Play all audios:
ABSTRACT The serious problems of environmental pollution and energy shortage have pushed the green economy photocatalysis technology to the forefront of research. Therefore, the development
of an efficient and environmentally friendly photocatalyst has become a hotpot. In this work, magnetic Fe3O4/C/MnO2/C3N4 composite as photocatalyst was synthesized by combining in situ
coating with low-temperature reassembling of CN precursors. Morphology and structure characterization showed that the composite photocatalyst has a hollow core–shell flower-like structure.
In the composite, the magnetic Fe3O4 core was convenient for magnetic separation and recovery. The introduction of conductive C layer could avoid recombining photo-generated electrons and
holes effectively. Ultra-thin g-C3N4 layer could fully contact with coupled semiconductor. A Z-type heterojunction between g-C3N4 and flower-like MnO2 was constructed to improve
photocatalytic performance. Under the simulated visible light, 15 wt% photocatalyst exhibited 94.11% degradation efficiency in 140 min for degrading methyl orange and good recyclability in
the cycle experiment. SIMILAR CONTENT BEING VIEWED BY OTHERS EFFECTS OF IRON OXIDE CONTENTS ON PHOTOCATALYTIC PERFORMANCE OF NANOCOMPOSITES BASED ON G-C3N4 Article Open access 17 April 2023
ROOM-TEMPERATURE SYNTHESIS OF FE3O4@MOF-5 MAGNETIC HYBRID AS AN EFFICIENT CATALYST FOR THE ONE-POT GREEN SYNTHESIS OF TETRAHYDROPYRIDINES Article Open access 28 December 2024 GREEN
SYNTHESIS, CHARACTERIZATION, AND PHOTOCATALYTIC ACTIVITY OF SUPERPARAMAGNETIC MGFE2O4@ZNAL2O4 NANOCOMPOSITES Article Open access 19 July 2024 INTRODUCTION In recent years, with the increase
of wastewater discharge, a large amount of toxic and harmful organic pollutants are put into the water, which are difficult to completely degrade1,2,3,4,5,6. Advanced photocatalytic
oxidation technology with strong oxidation capacity and high efficiency was considered as a very promising wastewater treatment means for the degradation of organic
pollutants7,8,9,10,11,12,13,14. Among them, semiconductor-based photocatalysts with mild reaction conditions were most widely used15,16,17,18,19,20,21,22. In addition, various styles of
self-assembled photocatalyst nanostructures were synthesized, such as one-dimensional nanotubes23, two-dimensional layered structure24,25, three-dimensional network structure26,
three-dimensional flower-like structure27 and etc. MnO2 has some advantages including low cost, high stability and environmental friendliness. Besieds, its narrow band gap can increase the
utilization of visible light, which is very promising as a photocatalyst28,29,30,31,32. The layered structure of δ-MnO2 is formed by the MnO6 octahedral layer with shared edges, and there
are some cations and H2O molecules between the layers to maintain the charge balance33. δ-MnO2 has been widely used as a catalyst to purify the environment34,35,36. However, while the narrow
band gap increases the usage of visible light, it is also accompanied by the rapid recombination of photo-generated electrons and holes. The low photocatalytic efficiency restricts pure
MnO2 for practical applications37,38,39. Coupling MnO2 with other semiconductors to build a heterojunction is an effective method to prevent the recombination of photo-generated carriers.
Wang et al.40 first reported that g-C3N4 could decompose water to generate hydrogen by visible light irradiation. Subsequently, owing to excellent visible light activity and chemical
stability, g-C3N4 as photocatalyst has attracted a lot of attention41,42,43. Recently, Zhu et al.44 coated g-C3N4 on the semiconductor surface to form an ultra-thin g-C3N4 layer, avoiding
the low contact rate between the bulk g-C3N4 and the coupling semiconductor interface. Therefore, the design of the composite with ultra-thin g-C3N4 layer and MnO2 is expected to achieve
significant charge transfer at their interface by facilitating the separation of photo-generated carriers, thereby enhancing photocatalytic activity. In our previous research, Fe3O4 was
introduced as a magnetic core to construct a magnetically recyclable core–shell structure photocatalyst. The existence of magnetic core was beneficial to the recycle of the catalyst45,46. In
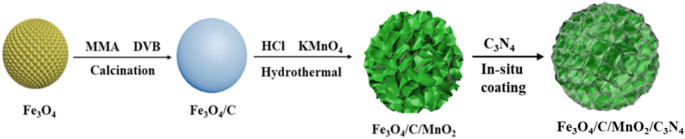
this paper, hollow Fe3O4 microspheres were also synthesized by using a hydrothermal method as magnetic cores. Subsequently, the carbon layer was obtained by calcining the polymer layer
formed on the periphery of Fe3O4 microspheres, which can protect the core and act as an electronic conductor simultaneously. Then, flower-like MnO2 was grown on the periphery of Fe3O4/C
microspheres through the hydrothermal method. Finally, the transparent CN precursor produced with the aid of the neutral hydrothermal process was polymerized in situ on the surface of above
microspheres at a low temperature. A flower-like Fe3O4/C/MnO2/C3N4 composite photocatalyst with core–shell structure was obtained. The photocatalyst displayed remarkable photocatalytic
activity for degrading the organic dye methyl orange (MO). The specific synthetic steps are shown in Fig. 1. EXPERIMENTAL SECTION PREPARATION OF FE3O4/C CORE–SHELL COMPOSITE First, hollow
Fe3O4 microspheres were obtained by the hydrothermal method47. Then the polymer layer was prepared through a distillation precipitation process. 0.10 g Fe3O4 microspheres were ultrasonically
dispersed in 80 mL acetonitrile. 1.0 mL divinylbenzene (DVB), 1.0 mL methyl methacrylate (MMA), and 0.040 g 2,2-azobisisobutyronitrile (AIBN) were also added into the above solution. The
system was heated in 90 °C water bath for 2 h. Afterwards, Fe3O4/P(MMA-DVB) microspheres were obtained by using an external magnet, and washed three times. Then, the Fe3O4/P(MMA-DVB) sample
was calcined at 600 °C for 2.0 h to obtain Fe3O4/C microspheres. PREPARATION OF FE3O4/C/MNO2 FLOWER-LIKE COMPOSITE 0.30 g Fe3O4/C microspheres were fully dispersed to 80 mL 0.055 M KMnO4
solution. Next, 1.0 mL HCl was added to the mixture dropwise. Thereafter, the mixture was transferred to an autoclave and heated to 100 °C for 6.0 h. At last, flower-like Fe3O4/C/MnO2
microspheres with core–shell structure were obtained by using an external magnet, and washed three times and lyophilized. PREPARATION OF FE3O4/C/MNO2/C3N4 COMPOSITE 6.0 g dicyandiamide was
calcined at 550 °C for 4.0 h to produce g-C3N4. Thereafter, 2.0 g g-C3N4 powder was dispersed in 80 mL deionized water, and then heated at 210 °C for 6 h to form a CN transparent precursor.
Fe3O4/C/MnO2 microspheres were added to the precursor (5.0, 10, 15, 20, 30 wt%). The solvent was slowly removed through a lyophilized process. Finally, Fe3O4/C/MnO2/C3N4 flower-like
photocatalyst was obtained via annealing at 200 °C for 4.0 h in a tube furnace under N2 protection. CHARACTERIZATION Scanning electron microscope (SEM, JSM-6700F, JEOL Ltd., Japan) was
employed to obtain a surface topography image of the samples. Transmission images were gotten by using a high-resolution transmission electron microscope (TEM, JEM-3010, Hitachi Co., Japan).
X-ray diffraction patterns of samples were obtained by the use of an X-ray diffractometer (XRD, Shimadzu XRD-7000, Shimadzu Co., Japan). X-ray photoelectron spectrometer (XPS, JPS-9010 MC,
JEOL Ltd., Japan) was utilized to obtain the samples’ surface elemental composition of the samples. Brunauer–Emmett–Teller (BET, ASAP 2020, Quantachrome, US) means was used to test the pore
size and specific surface area of the catalyst. The saturation magnetization of the samples was obtained by employing a vibrating sample magnetometer (VSM, Lake Shore 7307, Lake Shore Ltd.,
USA). A photochemical reactor (BL-GHX-V, Shanghai Bilang Instruments Co., Ltd., China) was used to simulate the illumination. The ultraviolet–visible absorption spectra were measured on an
ultraviolet–visible spectrophotometer (UV–vis, UV-5200PC, YuanXi, China). PHOTOCATALYTIC EXPERIMENT Firstly, 20 mg Fe3O4/C/MnO2/C3N4 photocatalyst were added to 65 mL, 10 mg/L MO solution.
Under dark environment, the mixture was agitated to reach adsorbed-desorbed equilibrium. Secondly, photocatalytic reaction was carried out with simulate light stemming from a 400 W metal
halide lamp. The absorbance of the solution at intervals was monitored with the help of UV–visible spectrophotometer. Ultimately, the degradation curves of the MO solution were recorded,
followed by the calculation of photocatalytic degradation rate. RESULTS AND DISCUSSION SEM and TEM images of samples are shown in Fig. 2. In Fig. 2a, Fe3O4 microspheres prepared by the
hydrothermal method have good dispersibility and uniform size of about 200 nm. Figure 2b shows Fe3O4/P(MMA-DVB) microspheres prepared by distillation precipitation process. Compared with the
former, the surface of the latter becomes much smoother, which proves the successful formation of polymer coating. And these polymer core–shell microspheres have a diameter of 225 nm. To
obtain the conductive carbon layer, the polymer microspheres were calcined and carbonized. The SEM image of Fe3O4/C microspheres is displayed in Fig. 2c. One can see that the original
core–shell structure of the material is not destroyed after the calcination treatment. And the agglomeration that originally occurred in Fe3O4/P(MMA-DVB) polymer microspheres has been
slightly weakened due to the carbonization treatment. From Fig. 2d,e, it can be found out that the flower-like morphology of the composite microspheres produced by the hydrothermal method is
composed of MnO2 intersecting sheets. And the overall particle size is about 480 nm. As shown in Fig. 2f, the overall flower-like morphology has not changed, but the thickness of the MnO2
flower sheets has increased significantly. This case indicates that the ultra-thin C3N4 layer is successfully formed on the surface of MnO2 to form a flower-like Fe3O4/C/MnO2/C3N4 composite
photocatalyst. It can be seen from Fig. 2g that the synthesized magnetic microspheres have a clear hollow structure with a particle size of about 200 nm. Figure 2h shows the TEM image of the
Fe3O4/C microspheres, which have a core–shell structure with 13 nm thickness of C shell. Figure 2i is the TEM image of the flower-like Fe3O4/C/MnO2/C3N4 microspheres. It can be found out
that the composite photocatalyst with a complete magnetic core and flower-like shell exhibits the diameter of around 480 nm. According to these results, the composite photocatalyst with a
magnetic core and flower-like shell was successfully prepared. The crystal phase composition of the composite was demonstrated by XRD characterization, as shown in Fig. 3I. Figure 3I-a is
the diffraction curve of the bulk g-C3N4 obtained by pyrolysis of dicyandiamide. The strong peak near 27.4° belongs to the (002) plane, corresponding to the crystal plane stack of the CN
aromatic system48. The broad peak at 13.0° belongs to the (100) plane ascribed to the triazine repeat unit44. Figure 3I-b is the diffraction curve of Fe3O4 microspheres showing the
diffraction peaks at 30.12°, 35.41°, 43.10°, 53.43°, 57.11°, and 62.52°, which are attributed to (220), (311), (400), (422), (511) and (440) crystal planes of Fe3O4 (JCDPS 85-1436)49. The
sharp peaks indicate that the synthesized Fe3O4 are well crystallized. In Fig. 3I-c, in addition to the diffraction peaks of Fe3O4 component, the diffraction peaks at 12.20°, 36.70°, and
65.70° correspond to (002), (006), and (119) crystal planes, in consistence with the crystal planes of MnO2 (JCDPS 18-0802)50. This case indicates that the MnO2 is successfully synthesized.
The diffraction curve of the Fe3O4/C/MnO2/C3N4 photocatalyst in Fig. 3I-d reveals that except for the diffraction peaks of Fe3O4 and MnO2, the diffraction peaks appear at 13.00° and 27.40°
are separately assigned to the (100) and (002) crystal planes of g-C3N4. This situation indicates that CN precursor successfully becomes g-C3N4 after low temperature polymerization and high
temperature calcination. XRD results show that the Fe3O4/C/MnO2/C3N4 composite photocatalyst were successfully synthesized. The XRD patterns cannot verify the existence of the C layer. For
further confirming the formation of the C layer, the Raman test was used to characterize the Fe3O4/C sample. The spectrum in Fig. 3II indicates two different peaks at 1344 cm−1 and 1596
cm−1, corresponding to D-band and G-band of carbon material, respectively. These results confirm the carbonization of Fe3O4/P(MMA-DVB) material, and Fe3O4/C microspheres are successfully
obtained. These two bands are related to the A1g phonon of _sp_3 carbon atoms in disordered graphite and the in-plane vibration of _sp_2 carbon atoms in the crystalline graphite,
respectively51. The peak intensity ratio (ID/IG) can evaluate the carbon material’s crystallinity. The smaller the value is, the higher the degree of atomic order is52. Herein, the value is
0.79, meaning that the carbon material is graphitized partially. Therefore, the presence of the carbon matrix can improve the electronic conductivity and help avoid the recombination of
photo-generated electron holes. The surface chemical composition and the chemical state of the products were demonstrated by XPS characterization. Figure 4a is the full-scan spectrum of the
photocatalyst, presenting the peaks of Mn, O, N, and C elements. From Fig. 4b, as for the Mn 2_p_ spectrum, two peaks at 653.9 eV and 642.3 eV correspond to Mn 2_p_1/2 and Mn 2_p_3/2. With
respect to the O1_s_, as illustrated in Fig. 4c, three peaks at 529.7 eV, 531.3 eV, 533.2 eV are fitted, which are separately attributed to the Mn–O–Mn lattice oxygen, surface hydroxyl and
surface adsorbed oxygen. The C1_s_ spectrum in Fig. 4d shows the sub-bands centered at 284.8 eV and 288.5 eV, which are ascribed to the C–C coordination of the surface-unstable carbon and
N=C–N2 of g-C3N4. In addition, there is another peak centering at 286.3 eV, which is assigned to the C-O bond formed between the C of C3N4 and the O of MnO2. This result indicates that MnO2
and g-C3N4 are closely connected and form a solid MnO2/g-C3N4 interface, promoting the transfer and separation of photo-generated carriers. In the case of the N1_s_ spectrum (Fig. 4e), the
peaks at 399.4 eV, 400.5 eV, 401.8 eV, and 405.3 eV are separately designated as carbon-bonded _sp_2 hybrid aromatic C=N–C, a tertiary nitrogen bonded to a carbon atom in the form N–(C)3, NH
and the charge effect or positive charge localization in the heterocyclic ring. The XPS spectra powerfully verify the surface chemical composition of the Fe3O4/C/MnO2/C3N4 photocatalyst.
The specific surface area and the pore structure of Fe3O4, Fe3O4/C, Fe3O4/C/MnO2 and Fe3O4/C/MnO2/C3N4 products are listed in Table 1. The former of the Fe3O4/C/MnO2 and Fe3O4/C/MnO2/C3N4
products are 119.56 m2/g and 120.25 m2/g, and the latter of them are 0.35 cm3/g and 0.31 cm3/g. Since C3N4 does not significantly affect the morphology of the composite structure, these
parameters of the two samples are almost similar. The higher values are owing to the flower-like structure of the composite photocatalyst. The increase in specific surface area is conducive
to exposing more active sites and increasing more surface adsorption, followed by improving catalytic performance. To evaluate the saturation magnetization value of Fe3O4, Fe3O4/C,
Fe3O4/C/MnO2 and Fe3O4/C/MnO2/C3N4, VSM measurement is conducted. It can be seen from Fig. 5a that the magnetization value of the Fe3O4 microspheres is 70.58 emu/g. After the carbon layer is
recombined, the value of Fe3O4/C microspheres decreases to 56.97 emu/g (Fig. 5b). After the flower-like MnO2 was fabricated, the content of Fe3O4 component is decreasing, which leads to the
value of Fe3O4/C/MnO2 microspheres decreases obviously to 37.62 emu/g (Fig. 5c). With the further formation of g-C3N4, the value is 30.02 emu/g (Fig. 5d). This value still meets the needs
of magnetic separation. As shown in the illustration, when the magnet is placed next to the Fe3O4/C/MnO2/C3N4 photocatalyst suspension, the photocatalyst can be quickly attracted to the side
of the cuvette in a short time. The results show that the photocatalyst has a good magnetic response to the magnetic field, favoring the magnetic separation from the mixed solution.
Determining the adsorption capacity of the photocatalyst in dark reaction, then degrading MO under simulated light is used to investigate the photocatalytic activity of the prepared
photocatalyst, and the results are shown in Fig. 6. Figure 6a reveals the mixture reached adsorption–desorption equilibrium within 60 min. And Fe3O4/C/MnO2/C3N4 can adsorb about 22% of MO
within 60 min, which is related to its higher specific surface area (120.25 m2/g). Figure 6b displays that UV–Vis is employed to monitor the change in the absorbance of the solution during
the photocatalytic reaction. In Fig. 6b, one can clearly view that MO was almost completely degraded with adding Fe3O4/C/MnO2/C3N4 composite photocatalyst after 140 min. The photocatalytic
degradation MO over Fe3O4/C/MnO2/C3N4 could be described by the following reactions: $${\text{photocatalyst}} + hv \to {\text{photocatalyst}}\left( {e^{ - } } \right) +
{\text{photocatalyst}}\left( {h^{ + } } \right)$$ (1) $${\text{H}}_{2} {\text{O}} + h^{ + } \to ^{ \cdot } {\text{OH}} + {\text{H}}^{ + }$$ (2) $${\text{O}}_{2} + e^{ - } \to ^{ \cdot }
{\text{O}}_{2}^{ - }$$ (3) $${\text{MO}} +^{ \cdot } {\text{OH/O}}_{2}^{ - } \to {\text{Mineralisation}}\;{\text{products}}$$ (4) Figure 6c indicates the change of the MO concentration ratio
Ct/C0 with varying the light time, in which C0 and Ct are the initial concentration of MO and the concentration of MO during the reaction, respectively. The degradation rate of MO solution
with Fe3O4/C/MnO2/C3N4 photocatalyst reaches 94.11%. From Fig. 6d, this reaction is attributed to a pseudo first-order reaction, which belongs to the Langmuir–Hinshelwood model with ln
(Ct/C0) = −_k_t. In the formula, _k_ is the apparent first-order rate constant. The calculated rate constant _k_ of Fe3O4/C/MnO2/C3N4 photocatalyst is 0.022 min−1. The excellent
photocatalytic performance of Fe3O4/C/MnO2/C3N4 composite material benefits from the synergistic effect between the various components. In order to find the optimal ratio, the effect of
amount of g-C3N4 on the photocatalytic efficiency was investigated. Meanwhile, determining the minimum optimal amount of photocatalyst in practical applications is important to reduce the
costs. The composite photocatalyst containing different amounts of g-C3N4 (5%, 10%, 15%, 20%, 30%) were used to degrade MO dyes under the same conditions. From Fig. 7a,b, when the amount of
g-C3N4 is 15%, the Fe3O4/C/MnO2/C3N4 composite photocatalyst has the highest value. In Fig. 7c, the effect of the amount of photocatalyst on the degradation efficiency is examined. The
results show that the photocatalytic efficiency gradually increases when the amount of photocatalyst increases in the range of 0–20 mg, due to the effective reaction area and the reactive
site increase. When the amount of photocatalyst continues to increase, the photocatalytic efficiency does not change significantly, which may be caused by the particle agglomeration
affecting the increase of active sites. Therefore, the optimal dosage of Fe3O4/C/MnO2/C3N4 photocatalyst is 20 mg. Considering the industrial application of Fe3O4/C/MnO2/C3N4 nanoparticles,
it is essential to investigate the recyclability and stability of the photocatalyst. The Fe3O4/C/MnO2/C3N4 was reused four times to examine their performances. And Fig. 7d reveals the
results that the degradation rates for the four cycles are 94.11%, 90.42%, 88.37% and 79.69%, respectively. There is no doubt that after the photocatalyst is recycled, the conversion rate
will decrease, which might result from the loss of sample during the cycle. However, even after four cycles, the value still has 79.69% that might be related with the structure stability of
the used photocatalysts, strongly demonstrating that the designed photocatalyst has excellent recyclability. In this study, Fe3O4/C/MnO2/C3N4 photocatalyst was synthesized by compounding
g-C3N4 on the surface of MnO2. In terms of enhanced photocatalytic activity, it is assumed that the charge transfer in the photocatalyst uses the Z-type mechanism, as shown in Fig. 8. For
the individual g-C3N4 or MnO2 component, due to thermodynamic effects, photo-generated holes in g-C3N4 cannot oxidize OH- to form •OH radicals, while photo-generated electrons in MnO2 cannot
generate ·O2− radicals effectively. Therefore, individual g-C3N4 or MnO2 material cannot possess good photocatalytic performances. However, after a heterojunction was fabricated between
these two components, the photo-generated electrons in the conduction band of MnO2 can be transferred to the valence band of g-C3N4 and combined with the photo-generated holes there. This
configuration of the Z-type scheme makes the utilization of holes from MnO2 and electrons from g-C3N4 remarkably enhanced. In addition, the conductive C layer can also increase the
photo-generated electron–hole pairs’ separation in MnO2, which effectively prevents the recombination of photo-generated carriers. In the meantime, the higher specific surface area supplies
much more active sites for photocatalytic activities. The prepared flower-like Fe3O4/C/MnO2/C3N4 photocatalyst forms a Z-type photocatalytic system, which effectively enhances the separation
of carrier, so that the composite material has excellent photocatalytic degradation efficiency. CONCLUSIONS In summary, a magnetic recyclable flower-like Fe3O4/C/MnO2/C3N4 heterojunction
photocatalyst was prepared for degrading organic dyes. The Fe3O4 core was used to facilitate magnetic separation and recovery. The C layer could conduct photo-generated electrons in MnO2 and
protect the core. The thin g-C3N4 layer was compounded on the surface of MnO2, which greatly improved the specific surface area and the reactive sites of the material. The obtained
Fe3O4/C/MnO2/C3N4 composites exhibited enhanced photocatalytic performance for the degradation of MO solution (65 mL, 10 mg/L) under simulated light irradiation. The maximum photocatalytic
degradation efficiency was 94.11% within 140 min. It was assumed that a Z-type heterojunction was fabricated between MnO2 and g-C3N4, which stimulated the electron transfer from the valence
band of MnO2 to the conduction band of g-C3N4. This structure promoted the photo-generated electron–hole pairs’ separation, inhibited the free charges’ recombination, and improved effective
use of visible light. In here, an effective method to construct heterostructure nanomaterials was provided for efficient photocatalytic degradation. REFERENCES * Masuda, Y. Bio-inspired
mineralization of nanostructured TiO2 on PET and FTO films with high surface area and high photocatalytic activity. _Sci. Rep._ 10, 13499 (2020). Article ADS CAS PubMed PubMed Central
Google Scholar * Lee, M. Y. _et al._ Comparison of UV/H2O2 and UV/PS processes for the treatment of reverse osmosis concentrate from municipal wastewater reclamation. _Chem. Eng. J._ 388,
124260 (2020). Article CAS Google Scholar * Liu, N. _et al._ Magnetic Fe3O4@MIL-53(Fe) nanocomposites derived from MIL-53(Fe) for the photocatalytic degradation of ibuprofen under visible
light irradiation. _Mater. Res. Bull._ 132, 111000 (2020). Article CAS Google Scholar * Takanabe, K. Photocatalyticwater splitting: quantitative approaches toward photocatalyst by
design. _ACS Catal._ 7, 8006–8022 (2017). Article CAS Google Scholar * Kumar, M., Basera, P., Saini, S. & Bhattacharya, S. Theoretical insights of codoping to modulate electronic
structure of TiO2 and SrTiO3 for enhanced photocatalytic efficiency. _Sci. Rep._ 10, 15372 (2020). Article ADS CAS PubMed PubMed Central Google Scholar * Fu, J., Xu, Q., Low, J.,
Jiang, C. & Yu, J. Ultrathin 2D/2D WO3/g-C3N4 step-scheme H2 production photocatalyst. _Appl. Catal. B Environ._ 243, 556–565 (2019). Article CAS Google Scholar * Trojanowicz, M.,
Bojanowska-Czajka, A., Bartosiewicz, I. & Kulisa, K. Advanced oxidation/reduction processes treatment for aqueous perfluorooctanoate (PFOA) and perfluorooctanesulfonate (PFOS): a review
of recent advances. _Chem. Eng. J._ 336, 170–199 (2018). Article CAS Google Scholar * Hodges, B. C., Cates, E. L. & Kim, J.-H. Challenges and prospects of advanced oxidation water
treatment processes using catalytic nanomaterials. _Nat. Nanotechnol._ 13, 642–650 (2018). Article ADS CAS PubMed Google Scholar * Xu, J., Olvera-Vargas, H., Loh, B. J. H. &
Lefebvre, O. FTO-TiO2 photoelectrocatalytic degradation of triphenyltin chloride coupled to photoelectro-Fenton: a mechanistic study. _Appl. Catal. B Environ._ 271, 118923 (2020). Article
CAS Google Scholar * Zhang, G. _et al._ Layered double hydroxides-based photocatalysts and visible-light driven photodegradation of organic pollutants: a review. _Chem. Eng. J._ 392,
123684 (2020). Article CAS Google Scholar * Sakhare, P. A. _et al._ Magnetically recoverable BiVO4/NiFe2O4 nanocomposite photocatalyst for efficient detoxification of polluted water under
collected sunlight. _Mater. Res. Bull._ 129, 110908 (2020). Article CAS Google Scholar * Zhang, Z. _et al._ Synthesis of 1D Bi12O17ClxBr2–x nanotube solid solutions with rich oxygen
vacancies for highly efficient removal of organic pollutants under visible light. _Appl. Catal. B Environ._ 269, 118774 (2020). Article CAS Google Scholar * Ma, Y. _et al._ Tuning
polyaniline nanostructures via end group substitutions and their morphology dependent electrochemical performances. _Polymer_ 156, 128–135 (2018). Article CAS Google Scholar * Ma, Y. _et
al._ Morphology-dependent electrochemical supercapacitors in multi-dimensional polyaniline nanostructures. _J. Mater. Chem. A_ 5, 14041–14052 (2017). Article CAS Google Scholar * Ran, J.,
Jaroniec, M. & Qiao, S. Z. Cocatalysts in semiconductor-based photocatalytic CO2 reduction: achievements, challenges, and opportunities. _Adv. Mater._ 30, 1704649 (2018). Article CAS
Google Scholar * Liu, D. _et al._ In-situ fabrication of atomic charge transferring path for constructing heterojunction photocatalysts with hierarchical structure. _Appl. Catal. B
Environ._ 248, 459–465 (2019). Article CAS Google Scholar * Jiang, D. _et al._ MoS2/SnNb2O6 2D/2D nanosheet heterojunctions with enhanced interfacial charge separation for boosting
photocatalytic hydrogen evolution. _J. Colloid Interface Sci._ 536, 1–8 (2019). Article ADS CAS PubMed Google Scholar * Zhong, J. _et al._ Opposite photocatalytic oxidation behaviors of
BiOCl and TiO2: direct hole transfer vs. indirect (OH)–O-center dot oxidation. _Appl. Catal. B Environ._ 241, 514–520 (2019). Article CAS Google Scholar * Weng, B., Qi, M.-Y., Han, C.,
Tang, Z.-R. & Xu, Y.-J. Photocorrosion inhibition of semiconductor-based photocatalysts: basic principle, current development, and future perspective. _Acs Catal._ 9, 4642–4687 (2019).
Article CAS Google Scholar * Wang, F., Li, Q. & Xu, D. Recent progress in semiconductor-based nanocomposite photocatalysts for solar-to-chemical energy conversion. _Adv. Energy
Mater._ 7, 1700529 (2017). Article CAS Google Scholar * Ma, Y. _et al._ Solid polyaniline dendrites consisting of high aspect ratio branches self-assembled using sodium lauryl sulfonate
as soft templates: synthesis and electrochemical performance. _Polymer_ 182, 121808 (2019). Article CAS Google Scholar * Ma, Y. _et al._ Three-dimensional core-shell Fe3O4/polyaniline
coaxial heterogeneous nanonets: preparation and high performance supercapacitor electrodes. _Electrochim. Acta_ 315, 114–123 (2019). Article CAS Google Scholar * Geng, R. _et al._ In situ
construction of Ag/TiO2/g-C3N4 heterojunction nanocomposite based on hierarchical co-assembly with sustainable hydrogen evolution. _Nanomaterials_ 10, 1 (2020). Article CAS Google Scholar
* Li, K. K. _et al._ Fabrication of tunable hierarchical MXene@AuNPs nanocomposites constructed by self-reduction reactions with enhanced catalytic performances. _Sci. China Mater._ 61,
728–736 (2018). Article CAS Google Scholar * Yin, J. J. _et al._ Facile preparation of self-assembled MXene@Au@CdS nanocomposite with enhanced photocatalytic hydrogen production activity.
_Sci. China Mater._ 63, 2228–2238 (2020). Article CAS Google Scholar * Jiao, T. F. _et al._ Self-assembly reduced graphene oxide nanosheet hydrogel fabrication by anchorage of
chitosan/silver and its potential efficient application toward dye degradation for wastewater treatments. _ACS Sustain. Chem. Eng._ 3, 3130–3139 (2015). Article CAS Google Scholar * Gao,
S. _et al._ A novel 3D hollow magnetic Fe3O4/BiOI heterojunction with enhanced photocatalytic performance for bisphenol A degradation. _Chem. Eng. J._ 307, 1055–1065 (2017). Article CAS
Google Scholar * Das, S., Sarnanta, A. & Jana, S. Light-assisted synthesis of hierarchical flower-like MnO2 nanocomposites with solar light induced enhanced photocatalyticactivity. _ACS
Sustain. Chem. Eng._ 5, 9086–9094 (2017). Article CAS Google Scholar * Wang, H., Chen, S., Wang, Z., Zhou, Y. & Wu, Z. A novel hybrid Bi2MoO6 MnO2 catalysts with the superior plasma
induced pseudo photocatalytic-catalytic performance for ethyl acetate degradation. _Appl. Catal. B Environ._ 254, 339–350 (2019). Article CAS Google Scholar * Liu, Y. _et al._ Promoted
oxygen activation of layered micro-mesoporous structured titanium phosphate nanoplates by coupling nano-sized delta-MnO2 with surface pits for efficient photocatalytic oxidation of CO.
_Appl. Catal. B Environ._ 254, 260–269 (2019). Article CAS Google Scholar * Ding, Y. _et al._ Rational design of Z-scheme PtS-ZnIn2S4/WO3-MnO2 for overall photo catalytic water splitting
under visible light. _Appl. Catal. B Environ._ 258, 117948 (2019). Article CAS Google Scholar * Yang, Y. _et al._ UV–vis-infrared light-driven photothermocatalytic abatement of CO on Cu
doped ramsdellite MnO2 nanosheets enhanced by a photoactivation effect. _Appl. Catal. B Environ._ 224, 751–760 (2018). Article CAS Google Scholar * Liang, X. _et al._ Coupled
morphological and structural evolution of delta-MnO2 to alpha-MnO2 through multistage oriented assembly processes: the role of Mn(iii). _Environ. Sci. Nano_ 7, 238–249 (2020). Article CAS
Google Scholar * Zhang, Y., Li, H., Zhang, L., Gao, R. & Dai, W.-L. Construction of highly efficient 3D/2D MnO2/g-C3N4 nanocomposite in the epoxidation of styrene with TBHP. _ACS
Sustain. Chem. Eng._ 7, 17008–17019 (2019). Article CAS Google Scholar * Huang, J. _et al._ Effects of MnO2 of different structures on activation of peroxymonosulfate for bisphenol A
degradation under acidic conditions. _Chem. Eng. J._ 370, 906–915 (2019). Article CAS Google Scholar * Xu, Y. _et al._ Selective conversion of syngas to aromatics over Fe3O4@MnO2 and
hollow HZSM-5 bifunctional catalysts. _ACS Catal._ 9, 5147–5156 (2019). Article CAS Google Scholar * Zhao, J. _et al._ Energy-efficient fabrication of a novel multivalence Mn3O4–MnO2
heterojunction for dye degradation under visible light irradiation. _Appl. Catal. B Environ._ 202, 509–517 (2017). Article CAS Google Scholar * Chen, M. _et al._ FeOOH-loaded MnO2
nano-composite: an efficient emergency material for thallium pollution incident. _J. Environ. Manag._ 192, 31–38 (2017). Article CAS Google Scholar * Zhang, Z. _et al._ Conversion of
CaTi1−xMnxO3 delta based photocatalyst for photocatalytic reduction of NO via structure-reforming of Ti-bearing blast furnace slag. _ACS Sustain. Chem. Eng._ 7, 10299–10309 (2019). Article
CAS Google Scholar * Wang, X. _et al._ A metal-free polymeric photocatalyst for hydrogen production from water under visible light. _Nat. Mater._ 8, 76–80 (2009). Article ADS CAS PubMed
Google Scholar * Lin, L. _et al._ Crystalline carbon nitride semiconductors prepared at different temperatures for photocatalytic hydrogen production. _Appl. Catal. B Environ._ 231,
234–241 (2018). Article CAS Google Scholar * Fu, J., Yu, J., Jiang, C. & Cheng, B. g-C3N4 based heterostructured photocatalysts. _Adv. Energy Mater._ 8, 1701503 (2018). Article CAS
Google Scholar * Yang, C., Wang, B., Zhang, L., Yin, L. & Wang, X. Synthesis of layered carbonitrides from biotic molecules for photoredox transformations. _Angew. Chem. Int. Ed. Engl._
56, 6627–6631 (2017). Article CAS PubMed Google Scholar * Wang, Y., Yang, W., Chen, X., Wang, J. & Zhu, Y. Photocatalytic activity enhancement of core-shell structure g-C3N4@TiO2
via controlled ultrathin g-C3N4 layer. _Appl. Catal. B Environ._ 220, 337–347 (2018). Article CAS Google Scholar * Boruah, P. K. & Das, M. R. Dual responsive magnetic
Fe3O4–TiO2/graphene nanocomposite as an artificial nanozyme for the colorimetric detection and photodegradation of pesticide in an aqueous medium. _J. Hazard Mater._ 385, 121516 (2020).
Article CAS PubMed Google Scholar * Yuan, D. _et al._ Superparamagnetic polymer composite microspheres supported Schiff base palladium complex: an efficient and reusable catalyst for the
Suzuki coupling reactions. _Chem. Eng. J._ 287, 241–251 (2016). Article CAS Google Scholar * Liu, Y. _et al._ One-pot hydrothermal synthesis of highly monodisperse water-dispersible
hollow magnetic microspheres and construction of photonic crystals. _Chem. Eng. J._ 259, 779–786 (2015). Article CAS Google Scholar * Dong, Z., Wu, Y., Thirugnanam, N. & Li, G. Double
Z-scheme ZnO/ZnS/g-C3N4 ternary structure for efficient photocatalytic H2 production. _Appl. Surf. Sci._ 430, 293–300 (2018). Article ADS CAS Google Scholar * Wang, D., Duan, H., Lü, J.
& Lü, C. Fabrication of thermo-responsive polymer functionalized reduced graphene oxide@Fe3O4@Au magnetic nanocomposites for enhanced catalytic applications. _J. Mater. Chem. A_ 5,
5088–5097 (2017). Article CAS Google Scholar * Zhang, H., Cao, D. & Bai, X. High rate performance of aqueous magnesium-ion batteries based on the δ-MnO2@carbon molecular sieves
composite as the cathode and nanowire VO2 as the anode. _J. Power Sources_ 444, 227299 (2019). Article CAS Google Scholar * Schuepfer, D. B. _et al._ Assessing the structural properties
of graphitic and non-graphitic carbons by Raman spectroscopy. _Carbon_ 161, 359–372 (2020). Article CAS Google Scholar * Tang, J. & Yamauchi, Y. MOF morphologies in control. _Nat.
Chem._ 8, 638–639 (2016). Article CAS PubMed Google Scholar Download references ACKNOWLEDGMENTS This work is financially supported by National Natural Science Foundation of China (Grant
No. 51503116), the Shandong Provincial Natural Science Foundation (Grant No. ZR2019BB063) and the Applied Basic Research Foundation of Qingdao City (Grant No. 19-6-2-13-cg). AUTHOR
INFORMATION AUTHORS AND AFFILIATIONS * School of Civil Engineering, Qingdao University of Technology, Qingdao, 266033, People’s Republic of China Mingliang Ma, Yuying Yang, Yan Chen, Jiabin
Jiang, Zunfa Wang, Weibo Huang, Mingqing Liu, Dongxue Ma & Xiaoning Yan * School of Material Science and Engineering, Shandong University of Science and Technology, Qingdao, 266590,
People’s Republic of China Yong Ma * Key Laboratory of Marine Environmental Corrosion and Bio-Fouling, Institute of Oceanology, Chinese Academy of Sciences, Qingdao, 266071, People’s
Republic of China Yuying Yang * School of Environmental and Municipal Engineering, Qingdao University of Technology, Qingdao, 266033, People’s Republic of China Shasha Wang Authors *
Mingliang Ma View author publications You can also search for this author inPubMed Google Scholar * Yuying Yang View author publications You can also search for this author inPubMed Google
Scholar * Yan Chen View author publications You can also search for this author inPubMed Google Scholar * Jiabin Jiang View author publications You can also search for this author inPubMed
Google Scholar * Yong Ma View author publications You can also search for this author inPubMed Google Scholar * Zunfa Wang View author publications You can also search for this author
inPubMed Google Scholar * Weibo Huang View author publications You can also search for this author inPubMed Google Scholar * Shasha Wang View author publications You can also search for this
author inPubMed Google Scholar * Mingqing Liu View author publications You can also search for this author inPubMed Google Scholar * Dongxue Ma View author publications You can also search
for this author inPubMed Google Scholar * Xiaoning Yan View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS M.M., Y.Y. and Y.C. wrote the main
manuscript text. Y.Y. drew the flow chart and mechanism diagram. Y.M. ananlyzed the data. J.J.,Y.M., Z.W., W.H., S.W., M.L., D.M. and X.Y. collected data. All authors reviewed the
manuscript. CORRESPONDING AUTHOR Correspondence to Yong Ma. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a
Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit
to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are
included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons
licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of
this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Ma, M., Yang, Y., Chen, Y. _et al._ Fabrication of hollow
flower-like magnetic Fe3O4/C/MnO2/C3N4 composite with enhanced photocatalytic activity. _Sci Rep_ 11, 2597 (2021). https://doi.org/10.1038/s41598-021-81974-2 Download citation * Received: 22
November 2020 * Accepted: 14 January 2021 * Published: 28 January 2021 * DOI: https://doi.org/10.1038/s41598-021-81974-2 SHARE THIS ARTICLE Anyone you share the following link with will be
able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative