Play all audios:
ABSTRACT Reproductive investment generally involves a trade-off between somatic growth and energy allocation for reproduction. Previous studies have inferred that jumbo squid _Dosidicus
gigas_ support growth during maturation through continuous feeding (an “income” source). However, our recent work suggests possible remobilization of soma during maturation (a “capital”
source). We used fatty acids as biochemical indicators to investigate energy acquisition and allocation to reproduction for female _D. gigas_. We compared the fatty acid profiles of the
ovary to those of the mantle muscle (slow turnover rate tissue, representing an energy reserve) and the digestive gland (fast turnover rate organ, reflecting recent consumption). For each
tissue, the overall fatty acids among maturity stages overlapped and were similar. The changes with maturation in fatty acid composition in the ovary consistently resembled those of the
digestive gland, with the similarity of fatty acids in the mantle muscle and the ovary increasing during maturation, indicating some energy reserves were utilized. Additionally, squid
maintained body condition during maturation regardless of increasing investment in reproduction and a decline in feeding intensity. Cumulatively, _D. gigas_ adopt a mixed income-capital
breeding strategy in that energy for reproduction is mainly derived from direct food intake, but there is limited somatic reserve remobilization. SIMILAR CONTENT BEING VIEWED BY OTHERS
SIZE-DEPENDENT RESOURCE ALLOCATION TO REPRODUCTION IN JAPANESE ANCHOVIES (_ENGRAULIS JAPONICUS_) Article Open access 29 April 2025 FASTER JUVENILE GROWTH PROMOTES EARLIER SEX CHANGE IN A
PROTANDROUS HERMAPHRODITE (BARRAMUNDI _LATES CALCARIFER_) Article Open access 26 January 2021 FEMALES INCREASE REPRODUCTIVE INVESTMENT WHEN MATED TO LESS SEXUALLY ATTRACTIVE MALES IN A
SERIALLY MONOGAMOUS FISH Article Open access 16 August 2024 INTRODUCTION Life-history theory predicts that individuals should trade-off energy allocation between reproduction and somatic
growth or even survival to maximize lifetime reproductive success1. Squids are characterized by short lifespan, fast growth and considerable flexibility in reproductive characteristics2,3.
Although reproduction is typically semelparous, some species spawn multiple times and others continuously2, and reproductive behavior relates to how energy is allocated to reproduction
during maturation4,5,6,7. For example, the deep-sea squid _Onykia ingens_ reduces somatic growth by utilizing mantle muscle as an energy source to fuel reproduction, which then results in
ovarian development for a terminal spawning event6. In contrast, the purpleback squid _Sthenoteuthis oualaniensis_ apparently supports reproduction using energy acquired directly from food
intake, leading to multiple spawning events and continuous growth before death4. The former is referred to as a capital breeder, and the latter as an income breeder8. Since how energy is
allocated during life is central to life-history theory1, an optimal trade-off between investment in reproduction and somatic growth has been found to maximize reproductive success, and
ultimately determine population size and stability over time9,10,11. The jumbo squid, _Dosidicus gigas_ is one of the most abundant nektonic squid in the eastern Pacific12, as well as the
target species of major cephalopod fisheries13. It plays an important role in pelagic ecosystems locally14, not only because it preys on a wide spectrum of organisms during ontogenesis, but
also because it is prey for other predators, including marine mammals15,16. Similar to other squid species, _D. gigas_ is short-lived, usually 1–2 years17,18, with a single reproductive
episode and multiple spawning events19,20,21. _D. gigas_ also responds to environmental conditions22,23, which influences how it invests in reproductive development, and hence annual
variation of recruitment biomass24,25. Rocha _et al_.2, Nigmatullin and Markaida19 and Hernández-Muñoz _et al_.20 suggested that energy allocation to reproduction in _D. gigas_ is directly
derived from the intake of food, evidenced by the multiple spawning events, non-stop feeding and somatic growth in adults between egg batches. However, recent work by Han _et al_.26 on body
condition and reproductive investment, involving estimates of the gonadosomatic index and the residuals of a regression of gonad weight on mantle length, suggested that _D. gigas_ may also
use energy reserves to support reproductive growth. Fatty acid analyses have been used widely to infer dietary history and trophic ecology for marine species27,28,29, and to some extent, to
provide information on how energy is acquired and allocated to tissue types30,31. In marine environments, many fatty acids, particularly polyunsaturated fatty acids, can be biosynthesized by
certain phytoplankton and microalgae species32. In contrast, marine animals are subject to biochemical limitations in biosynthesis and modification of fatty acids, and directly assimilate
dietary fatty acids in their basic form without modification33,34,35,36. In cephalopods, the digestive gland is important for digestion and absorption37,38, and has a fast turnover of
dietary fatty acids, reflecting more recent food intake (10–14 days39,40), and is hence considered a good indicator of nutritional status due to the high lipid concentration41. In contrast,
tissues such as the mantle muscle are considered the most important energy reserve, with a slower fatty acid turnover rate, that reflects diet over a longer period of time (~4 weeks or
longer39). Thus, whether gonads are formed using income sources or energy stored in the somatic tissues can be evaluated by comparing fatty acid profiles of these fast and slow turnover
tissues with those of the gonads42. This comparison could also reveal whether individuals change feeding habits with maturation to obtain greater energy for reproduction43. Following Lin _et
al_.42 who used fatty acids as biomarkers to determine the mixed income-capital breeding strategy for the female Argentinean shortfin squid _Illex argentinus_, we used fatty acids to
investigate the breeding strategy of female _D. gigas_ with respect to energy acquisition and allocation. More specifically, we analyzed fatty acids in the digestive gland, the mantle muscle
and the ovary to (a) assess whether _D. gigas_ shifts its diet to acquire more energy with maturation, (b) determine the pathway of energy sources for reproduction, and (c) justify whether
the energy reserve in the somatic tissues are used for reproduction. The results of this work will lead to a better understanding of the breeding strategy of _D. gigas_, and also further
support the use of fatty acids to study energy allocation to reproduction for oceanic squid and other species. RESULTS FATTY ACIDS WITHIN TISSUES Twenty-eight fatty acids were found in
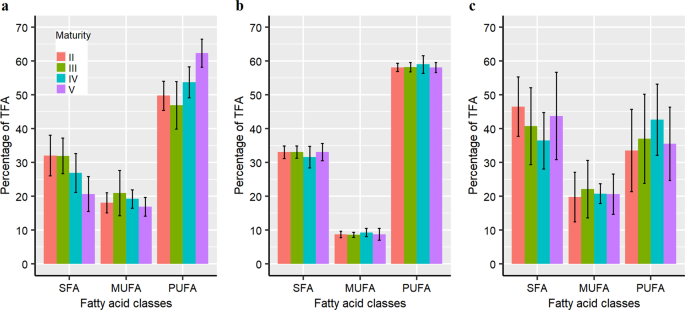
female _D. gigas_, of which 19 had relative mean values greater than 0.5% and in total made up 92–98% of total fatty acids (Table 1). For each tissue, most of the saturated fatty acid (SFA)
content was 16:0 and 18:0, most of the monosaturated fatty acid (MUFA) content 18:1n9c and 20:1, and most of the PUFA content 20:5n3 and 22:6n3 (Table 1). The total fatty acid content was
higher for functionally mature animals in all tissues analysed, with the highest values consistently observed in the digestive gland (Supplementary Tables 1–3). SFA content was significantly
lower and PUFA significantly higher in the ovaries of mature animals (stages IV and V) than in those of immature animals (stages II and III) (SFA _F_ = 5.19, _P_ = 0.008; PUFA _F_ = 9.29,
_P_ = 0.0005; Fig. 1a). The higher PUFA content in the ovary of mature animals is expected given it is essential for egg and larval quality35,44,45. However, no individual fatty acid in the
ovary differed significantly between animals at different maturity stages (Supplementary Tables 1 and 5). There were no significant differences in the proportion of the main fatty acid
classes (SFA, MUFA and PUFA), nor in the relative amount of each fatty acid except 20:5n3, between maturity stages in the mantle muscle (Fig. 1b; Supplementary Tables 2 and 5). Similarly,
the content of SFA and PUFA in the digestive gland was found vary, but not differ significantly, among maturity stages (SFA _F_ = 1.05, _P_ = 0.39; PUFA _χ_2 = 1.81, _P_ = 0.61; Fig. 1c,
Supplementary Table 5), and MUFA content and the relative amount of each fatty acid were also not significantly different among maturity stages (Supplementary Tables 3 and 5). Multivariate
analyses revealed considerable overlap in the overall fatty acids between maturity stages for each tissue (Fig. 2). A small but insignificant difference was detected for overall fatty acids
between the ovaries of the four maturity stages (ANOSIM _R_-value = 0.11, _P_ = 0.07), but no significant differences were found for the mantle muscle (ANOSIM _R_-value = 0.04, _P_ = 0.28)
and the digestive gland (ANOSIM _R_-value = 0.02, _P_ = 0.36). SIMILARITY OF FATTY ACID COMPOSITION BETWEEN TISSUES Paired Tests revealed that 11, 9 and 13 of the comparisons of the relative
amount of each fatty acid between the ovary and the mantle muscle, between the ovary and the digestive gland, and between the mantle muscle and the digestive gland, respectively were
significant for animals at maturity stage II (Supplementary Table 7). These numbers were 9, 7 and 12 for animals at maturity stage III (Supplementary Table 9), 6, 5 and 4 for animals at
maturity stage IV (Supplementary Table 11), and 5, 4 and 7 for animals at maturity stage V (Supplementary Table 13). Multivariate analyses revealed that the fatty acid profiles for the ovary
overlapped those for the digestive gland (ANOSIM _R_-value = 0.32, _P_ = 0.001), but not those for the mantle muscle (ANOSIM _R_-value = 0.54, _P_ = 0.001), and that changes in the fatty
acid composition for the ovary and the digestive gland showed a similar dispersed distribution pattern compared to a concentrated pattern for the mantle muscle (Fig. 3). There is greater
similarity in fatty acid compositions between the ovary and the digestive gland during physiological maturation (stage III, _R_-value = 0.26, _P_ = 0.012) and for physiologically mature
animals (stage IV, _R_-value = 0.20, _P_ = 0.026). This is also the case for the ovary and the mantle muscle, even though the extent of similarity was consistently less than that between the
ovary and the digestive gland (Table 2). The extent of dissimilarity between the mantle muscle and the digestive gland was less for physiologically mature animals than animals at other
maturity stages (Table 2). BODY CONDITION, GONADOSOMATIC INDEX AND FEEDING INTENSITY There was a significant positive correlation between body weight excluding ovary weight and mantle length
(log(_BW-OvaW_) = −7.18 + 2.43 × log(_ML_); _r_2 = 0.90, _P_ = 7.08e-13). Most functionally mature individuals (stage V) were heavier for a given length (Fig. 4a). Consequently, significant
differences in body condition (represented by the residuals of body weight excluding ovary weight regressed on the mantle length) were found between maturity stages (ANOVA, _F_ = 6.67, _P_
= 0.003; Fig. 4b). The gonadosomatic index (GSI) increased significantly following maturation (K-W test, _χ_2 = 19.05, _P_ = 0.0002; II: 0.58 ± 0.17 (range 0.39–0.92); III: 1.12 ± 0.52
(range 0.67–1.81); IV: 2.86 ± 1.69 (range 1.63–5.84); V: 11.31 ± 6.57 (range 4.25–19.00)). There was a weak but significant correlation between GSI and body condition (GSI = 2.43 + 3.54 ×
BC; _r_2 = 0.41, _P_ = 0.0004; Fig. 5a), suggesting that reproductive allocation is higher when animals are heavier than expected given their lengths. In contrast, the digestive gland index
(DGI) was lower following maturation (ANOVA, _F_ = 4.64_, P_ = 0.012; II: 7.28 ± 1.17 (range 5.68–9.16); III: 7.41 ± 3.36 (range 3.25–11.93); IV: 4.72 ± 0.78 (range 3.82–5.68); V: 3.75 ±
2.00 (range 1.96–6.94)). DGI was also negatively related to body condition (DGI = 6.41–1.29 × BC; _r_2 = 0.21, _P_ = 0.014; Fig. 5b). These observations indicate that individuals reduce
feeding prior to reproduction, but maintain body condition. DISCUSSION Data on fatty acids show that _D. gigas_ preys on similar organisms during ontogeny, given the same pattern of fatty
acid profiles for animals at different maturity stages in all tissues. Energy for reproduction appears to be driven primarily by concurrent food intake, since the changes in fatty acids in
the ovary closely resemble those in the digestive gland. The fatty acid compositions of the ovary and the mantle muscle change similarity with maturation, most notably from the developing to
physiologically mature stages, indicating that energy reserves are also involved in reproduction. Female _D. gigas_ appear to maintain somatic fitness, although the investment in
reproduction increases with maturation along with a major reduction in feeding intensity. As such, female _D. gigas_ adopt a mixed income-capital breeding strategy, which mostly relies on
continual food intake, coupled with the limited use of stored energy during sexual maturation. Of the tissues analysed in female _D. gigas_, the digestive gland has the greatest fatty acid
content regardless of maturity stage (Table 1, Supplementary Tables 1–3). However, the predominant fatty acids in the main fatty acid classes (SFA, MUFA, PUFA) are similar for each tissue,
with 16:0 and 18:0 most prevalent in SFA, 18:1n9c and 20:1 most prevalent in MUFA, and 20:5n3 and 22:6n3 most prevalent in PUFA (Table 1). The most prevalent fatty acids may be the result of
the fatty acid levels of the diet sources given the limited capacity for biosysnthesis of fatty acids33,34,36. On the other hand, these oberservations are in accordance with the findings of
Saito _et al_.46 and Gong _et al_.47, and are also very similar to the results of studies for other squids such as _Loligo vulgaris_48 and _Todarodes filippovae_49. This may imply that
these fatty acids are the common nutrients for squids, presumably owing to their important roles in cell and organelle function31,50,51, as well as energy sources for rapid growth and
development41,52,53. The significantly lower SFA content in the ovaries of mature animals could be due to energy mobilization for reproductive growth53,54. Studies on trophic relationships
have shown that many species, including cephalopods change feeding habits with increasing size or during maturation to maximize energy intake, enhance growth rate and minimize the risk of
predation55,56,57,58,59,60. In the present study, however, the female _D. gigas_ appear to prey on similar prey items before and after maturation because no significant differences were
found in the relative abundance of each fatty acid among maturity stages for the ovary, mantle muscle (except 20:5n3) and digestive gland (Supplementary Table 5). Further evidence is
provided by the clear overlap and similarity of the overall fatty acid profiles between maturity stages for each tissue (Fig. 2). These findings indicate that the female _D. gigas_ may adopt
a foraging strategy that focuses on the amount and not quality of food, which is not unexpected, as squids are well known for their voracious and opportunistic feeding3,12. Although squids
seem to become more active and successful predators as they mature3, their energy expenditure is higher given the need for increased metabolism with maturation for basic maintenance,
predation and reproductive growth61,62. Preying on species that are caught more easily may be a successful tactic to balance energy expenditure during the period of reproduction when energy
requirements are relatively high6,7,26,42. Indeed, studies have showed that squids including _D. gigas_ are opportunitistic predators at all maturity stages12,63,64, presumably related to
their “live for today” lifestyle10. Among the tissues analysed, consistently fewer fatty acids differed significantly between the ovary and the digestive gland than between the ovary and the
mantle muscle for any maturity stage (Supplementary Tables 7, 9, 11 and 13), and this was supported by the multivariate analyses (Fig. 3, Table 2). These lines of evidence indicate that
there is an energy trade-off between gonad development and resource uptake, with the energy sources for reproduction derived primarily from concurrent intake of prey. The similar despersed
distribution pattern of the fatty acid composition for the ovary and the digestive gland revealed by the NMDS analyses (Fig. 3) might indirectly provide futher evidence of energy allocation
to reproduction acquired directly from food intake, as the fatty acids in the digestive gland reflect the corresponding diets within a more recent period of 10–14 days39,40. Furthermore, the
fatty acid composition between the ovary and the digestive gland is more similar for mature animals (particularly those that are physiologically mature) (Table 2), suggesting an increase in
energy allocation to reproduction from food intake, which is consistent with the gonadosomatic index (GSI) being significantly higher for mature animals (K-W test, _χ_2 = 19.05, _P_ =
0.0002). It is worth noting that the fatty acid composition in the ovary and the digestive gland appears to vary at the individual level, especially for the ovary at the functionally mature
stage (Fig. 3). A possible reason for this is the fact that squids prey on a wide spectrum of prey items12,63,64. Variation in the fatty acids of the ovary at the mature stage may be also
related to the accumulation of essential fatty acids such as long-chain polyunsaturated fatty acids for egg quality35,44,45 and possible remobilization of short-chain saturated fatty acids
for energy use53,54, since the ovary showed a significant increase of PUFA content and decrease of SFA content with maturation (Fig. 1). However, future studies on the specific fatty acid
requirements for gonad development are needed to address these hypotheses. Reproduction generally constitutes a major fraction of the total energy budget of an adult organism1, and gonad
development in many organisms is fuelled by increased food intake as well as mobilization of previously stored reserves65. In the present study, although the fatty acid composition in the
ovary differed from that in the mantle muscle (Fig. 3), the similarity in fatty acid composition between these two tissues increased from the developing to physiologically mature stage
(Table 2). Meanwhile, the significant reduction of the digestive gland index (ANOVA, _F_ = 4.64_, P_ = 0.012), an index of feeding activity38,66, for mature females suggested a possible
reduction in feeding intensity during maturation. It is therefore reasonable to expect that mature female _D. gigas_ remobilize some of their somatic reserve to provide energy for
reproduction. The energy remobilization of somatic reserves for reproduction is limited and probably only occurs as a complementary source during maturation when the development of the
reproductive organs is significant. This is because the dissimilarity (represented by the ANOSIM _R_-value) in the fatty acid composition between the ovary and the mantle muscle is larger
than that between the ovary and the digestive gland (_R_-value, 0.54 _vs_. 0.32). Meanwhile, female _D. gigas_ have better body condition when mature (Fig. 4B), indicating that the adults
have not used up much somatic tissue. Further, the animals with higher reproductive investment appeared to be in good condition (Fig. 5A), although they fed less given the negative
relationship between body condition and the digestive gland index (Fig. 5B). These lines of evidence suggest that female _D. gigas_ maintain somatic fitness even if some energy reserves are
mobilized. Indeed, the fatty acid composition in the mantle muscle more closely resembles that of the digestive gland for mature animals (Table 2), suggesting that the somatic tissues
continue to incorporate nutrients from feeding. This is in stark contrast to species with synchronous ovarian development, such as _O. ingens_ that mobilizes much of its somatic tissues to
support reproduction6. The pattern of limited use of somatic reserve for reproduction may be an evolutionary tactic to adapt to the asynchronous ovarian development of _D. gigas_2,19, as the
maintenance of somatic condition appears to be important for this species to develop the multiple cohorts of oocytes during the protracted spawning period20,21. CONCLUSIONS Female _D.
gigas_ feed on similar prey items during ontogeny, and adopt a mixed income-capital breeding strategy, in which energy for reproduction is mainly derived from direct feeding, coupled with
limited mobilization of somatic energy. The results confirm the recent suggestion by Han _et al_.26 that the energy reserves in the somatic tissues are remobilized to support reproduction
during maturation. The energy trade-off between reproduction and limited use of energy reserve warrants further research to better understand the life-history strategy of _D. gigas_ in terms
of energy acquisition and allocation both within and across taxa. This study could also contribute to the use of fatty acids as biochemical markers to identify the breeding strategy for
oceanic squids as suggested by Lin _et al_.42. METHODS ETHICS STATEMENT Specimens were collected as dead squids from the commercial jigging fisheries landings, during the fishing season from
June to August 2017. The specimens were analyzed in the laboratory using methods that are in line with current Chinese national standards, namely Laboratory Animals - General Requirements
for Animal Experiment (GB/T 35823-2018). As all material sampled in this work was obtained from commercial fishermen and already dead, there was no requirement for ethical approval of
sampling protocols as it did not include live organisms. SAMPLE COLLECTION Samples were collected from the landings from commercial jig fishery in the eastern Pacific (longitude:
84°07′W~102°27′W, latitude: 00°47′S~08°26′S), from June to August 2017. These were immediately frozen at −30 °C for further analyses in the laboratory. A total of 24 females (14 immature and
10 mature) were randomly selected for the following fatty acids analyses after defrosting at room temperature in the laboratory. Each specimen was assigned a maturity stage following the
scheme proposed by Arkhipkin67, Arkhipkin and Laptikhovsky68 and ICES69, with maturity stages: I immature, II developing, III physiologically maturing, IV physiologically mature, V
functionally mature, VI spawning, and VII spent. Specimens were in maturity stages II to V (Table 3). Specimens at maturity stages II and III were categorized as immature, and specimens at
maturity stages IV and V as mature. The following parameters were also recorded for each specimen: mantle length (ML, mm), body weight (BW, g), ovary weight (OvaW, g) and digestive gland
weight (DgW, g) (Table 3). The gonadosomatic index (GSI; ovary weight/body weight × 100) and the digestive gland index (DGI; digestive gland weight/body weight × 100) were also determined
for each specimen38,70. The ventral mantle muscle (~10.0 g), whole gonad and whole digestive gland were collected for each individual, and separately lyophilized to a constant weight in a
freeze-drying system (Christ Alpha 1–4/LDplus). The digestive gland is a site of digestive absorption and intracellular digestion37,38, and deposits recent intake of dietary fatty acids
(10–14 days) without modification36,39,40,71. The mantle muscle is the most important energy reserve organ7,42,72, and reflects dietary information over a time scale of 4 weeks or
longer39.The dried tissues were ground to fine powder individually, and a 0.2 g subsample was used for fatty acid analysis. FATTY ACID ANALYSES Fatty acid methyl esters (FAME) were analyzed
for each tissue sample of each specimen following the “Determination of total fat, saturated fat, and unsaturated fat in foods - Hydrolytic extraction-gas chromatography” protocol73. Lipids
were extracted by using a mixture of chloroform and methanol 2:1 (v/v)74. To esterify the fatty acids, lipids were introduced into a 25 mL vial with 4 mL of 0.5 mol/L KOH-MeOH, which was
incubated at 90 °C for 10 minutes, shaking for 5 seconds every 2 minutes. Then, 4 ml BF3/MeOH was added and the sample incubated at 90 °C for 30 minutes, shaking for 5 seconds every 5
minutes, followed by the addition of 4 mL n-Hexane for 2 minutes incubation at a similar temperature. Thirdly, 10 mL saturated NaCl was added and shaken gently, followed by introduction into
a 20 mL centrifuge tube for stratification at room temperature. Finally, the upper hexane layer, which contained the FAME, was transferred to a vial, and evaporated under nitrogen current
with 19:0 as an internal standard. Fatty acids were determined using an Agilent 7890B Gas Chromatography (GC) coupled to a 5977 A series Mass Spectrometer Detector (MSD, Agilent
Technologies, Inc. USA), equipped with a fused silica 60 m × 0.25 nm open tubular column (HB-88: 0.20 μm, Agilent Technologies, Inc. USA). The separation was carried out with helium as the
carrier gas, and a thermal gradient programed from 125 °C to 250 °C, with the auxiliary heater at 280 °C. Individual fatty acid peaks were identified by comparing their retention times with
those of chromatographic Sigma standards. Total fatty acids (total FAs) were determined as mg/g, and individual fatty acids were expressed as percentages of total fatty acids (% of total
fatty acids)36. The individual fatty acids were also grouped into saturated fatty acids (SFA), monounsaturated fatty acids (MUFA), and polyunsaturated fatty acids (PUFA). Fatty acids that
accounted for <0.5% were excluded from statistical analyses. STATISTICAL ANALYSIS The results were expressed as means ±standard deviation. The fatty acid data for each tissue were checked
for normality using the one-sample Kolmogorov-Smirnov test75 (Supplementary Tables 4, 6, 8, 10 and 12). Thereafter, one-way analysis of variance (ANOVA) was used to detect significant
differences in the means of the main fatty acid classes (SFA, MUFA, PUFA) and each fatty acid between maturity stages for each tissue75. When normality was rejected, the data were analyzed
using Kruskal-Wallis tests (K-W test)75. Paired _t_-Tests were used to investigate significant differences for each fatty acid within matched pairs of tissues given maturity stage, and
paired Wilcoxon tests were used when normality was rejected75. Non-metric multidimensional scaling (NMDS) and analysis of similarities (ANOSIM) were applied to assess the differences in the
overall fatty acid profiles between immature and mature stages for each tissue, and to determine the differences in the overall fatty acids between the ovary, the mantle muscle and the
digestive gland. These multivariate analyses of fatty acids have the advantage of pattern recognition28,76, and can be used to determine whether energy for reproduction is from energy
reserves (mantle tissue) or consumption of prey (digestive gland)42. The fatty acid data were square root transformed and Euclidean dissimilarity matrices were used in the NMDS and ANOSIM77.
NMDS and ANOSIM analyses were conducted using the vegan package in R78. The relationship between mantle length (ML) and body weight (BW) excluding ovary weight (OvaW) was examined after
log-transformation, and the standardized residuals of the regression used as an index of body condition6,22, where the residuals provide a size-independent measure of the somatic condition
of an individual at the whole animal level5,6. ANOVA was used to detect differences in the means of body condition, GSI and DGI between maturity stages, and these data were analyzed using
Kruskal-Wallis tests when the normality assumption was not satisfied75. To some extent, the GSI can be used as an indicator of reproductive investment5, while the DGI can be used as an
indicator of feeding intensity38. The linear relationships among body condition, GIS and DGI were investigated to assess the interactions between the soma reserve, reproduction and energy
acquisition. Statistical analyses were carried out using SPSS 20.0 and R version 3.5.078. A test was considered significant when _P_ < 0.05. DATA AVAILABILITY The biological measurement
data and biochemical data (fatty acids) that support the findings of this study are available from the Distant Squid Fisheries Sci-Tech Group (SHOU), but restrictions apply to the
availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and
with permission of the Distant Squid Fisheries Sci-Tech Group (SHOU). REFERENCES * Stearns, S. C. _The Evolution of Life Histories_. Vol. VII 249 (Oxford University Press, 1992). * Rocha,
F., Guerra, Á. & González, Á. F. A review of reproductive strategies in cephalopods. _Biol. Rev._ 76, 291–304, https://doi.org/10.1017/S1464793101005681 (2001). Article CAS PubMed
Google Scholar * Boyle, P. & Rodhouse, P. _Cephalopods: ecology and fisheries_. 464 (Wiley-Blackwell, 2005). * Harman, R. F. _et al_. Evidence for multiple spawning in the tropical
oceanic squid _Sthenoteuthis oualaniensis_ (Teuthoidea: Ommastrephidae). _Mar. Biol._ 101, 513–519, https://doi.org/10.1007/BF00541653 (1989). Article Google Scholar * McGrath, B. &
Jackson, G. Egg production in the arrow squid _Nototodarus gouldi_ (Cephalopoda: Ommastrephidae), fast and furious or slow and steady? _Mar. Biol._ 141, 699–706,
https://doi.org/10.1007/s00227-002-0864-z (2002). Article Google Scholar * Jackson, G. D., Semmens, J. M., Phillips, K. L. & Jackson, C. H. Reproduction in the deepwater squid
_Moroteuthis ingens_, what does it cost? _Mar. Biol._ 145, 905–916, https://doi.org/10.1007/s00227-004-1375-x (2004). Article Google Scholar * Moltschaniwskyj, N. A. & Carter, C. G.
The adaptive response of protein turnover to the energetic demands of reproduction in a cephalopod. _Physiological Biochemical Zool._ 86, 119–126, https://doi.org/10.1086/667799 (2013).
Article CAS Google Scholar * McBride, R. S. _et al_. Energy acquisition and allocation to egg production in relation to fish reproductive strategies. _Fish. Fish._ 16, 23–57,
https://doi.org/10.1111/faf.12043 (2015). Article Google Scholar * Boggs, C. L. Dynamics of reproductive allocation from juvenile and adult feeding: Radiotracer studies. _Ecology_ 78,
192–202, https://doi.org/10.1890/0012-9658(1997)078[0192:DORAFJ]2.0.CO;2 (1997). Article Google Scholar * Pecl, G. T. & Moltschaniwskyj, N. A. Life history of a short-lived squid
(Sepioteuthis australis): resource allocation as a function of size, growth, maturation, and hatching season. _ICES J. Mar. Sci._ 63, 995–1004, https://doi.org/10.1016/j.icesjms.2006.04.007
(2006). Article Google Scholar * Kuipers, M. R., Pecl, G. T. & Moltschaniwskyj, N. A. Batch or trickle: understanding the multiple spawning strategy of southern calamary, _Sepioteuthis
australis_ (Mollusca: Cephalopoda). _Mar. Freshw. Res._ 59, 987–997, https://doi.org/10.1071/MF07200 (2008). Article Google Scholar * Nigmatullin, C. M., Nesis, K. & Arkhipkin, A. A
review of the biology of the jumbo squid _Dosidicus gigas_ (Cephalopoda: Ommastrephidae). _Fish. Res._ 54, 9–19 (2001). Article Google Scholar * FAO. _The State of World Fisheries and
Aquaculture_ 2_016. Contributing to food security and nutrition for all_. 200 (FAO, 2016). * Rosas-Luis, R. _et al_. Importance of jumbo squid _Dosidicus gigas_ (Orbigny, 1835) in the
pelagic ecosystem of the central Gulf of California. _Ecol. Model._ 218, 149–161, https://doi.org/10.1016/j.ecolmodel.2008.06.036 (2008). Article Google Scholar * Field, J. C. _et al_.
Foraging ecology and movement patterns of jumbo squid (_Dosidicus gigas_) in the California Current System. _Deep-Sea Res. II_ 95, 37–51, https://doi.org/10.1016/j.dsr2.2012.09.006 (2013).
Article Google Scholar * Rosa, R. _et al_. In _Advances in Squid Biology, Ecology and Fisheries Part II - Oegopsid squids_ (eds Rui Rosa, Graham Pierce, & Ron O’Dor) Ch. VI, 169–206
(Nova Science Publishers, 2013). * Velázquez, C. Q., Herrera, A. H., Velázquez-Abunader, I. & Valencia, N. F. Maturation, Age, and Growth Estimation of the Jumbo Squid _Dosidicus gigas_
(Cephalopoda: Ommastrephidae) in the Central Region of the Gulf of California. _J. Shellfish. Res._ 32, 351–359, https://doi.org/10.2983/035.032.0214 (2013). Article Google Scholar * Hu,
G. _et al_. Age, growth and population structure of jumbo flying squid _Dosidicus gigas_ off the Peruvian Exclusive Economic Zone based on beak microstructure. _Fish. Sci._ 82, 597–604,
https://doi.org/10.1007/s12562-016-0991-y (2016). Article ADS CAS Google Scholar * Nigmatullin, C. M. & Markaida, U. Oocyte development, fecundity and spawning strategy of large
sized jumbo squid _Dosidicus gigas_ (Oegopsida: Ommastrephinae). _J. Mar. Biol. Assoc. UK_ 89, 789–801, https://doi.org/10.1017/S0025315408002853 (2009). Article Google Scholar *
Hernández-Muñoz, A. T., Rodríguez-Jaramillo, C., Mejía-Rebollo, A. & Salinas-Zavala, C. A. Reproductive strategy in jumbo squid _Dosidicus gigas_ (D’Orbigny, 1835): A new perspective.
_Fish. Res._ 173, 145–150, https://doi.org/10.1016/j.fishres.2015.09.005 (2016). Article Google Scholar * Pérez-Palafox, X. A. _et al_. Evidence of Iteroparity in Jumbo Squid _Dosidicus
gigas_ in the Gulf of California, Mexico. _J. Shellfish. Res._ 38, 149–162, https://doi.org/10.2983/035.038.0114 (2019). Article Google Scholar * Argüelles, J. & Tafur, R. New insights
on the biology of the jumbo squid _Dosidicus gigas_ in the Northern Humboldt Current System: Size at maturity, somatic and reproductive investment. _Fish. Res._ 106, 185–192,
https://doi.org/10.1016/j.fishres.2010.06.005 (2010). Article Google Scholar * Seibel, B. A. Environmental Physiology of the Jumbo Squid, _Dosidicus gigas_ (d’Orbigny, 1835) (Cephalopoda:
Ommastrephidae): Implications for Changing Climate. _Am. Malacological Bull._ 33, 161–173, https://doi.org/10.4003/006.033.0113 (2015). Article Google Scholar * Keyl, F., Argüelles, J.
& Tafur, R. Interannual variability in size structure, age, and growth of jumbo squid (_Dosidicus gigas_) assessed by modal progression analysis. _ICES J. Mar. Sci._ 68, 507–518,
https://doi.org/10.1093/icesjms/fsq167 (2011). Article Google Scholar * Ibáñez, C. M. _et al_. Population dynamics of the squids _Dosidicus gigas_ (Oegopsida: Ommastrephidae) and
_Doryteuthis gahi_ (Myopsida: Loliginidae) in northern Peru. _Fish Res_ 173, Part 2, 151–158, https://doi.org/10.1016/j.fishres.2015.06.014 (2016). * Han, F., Chen, X., Lin, D. & Xuan,
S. The body condition and reproductive investment of Dosidicus gigas in the equatorial waters of eastern Pacific Ocean. _Journal of Fisheries of China_, 10.11964/jfc. 20180611323 (2019). *
Graeve, M., Kattner, G., Wiencke, C. & Karsten, U. Fatty acid composition of Arctic and Antarctic macroalgae: indicator of phylogenetic and trophic relationships. _Mar. Ecol. Prog. Ser._
231, 67–74, https://doi.org/10.3354/meps231067 (2002). Article ADS CAS Google Scholar * Dalsgaard, J. _et al_. Fatty acid trophic markers in the pelagic marine environment. _Adv. Mar.
Biol._ 46, 225–340, https://doi.org/10.1016/S0065-2881(03)46005-7 (2003). Article PubMed Google Scholar * Sargent, J. R., Tocher, D. R. & Bell, J. G. The Lipids in _Fish Nutrition
(Third Edition)_ (ed. Halver, J. E.) 181–257 (Academic Press, 2003). * Lourenço, S. _et al_. Feeding Relationship between _Octopus vulgaris_ (Cuvier, 1797) Early Life-Cycle Stages and Their
Prey in the Western Iberian Upwelling System: Correlation of Reciprocal Lipid and Fatty Acid Contents. _Front. Physiol._ 8, 1–11, https://doi.org/10.3389/fphys.2017.00467 (2017). Article
Google Scholar * Roo, J. _et al_. Effects of supplementation of decapod zoea to Artemia basal diet on fatty acid composition and digestive gland histology in common octopus (_Octopus
vulgaris_) paralarvae. _Aquaculture Res._ 48, 633–645, https://doi.org/10.1111/are.12910 (2017). Article CAS Google Scholar * Pond, D. W., Bell, M. V., Harris, R. P. & Sargent, J. R.
Microplanktonic Polyunsaturated Fatty Acid Markers: a Mesocosm Trial. _Estuar. Coast. Shelf. Sci._ 46, 61–67, https://doi.org/10.1006/ecss.1998.0334 (1998). Article ADS CAS Google Scholar
* Sargent, J. R. _et al_. Requirement criteria for essential fatty acids. _J. Appl. Ichthyology_ 11, 183–198, https://doi.org/10.1111/j.1439-0426.1995.tb00018.x (1995). Article CAS
Google Scholar * Ackman, R. G. In _Lipids in Freshwater Ecosystems_ (eds Arts, M. T. & Wainman, B. C.) 263–298 (Springer New York, 1999). * Li, Y.-Y. _et al_. Effects of n-3 HUFA
content in broodstock diet on spawning performance and fatty acid composition of eggs and larvae in _Plectorhynchus cinctus_. _Aquaculture_ 245, 263–272,
https://doi.org/10.1016/j.aquaculture.2004.12.016 (2005). Article CAS Google Scholar * Iverson, S. J. Tracing aquatic food webs using fatty acids: from qualitative indicators to
quantitative determination in _Lipids in Aquatic Ecosystems_ (eds Martin Kainz, Michael T. Brett, & Michael T. Arts) 281–308 (Springer New York, 2009). * Semmens, J. M. Changes in the
digestive gland of the loliginid squid _Sepioteuthis lessoniana_ (Lesson 1830) associated with feeding. _J. Exp. Mar. Bio Ecol._ 274, 19–39, https://doi.org/10.1016/S0022-0981(02)00165-X
(2002). Article Google Scholar * Swift, K., Johnston, D. & Moltschaniwskyj, N. The digestive gland of the Southern Dumpling Squid (_Euprymna tasmanica_): structure and function. _J.
Exp. Mar. Bio Ecol._ 315, 177–186, https://doi.org/10.1016/j.jembe.2004.09.017 (2005). Article Google Scholar * Stowasser, G. _et al_. Experimental study on the effect of diet on fatty
acid and stable isotope profiles of the squid Lolliguncula brevis. _J. Exp. Mar. Bio Ecol._ 333, 97–114, https://doi.org/10.1016/j.jembe.2005.12.008 (2006). Article CAS Google Scholar *
Fluckiger, M. _et al_. An experimental study of the effect of diet on the fatty acid profiles of the European Cuttlefish (_Sepia officinalis_). _Mar. Biol._ 154, 363–372,
https://doi.org/10.1007/s00227-008-0932-0 (2008). Article CAS Google Scholar * García, S. _et al_. Growth, partial energy balance, mantle and digestive gland lipid composition of _Octopus
vulgaris_ (Cuvier, 1797) fed with two artificial diets. _Aquaculture Nutr._ 17, e174–e187, https://doi.org/10.1111/j.1365-2095.2009.00746.x (2011). Article Google Scholar * Lin, D., Han,
F., Xuan, S. & Chen, X. Fatty acid composition and the evidence for mixed income–capital breeding in female Argentinean short-fin squid _Illex argentinus_. _Mar. Biol._ 166, 90,
https://doi.org/10.1007/s00227-019-3534-0 (2019). Article Google Scholar * Meyer, L. _et al_. Abiotic and biotic drivers of fatty acid tracers in ecology: A global analysis of
chondrichthyan profiles. _Funct. Ecol._ 33(33), 1243–1255, https://doi.org/10.1111/1365-2435.13328 (2019). Article Google Scholar * Reis, D. B. _et al_. An insight on _Octopus vulgaris_
paralarvae lipid requirements under rearing conditions. _Aquaculture Nutr._ 21, 797–806, https://doi.org/10.1111/anu.12205 (2015). Article CAS Google Scholar * Garrido, D. _et al_. Fatty
acid composition and age estimation of wild _Octopus vulgaris_ paralarvae. _Aquaculture_ 464, 564–569, https://doi.org/10.1016/j.aquaculture.2016.07.034 (2016). Article CAS Google Scholar
* Saito, H., Sakai, M. & Wakabayashi, T. Characteristics of the lipid and fatty acid compositions of the Humboldt squid, _Dosidicus gigas_: The trophic relationship between the squid
and its prey. _Eur. J. Lipid Sci. Technol._ 116, 360–366, https://doi.org/10.1002/ejlt.201300230 (2014). Article CAS Google Scholar * Gong, Y. _et al_. A comparative analysis of fatty
acid profiles in muscle of _Dosidicus gigas_ from different harvest locations in the eastern Pacific Ocean. _Progress in Fishery_. _Sciences_ 39, 147–154,
https://doi.org/10.19663/j.issn2095-9869.20171208001 (2018). Article Google Scholar * Salman, Y., Salman, A. & Ozkizilcik, S. The fatty acid profile of the marine cephalopod _Loligo
vulgaris_. _Israeli Journal of Aquaculture-Bamidgeh_ 59, 133-136, http://hdl.handle.net/10524/19226 (2007). * Pethybridge, H. R., Nichols, P. D., Virtue, P. & Jackson, G. D. The foraging
ecology of an oceanic squid, _Todarodes filippovae_: The use of signature lipid profiling to monitor ecosystem change. _Deep-Sea Res. II_ 95, 119–128,
https://doi.org/10.1016/j.dsr2.2012.07.025 (2013). Article CAS Google Scholar * Navarro, J. C. & Villanueva, R. The fatty acid composition of _Octopus vulgaris_ paralarvae reared with
live and inert food: deviation from their natural fatty acid profile. _Aquaculture_ 219, 613–631, https://doi.org/10.1016/S0044-8486(02)00311-3 (2003). Article CAS Google Scholar *
Tocher, D. R. M. and Functions of Lipids and Fatty Acids in Teleost Fish. _Rev. Fish. Sci._ 11, 107–184, https://doi.org/10.1080/713610925 (2003). Article CAS Google Scholar * Ferreira,
A. _et al_. The use of alternative diets to culture juvenile cuttlefish, Sepia officinalis: effects on growth and lipid composition. _Aquaculture Nutr._ 16, 262–275,
https://doi.org/10.1111/j.1365-2095.2009.00661.x (2010). Article CAS Google Scholar * García-Garrido, S. _et al_. Lipid composition of the mantle and digestive gland of _Octopus vulgaris_
juveniles (Cuvier, 1797) exposed to prolonged starvation. _Aquacult Int._ 18, 1223–1241, https://doi.org/10.1007/s10499-010-9335-6 (2010). Article CAS Google Scholar * Sargent, J. _et
al_. Recent developments in the essential fatty acid nutrition of fish. _Aquaculture_ 177, 191–199, https://doi.org/10.1016/S0044-8486(99)00083-6 (1999). Article CAS Google Scholar *
Brown, J. A. The adaptive significance of behavioural ontogeny in some. centrarchid fishes. _Env. Biol. Fish._ 13, 25–34, https://doi.org/10.1007/bf00004853 (1985). Article Google Scholar
* Parry, M. Trophic variation with length in two ommastrephid squids, _Ommastrephes bartramii_ and _Sthenoteuthis oualaniensis_. _Mar. Biol._ 153, 249–256,
https://doi.org/10.1007/s00227-007-0800-3 (2008). Article Google Scholar * Paul, M. _et al_. Trophic Ecology of Eight Sympatric Nemipterid Fishes (Nemipteridae) in the Lower Part of the
South China Sea. _Turkish J. Fish. Aquat. Sci._ 18, 277–287, https://doi.org/10.4194/1303-2712-v18_2_07 (2018). Article Google Scholar * Ruiz-Cooley, R. I., Markaida, U., Gendron, D. &
Aguíñiga, S. Stable isotopes in jumbo squid (_Dosidicus gigas_) beaks to estimate its trophic position: comparison between stomach contents and stable isotopes. _J. Mar. Biol. Assoc. UK_
86, 437–445, https://doi.org/10.1017/S0025315406013324 (2006). Article Google Scholar * Ruiz-Cooley, R. I., Ballance, L. T. & McCarthy, M. D. Range Expansion of the Jumbo Squid in the
NE Pacific: δ15N Decrypts Multiple Origins, Migration and Habitat Use. _PLoS ONE_ 8, e59651, https://doi.org/10.1371/journal.pone.0059651 (2013). Article ADS CAS PubMed PubMed Central
Google Scholar * Gong, Y. _et al_. Sexual dimorphism in feeding apparatus and niche partitioning in juvenile jumbo squid _Dosidicus gigas_. _Mar. Ecol. Prog. Ser._ 607, 99–112,
https://doi.org/10.3354/meps12768 (2018). Article ADS CAS Google Scholar * Sokolova, I. M. _et al_. Energy homeostasis as an integrative tool for assessing limits of environmental stress
tolerance in aquatic invertebrates. _Mar. Environ. Res._ 79, 1–15, https://doi.org/10.1016/j.marenvres.2012.04.003 (2012). Article CAS PubMed Google Scholar * Boratyński, Z. Energetic
constraints on mammalian home range size. _Functional Ecology_ N/A, 1–7, https://doi.org/10.1111/1365-2435.13480 (2019). * Rosas-Luis, R., Sánchez, P., Portela, J. M. & del Rio, J. L.
Feeding habits and trophic interactions of _Doryteuthis gahi_, _Illex argentinus_ and _Onykia ingens_ in the marine ecosystem off the Patagonian Shelf. _Fish. Res._ 152, 37–44,
https://doi.org/10.1016/j.fishres.2013.11.004 (2014). Article Google Scholar * Rosas-Luis, R., Navarro, J., Martínez-Baena, F. & Sánchez, P. Differences in the trophic niche along the
gladius of the squids _Illex argentinus_ and _Doryteuthis gahi_ based on their isotopic values. _Regional Stud. Mar. Sci._ 11, 17–22, https://doi.org/10.1016/j.rsma.2017.02.003 (2017).
Article Google Scholar * Clarke, A., Rodhouse, P. G. & Gore, D. J. Biochemical Composition in Relation to the Energetics of Growth and Sexual Maturation in the Ommastrephid Squid
_Illex argentinus_. _Philos. Trans. R. Soc. Lond. B Biol. Sci._ 344, 201–212, https://doi.org/10.1098/rstb.1994.0061 (1994). Article ADS Google Scholar * Rosa, R., Costa, P. R. &
Nunes, M. L. Effect of sexual maturation on the tissue biochemical composition of _Octopus vulgaris_ and _O. defilippi_ (Mollusca: Cephalopoda). _Mar. Biol._ 145, 563–574,
https://doi.org/10.1007/s00227-004-1340-8 (2004). Article CAS Google Scholar * Arkhipkin, A. Reproductive system structure, development and function in cephalopods with a new general
scale for maturity stages. _J. Northwest. Atl. Fish. Sci._ 12, 63–74 (1992). Article Google Scholar * Arkhipkin, A. & Laptikhovsky, V. Seasonal and interannual variability in growth
and maturation of winter-spawning _Illex argentinus_ (Cephalopoda, Ommastrephidae) in the Southwest Atlantic. _Aquat. Living Resour._ 7, 221–232 (1994). Article Google Scholar * ICES.
Report of the Workshop on Sexual Maturity Staging of Cephalopods, 8-11 November 2010, Livorno, Italy. ICES CM 2010/ACOM:49. 97 (ICES 2010). * Villegas, P. G. life cycle and fishery biology
of _Loligo gahi_ (d’Orbigny, 1835) off the Peruvian coast. _Fish. Res._ 54, 123–131, https://doi.org/10.1016/S0165-7836(01)00376-9 (2001). Article Google Scholar * Reis, D. B. _et al_. _In
vivo_ metabolism of unsaturated fatty acids in _Sepia officinalis_ hatchlings. _Aquaculture_ 450, 67–73, https://doi.org/10.1016/j.aquaculture.2015.07.012 (2016). Article CAS Google
Scholar * Lin, D., Chen, X., Wei, Y. & Chen, Y. The energy accumulation of somatic tissue and reproductive organs in post-recruit female _Illex argentinus_ and the relationship with sea
surface oceanography. _Fish. Res._ 185, 102–114, https://doi.org/10.1016/j.fishres.2016.09.023 (2017). Article Google Scholar * GAQSIQ. Determination of total fat, saturated fat, and
unsaturated fat in foods: Hydrolytic extraction-Gas chromatography (GB/T 22223-2008). 16 (Standards Press of China, 2008). * Parrish, C. C. _et al_. Lipid and Phenolic Biomarkers in Marine
Ecosystems: Analysis and Applications in _Marine Chemistry_ (ed.Wangersky, P. J.) 193–223 (Springer, 2000). * Zar, J. H. _Biostatistical Analysis, fourth Edition_. 960 (Prentice Hall, 1999).
* Bromaghin, J. F. qfasar: quantitative fatty acid signature analysis with R. _Methods Ecol. Evol._ 8, 1158–1162, https://doi.org/10.1111/2041-210X.12740 (2017). Article Google Scholar *
Zuur, A., Ieno, E. N. & Smith, G. M. _Analyzing Ecological Data_. First edition. 672 (Springer-Verlag, 2007). * R Core Team. R: A language and environment for statistical computing v.
3.5.0 (R Foundation for Statistical Computing, Vienna, Austria, 2018). Download references ACKNOWLEDGEMENTS This is a contribution of the Distant Squid Fisheries Sci-Tech Group, SHOU. We
thank the staff members of the Key Laboratory of Sustainable Exploitation of Oceanic Fisheries Resources, Ministry of Education, Shanghai Ocean University for providing assistance at the
laboratory. We are grateful to technician Shaoqin Wang for the fatty acids determination and Sipeng Xuan for collecting the biological data. We also thank the Editor and an anonymous
reviewer for their insightful comments on the manuscript. Funding for this project was provided by the National Natural Science Foundation of China (41876144) and the Natural Science
Foundation of Shanghai (16ZR1415400) to Dongming Lin, and the National Key Research and Development Project of China (2019YFD0901404), National Natural Science Foundation of China (41876141)
and Shanghai Science and Technology Innovation Program (19DZ1207502) to Xinjun Chen. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * College of Marine Sciences, Shanghai Ocean University,
Shanghai, 201306, China Xinjun Chen, Fei Han, Kai Zhu & Dongming Lin * Key Laboratory of Sustainable Exploitation of Oceanic Fisheries Resources, Ministry of Education, Shanghai, 201306,
China Xinjun Chen & Dongming Lin * National Engineering Research Center for Oceanic Fisheries, Ministry of Science and Technology, Shanghai, 201306, China Xinjun Chen & Dongming Lin
* Key Laboratory of Oceanic Fisheries Exploration, Ministry of Agriculture and Rural Affairs, Shanghai, 201306, China Xinjun Chen & Dongming Lin * Scientific Observing and Experimental
Station of Oceanic Fishery Resources, Ministry of Agriculture and Rural Affairs, Shanghai, 201306, China Xinjun Chen & Dongming Lin * Laboratory for Marine Fisheries Science and Food
Production Processes, Qingdao National Laboratory for Marine Science and Technology, Qingdao, 266071, China Xinjun Chen * School of Aquatic and Fishery Sciences, University of Washington,
Seattle, WA, 98195-5020, USA André E. Punt Authors * Xinjun Chen View author publications You can also search for this author inPubMed Google Scholar * Fei Han View author publications You
can also search for this author inPubMed Google Scholar * Kai Zhu View author publications You can also search for this author inPubMed Google Scholar * André E. Punt View author
publications You can also search for this author inPubMed Google Scholar * Dongming Lin View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS
X.J.C. and F.H. designed the study, acquired data by performing the majority of laboratory experiments and drafted the manuscript; K.Z. acquired data by performing and interpreting some
experiments; A.E.P. reviewed and re-edited the manuscript; D.M.L. conceptualized the study, supervised the whole work and interpreted all the data. All authors finally approved the paper in
the present form. All authors contributed to the writing of the manuscript. CORRESPONDING AUTHOR Correspondence to Dongming Lin. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare
no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits
use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the
Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated
otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds
the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and
permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Chen, X., Han, F., Zhu, K. _et al._ The breeding strategy of female jumbo squid _Dosidicus gigas_: energy acquisition and allocation. _Sci
Rep_ 10, 9639 (2020). https://doi.org/10.1038/s41598-020-66703-5 Download citation * Received: 30 January 2020 * Accepted: 22 May 2020 * Published: 15 June 2020 * DOI:
https://doi.org/10.1038/s41598-020-66703-5 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative