on consumption of 1.7 litres per adult per day. Within this study, Ca in water was found to contribute up to 8% of Ca supply per day, based on an estimated
consumption of 200 litres of water per elephant per day. Zoos were selected to provide a geographical and geological spread with variance within the soil make up, therefore the variation
seen in the elemental analysis of soils sampled was not unexpected. The linear mixed model determined that soil minerals were a significant predictor of Mg and P levels in toenail and Fe and
Mn in plasma. For these elements, provision from soil may be important to consider when looking at elemental reflection in these sample matrices. There are limited published recommendations
for elephant dietary mineral provision. Generally the domestic horse is considered to be an acceptable physiological model for elephants4,51. Additionally, the BIAZA (British and Irish
Association of Zoos and Aquaria) Elephant Management Guidelines (2019)4 provides recommendations on dietary management, which must be followed by BIAZA member zoos as part of the BIAZA
Elephant Management Policy. All zoos within this study fed browse daily throughout the year and provided some grazing access to all animals, in line with these BIAZA recommendations. Mineral
provision from the keeper-fed diet within this study was found to be similar to previous work. Partington (2012)52 found all UK elephant diets to be excessive in Ca, as was the case in this
study, and also detected some possible Zn deficiencies. The study conducted by Partington used intake data from all UK elephant holding zoos at the time of writing but used published
elemental data for each food item and did not include elemental provision from browse as was included in the current study. The lowest Zn dietary intake identified by Partington (2012)52 was
22 mg/kg DM, whereas in this study, the lowest Zn dietary intake was 28 mg/kg DM (Zoo B) as shown in Fig. 1, but still below the recommended 40 mg/kg DM. Published reference ranges for
elephant plasma, urine and faecal mineral levels are very limited. The largest database of this information resides with Species 360, USA27. All member zoos internationally are encouraged to
submit data as available. Sample sizes of these datasets are often small and animals may be health-compromised at the time of sampling. Results for elemental levels in plasma, urine and
faeces in this study (Tables 4 and 5 and Supplementary Information Table 10) were within reported reference ranges, Species 360, 201727. Published data on elemental analysis in elephant tail
hair or toenails is absent from the literature. Feed costs are the second largest day-to-day running costs of a captive elephant herd4,22. It is therefore essential that zoos are feeding
appropriate items of acceptable quality to their animals. The BIAZA Elephant Management Guidelines recommends the use of fruits and vegetables in very limited quantities, less than 1 kg per
elephant per day53. All zoos in the current study were feeding greatly in excess of this recommendation as shown in Supplementary Information Tables 4–8. Given the high incidence of obesity
in UK zoo elephants, with estimations of up to 75% of the population being recorded as ‘overweight’54, a seasonal reduction in hay is recommended to offset increased grass from pasture and
browse availability during summer months, this is currently only practiced in Zoo C. Finally, the elephants in this study are under human care, and could arguably be variably stressed or
compromised55, which may alter mineral metabolism. For example, plasma Zn levels can be artificially increased when an animal is stressed or suffering from an inflammatory condition13.
Caution must be used when comparing these values to wild elephants, or as target values for elephants. Likewise, as seen within this current study, UK zoo elephants are unlikely to be
experiencing nutritional compromise or substantial mineral deficiency. Diets fed in these five UK zoos, in general, were appropriate to meet species’ documented mineral needs. CONCLUSION The
results from the current study indicate that no single sample matrix from elephants are sufficient to reflect elemental intake within the animal, and thus be a good proxy for elemental
status, a variety of sample matrices are needed. Of the five sample matrices investigated in this study, toenail reflected inputs for the largest number of elements assessed, and is likely
to be the best reflection of status for these elements. Faeces and tail hair were also found to significantly correlate to inputs into the elephant. Plasma was of limited value with a small
number of elements being responsive to dietary variation. Urine did not correlate with any inputs for any element and thus was not a useful bio-indicator. Predicting how elemental status is
reflected in various sample matrices presents a challenge, as the sample matrix concentrations may not be indicative unless levels are below an excess threshold. Sample availability may also
influence sample matrix choice when investigating mineral status. Finally, mineral provision from water should never be overlooked when assessing zoo animal diets, especially for species
that consume such large volumes as elephants. Future work should investigate how the methods described in this study could be applied to free-living populations of elephants, especially
those within smaller fenced reserves, to identify individuals with mineral deficiencies, or elephants exposed to uncharacteristically high levels of trace metal intake. Opportunities exist
to address the United Nations Sustainable Development Goals (SDGs) 3 (Good Health and Well-Being), 15 (Life on Land) and 17 (Partnerships for the Goals) from this work. Advancing zoo animal
health and welfare will increase opportunities to (re) connect people with nature and promote well-being through the visiting of zoos (SDG 3), with further potential for education and
creation of livelihoods (SDG 4, 8). Secondly, application of this work provides the opportunity to protect ecosystems through benefitting wildlife management (SDG 15). Finally, global
partnerships can be developed between North and South with the opportunity for studies on captive animals in a controlled environment to inform research and welfare of wild counterparts (SDG
17). REFERENCES * Dierenfeld, E. S. 1997. Captive wild animal nutrition: a historical perspective. In: Proceedings of the Nutrition Society Symposium on “ Nutrition of wild and captive wild
animals” Plenary Lecture. 989–999, https://doi.org/10.1079/PNS19970104. * Combs, G. F. _et al_. Biomarkers in nutrition: New frontiers in research and application. _Annals of the New York
Academy of Sciences._ 1278, 1–10, https://doi.org/10.1111/nyas.12069 (2013). Article ADS CAS PubMed PubMed Central Google Scholar * Zoo Licensing Act 1981.,
http://www.licensingresource.co.uk/sites/all/files/animal/zoo1.pdf (accessed July 19, 2018). * Sach, F., Dierenfeld, E., Langley-Evans, S., Watts, M. & Yon, L. African elephants
(Loxodonta africana) as an example of a mega-herbivore making movement choices based on nutritional needs. _Peer J_ 7, e6260, https://doi.org/10.7717/peerj.6260 (2019a). Article CAS PubMed
PubMed Central Google Scholar * Ullrey D., Roocroft A., Bernard J., Oosterhuis J., Magee W. Biological value of vitamin E forms for elephants. _Report to the Zoological Society of San
Diego_ (1991). * Jansman, A. J. M. & Te Pas, M. F. W. 2015. Techniques for evaluating nutrient status in farm animals. Wageningen UR (University & Research centre) Livestock
Research, Livestock Research Report 846 * McDonald, P. _et al_. 2010. Animal Nutrition. Gosport: Ashford Colour Press Ltd. * Suttle, N. F. Mineral Nutrition of Livestock. _Wallingford: MPG
Books Group_ (2010). * Assane, M., Gongnet, G., Coulibaly, A. & Sere, A. Influence of dietary calcium/phosphorus ratio on blood calcium, phosphate and magnesium during gestation in the
rabbit. _Reproduction Nutrition Development_ 33, 223–8 (1993). Article CAS Google Scholar * Steevens, B. J., Bush, L., Stout, J. & Williams, E. Effects of varying amounts of calcium
and phosphorus in rations for dairy cows. _Journal of Dairy Science_ 54, 655–661, https://doi.org/10.3168/jds.S0022-0302(71)85902-7 (1971). Article CAS PubMed Google Scholar * Olson D.
2004.Elephant Husbandry Resource Guide., http://www.elephantconservation.org/iefImages/2015/06/CompleteHusbandryGuide1stEdition.pdf accessed June 1, 2018. * Mills, C. Biochemical and
physiological indicators of mineral status in animals: copper, cobalt and zinc. _Journal of Animal Science_ 65, 1702–11 (1987). Article CAS PubMed Google Scholar * Kincaid, R. L. 1999.
Assessment of trace mineral status of ruminants: A review. Proceedings of the American Society of Animal Science, https://doi.org/10.2527/jas2000.77E-Suppl1x. * Caple, I., Doake, P. &
Ellis, P. Assessment of the calcium and phosphorus nutrition in horses by analysis of urine. _Australian Veterinary Journal_ 58, 125–31. (1982). Article CAS PubMed Google Scholar *
Herdt, T., Rumbeiha, W. & Braselton, W. The use of blood analyses to evaluate mineral status in livestock. _Veterinary Clinics of North America: Food Animal Practice_ 16, 423–444,
https://doi.org/10.1016/S0749-0720(15)30078-5 (2000). Article CAS Google Scholar * Bourne D. 2005.Blood sampling from the auricular (ear) vein.,
http://wildpro.twycrosszoo.org/S/00Man/VeterinaryTechniques/EleIndTech/Ele_Bloodsample.htm accessed February 11, 2019. * Middleton, D. R. S. _et al_. 2016a. Urinary arsenic profiles reveal
exposures to inorganic arsenic from private drinking water supplies in Cornwall, UK. Nature Publishing Group:1–11, https://doi.org/10.1038/srep25656. * Middleton, D. R. S., Watts, M. J.,
Lark, R. M., Milne, C. J. & Polya, D. A. Assessing urinary flow rate, creatinine, osmolality and other hydration adjustment methods for urinary biomonitoring using NHANES arsenic,
iodine, lead and cadmium data. Environmental Health:1–13. https://doi.org/10.1186/s12940-016-0152-x (2016b). * Watts, M. J. _et al_ Iodine status in western Kenya: a community-based
cross-sectional survey of urinary and drinking water iodine concentrations. _Environmental Geochemistry and Health_. https://doi.org/10.1007/s10653-019-00352-0 (2019a). * Cerling, T. E. _et
al_. Orphans’ tales: Seasonal dietary changes in elephants from Tsavo National Park, Kenya. _In: Palaeogeography, Palaeoclimatology, Palaeoecology_ 206, 367–376,
https://doi.org/10.1016/j.palaeo.2004.01.013 (2004). Article ADS Google Scholar * Sponheimer, M. _et al_. An experimental study of carbon-isotope fractionation between diet, hair, and
feces of mammalian herbivores. _Canadian Journal of Zoology_ 81, 871–876, https://doi.org/10.1139/z03-066 (2003). Article CAS Google Scholar * Sach F., Fitzpatrick M., Masters N. &
Field D. Financial planning required to keep elephants in zoos in the United Kingdom in accordance with the Secretary of State’ s Standards of Modern Zoo Practice for the next 30 years.
24:1–11. https://doi.org/10.1111/izy.12213 (2019b). * Bencko, V. Use of human hair as a biomarker in the assessment of exposure to pollutants in occupational and environmental settings.
_Toxicology_ 101, 29–39, https://doi.org/10.1016/0300-483X(95)03018-B (1995). Article CAS PubMed Google Scholar * Button, S. & Monit, J. E. Human toenails as a biomarker of exposure
to elevated environmental arsenic. _Journal of Environmental Monitoring_ 11(3), 610–617, https://doi.org/10.1039/b817097e (2009). Article CAS PubMed Google Scholar * Middleton, D. R. S.
_et al_. Environmental Science Processes & Impacts supplies: toenail, hair and drinking water. _Environmental Science: Processes & Impacts_ 18, 562–574,
https://doi.org/10.1039/C6EM00072J (2016c). Article CAS Google Scholar * Young, V. R., Lofgreen, G. P. & Luick, J. R. The effects of phosphorus depletion, and of calcium and
phosphorus intake, on the endogenous excretion of these elements by sheep. _BY. J. Nub_ 20, 795–805, https://doi.org/10.1079/BJN19660081 (1966). Article CAS Google Scholar * Species 360:
Zoological Information Management System (ZIMS) 2019. www.zims.Species360.org. Accessed Jan 2020. * Watts, M. J. _et al_. Source apportionment of micronutrients in the diets of Kilimanjaro,
Tanzania and Counties of Western Kenya. _Scientific Reports_ 9, 14447 (2019b). Article ADS PubMed Central PubMed Google Scholar * Phiri, F. P. _et al_. The risk of selenium deficiency
in Malawi is large and varies over multiple spatial scales. _Scientific Reports_ 9, 6566, https://doi.org/10.1038/s41598-019-43013-z (2019). Article ADS CAS PubMed PubMed Central Google
Scholar * Delanghe, J. R. & Speeckaert, M. M. Creatinine determination according to Jaffe - What does it stand for? _NDT Plus_ 4, 83–86, https://doi.org/10.1093/ndtplus/sfq211 (2011).
Article PubMed PubMed Central Google Scholar * Blanc J. 2008. Loxodonta africana. The IUCN Red List of Threatened Species., http://www.iucnredlist.org/details/12392/0 accessed May 24,
2018. * Sukumar R. The Asian elephant. Cambridge University Press (1989). * Dierenfeld, E. S. 2017. Zootrition. * Ullrey D., Crissey S. & Hintz H. Elephants: nutrition and dietary
husbandry. In: Allen M, Edwards M, Roocroft A eds. Nutrition Advisory Group Handbook. 1–20 (1997). * R Core Team. R: A language and environment for statistical computing. R Foundation for
Statistical Computing. (2017). www.rstudio.com Accessed February 2020. * Pinheiro, J., Bates, D., DebRoy, S., Sarkar, D. & Core Team, R. nlme: Linear and Nonlinear Mixed Effects Models.
_R package version_ 3, 1–131 (2017). Google Scholar * Verbeke G. & Molenberghs G. Linear Mixed Models for Longitudinal Data. _New York_ (2000). * Benjamini, Y. & Hochberg, Y.
Controlling the false discovery rate: a practical and powerful approach to multiple testing. _Journal of the Royal Statistical Society Series B_ 57, 289–300 (1995). MathSciNet MATH Google
Scholar * Foster, D. & Stine, R. A. 𝛼 investing: a procedure for sequential control of expected false discoveries. _Journal of the Royal Statistical Society B_ 70, 429–444 (2008).
Article MathSciNet Google Scholar * Lark, R. Controlling he marginal false discovery rate in inferences from a soil dataset with 𝛼-investment. _European Journal of Soil Science_ 68,
221–23. (2017). Article CAS Google Scholar * Aharoni, E. & Rosset, S. Generalized 𝛼-investing: definitions, optimality results and application to public databases. _Journal of the
Royal Statistical Society B_ 76, 771–794 (2014). Article MathSciNet Google Scholar * Hill, W. & Thompson, R. Probabilities of Non-Postive Definite Between-Group or Genetic Covariance
Matrices. _Biometrics_ 34, 429–439 (1978). Article Google Scholar * World Health Organisation 2017.Guidelines for drinking-water quality, 4th edition, incorporating the 1st addendum.,
https://www.who.int/water_sanitation_health/publications/drinking-water-quality-guidelines-4-including-1st-addendum/en/ Accessed Febuary 2020. * G-Base, Avaliable at,
https://www.bgs.ac.uk/gbase/home.html. Accessed November 2019. * Wittemyer, G., Cerling, T. & Douglas-Hamilton, I. Establishing chronologies from isotopic profiles in serially collected
animal tissues: An example using tail hairs from African elephants. _Chemical Geology_ 267(1-2), 3–11 (2009). Article ADS CAS Google Scholar * Geyer H. & Benz A. The elephant’s hoof:
Macroscopic and microscopic morphology of defined locations under consideration of pathological changes. Inaugural-Dissertation, Zurich (2005). * Satia, A., King, I., Morris, J., Stratton,
K. & White, E. Toenail and Plasma Levels as Biomarkers of Selenium Exposure. _Annals of Epidemiology. Annals of Epidemiology_ 16, 53–58 (2006). Article PubMed Google Scholar *
Dierenfeld, E. S. & Dolensek, E. P. Circulating levels of vitamin E in captive Asian elephants (Elephas maximus). _Zoo Biology_ 7, 165–172, https://doi.org/10.1002/zoo.1430070210 (1988).
Article CAS Google Scholar * Anon 2017 DEFRA Secretary of State’s Standards of Modern Zoo Practice Appendix 8 – Specialist exhibits, Elephants.,
https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/654713/zoo-practice-elephants.pdf (accessed July 19, 2018). * Beal, T., Massiot, E.,
Arsenault, J. E., Smith, M. R. & Hijmans, R. J. Global trends in dietary micronutrient supplies and estimated prevalence of inadequate intakes. _PLoS One_ 12(4), e0175554,
https://doi.org/10.1371/journal.pone.0175554 (2017). Article CAS PubMed PubMed Central Google Scholar * Clauss, M., Loehlein, W., Kienzle, E. & Wiesner, H. Studies on feed
digestibilities in captive Asian elephants (Elephas maximus). _Journal of animal physiology and animal nutrition_ 87, 160–173, https://doi.org/10.1046/j.1439-0396.2003.00429.x (2003).
Article CAS PubMed Google Scholar * Partington C. 2012. MSc Thesis: Feeding, nutrition and body condition of UK elephants. University of Liverpool. * Sach F., Tatchley C., Needham N.
& Pullen K. Guidelines for the Management of Elephants within BIAZA Zoos 4th edition Incorporating BIAZA’s Policy on the Management of Elephants (2019c). * Harris M., Sherwin C., Harris
S. 2008. The welfare, housing and husbandry of elephants in UK zoos, https://www.idausa.org/wp-content/uploads/2013/05/U-of-Bristol-Report.pdf accessed Feburary 2020 * Meehan,C. L., Mench,
J. A., Carlstead, K. & Hogan, J. N. Determining connections between the daily lives of zoo elephants and their welfare: An epidemiological approach. _PLoS One_.
https://doi.org/10.1371/journal.pone.0158124 (2016). Download references ACKNOWLEDGEMENTS The authors would like to thank all the keepers, veterinary staff and research staff at the five UK
zoos who assisted with collecting samples from the elephants for this study; Knowsley Safari, Colchester Zoo, Noah’s Ark Zoo Farm, Twycross Zoo and ZSL Whipsnade Zoo. Additionally, Amanda
Gardner, John Wheeler, Lee Evans and Sophia Dowell at the British Geological Survey and Stephanie Taylor from the University of Nottingham for their assistance with sample preparation and
laboratory analysis. This work was supported by the Natural Environment Research Council (grant number NE/L002604/1) through the Envision Doctoral Training Partnership. Envision DTP is a
consortium consisting of Bangor University, British Geological Survey, Centre for Ecology and Hydrology, Lancaster University, Rothamsted Research and the University of Nottingham.
Additionally, the British Geological Survey University Funding Initiative (BUFI) supported the work. The funders had no role in study design, data collection and analysis, decision to
publish or preparation of the manuscript.The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. The authors disclosed
the following grant information: Natural Environment Research Council: NE/L002604/1. British Geological Survey University Funding Initiative (BUFI). AUTHOR INFORMATION AUTHORS AND
AFFILIATIONS * Inorganic Geochemistry, Centre for Environmental Geochemistry, British Geological Survey, Nicker Hill, Keyworth, Nottingham, United Kingdom Fiona Sach, Elliott Hamilton, Lisa
Yon & Michael J. Watts * School of Biosciences, University of Nottingham, Sutton Bonington, United Kingdom Fiona Sach, Simon C. Langley-Evans, Elliott Hamilton & R. Murray Lark *
Ellen S. Dierenfeld, LLC, Saint Louis, MO, 63128, USA Ellen S. Dierenfeld * School of Animal, Rural & Environmental Sciences, Nottingham Trent University, Southwell, United Kingdom Ellen
S. Dierenfeld * School of Veterinary Medicine and Science, University of Nottingham, Sutton Bonington, United Kingdom Lisa Yon Authors * Fiona Sach View author publications You can also
search for this author inPubMed Google Scholar * Ellen S. Dierenfeld View author publications You can also search for this author inPubMed Google Scholar * Simon C. Langley-Evans View author
publications You can also search for this author inPubMed Google Scholar * Elliott Hamilton View author publications You can also search for this author inPubMed Google Scholar * R. Murray
Lark View author publications You can also search for this author inPubMed Google Scholar * Lisa Yon View author publications You can also search for this author inPubMed Google Scholar *
Michael J. Watts View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Fiona Sach Project administration, Formal analysis, Investigation, Writing
- Original Draft, Visualization, Project administration. Ellen S. Dierenfeld Conceptualization, Writing - Review & Editing. Simon C Langley-Evans Writing - Review & Editing. Elliott
Hamilton Validation, Investigation. R. Murray Lark Methodology, Software, Formal analysis. Lisa Yon Conceptualization, Writing - Review & Editing. Michael J Watts Funding acquisition,
Conceptualization, Resources, Writing - Review & Editing. CORRESPONDING AUTHOR Correspondence to Michael J. Watts. ETHICS DECLARATIONS COMPETING INTERESTS Ellen Dierenfeld is employed by
Ellen S. Dierenfeld, LLC. Fiona Sach is employed by the Zoological Society of London. Simon Langley-Evans, Michael Watts, R.Murray Lark, Elliott Hamilton and Lisa Yon have no competing
interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY
INFORMATION SUPPLEMENTARY INFORMATION. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing,
adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons
license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a
credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted
use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT
THIS ARTICLE CITE THIS ARTICLE Sach, F., Dierenfeld, E.S., Langley-Evans, S.C. _et al._ Potential bio-indicators for assessment of mineral status in elephants. _Sci Rep_ 10, 8032 (2020).
https://doi.org/10.1038/s41598-020-64780-0 Download citation * Received: 22 November 2019 * Accepted: 22 April 2020 * Published: 15 May 2020 * DOI: https://doi.org/10.1038/s41598-020-64780-0
SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy
to clipboard Provided by the Springer Nature SharedIt content-sharing initiative