Play all audios:
ABSTRACT Rho GTPases are important regulators of many cellular functions like cell migration, adhesion and polarity. The molecular switches are often dysregulated in cancer. We detected
Rho-dependent upregulation of the orphan seven-transmembrane receptor G-protein-coupled receptor family C group 5 member A (GPRC5A). GPRC5A is highly expressed in breast cancer whereas in
lung cancer, it is often downregulated. Here, we analyzed the function of GPRC5A in breast epithelial and breast cancer cells. Activation or expression of RhoA/C led to GPRC5A-dependent
inhibition of proliferation and reduction of the colony forming capacity of benign breast epithelial cells. This effect is based on an inhibition of EGFR signalling. Knockout of retinoic
acid induced 3 (RAI3, the gene for GPRC5A) in breast cancer cells increased cell division, whereas Rho activation had no effect on proliferation. Knockout of RAI3 in benign breast epithelial
cells led to decrease of EGFR expression and diminished proliferation. SIMILAR CONTENT BEING VIEWED BY OTHERS EFFECTS OF RARΑ LIGAND BINDING DOMAIN MUTATIONS ON BREAST FIBROEPITHELIAL TUMOR
FUNCTION AND SIGNALING Article Open access 03 January 2025 GREMLIN-1 AUGMENTS THE OESTROGEN-RELATED RECEPTOR Α SIGNALLING THROUGH EGFR ACTIVATION: IMPLICATIONS FOR THE PROGRESSION OF BREAST
CANCER Article Open access 23 June 2020 THE TRANSCRIPTION FACTOR PBX3 PROMOTES TUMOR CELL GROWTH THROUGH TRANSCRIPTIONAL SUPPRESSION OF THE TUMOR SUPPRESSOR P53 Article 01 February 2021
INTRODUCTION Rho GTPases are molecular switches regulating important cellular functions like gene transcription and proliferation1. They are well known regulators of the cytoskeleton and are
essentially involved in cell migration, adhesion and polarity. Recent studies showed that dysregulation of Rho GTPases plays a pivotal role in cancer development2 regulating proliferation,
invasion and metastasis of various types of tumor cells3,4,5. In epithelia, progression from a persistent to an invasive phenotype requires loss of epithelial polarity and of cellular
adhesion. This epithelial-to-mesenchymal transition (EMT) includes a change in gene expression pattern induced by several transcription factors, like Snail, ZEB1 or Twist6, 7. Recently, we
showed that pro-migratory genes like PTGS2 and serpine1 are upregulated in a RhoA/C specific manner8. Moreover, RhoC-dependent expression of Cox2 was involved in migration and invasion. In
our studies, we noticed that expression or activation of Rho GTPases dramatically inhibited proliferation of MCF10A breast epithelial cells. Activation of Rho GTPases led to upregulation of
the G-protein-coupled receptor family C group 5 member A (GPRC5A). GPRC5A is an orphan seven-transmembrane receptor identified in 1998 to be encoded by the retinoic acid (RA)-induced gene 3
(RAI3)9. RAI3 is dysregulated in several human cancer entities. Interestingly, in tissues with high GPRC5A expression (lung), malignant cells are associated with reduced expression,
indicating a tumor-suppressive role of the membrane protein. Studies with GPRC5A knockout mice suggested a tumor-suppressive function of the protein in lung adenocarcinoma. It was shown that
GPRC5A interacts with the epidermal growth factor receptor (EGFR) thereby preventing its signaling and reducing proliferation of lung cancer cells10. Consistently, RAI3 was downregulated in
more than 60% of lung tumors11. In sharp contrast, GPRC5A is highly expressed in breast cancer, colorectal and pancreatic carcinoma while its expression is low in the respective healthy
tissues (for review see12). In line with the contrasting expression, recent analysis of GPRC5A function revealed a controversial role in different cancer entities: Expression of GPRC5A in
non-tumorigenic pancreatic epithelial cells promoted colony formation13. Consistently, knockdown of RAI3 in pancreatic cancer cells led to decreased proliferation and reduced migration,
indicating a pro-metastatic role for GPRC5A in pancreatic cancer14. In colorectal cancer, elevated GPRC5A expression is associated with worse prognosis and induces cell proliferation and
tumorigenesis in a colitis-associated cancer model15. A tumor-suppressive effect of GPRC5A has been shown in MDA-MB-231 breast cancer cells16. In these cells knockdown of RAI3 induced
proliferation, migration and invasion. In contrast, silencing of GPRC5A had no effect on MCF7 cells with low EGFR levels, indicating a direct effect of GPRC5A on the EGFR stability and/or
EGF-induced proliferation16. We intended to analyze the connection between Rho GTPases, GPRC5A expression and proliferation in breast epithelial and cancer cells. In our studies we used the
benign breast epithelial cell line MCF10A with inducible expression of Rho proteins. Moreover, we treated the cells with the bacterial toxins Cytotoxic Necrotizing Factor 1 or Y (CNF1 or
CNFY) to activate the endogenous pool of Rho GTPases. The toxins are taken up into mammalian cells by receptor-mediated endocytosis and are released from the endosome into the cytoplasm17.
Rho proteins are constitutively activated by the bacterial protein toxins which catalyze the deamidation of a specific glutamine residue in Rho proteins and thereby lead to constitutive
activation of the GTPases (for review, see18). Moreover, we knocked out RAI3 in MDA-MB-231 breast cancer cells and in benign MCF10A breast epithelial cells to study the effects of G-protein
receptor deficiency in the absence and presence of Rho activation. MATERIALS AND METHODS CELL CULTURE AND REAGENTS MCF10A wild-type cell line was purchased from ATCC. MCF-10Atet cells
allowing inducible expression of RhoA or RhoC together with GFP under the control of a second generation Tet-regulated transcriptional trans-activator and silencer were generated via
nucleofection and have been described previously8. All cells were grown in DMEM/F12 medium containing 5% horse serum, 100 U/ml penicillin, 100 µg/ml streptomycin, 20 ng/ml epidermal growth
factor, 0.5 µg/ml hydrocortisone, 100 ng/ml cholera toxin and 10 µg/ml insulin. MDA-MB-231 culture medium contains DMDM/F12, 10% FCS, 100 U/ml penicillin and 100 µg/ml streptomycin. The
cells were incubated at 37 °C and 5% CO2. For the induction of the transgenic overexpression of RhoA and RhoC doxycycline was used at 2 µg/ml. Staurosporine was dissolved in DMSO.
Purification of CNF toxins was performed as described previously19 and were used at 1 nM. Every 2–3 days, all inhibitors, inducers and toxins were re-added with new medium. COLONY FORMATION
ASSAY To check for the colony formation capacity cells were seeded in a 6-well plate (500 cells per well). Following overnight attachment, doxycycline was added where indicated and cultured
for at least 6 days at 37 °C and 5% CO2. The medium was exchanged every three days. The colonies were fixed with glutaraldehyde (6,0% (v/v)) and stained with crystal violet (0,5% (w/v)) for
30 min. Afterwards the fixation staining solution was removed, the colonies were washed carefully with distilled water and dried at room temperature. BRDU PROLIFERATION ASSAY As an indicator
for proliferation DNA synthesis was measured using the chemiluminescent Cell Proliferation ELISA Kit (Roche) for quantifying the incorporation of 5-bromo-2′-deoxyuridine (BrdU). The cells
were seeded in a black flat bottom 96-well plate (5,000 cells per well), allowed to adhere overnight and incubated for 48 h with doxycycline where indicated. Then BrdU was added for 4 h
(final concentration 10 µM) and the assay was performed according to the manufacturers protocol. The chemiluminescent signal was detected using a 96-well plate reader (Tecan infinite M200,
Tecan Trading AG). Each assay was performed in technical triplicates. Percentage of BrdU incorporation was calculated with the following equation: % BrdU incorporation = (experimental
signal-background signal)/(control signal-background signal) × 100. CELL VIABILITY ASSAY Metabolic activity was detected measuring the cellular capacity to reduce the indicator dye resazurin
to resafurin with the CellTiter-Blue® Cell Viability Assay Kit (Promega). The cells were seeded in a black flat bottom 96-well plate (5,000 cells per well), allowed to adhere overnight and
incubated for 48 h with doxycycline, where indicated. After incubation the CellTiter-Blue® Reagent was added for 3 h and fluorescence was detected using a 96-well plate reader (Tecan
infinite M200, Tecan Trading AG). Each assay was performed in technical triplicates. Percentage of viable cells was calculated with the following equation: % viable cells = (experimental
absorbance-background absorbance)/(control absorbance-background absorbance) × 100. QRT-PCR RNA was isolated from 2D cultures at indicated time points using the RNeasy Mini Kit (Qiagen)
according to the manufactures protocol. Total RNA was eluted in RNAse-free distilled H20 and the final concentration was determined on a photometer at 260 nm. For each sample, 1 µg RNA was
applicated for cDNA synthesis using QuantiTect Reverse Transcription Kit (Qiagen) according to the manufacturers instructions. Finally, cDNA was diluted 1:10 and amplified using the GoTaq®
qPCR Master-Mix (Promega) on a Mastercycler® Realplex (Eppendorf). Raw data were analyzed with LinRegPCR 2012. S29 served as a housekeeping gene reference. (Primers: GPRC5A forward:
5′-GCACTAGGGTCCAGAATGG-3′, GPRC5A reverse: 5′-ACCGTTTCTAGGACGATGC-3′, S29 forward: 5′-GGTTCTCGCTCTTGTCGTGTC-3′, S29 reverse: 5′-ATATCCTTCGCGTACTGACGG-3′). WESTERN BLOT ANALYSIS Western Blot
analysis were performed using standard techniques. After removing the medium, the cells were washed once with PBS and then lysed in NP-40 lysis buffer (50 mM Tris–HCL (pH 8.0), 150 mM NaCl
and 1% NP-40) containing protease inhibitor (Complete, Roche) and phosphatase inhibitor (Sigma-Aldrich) if necessary. The samples were separated with a 12.5% or 7% SDS-PAGE and blotted using
the wet blot method (25 mM Tris–HCl, 192 mM glycine, 20% (v/v) methanol, 100 V, 75 min). Incubation with the primary antibodies were performed overnight. Used antibodies are: anti-RhoA
(67B9, Cell Signaling Technologies), anti-RhoC (D40E, Cell Signaling Technologies), anti-GAPDH (6C5, EMD-Millipore), anti-tubulin (DM1A, Santa Cruz), anti-GPRC5A (HPA007928, Atlas
Antibodies), anti-EGFR (D38B1, Cell Signaling Technologies), anti-P-EGFR (Y1068, 1H12, Cell Signaling Technologies) and a suitable secondary antibody coupled to horseradish peroxidase (HRP).
VIRAL TRANSDUCTION For virus production, HEK phoenix cells were transfected with pMIBerry empty vector or with pMiBerry containing the RAI3 gene and stimulated with 5 mM sodium butyrate
overnight. Transfection was controlled using fluorescent microscopy. Virus containing supernatant was used directly or stored at 4 °C. MCF10A cells were treated with virus containing
supernatant (1 ml + 9 ml fresh culture medium) four times for 1 day each. Sufficient transduction was analyzed by red fluorescence before cells were serum starved. CRISPR-CAS9 MEDIATED
GPRC5A KNOCKOUT We performed a knockout of GPRC5A in MDA-MB-231 and MCF10A cells using the CRISPR-Cas9 system. We followed the protocol from Ref.1. To design the targeting components and
determine the 20-nt guide sequence (5ʹ GTCCCTGATGGTTGCCGCAA 3ʹ) within the sgRNA including a 5′-NGG PAM (5ʹ TGG 3ʹ), we used the online CRISPR-Cas9 Design tool provided by
https://tools.genome-engineering.org. We selected a target site within Exon 2 of the human GPRC5A gene. For construction of an expression plasmid for sgRNA and Cas9 we used the
pSpCas9(BB)-2A-Puro (PX459) V2.0 Vector (AddGene Plasmid #62988). For co-expression of sgRNA and Cas9, the partially complementary oligonucleotides encoding the 20-nt guide sequences were
phosphorylated, annealed and ligated into the plasmid. The plasmid was then transformed into competent _E. coli_ strain. To verify the sequence of the plasmid we isolated the plasmid DNA
from several bacterial cultures and performed sequencing from the U6 promoter. TRANSFECTION OF MDA-MB-231 AND MCF10A CELLS To perform the knockout, MDA-MB-231 and MCF10A cells were
transfected with the sequence verified plasmid. For the Insertion of DNA in mammalian cells Lipofectamin 2000 (Invitrogen, Thermo Fisher Scientific) was used according to the
manufacturer's instructions. For transfection 1 million cells per well were seeded into a 10 cm dish. The confluency was 60–80%. Due to the selectable marker on the pSpCas9(BB)-2A-Puro
(PX459) V2.0 Vector the cells were selected through a Puromycin treatment with 1,0 µg/ml Puromycin over two days. CLONAL ISOLATION OF CELL LINES After transfection and selection, isolation
of clonal cell lines was achieved by serial dilution. After an expansion period the new single cell lines where each tested for a GPRC5A knockout through PCR and Western Blot. STATISTICAL
ANALYSIS For all statistical analysis GraphPad Prism 5.0 was used. All values, bars and error bars represent mean + standard deviation (SD). A p-value of < 0.05 was considered as
statistically significant. RESULTS RHOA/C EXPRESSION OR ACTIVATION INHIBITS PROLIFERATION OF MCF10A HUMAN BREAST EPITHELIAL CELLS We intended to study the effect of RhoA/C expression or
activation on the proliferation of breast epithelial cells. Therefore, we used sublines of human benign MCF10A cells, in which expression of either GFP, simultaneous expression of GFP and
RhoA or expression of GFP and RhoC can be induced by addition of doxycycline. Time- and dose-dependent expression of the proteins following addition of doxycycline (+ dox) was analyzed by
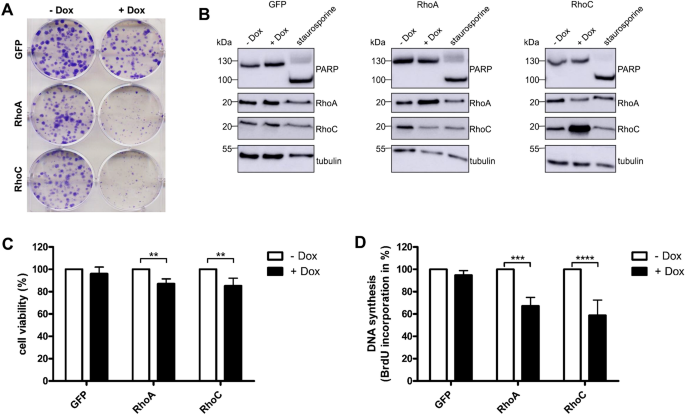
Western Blotting previously8. In a first set of experiments, colony formation assays were performed by growing the cell-lines in the absence or presence of doxycycline for 6 days. Expression
of GFP had no effect on colony formation. In contrast, we detected a severe inhibition of the colony formation capacity of MCF10A cells following expression of GFP and RhoA or expression of
GFP and RhoC, respectively (Fig. 1A). Colony formation depends on proliferation and viability as well as on differences of the cell size, contact inhibition and other cellular properties.
First, we studied apoptosis. As expected, expression of RhoA or RhoC did not induce cell death. Staurosporine was used as positive control (Fig. 1B). Measurements of the metabolic activity
as an indicator for cell viability showed a slight reduction to 90 or 80% in consequence of RhoA or RhoC overexpression. This moderate effect indicates that the reduced colony forming
capacity of Rho expressing cells was not exclusively based on reduced viability (Fig. 1C). To measure proliferation, BrdU incorporation into newly synthesized DNA was detected.
Doxycycline-induced expression of RhoA or RhoC reduced cell proliferation to about 50 to 60% compared to non-induced cells (Fig. 1D). In all experiments, RhoC had stronger effects than RhoA
(compare Fig. 1B middle and right). To study whether the reduced metabolic activity and proliferation was based on the strong protein expression per se, we stimulated the endogenous pool of
Rho GTPases by treatment of MCF10A cells with two bacterial toxins: CNFY predominantly activates RhoA,B,C whereas CNF1 activates Rac1, Cdc42 and RhoA,B,C. As controls, we used the respective
catalytically inactive mutants of the toxins (CNF1 C866S and CNFY C865S). Effective uptake of the toxins into MCF10A cells and Rho activation was shown previously8. The colony formation
assay was performed with MCF10A cells in the presence or absence of CNFs for 6 days. As shown in Fig. 2, a similar inhibitory effect on colony formation (A), metabolic activity (C) and
proliferation (D) was achieved by activation of Rho GTPases due to treatment of the cells with CNFY. However, treatment with CNF1 had no effect on colony formation, indicating that
activation of other Rho GTPases like Rac and/or Cdc42 may counteract the RhoA,B,C-induced inhibition of proliferation/colony formation20. To exclude an effect of the toxins on cell death, we
additionally analyzed PARP-cleavage. Both toxins did not induce apoptosis of MCF10A cells (Fig. 2B). Metabolic activity was even slightly increased in the presence of CNF1 (Fig. 2C). To
measure proliferation exclusively, BrdU incorporation was analyzed in the presence of the toxins or their inactive mutants, respectively. CNFY but not CNF1 reduced cell proliferation to
about 60% compared to untreated controls (Fig. 2D). RHO-DEPENDENT EXPRESSION OF GPRC5A IN MCF10A CELL LINES Recently, we performed a genetic screen to analyze genes regulated by expression
of Rho proteins in MCF10A cells. We detected several pro-migratory genes upregulated following RhoA and/or RhoC expression8. Additionally, one of the genes with higher expression was RAI38.
It encodes for an orphan G-protein coupled receptor GPRC5A differently expressed in several human cancer entities. Interestingly, RAI3 was recently identified as a protein with a significant
influence on proliferation of EGFR expressing cells16. Therefore, we asked whether upregulation of RAI3 might be involved in the Rho-dependent inhibition of proliferation of MCF10A cells.
First, we validated the Rho-dependent induction of RAI3 mRNA levels by qRT-PCR and additionally studied the respective GPRC5A protein levels by Western Blotting. In line with the genetic
screen, the amount of RAI3 mRNA increased about two-fold following induction of RhoA or RhoC expression by doxycycline for 24 h (Fig. 3A). Consistently, GPRC5A expression correlates with
mRNA synthesis. It increased following induction of RhoA/C expression, whereas the level of EGFR did not change (Fig. 3B, quantification in Fig. 3C). We additionally studied the effect of
Rho activation by toxin treatment and detected the same increase of RAI3 mRNA and GPRC5A protein in cells treated with CNF1 or CNFY, respectively (Fig. 3D,E, quantification in Fig. 3F). As
expected, treatment of the cells with catalytically inactive toxin mutants had no effect. The data show that GPRC5A expression is upregulated downstream of RhoA and RhoC. EFFECT OF GPRC5A
EXPRESSION ON LIGAND-INDUCED EGFR PHOSPHORYLATION In former studies, an inhibition of EGFR signaling by direct interaction with GPRC5A was shown10. To analyze the effect of Rho activation
solely on EGF-dependent proliferation, we studied colony formation and DNA synthesis using serum starved MCF10A cells. As revealed by dose response analysis of BrdU incorporation into newly
formed DNA, the optimal EGF concentration necessary to maximally stimulate proliferation of serum starved MCF10A cells is 20 ng/ml (EC50 = 1.3 ng/ml, Fig. 4A). Therefore, colony formation
assays were performed with 20 ng/ml EGF in the presence or absence of the bacterial toxins or their catalytically inactive mutants, as indicated in Fig. 4B. In contrast to the experiments in
full medium (containing 5% serum, Fig. 1A), colony formation was blocked in medium with low serum (1%, supplemented with EGF) in the presence of CNF1 or CNFY, respectively. In line with the
colony formation assay, both toxins reduced basal and EGF-stimulated BrdU incorporation, whereas the catalytically inactive mutants had no effect (Fig. 4C). These data indicate that Rho
activation blocked EGF-dependent proliferation. Therefore, we studied direct phosphorylation of the EGFR following EGF stimulation in the presence and absence of the toxins by Western
Blotting. For detection of EGFR phosphorylation, we used an antibody against phospho-EGFR (Fig. 4D, top lane) and a second antibody, which detects only the non-phosphorylated EGFR (Fig. 4D,
middle lane). EGF-stimulated phosphorylation was reduced by treatment with the toxins. Rho stimulation by CNFs led to reduced EGF-dependent receptor phosphorylation and proliferation
probably by enhanced expression of GPRC5A. CNF1 and CNFY led to reduced basal DNA synthesis and impaired the EGF-dependent proliferation, respectively. EXPRESSION OF GPRC5A IS SUFFICIENT TO
INHIBIT PROLIFERATION To analyze, whether expression of GPRC5A is sufficient to influence EGFR signaling, we transiently expressed the hepta-helical receptor in MCF10A cells by viral
transduction. Following addition of virus-containing supernatants (empty vector control and GPRC5A, respectively), cells were serum starved for 2 days, stimulated with EGF (1.3 and 20 ng/ml,
respectively) for 5 min. Cleared lysates were analyzed for expression of GPRC5A, phospho-EGFR and total EGFR by Western Blotting. As shown in Fig. 5A, the cells show about 2 to 3-fold
higher expression compared to the empty vector-transduced MCF10A cells. Phosphorylation of the EGFR following stimulation with EGF was significantly reduced (by about 50%) in GPRC5A
expressing cells (Fig. 5B). Additionally, to measure proliferation, BrdU incorporation into newly synthesized DNA was detected in GPRC5A overexpressing cells. Therefore, transduced cells
(empty vector control and GPRC5A, respectively) were seeded into 96 well plates, serum starved for two days and stimulated with EGF (20 ng/ml) for 4 h in the presence of BrdU. As shown in
Fig. 5C, EGF-stimulated proliferation of the empty vector transduced cells was increased about 1.5 times compared to the unstimulated cells (set to 1). In contrast, there was no increased
BrdU incorporation detectable in GPRC5A expressing cells following exposure to EGF. The data show that EGF-stimulated proliferation was inhibited due to enhanced expression of GPRC5A. GPRC5A
IS REQUIRED FOR STABILIZATION OF MONOMERIC EGFR To study the effect of Rho activation per se on EGFR signaling and proliferation, we performed a knockout of GPRC5A in MCF10A cells proven by
Western Blot (Fig. 6A, quantification in Fig. 6B). Interestingly, the expression of EGFR decreased to 20 and 55% and under serum starvation to 15 and 20%, respectively. To analyze the
effect of Rho activation on EGF-dependent proliferation, we studied EGFR phosphorylation and DNA synthesis using serum starved MCF10A cells as described above and stimulated them with 20
ng/ml EGF. In GPRC5A knockout cells, the amount of phosphorylated EGFR was significantly diminished and additional activation of Rho GTPases by CNF1 or CNFY had no effect (Fig. 6C). BrdU
incorporation in RAI3 depleted cells was significantly reduced under serum starvation and almost blocked, even after EGF stimulation. As expected, intoxication with CNF1 or CNFY was not
sufficient to reactivate proliferation (Fig. 6D). Our data show that the amount of EGFR is influenced by the expression of GPRC5A and not mediated by toxin-induced Rho activation. The
monomer seems to be stabilized in the presence of GPRC5A, which on the one hand inhibits degradation and on the other hand negatively influences dimerization and signaling of the receptor.
RHO ACTIVATION IN BREAST CANCER CELLS CARRYING AN ACTIVATING RAS MUTATION HAD NO EFFECT ON PROLIFERATION, WHEREAS KNOCKOUT OF GPRC5A INCREASED CELL DIVISION Our data suggest an inhibitory
effect of GPRC5A on proliferation of breast epithelial cells most likely by diminished EGFR stimulation. To verify that this influence on proliferation was predominantly based on EGFR
signaling, the effect of the toxins was analyzed on MDA-MB-231 breast cancer cells bearing an activating Ras mutation and are therefore independent on EGFR signaling. We induced a knockout
of RAI3 in MDA-MB-231 cells by CRISPR-Cas9 and verified the functional gene knockout by Western Blot as shown in Fig. 7A (quantification in Fig. 7B). As expected, knockout of GPRC5A
increased the colony forming capacity of MDA-MB-231-cells (Fig. 7C) and stimulated BrdU incorporation by about 20% compared to the wild-type cells but did not affect cell viability (Fig.
7D,E). The data indicate an anti-proliferative effect of GPRC5A also in cells with dominant active Ras. Our data are in line with recent experiments in which knockdown of GPRC5A in
MDA-MB-231 breast cancer cells promoted colony formation and proliferation16. Neither cell viability nor proliferation of MDA-MB-231 wild-type and GPRC5A knockout cells was affected by CNF1
or CNFY most likely because EGFR downstream signaling was already activated in MDA-MB-231 cells (Fig. 7F,G). The data show that it is not the effect of the Rho-activating toxins which
influences the proliferation and colony formation but Rho-induced expression of GPRC5A and inhibition of EGFR signaling. DISCUSSION Cancer is an extremely heterogeneous disease and even
cells of one cancer entity often show a wide variety of different gene profiles and morphological characteristics. The epidermal growth factor receptor (EGFR) plays a critical role in cancer
since it mediates proliferation by activation of Ras and STAT. EGFR kinase inhibitors have successfully developed. Recently, it was shown that an orphan G protein coupled receptor (GPCR)
interacts with EGFR, sequestering it as a monomer and thereby inhibiting receptor signaling. In line with this, expression of GPRC5A is low in non small cell lung cancer (NSCLC)21. Moreover,
GPRC5A knockout mice developed spontaneous lung cancer11 and GPRC5A loss was associated with increased cell proliferation and resistance to cell death22. The gene was thus designated a
tumor suppressor. In pancreatic cancer however, knockdown of RAI3 (the gene for GPRC5A) led to decreased proliferation and reduced migration, indicating a pro-metastatic role for GPRC5A in
pancreatic cancer14. In breast cancer, the picture is diverse: According to the “bioportal” website, GPRC5A expression analysis revealed more breast tumor tissues with protein amplification
than with deletions. However, the diverse role of GPRC5A in tumor formation is reflected by recent studies with breast cancer cell lines. Knockdown of GPRC5A promotes colony formation and
proliferation by activation of EGFR in MDA-MB-231 cells but showed no effect in MCF7 cells expressing only low amounts of EGFR16. In cells carrying an activating Ras mutation, GPRC5A has
less effect on proliferation and survival. This proves that the effect of GPRC5A on proliferation is mediated by its influence on EGFR, which also activates other signaling pathways as for
example PI3K. Our data indicate that only the EGFR dimer is stably expressed at the cell membrane, whereas the empty receptor needs GPRC5A to be stabilized, suggesting that the level of
GPRC5A on the one hand interferes with dimerization and signaling of EGFR but on the other hand stabilizes the EGFR monomer against degradation (model depicted in Fig. 8). The inhibitory
effect of GPRC5A on proliferation may vary, if other EGFR family members are expressed in that sense that Her2 stabilizes EGFR. It is not known whether human EGF receptor 2 (Her2) also
interacts with GPRC5A and whether its signaling is also blocked. In a recent publication by Fichter et al. it is shown that homo- and heterodimers of EGFR and Her2 form differently in
diverse tissues, which may explain varying effects of GPRC5A expression23. Here, we identified RAI3 as a gene upregulated by Rho GTPase signaling in breast epithelial cells. Expression of
GPRC5A significantly reduced proliferation of the cells. Moreover, knockout of RAI3 also inhibited EGF-dependent proliferation due to EGFR downregulation. The GPCR is an orphan receptor.
Neither the ligand nor its intracellular signaling partner is known. However, recent deletion studies showed that it is not the signaling of the GPCR to heterotrimeric G-proteins, which
influences EGFR activity. Rather the transmembrane part of GPRC5A seems to be important because deletion of the N-terminus, or deletion of the C-terminus of the hepta-helical receptor did
not affect its inhibitory action on EGFR signaling10. REFERENCES * Vega, F. M. & Ridley, A. J. Rho GTPases in cancer cell biology. _FEBS Lett._ 582, 2093–2101 (2008). Article CAS
Google Scholar * Raftopoulou, M. & Hall, A. Cell migration: Rho GTPases lead the way. _Dev. Biol._ 265, 23–32 (2004). Article CAS Google Scholar * Wang, M., Wang, X. J. & Liu, B.
R. Effect of shRNA targeted against RhoA on proliferation and migration of human colonic cancer cells. _Int. J. Clin. Exp. Pathol._ 8, 7040–7044 (2015). CAS PubMed PubMed Central Google
Scholar * Clark, E. A., Golub, T. R., Lander, E. S. & Hynes, R. O. Genomic analysis of metastasis reveals an essential role for RhoC. _Nature_ 406, 532–535 (2000). Article ADS CAS
Google Scholar * Lin, M., DiVito, M. M., Merajver, S. D., Boyanapalli, M. & van Golen, K. L. Regulation of pancreatic cancer cell migration and invasion by RhoC GTPase and Caveolin-I.
_Mol. Cancer_ 4, 20 (2005). Article Google Scholar * Moody, S. E. _et al._ The transcriptional repressor Snail promotes mammary tumor recurrence. _Cancer Cell_ 8, 197–209 (2005). Article
CAS Google Scholar * Mock, K. _et al._ The EMT-activator ZEB1 induces bone metastasis associated genes including BMP-inhibitors. _Oncotarget_ 6, 14399–14412 (2015). Article Google Scholar
* Lang, S. _et al._ Specific role of RhoC in tumor invasion and metastasis. _Oncotarget_ 8, 87364–87378 (2017). Article Google Scholar * Cheng, Y. & Lotan, R. Molecular cloning and
characterization of a novel retinoic acid-inducible gene that encodes a putative G protein-coupled receptor. _J. Biol. Chem._ 273, 35008–35015 (1998). Article CAS Google Scholar * Zhong,
S. _et al._ Lung tumor suppressor GPRC5A binds EGFR and restrains its effector signaling. _Cancer Res._ 75, 1801–1814 (2015). Article CAS Google Scholar * Tao, Q. _et al._ Identification
of the retinoic acid-inducible Gprc5a as a new lung tumor suppressor gene. _J. Natl. Cancer Inst._ 99, 1668–1682 (2007). Article CAS Google Scholar * Jiang, X. _et al._ GPRC5A: An
emerging biomarker in human cancer. _Biomed Res. Int._ 2018, 1823726 (2018). PubMed PubMed Central Google Scholar * Zhou, H. _et al._ GPRC5A is a potential oncogene in pancreatic ductal
adenocarcinoma cells that is upregulated by gemcitabine with help from HuR. _Cell Death Dis_ 7, 20 (2016). Google Scholar * Jahny, E. _et al._ The G protein-coupled receptor RAI3 is an
independent prognostic factor for pancreatic cancer survival and regulates proliferation via STAT3 phosphorylation. _PLoS ONE_ 12, 20 (2017). Article Google Scholar * Zhang, L. _et al._
Elevation of GPRC5A expression in colorectal cancer promotes tumor progression through VNN-1 induced oxidative stress. _Int. J. Cancer_ 140, 2734–2747 (2017). Article CAS Google Scholar *
Yang, L., Ma, T. & Zhang, J. GPRC5A exerts its tumor-suppressive effects in breast cancer cells by inhibiting EGFR and its downstream pathway. _Oncol. Rep._ 36, 2983–2990 (2016).
Article CAS Google Scholar * Blumenthal, B., Hoffmann, C., Aktories, K., Backert, S. & Schmidt, G. The cytotoxic necrotizing factors from Yersinia pseudotuberculosis and from
_Escherichia coli_ bind to different cellular receptors but take the same route to the cytosol. _Infect. Immun_ 75, 3344–3353 (2007). Article CAS Google Scholar * Schmidt, G. &
Aktories, K. in _Regulators and Effectors of Small GTPases_ Vol. 325 _Methods in Enzymology_ (eds W.E. Balch, C.J. Der, & A. Hall) Ch. 12, 125–136 (Academic Press, New York, 2000). *
Knust, Z., Blumenthal, B., Aktories, K. & Schmidt, G. Cleavage of _Escherichia coli_ cytotoxic necrotizing factor 1 is required for full biologic activity. _Infect. Immun_ 77, 1835–1841
(2009). Article CAS Google Scholar * Hoffmann, C. _et al._ The Yersinia pseudotuberculosis cytotoxic necrotizing factor (CNFY) selectively activates RhoA. _J. Biol. Chem_ 279, 16026–16032
(2004). Article CAS Google Scholar * Fujimoto, J. _et al._ G-protein coupled receptor family C, group 5, member A (GPRC5A) expression is decreased in the adjacent field and normal
bronchial epithelia of patients with chronic obstructive pulmonary disease and non-small-cell lung cancer. _J. Thorac. Oncol._ 7, 1747–1754 (2012). Article CAS Google Scholar * Deng, J.
_et al._ Knockout of the tumor suppressor gene Gprc5a in mice leads to NF-kappaB activation in airway epithelium and promotes lung inflammation and tumorigenesis. _Cancer Prev. Res. (Phila)_
3, 424–437 (2010). Article CAS Google Scholar * Fichter, C. D. _et al._ ErbB targeting inhibitors repress cell migration of esophageal squamous cell carcinoma and adenocarcinoma cells by
distinct signaling pathways. _J. Mol. Med. (Berl)_, (2014). Download references ACKNOWLEDGEMENTS We thank Jürgen Dumbach for excellent technical assistance and Sarah Lang for scientific
discussion. We thank the MOTI-VATE program of the medical faculty for support of L.R.. The project was funded by the Deutsche Forschungsgemeinschaft (SFB 850 - C2). Open access funding
provided by Projekt DEAL. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Institute for Experimental and Clinical Pharmacology and Toxicology, Albert-Ludwigs-University of Freiburg,
Albert-Str. 25, 79104, Freiburg, Germany Lukas Richter, Viktoria Oberländer & Gudula Schmidt Authors * Lukas Richter View author publications You can also search for this author inPubMed
Google Scholar * Viktoria Oberländer View author publications You can also search for this author inPubMed Google Scholar * Gudula Schmidt View author publications You can also search for
this author inPubMed Google Scholar CONTRIBUTIONS L.R., V.O. and G.S. performed experiments and prepared figures, G.S. wrote the manuscript and supervised the project. All authors reviewed
the manuscript. CORRESPONDING AUTHOR Correspondence to Gudula Schmidt. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION
PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article
is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give
appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in
this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative
Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a
copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Richter, L., Oberländer, V. & Schmidt, G. RhoA/C
inhibits proliferation by inducing the synthesis of GPRC5A. _Sci Rep_ 10, 12532 (2020). https://doi.org/10.1038/s41598-020-69481-2 Download citation * Received: 08 May 2019 * Accepted: 09
July 2020 * Published: 27 July 2020 * DOI: https://doi.org/10.1038/s41598-020-69481-2 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get
shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative