Play all audios:
ABSTRACT Triple-negative breast cancer (TNBC) patients who do not achieve pathologic complete response post neoadjuvant chemotherapy have a poor prognosis. Alteration in PI3K/mTOR plus DNA
repair pathways are some of the major mechanisms of chemotherapy resistance. We designed an open-label phase II clinical trial to evaluate if the combination of everolimus (mTOR inhibitor)
plus cisplatin (interferes with DNA function) will improve the rate of pathologic response, as assessed by residual cancer burden (RCB). Twenty-four Stage II/III TNBC patients with residual
cancer > 1 cm post neoadjuvant anthracycline and taxane-based chemotherapy were enrolled. Patients received everolimus daily orally at 10 mg for 12 weeks and cisplatin IV at 20 mg/m2
weekly for 4 cycles (21-day cycle), until definitive surgery. The primary endpoint was the rate of RCB-0-I at the surgery. The median age of the whole cohort was 50.1 years, with 66.7%
non-Hispanic Caucasians. Of the 24 patients enrolled, 22 were included in the efficacy analysis. Twenty-one patients underwent definitive surgery while one patient developed distant
metastasis. Five patients had RCB-I at surgery, a response rate of 23% (5/22). Patients with germline PALB2 mutation or somatic PI3KCA mutation had a pathologic response, achieving RCB-I at
the surgery. Three patients had metaplastic histology achieving RCB-I at the surgery. Estimated OS at 1 year was 100% in the RCB-I group vs. 76.5% in others, which was not statistically
significant due to the small sample size. Certain cohorts including PALB2 germline mutation carrier and somatic PI3KCA mutations warrant further investigation. TRIAL REGISTRATION:
Clinicaltrials.gov identifier: NCT01931163. https://clinicaltrials.gov/ct2/show/NCT01931163. SIMILAR CONTENT BEING VIEWED BY OTHERS CISPLATIN +/− RUCAPARIB AFTER PREOPERATIVE CHEMOTHERAPY IN
PATIENTS WITH TRIPLE-NEGATIVE OR BRCA MUTATED BREAST CANCER Article Open access 22 March 2021 DATOPOTAMAB–DERUXTECAN IN EARLY-STAGE BREAST CANCER: THE SEQUENTIAL MULTIPLE ASSIGNMENT
RANDOMIZED I-SPY2.2 PHASE 2 TRIAL Article 14 September 2024 A PHASE II STUDY OF PALBOCICLIB PLUS LETROZOLE PLUS TRASTUZUMAB AS NEOADJUVANT TREATMENT FOR CLINICAL STAGES II AND III ER+ HER2+
BREAST CANCER (PALTAN) Article Open access 06 January 2023 INTRODUCTION Triple-negative breast cancers (TNBC) are characterized by lack of expression of estrogen receptor (ER), progesterone
receptor (PR), and human epidermal growth factor receptor 2 (HER-2)1. TNBC occurs in 10–20% of all breast cancers and has a poor prognosis1,2. Patients who do not achieve pathological
complete response (pCR) to neoadjuvant chemotherapy have worse survival compared to patients who have pCR at surgery, with more than 30% of these patients relapsing within 3 years3,4. Thus,
there remains a need to improve outcomes for TNBC who have residual disease post neoadjuvant chemotherapy. Sporadic TNBC and germline mutated BRCA 1/2 associated cancer are both typically
basal-like by gene expression profiling5. Since BRCA1 and BRCA2 dysfunction leads to impaired homologous recombination repair of DNA6, drugs targeting DNA repair is a way to improve outcomes
in TNBC. Cisplatin which targets DNA function has previously shown clinical efficacy in the neoadjuvant setting in TNBC as a single agent7. Alterations in Phosphoinositide
3-kinase/mammalian target of rapamycin (PI3K/mTOR) pathway followed by DNA repair alterations have been described as the major mechanisms of chemotherapy resistance in TNBC patients with
residual disease post neoadjuvant chemotherapy8. Thus, we designed a rational Phase II trial of combination everolimus which targets the PI3K/mTOR pathway by inhibiting mTOR, and cisplatin
which targets DNA function in the neoadjuvant setting in TNBC patients who have residual disease post anthracycline or taxane-based chemotherapy. The primary objective of the trial was the
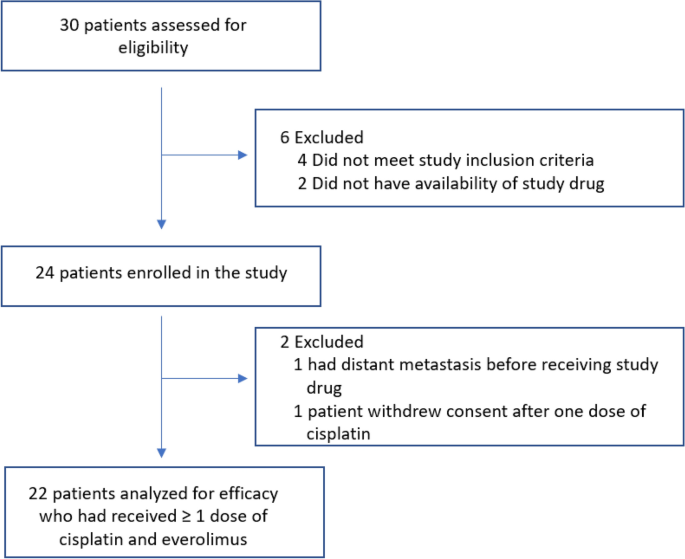
rate of pathologic response, as measured by the Residual Cancer Burden (RCB) after treatment with cisplatin plus everolimus. RESULTS PATIENT CHARACTERISTICS Twenty-four patients with
triple-negative chemorefractory breast cancer (post anthracycline plus taxane-based chemotherapy, with the biopsy-proven residual disease) were enrolled in the study from June 1, 2013, to
August 30, 2017, after obtaining informed written consent (Fig. 1). The median age of the whole cohort was 50.1 years (Table 1). The majority of patients were non-Hispanic Caucasian (66.7%)
followed by African American (25%) and Hispanic Caucasian (8.3%). Patients presented with both early-stage and locally advanced disease with 54.2% (13/24) stage II disease while 45.8%
(11/24) had stage III disease. Of these twenty-four patients, three had the rare subtype of metaplastic breast cancer (3/24, 12.5%). Patients underwent germline mutation testing, and only
10% (2/20) of those tested had germline genetic mutation, both of which were deleterious PALB2 mutations. Notably, there were no patients with germline BRCA1 or BRCA2 mutations in this
chemorefractory TNBC patient cohort. The most common prior standard neoadjuvant regimen received by the study cohort was dose-dense doxorubicin plus cyclophosphamide (60 mg/m2 IV doxorubicin
plus 600 mg/m2 IV cyclophosphamide on Day1 every 14 days × 4 cycles) followed by weekly paclitaxel (80 mg/m2 IV every 7 days × 12 cycles). TREATMENT RESPONSE Out of twenty-four patients
enrolled, one patient progressed/metastasized prior to receiving the study treatment and a second patient withdrew after 10 days on trial without toxicity or progression, and thus were not
evaluated for treatment response. The treatment response was assessed in twenty-two patients. Of these, 10 patients did not complete all four cycles of cisplatin/everolimus—six patients came
off the study due to disease progression (one patient developed distant metastasis while five had progression of the primary lesion) and four patients secondary to treatment toxicity.
Twenty-one patients out of twenty-two patients underwent definitive surgery. The predefined primary endpoint for these chemorefractory TNBC patients was residual cancer burden (RCB). Sample
size estimations were based on historical pathologic response (RCB 0–1) of less than 5%. Here, in this trial, the overall response rate for RCB 0-I was 23% (5/22) (95% confidence interval
(CI) 10.1–43.4%). While no patients achieved RCB 0, 5 patients did achieve RCB-I (Table 2). The characteristics of patients achieving RCB 0-I at the surgery are summarized in Table 3.
SURVIVAL ANALYSIS At a median follow-up of 29 months (95% CI 25–36.5 months) from study enrollment, 64% (14/22) of the patient remained free of distant metastasis while 46% (6/22) developed
metastatic disease. There were 5 deaths observed among the 22 patients. The estimated overall survival (OS) at 1 year was 81% and at 4 years was 65.5%. (Fig. 2A). The 1-year OS was 100% in
responders vs. 76.5% in non-responders, which is not statistically significant given the small sample size (Fig. 2B). TOXICITY The regimen had fair tolerance with only grade 1 and 2
toxicities in 95.4% (21/22) and grade 3 and 4 in 18.2% (4/22) of the patients. Three main toxicities with incidence > 20% were fatigue (45%), nausea (41%), and mucositis (23%) (see
Supplementary Table 1). One patient experienced grade 3 nausea, one patient had both grade 3 thrombocytopenia and grade 3 hyperglycemia, one patient had both grade 3 leucocytopenia and
neutropenia while one patient had grade 4 papilledema. NEXT-GENERATION EXOME SEQUENCING AND GERMLINE MUTATION TESTING Somatic mutation testing by next-generation exome sequencing was
performed in 45% (10/22) of the patients. Somatic mutation testing was performed on pre-treatment biopsy samples using FoundationOne companion diagnostic. Germline mutation testing was
available for all these 10 patients, germline testing was done using commercially available Myriad myRisk. Here, no germline BRCA1/2 mutations were detected in patients with triple-negative
breast cancer, contrary to expectations. Two patients had germline PALB2 mutations. The most predominant somatic mutation was in _TP53_ in 60% of the patients (6/10). Of 5 patients with RCB
0–1, two had deleterious PALB2 germline mutations. All five patients underwent somatic mutation testing. Of these, two patients had actionable somatic PI3KCA mutations. Of interest, of the
three patients with metaplastic breast cancer, two patients who had somatic PIK3CA mutation responded with a residual 4 mm (patient #1) and 8 mm (patient #8) tumor post-treatment. On
comparing mutation profile between responders and non-responders (see Supplementary Figure 2), mutations affecting PI3K/mTOR pathway and DNA repair mechanisms were enriched in the responder
cohort while non-responders had enrichment of _TP53_ alterations (all cases). The difference in PALB2 mutation in responders vs. non-responders (p = 0.44) and P1K3CA mutation in responders
vs. non-responders (p = 0.44) were not statistically significant while the difference in TP53 alteration in responders vs. non-responders (p = 0.04) was statistically significant using
Fisher’s exact test. DISCUSSION In this combination study of everolimus plus cisplatin in the patient’s refractory to standard chemotherapy, where any pathologic response is notable, we
report a 23% RCB-I pathologic response. In a long term follow up of patients achieving RCB-0 (pCR) or RCB-I in TNBC post neoadjuvant chemotherapy, prognoses was superior to patients with
RCB-II and RCB-III9. Relapse-free survival and overall survival were improved in the TNBC patient subset with RCB-0 or RCB-I at surgery. In a multivariate model of relapse-free survival in
TNBC, RCB was prognostic independent of other clinical pathologic variables9. Except for one patient that developed metastasis while on the study protocol, all others were able to get
definitive surgery. On analysis of characteristics of 5 patients who had RCB-I at surgery, 2 had PALB2 germline mutation, 2 had PIK3CA somatic mutation and one had CHEK1 mutation. PALB2 is a
tumor suppressor gene that interacts with BRCA2 and is required for DNA repair10. Women with PALB2 mutations are at increased risk of developing breast cancer11. More importantly, breast
cancer patients with known PALB2 mutations are known to have a poor prognosis12. Patients with germline PALB2 mutation may be sensitive to drugs that target DNA repair mechanisms like
cisplatin13 or PARP inhibitors, which are being tested in clinical trials in various tumor types including pancreatic cancers14,15. In our trial, both patients with germline PALB2 mutation
had RCB-I at the surgery. Patient #18 with germline PALB2 mutation presented with ~ 10 cm cancer, had large 3.5 cm residual cancer following neoadjuvant anthracyclines and taxanes. Notably,
after everolimus and cisplatin, she had no residual cancer in the breast and one small 0.3 cm focus in the axillary node at the time of surgery. The other germline PALB2 mutation carrier
(Patient #20) had the chemorefractory disease, with her primary tumor progressing while on standard neoadjuvant anthracycline and taxane. After 4 cycles of everolimus and cisplatin, she
responded clinically, and her pathologic residual cancer was under 1 cm. There was no germline BRCA 1 or 2 mutations cancer in this study of chemorefractory patients, reflective of more
chemosensitive disease in germline cancers. Two patients who had somatic PI3KCA mutation also responded to cisplatin plus everolimus, both had RCB-I at the surgery. Patient #1 with PI3KCA
mutation had only 0.45 cm disease left at surgery post combination therapy, responding even when concomitant _TP53_ mutation was present. Patient #8 also had PI3KCA mutation and had less
than 1 cm disease at surgery. Of the three patients with metaplastic cancers which are known to be highly refractory to most chemotherapy regimens and to have one of the worst prognosis, two
patients (Patient #1 and #8) who had PIK3CA mutation were able to achieve RCB-I at the surgery. The remaining one metaplastic patient (#7) did not have PIK3CA mutations but the CHEK1-ATR
pathway mutation also achieved RCB-I at the surgery. The overall survival of the whole cohort was 81% at 1 year and 65.5% at 4 years. Estimated OS at 1 year was higher in patients achieving
RCB-I vs. others at 100% vs. 76.5% respectively, although this was not statistically different due to a low number of patients in this trial. Patients in the responder group had enrichment
of mutation affecting DNA repair (PALB2, CHEK1, and ATR) and PI3KCA/mTOR pathway, compared to non-responders. Non-responders had enrichment of TP53 alterations compared to responders.
Everolimus has been previously studied in combination with chemotherapy in the upfront neoadjuvant setting in TNBC where its addition led to a lower pCR rate compared to chemotherapy with
increased toxicity16. In this trial, the regimen consists of everolimus in combination with cisplatin, after neoadjuvant chemotherapy where the residual tumors have been shown to have an
enrichment of both the PI3K/mTOR pathway along with DNA repair alterations as the major mechanistic pathways of chemotherapy resistance8. We report a noteworthy 23% of the patients achieved
RCB-I at the surgery. In this trial, patients who had mutations affecting the DNA repair pathway and patients with somatic PI3KCA mutations had a response to treatment with cisplatin plus
everolimus. There are limitations of this study, namely a low number of patients and a single-arm study with no randomization arm. Despite the limitations, these results add to the
understanding of targeting the PI3K/mTOR pathway in TNBC especially in patients who have residual disease post neoadjuvant chemotherapy where the current standard of care is the use of
adjuvant capecitabine17. Further studies evaluating cisplatin plus everolimus in cohorts with PI3K pathway alteration or PALB2 germline mutation is needed especially in poor prognosis
metaplastic breast cancer patients18. CONCLUSION The combination of everolimus plus cisplatin is active in the neoadjuvant setting in TNBC patients who have residual disease post standard
neoadjuvant chemotherapy with a response rate of 23% for RCB-I at the surgery. This combination was active in a subset of patients with germline PALB2 mutation or somatic PI3KCA mutation.
This is the first study using targeted therapy in a neoadjuvant setting for TNBC patients who had residual disease post standard anthracycline-taxane neoadjuvant chemotherapy. Responders to
this combination included patients with germline PALB2 mutation and metaplastic histology who are known historically have a poor prognosis. Further studies evaluating this regimen in cohorts
with PI3KCA mutation or PALB2 germline mutation is needed. PATIENTS AND METHODS PATIENTS All female patients > 18 years of age with TNBC defined as estrogen receptor-negative and
progesterone receptor-negative (< 10% staining by immunohistochemistry (IHC) for estrogen and progesterone receptor) plus HER2 negative (FISH ratio < 2.2 or IHC 0–1 + or IHC 2–3 + and
FISH ratio < 2.2) who had clinical and pathological documentation of residual disease of > 1 cm after neoadjuvant chemotherapy were eligible for the trial (Clinicaltrials.gov
identifier: NCT01931163. Registered on 29/08/2013). Trial was approved by Houston Methodist Hospital Institutional Review Board (IRB) and monitored by the Houston Methodist Hospital Data
Safety and Monitoring Board (DSMB). All methods were performed in accordance with the relevant guidelines and regulations. Written informed consent was obtained from all patients before
sample and data collection. Patients had to be Eastern Cooperative Oncology Group (ECOG) performance status 0 or 1. Exclusion criteria included women who were pregnant or breastfeeding,
history of malabsorption syndrome, allergy to everolimus or other rapamycin analogs, and previous cancer (except for non-melanoma skin cancer or cervical carcinoma in situ) in the past 5
years. STUDY DESIGN AND TREATMENT PLAN Patients with TNBC received standard neoadjuvant anthracycline and taxane-based chemotherapy regimen and had to have the biopsy-proven residual disease
before being eligible. Prior to enrollment to this clinical study, all patients underwent baseline biopsy from a primary tumor or an abnormal axillary lymph node. Everolimus was given daily
orally at 10 mg for 12 weeks and cisplatin was given intravenously at 20 mg/m2 on days 1, 8, 15 for 4 cycles (each cycle of 21 days). Patients then underwent definitive surgery, including
axillary lymph node dissection, if indicated. The specimen was evaluated for chemotherapy response. RCB analysis was performed on the specimen by a previously validated method19. Toxicity
was assessed by Common Terminology Criteria for Adverse Events (CTCAE) version 3.0 (see Supplementary Figure 1). STATISTICAL METHODS Median follow-up was estimated by the reverse
Kaplan–Meier method20. Survival was estimated using the Kaplan–Meier method and 95% confidence intervals for timepoint survival probabilities (e.g., 1-year survival) were calculated using a
log–log transformed pointwise method. The Wilson score method was used to construct 95% confidence intervals for the response. All analyses were conducted using SAS 9.4 (SAS Institute Inc.,
Cary, NC, USA). ETHICS APPROVAL AND CONSENT Trial was monitored by the institutional Data Safety and Monitoring Board (DSMB). Written informed consent was obtained from all patients before
sample and data collection. The trial was registered at Clinicaltrials.gov identifier: NCT01931163. Registered August 29th, 2013. https://clinicaltrials.gov/ct2/show/NCT01931163. ANIMAL
SUBJECTS This article does not contain any studies with animals performed by any of the authors. DATA AVAILABILITY The datasets supporting the conclusions for the current study are stored in
a secured shared drive and will be shared by the corresponding author upon reasonable request. REFERENCES * Bauer, K. R., Brown, M., Cress, R. D., Parise, C. A. & Caggiano, V.
Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: A
population-based study from the California cancer Registry. _Cancer_ 109, 1721–1728. https://doi.org/10.1002/cncr.22618 (2007). Article PubMed Google Scholar * Dent, R. _et al._
Triple-negative breast cancer: Clinical features and patterns of recurrence. _Clin. Cancer Res._ 13, 4429–4434. https://doi.org/10.1158/1078-0432.CCR-06-3045 (2007). Article PubMed Google
Scholar * Carey, L. A. _et al._ The triple negative paradox: Primary tumor chemosensitivity of breast cancer subtypes. _Clin. Cancer Res._ 13, 2329–2334.
https://doi.org/10.1158/1078-0432.CCR-06-1109 (2007). Article ADS CAS PubMed Google Scholar * Liedtke, C. _et al._ Response to neoadjuvant therapy and long-term survival in patients
with triple-negative breast cancer. _J. Clin. Oncol._ 26, 1275–1281. https://doi.org/10.1200/JCO.2007.14.4147 (2008). Article PubMed Google Scholar * Lehmann, B. D. _et al._
Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. _J. Clin. Investig._ 121, 2750–2767.
https://doi.org/10.1172/JCI45014 (2011). Article CAS PubMed Google Scholar * Tutt, A. N. _et al._ Exploiting the DNA repair defect in BRCA mutant cells in the design of new therapeutic
strategies for cancer. _Cold Spring Harb. Symp. Quant. Biol._ 70, 139–148. https://doi.org/10.1101/sqb.2005.70.012 (2005). Article CAS PubMed Google Scholar * Silver, D. P. _et al._
Efficacy of neoadjuvant cisplatin in triple-negative breast cancer. _J. Clin. Oncol._ 28, 1145–1153. https://doi.org/10.1200/JCO.2009.22.4725 (2010). Article CAS PubMed PubMed Central
Google Scholar * Balko, J. M. _et al._ Molecular profiling of the residual disease of triple-negative breast cancers after neoadjuvant chemotherapy identifies actionable therapeutic
targets. _Cancer Discov._ 4, 232–245. https://doi.org/10.1158/2159-8290.CD-13-0286 (2014). Article CAS PubMed Google Scholar * Symmans, W. F. _et al._ Long-term prognostic risk after
neoadjuvant chemotherapy associated with residual cancer burden and breast cancer subtype. _J. Clin. Oncol._ 35, 1049–1060. https://doi.org/10.1200/JCO.2015.63.1010 (2017). Article CAS
PubMed PubMed Central Google Scholar * Rahman, N. _et al._ PALB2, which encodes a BRCA2-interacting protein, is a breast cancer susceptibility gene. _Nat. Genet._ 39, 165–167.
https://doi.org/10.1038/ng1959 (2007). Article CAS PubMed Google Scholar * Antoniou, A. C. _et al._ Breast-cancer risk in families with mutations in PALB2. _N. Engl. J. Med._ 371,
497–506. https://doi.org/10.1056/NEJMoa1400382 (2014). Article CAS PubMed PubMed Central Google Scholar * Cybulski, C. _et al._ Clinical outcomes in women with breast cancer and a PALB2
mutation: A prospective cohort analysis. _Lancet Oncol._ 16, 638–644. https://doi.org/10.1016/S1470-2045(15)70142-7 (2015). Article CAS PubMed Google Scholar * Isaac, D., Karapetyan, L.
& Tamkus, D. Association of germline PALB2 mutation and response to platinum-based chemotherapy in metastatic breast cancer: A case series. 1–5, https://doi.org/10.1200/po.17.00258
(2018). * Binder, KAR, Mick, R, O’Hara, M _et al._ A phase II, single arm study of maintenance rucaparib in patients with platinum-sensitive advanced pancreatic cancer and a pathogenic
germline or somatic mutation in BRCA1, BRCA2 or PALB2. _Cancer Res._ 79(Suppl. 13), CT234 (2019). * O’Reilly, E. M. _et al._ Randomized, multicenter, phase II trial of gemcitabine and
cisplatin with or without veliparib in patients with pancreas adenocarcinoma and a germline BRCA/PALB2 mutation. _J. Clin. Oncol._ 38, 1378–1388 (2020). Article Google Scholar * Jovanovic,
B. _et al._ A randomized phase II neoadjuvant study of cisplatin, paclitaxel with or without everolimus in patients with stage II/III triple-negative breast cancer (TNBC): Responses and
long-term outcome correlated with increased frequency of DNA damage response gene mutations, tnbc subtype, AR status, and Ki67. _Clin. Cancer Res._ 23, 4035–4045.
https://doi.org/10.1158/1078-0432.CCR-16-3055 (2017). Article CAS PubMed PubMed Central Google Scholar * Masuda, N. _et al._ Adjuvant capecitabine for breast cancer after preoperative
chemotherapy. _N. Engl. J. Med._ 376, 2147–2159. https://doi.org/10.1056/NEJMoa1612645 (2017). Article CAS PubMed Google Scholar * Rayson, D., Adjei, A., Suman, V. J., Wold, L. &
Ingle, J. Metaplastic breast cancer: Prognosis and response to systemic therapy. _Ann. Oncol._ 10, 413–419 (1999). Article CAS Google Scholar * Symmans, W. F. _et al._ Measurement of
residual breast cancer burden to predict survival after neoadjuvant chemotherapy. _J. Clin. Oncol._ 25, 4414–4422. https://doi.org/10.1200/JCO.2007.10.6823 (2007). Article PubMed Google
Scholar * Facchini, F. & Pettener, D. Chemical and physical methods in dating human skeletal remains. _Am. J. Phys. Anthropol._ 47, 65–70. https://doi.org/10.1002/ajpa.1330470112
(1977). Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS Authors want to acknowledge Zannatul Ferdous, Houston Methodist Research Institute for technical editing of
the manuscript. Authors also want to acknowledge the patients who participated in the study and their caregivers. FUNDING Authors acknowledge the support from Novartis for the clinical
trial. Role of Funder: Novartis provided the study drug. Novartis was not involved with the implementation of the protocol, analysis of the data, or preparation of the manuscript. The
corresponding author had final responsibility for the decision to submit the manuscript for publication. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Houston Methodist Cancer Center/Weill
Cornell Medicine, OPC 24, 6445 Main Street, Houston, TX, 77030, USA Kartik Anand, Tejal Patel, Polly Niravath, Angel Rodriguez, Jorge Darcourt, Anna Belcheva, Toniva Boone & Jenny Chang
* Houston Methodist Research Institute, Houston, TX, 77030, USA Joe Ensor & Jenny Chang Authors * Kartik Anand View author publications You can also search for this author inPubMed
Google Scholar * Tejal Patel View author publications You can also search for this author inPubMed Google Scholar * Polly Niravath View author publications You can also search for this
author inPubMed Google Scholar * Angel Rodriguez View author publications You can also search for this author inPubMed Google Scholar * Jorge Darcourt View author publications You can also
search for this author inPubMed Google Scholar * Anna Belcheva View author publications You can also search for this author inPubMed Google Scholar * Toniva Boone View author publications
You can also search for this author inPubMed Google Scholar * Joe Ensor View author publications You can also search for this author inPubMed Google Scholar * Jenny Chang View author
publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS J.C. and T.P. had full access to all study data in the study and take full responsibility for the
integrity of the data and the accuracy of the data analysis. Conceptualization and design: J.C.; acquisition, analysis, and interpretation of data: K.A., T.P., P.N., J.E, T.B, and J.C.;
statistical analysis: J.E.; obtained funding: J.C.; drafting of manuscript: K.A., J.C.; critical revision of the manuscript: all authors. CORRESPONDING AUTHOR Correspondence to Jenny Chang.
ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional
claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative
Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the
original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in
the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your
intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence,
visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Anand, K., Patel, T., Niravath, P. _et al._ Targeting mTOR and DNA repair
pathways in residual triple negative breast cancer post neoadjuvant chemotherapy. _Sci Rep_ 11, 82 (2021). https://doi.org/10.1038/s41598-020-80081-y Download citation * Received: 14 July
2020 * Accepted: 11 December 2020 * Published: 08 January 2021 * DOI: https://doi.org/10.1038/s41598-020-80081-y SHARE THIS ARTICLE Anyone you share the following link with will be able to
read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative