Play all audios:
ABSTRACT Nutritional intake can promote early neonatal brain development in very preterm born neonates (< 32 weeks’ gestation). In a group of 7-year-old very preterm born children
followed since birth, we examined whether early nutrient intake in the first weeks of life would be associated with long-term brain function and neurocognitive skills at school age. Children
underwent resting-state functional MRI (fMRI), intelligence testing (Wechsler Intelligence Scale for Children, 5th Ed) and visual-motor processing (Beery-Buktenica, 5th Ed) at 7 years.
Relationships were assessed between neonatal macronutrient intakes, functional connectivity strength between thalamic and default mode networks (DMN), and neuro-cognitive function using
multivariable regression. Greater functional connectivity strength between thalamic networks and DMN was associated with greater intake of protein in the first week (β = 0.17; 95% CI 0.11,
0.23, p < 0.001) but lower intakes of fat (β = − 0.06; 95% CI − 0.09, − 0.02, p = 0.001) and carbohydrates (β = − 0.03; 95% CI − 0.04, − 0.01, p = 0.003). Connectivity strength was also
associated with protein intake during the first month (β = 0.22; 95% CI 0.06, 0.37, p = 0.006). Importantly, greater thalamic-DMN connectivity strength was associated with higher processing
speed indices (β = 26.9; 95% CI 4.21, 49.49, p = 0.02) and visual processing scores (β = 9.03; 95% CI 2.27, 15.79, p = 0.009). Optimizing early protein intake may contribute to promoting
long-term brain health in preterm-born children. SIMILAR CONTENT BEING VIEWED BY OTHERS BRAIN VOLUMES AND CORTICAL THICKNESS AND ASSOCIATIONS WITH COGNITION IN CHILDREN BORN EXTREMELY
PRETERM Article Open access 21 August 2024 ALTERED FUNCTIONAL CONNECTIVITY IN CHILDREN BORN VERY PRETERM AT SCHOOL AGE Article Open access 04 May 2022 EARLY LIFE FACTORS AND STRUCTURAL BRAIN
NETWORK IN CHILDREN WITH OVERWEIGHT/OBESITY: THE ACTIVEBRAINS PROJECT Article 08 December 2023 INTRODUCTION Very preterm birth (< 32 weeks’ gestational age) remains a significant
predictor of morbidity, with a sizable proportion of survivors developing motor difficulties1,2. Neonates born at younger gestational ages are at a higher risk of adverse outcomes3, and the
risk is highest in those born < 30 weeks’ gestation4,5. Those neonates who escape major deficits may also go on to have subtle deficits in cognitive ability that are revealed at preschool
and school age6,7. Cognitive and motor impairments in children born preterm have been associated with brain dysmaturation, particularly in the prefrontal cortices, which are essential for
higher order cognitive functions. Further, longitudinal and cross-sectional neuroimaging studies have reported alterations in brain structural and functional development in infants, children
and adolescents born preterm, including alterations in white matter8, decreases in brain volumes and cortical thickness in regionally specific locations in the thalamus, amygdala,
hippocampus, frontal, parietal and temporal cortices9,10,11,12, with greater degree of prematurity associated with larger changes13. Resting-state functional MRI has provided insight into
functional connectivity patterns in infants and children born preterm14,15,16. Alterations in structural and intrinsic functional connectivity affecting networks associated with higher-order
cognitive functions including default mode networks (DMN) have been reported in school-age children, adolescents and adults born preterm14,17,18. The DMN develops in the third trimester,
which is marked by substantial increases in both functional connectivity and development of white matter supporting a globally integrated network of brain regions19,20,21. The DMN is
separated into anterior and posterior subdivisions22. The anterior DMN includes portions of the medial and lateral prefrontal cortices as well as the anterior cingulate cortex, and anterior
temporal lobe. It is involved in higher order cognitive functions21 and early disruptions in this network may underlie cognitive deficits in children born preterm23,24. Some evidence
suggests that enhanced nutrition may protect brain structural and functional development in children born preterm25,26. For example, protein and energy intake during the first week after
birth positively predicted cognitive outcomes at 18 months in a cohort of extremely low birth weight infants27. In another cohort of very preterm born infants who underwent serial MRI scans
from birth, total energy, carbohydrates, lipids and protein intake in the first few weeks positively predicted larger brain volumes at term equivalent age as well as cognitive outcomes at 18
months28. Further, compared to preterm infants randomized to a standard diet, those randomized to a high nutrient diet had larger caudate nucleus volumes in adolescence which correlated
with higher verbal IQs, particularly in boys29. However, there have been no studies of the relationship between very early macronutrient intakes in very preterm infants and brain
connectivity and neurodevelopmental outcomes at school age, when different aspects of brain function can be more reliably assessed. We examined brain functional connectivity and cognitive
and visuomotor ability at 7 years of age in a group of children born very preterm in whom daily actual macronutrient intakes and clinical variables were collected prospectively. During the
course of the study, the amount of dietary intake of protein was increased as part of a planned change in clinical care to better meet international recommendations30, as previously
described31. We focused on frontal resting state networks, given previous research suggesting structural and functional alterations in the prefrontal cortices underlying cognitive32 and
visual perceptual abilities33 in children born preterm. Our a-priori hypotheses were that early neonatal macronutrient intakes would be associated with functional connectivity between the
thalamic and default mode networks (DMN) at 7 years, and that functional connectivity would be associated with cognitive ability and visual-motor function. METHODS PARTICIPANTS The PIANO
Study cohort has been reported previously34. In brief, all infants born at < 30 weeks’ gestation or < 1500 g between July 2005 and October 2008 inclusive and admitted to the neonatal
intensive care unit at National Women’s Hospital, Auckland, New Zealand for at least the first postnatal week were eligible for recruitment into a follow-up study at 7 years of age. Of the
128 children assessed at 7 years (28% of original cohort), 113 consented to MRI, 102 completed at least some MRI sequences, and 56 successfully completed resting-state functional MRI
sequences. The study was approved by the Northern B ethics committee (NTY/12/05/035) and the Auckland District Health Board (ADHB 5486). A statement to confirm that all methods were carried
out in accordance with relevant guidelines and regulations. Informed consent was obtained from a parent and/or legal guardian. Clinical data were collected from the medical records by
trained clinical research staff. All daily actual parenteral and enteral intakes (excluding blood products) were recorded for the first 28 days. Energy intake was computed with proteins and
carbohydrates providing 4 kilocalories (kcal) per gram and lipids providing 9 kcal per gram. Breast milk and formula composition was based on commercial notifications34. Intakes per kg were
calculated for each day using the most recent highest weight, and averaged for the first 7 days and first 28 days (first month). MAGNETIC RESONANCE IMAGING AND ANALYSIS Children underwent
anatomical and functional MRI on a 3 T scanner (Siemens, Skyra, Erlangen, Germany). Anatomical images were acquired using an MPRAGE pulse sequence ([repetition time]TR, 2000 ms, [echo
time]TE, 3510 ms, [inversion time]TI, 1010 ms, slice thickness, 0.85 mm, FOV, 210 × 210 mm). Resting-state fMRI was acquired using an echo-planar imaging sequence (TR, 3200 ms, TE, 40 ms,
FOV, 147 × 147 mm, matrix, 64 × 64, slice thickness, 3 mm). Resting-state fMRI data were preprocessed using the FMRIB Software Library (FSL; version 5.0.6). Preprocessing included: slice
timing and motion correction, spatial smoothing, band-pass filtering (suppressing physiological noise) and whole-brain tissue extraction. Data were nonlinearly registered to a standardized
MRI template in MNI (Montreal Neurological Institute) space. Whole-brain resting state connectivity was examined using a group temporal concatenation independent components analysis (ICA),
available in FSL using MELODIC (Multivariate Exploratory Linear Decomposition into Independent Components, version 4.0). This produced a set of independent components common to the whole
group. All resting-state components were visually inspected. Resulting ICA maps were thresholded at Z ≥ 4. The time courses from the independent components were extracted. NEUROPSYCHOLOGICAL
TESTING Children underwent neuropsychological testing by a trained assessor. Full scale IQ (FSIQ) was assessed using the Wechsler Intelligence Scale for Children 5th Ed (WISC-V) (standard
mean score 100, standard deviation [SD] 15)29. Subtests include the perceptual reasoning index (PRI), processing speed index (PSI), working memory index (WMI), and the verbal comprehension
index (VCI). Visuomotor integration was assessed using Beery–Buktenica Developmental Test of Visual–Motor Integration (Beery), 5th edition35, which assesses motor and perceptual skills. The
maximum raw score for each of the three sub-tests is 30. The scores were converted to standardized scores. STATISTICAL ANALYSIS All analyses were carried out using Statistical Package for
the Social Sciences (v.25 SPSS, Chicago, IL). The time courses from the thalamic and DMN were extracted. Correlation coefficients describing the connectivity strength between the thalamus
and the DMN time courses were calculated for each participant. To assess the relationship between early macronutrient intakes and functional connectivity strength between the subcortical and
cortical resting-state networks, we used a general linear model, with the correlation coefficients describing thalamocortical connectivity strength entered as the dependent variables and
macronutrient intakes (i.e., protein, carbohydrates, fats, total energy) as independent variables. Analyses were adjusted for gestational age (GA) at birth, birth weight z score, age at scan
and biological sex. Birthweight z score reflects growth before birth, and is expressed in standard deviation scores, which are independent of absolute weight. Since we had one _a-priori_
hypothesis (that neonatal macronutrient intake would be associated with functional connectivity between the thalamic and cortical networks at 7 years of age), we set the alpha level at 0.05.
To assess the relationships between functional connectivity strength and 7-year cognitive and visuomotor abilities, we used general linear models, with the correlation coefficients
describing the connectivity strength between the thalamic and cortical networks entered as independent variables and the scores on the WISC-V subtests and the Beery entered as dependent
variables. Analyses were adjusted for GA at birth, birth weight z score, age at scan and biological sex. Since we had two a-priori hypotheses (that both cognitive and visuomotor scores would
be associated with functional connectivity at 7 years), we set the alpha level at 0.025. RESULTS Of the total 113 children, 102 children underwent MRI, of whom 13 were unable to complete
the resting-state scans, and an additional 33 children moved excessively during the scans. One of these children had grade III/IV IVH as a neonate. Analyzable data were available for 56
children (Table 1). The neonatal macronutrient intake for the cohort is available in Table 2. No significant differences in sex (p = 0.30), adjusted age (t = − 0.9, p = 0.35), GA at birth (t
= 0.22, p = 0.08), or birth weight (t = 0.91, p = 0.37) were evident between the children with useable MRI data compared to the children in the full cohort. There were also no differences
in neonatal macronutrient intakes between the children with included MRI data and the other children in the full cohort (all p > 0.05). COGNITIVE OUTCOMES The majority of children
performed within the typical range (i.e., 70–130) on the WISC-V (Table 3). GA at birth was related to FSIQ, VCI, PRI, and PSI scores (all, p < 0.05), but sex was not related to any of the
WISC-V scores (p > 0.33). No significant differences were noted between the children whose MRI data were or were not included on the WISC-V subtests (all, p > 0.05), except for the
PSI (p = 0.04). VISUOMOTOR OUTCOMES Most scores on the Beery were also in the typical range (Table 3). GA was not associated with any of the visuomotor outcome scores (all, p > 0.06), but
girls had higher scores than boys (B = 0.21 [females], p = 0.003). No significant differences were noted between the children whose MRI data were or were not included on the Beery (all, p
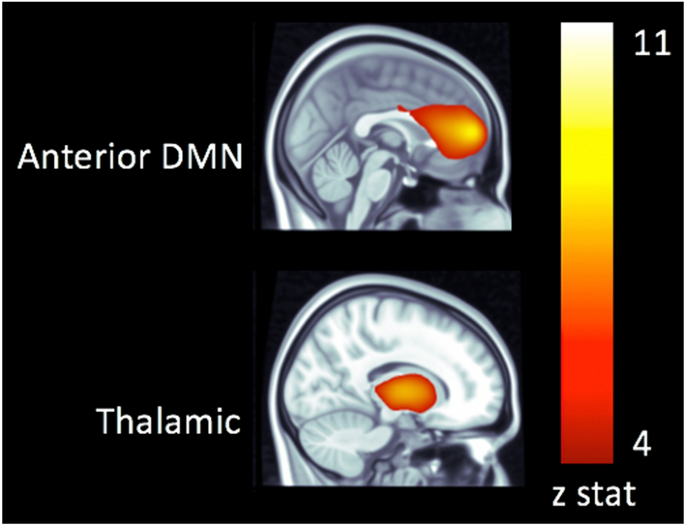
> 0.05). RESTING-STATE NETWORKS Independent components analysis produced whole-brain functional connectivity maps, with robust resting state activity in anterior default mode (aDMN) and
thalamic networks (Fig. 1). Greater functional connectivity strength between the thalamic networks and aDMN (range: 0–1) was predicted by greater intake of protein in the first week (β =
0.17; 95% CI 0.11, 0.23, p < 0.001, Fig. 2) but lower intakes of fat (β = − 0.06; 95% CI − 0.09, − 0.02, p = 0.001) and carbohydrates (β = − 0.03; 95% CI − 0.04, − 0.01, p = 0.003). Total
energy intake in the first week did not predict thalamocortical connectivity strength (β = -0.002; 95% CI − 0.006, 0.001, p = 0.1). Thalamocortical connectivity strength was also positively
associated with increased intake of protein in the first 28 days (β = 0.22; 95% CI 0.06, 0.37, p = 0.006) but not with intakes of fats, carbohydrates or total energy (all p > 0.1). In
turn, thalamocortical connectivity strength predicted higher scores for the PSI subtest of the WISC (β = 26.9; 95% CI 4.21, 49.49, p = 0.02, Fig. 3) but not for the WMI (β = 23.46; 95% CI
0.001, 46.9, p = 0.0.5), PRI (β = 23.25; 95% CI − 3.14, 49.64, p = 0.0.8), or VCI subtest scores (β = − 3.1; 95% CI − 29.91, 20.67, p = 0.8) or full-scale IQ (β = 20.2; 95% CI − 3.79, 44.18,
p = 0.1). Thalamocortical connectivity strength was also related to visual perceptual scores on the Beery (β = 9.03; 95% CI 2.27, 15.79, p = 0.009), but not the Beery visuomotor index (β =
− 2.39; 95% CI − 6.47, 1.7, p = 0.3) or motor scores (β = 2.52; 95% CI − 2.62, 7.66, p = 0.3). DISCUSSION Seven-year-old children born very preterm, who had higher protein intake in the
first week and first 28 days after birth positively predicted the strength of the connectivity between subcortical and cortical resting state networks at 7 years of age, and in turn, that
this connectivity is associated with specific neurocognitive skills such as processing speed index. Our findings are in line with a growing body of evidence indicating that very early
macronutrient intake has long-term effects on preterm brain development and functional outcomes at school age. EARLY NUTRIENTS AND OUTCOMES IN PRETERM INFANTS Our observation of an
association between early protein intake and functional connectivity between the thalamic and the anterior default mode networks at 7 years of age is consistent with previous reports that
nutrition is an important predictor of growth and outcome in preterm neonates36. Greater energy and lipid intake during the first two weeks after birth predicted larger subcortical
structures, cerebellum, and total brain, and accelerated white matter microstructural maturation over the course of neonatal intensive care to term age28. Protein intake in the first two
weeks also predicted 18-month cognitive and motor outcomes in very low birth weight neonates born preterm37. Stephens et al. reported that a 1 g/kg/day increase in protein intake up to
2.5–3.5 g/kg/day was independently associated with an ~ 8 point increase in cognitive scores at 18 months27. Protein intakes in our study cohort were largely within this range. In contrast
to these findings, another report noted that high protein intake (4 g/kg/day) was associated with neurodevelopmental impairments at 2 years in very low birth weight children38. These
findings suggest that adequate early protein intake is essential for optimal brain growth, although adverse effects of protein overfeeding cannot be excluded. The specific mechanisms
regarding early protein intake and the association with functional brain connectivity may be attributed to regional increases in brain growth, promotion of synaptic connectivity or a
combination of macrostructural and functional changes that are supportive of neuroplasticity25. Previous studies have reported that early intake of fat and energy rather than protein were
important predictors of brain maturation16 and later cognitive function28,39. However, other nutrients including protein, choline and zinc are also essential for healthy brain development40.
In our study only protein intake positively predicted 7-year thalamocortical connectivity. Protein is essential for cell proliferation and synaptogenesis, which may support developing
thalamocortical connectivity41, perhaps by supporting myelination of anterior white matter pathways in the preterm neonatal brain and hence connectivity between thalamic networks and the
anterior DMN. Compared with previous studies, our cohort had on average greater energy and fat intakes, and similar or greater protein intakes. Thus, it is possible that protein intake has
an additional effect on brain development only if energy intake is adequate. Remarkably, these relationships are seen following nutritional differences even in the first week after birth,
emphasising the importance of very early nutrition for optimising long-term outcomes. FUNCTIONAL CONNECTIVITY, COGNITIVE AND VISUOMOTOR ABILITIES Resting-state networks have been reliably
detected in neonates, infants and children born preterm during the first few weeks to years of life42,43. Previous research has demonstrated alterations in thalamic networks and
thalamocortical connectivity strength in preterm infants16,44,45 and that thalamocortical connectivity predicted cognitive scores in the second year of life45. Our findings suggest that
alterations in thalamocortical connectivity persist beyond the neonatal and early childhood period and have long lasting effects on brain function and specific cognitive skills. Indeed, the
association of functional connectivity with visual perceptual abilities is consistent with previous functional neuroimaging studies of school-age children born preterm showing that altered
functional connectivity in the prefrontal cortices is associated with visual-perceptual impairments33,46. Our results suggest that alterations in corticothalamic connectivity may contribute
to higher order vision deficits associated with preterm birth. CONCLUSIONS In a cohort of school aged children born very preterm, greater protein intake during the first week and the first
month of life were positively associated with more robust functional connectivity strength between thalamic networks and anterior DMN essential for higher order cognitive functioning.
Further, greater strength between the thalamocortical networks was associated with processing speed and visual perceptual abilities at 7 years of age. However, the generalizability of
findings from this small study requires confirmation. The full cohort recruited at birth were not assessed with MRI at 7 years. The subset of children included in the current study may not
be entirely be representative of the full sample. Future studies with larger samples will permit the examination of early nutrient intake on the development of functional connectivity
networks, and also the possible effects of social adversity. Early neonatal nutrition, particularly increasing early protein intake in very preterm neonates, may help mitigate the adverse
effects of prematurity and neonatal intensive care on brain development. REFERENCES * Barfield, W. D. Public health implications of very preterm birth. _Clin. Perinatol._ 45, 565–577.
https://doi.org/10.1016/j.clp.2018.05.007 (2018). Article PubMed PubMed Central Google Scholar * Frey, H. A. & Klebanoff, M. A. The epidemiology, etiology, and costs of preterm
birth. _Semin. Fetal Neonatal Med._ 21, 68–73. https://doi.org/10.1016/j.siny.2015.12.011 (2016). Article PubMed Google Scholar * Glass, H. C. _et al._ Outcomes for extremely premature
infants. _Anesth. Analg._ 120, 1337–1351. https://doi.org/10.1213/ANE.0000000000000705 (2015). Article PubMed PubMed Central Google Scholar * Isaacs, E. B., Morley, R. & Lucas, A.
Early diet and general cognitive outcome at adolescence in children born at or below 30 weeks gestation. _J. Pediatr._ 155, 229–234. https://doi.org/10.1016/j.jpeds.2009.02.030 (2009).
Article PubMed Google Scholar * Wocadlo, C. & Rieger, I. Developmental outcome at 12 months corrected age for infants born less than 30 weeks gestation: Influence of reduced
intrauterine and postnatal growth. _Early Hum. Dev._ 39, 127–137. https://doi.org/10.1016/0378-3782(94)90161-9 (1994). Article CAS PubMed Google Scholar * Nosarti, C. &
Froudist-Walsh, S. Alterations in development of hippocampal and cortical memory mechanisms following very preterm birth. _Dev. Med. Child Neurol._ 58(Suppl 4), 35–45.
https://doi.org/10.1111/dmcn.13042 (2016). Article PubMed PubMed Central Google Scholar * Loe, I. M., Heller, N. A. & Chatav, M. Behavior problems and executive function impairments
in preterm compared to full term preschoolers. _Early Hum. Dev._ 130, 87–95. https://doi.org/10.1016/j.earlhumdev.2019.01.014 (2019). Article PubMed PubMed Central Google Scholar *
Cainelli, E., Arrigoni, F. & Vedovelli, L. White matter injury and neurodevelopmental disabilities: A cross-disease (dis)connection. _Prog. Neurobiol._ 193, 101845.
https://doi.org/10.1016/j.pneurobio.2020.101845 (2020). Article PubMed Google Scholar * Chau, C. M. Y. _et al._ Hippocampus, amygdala, and thalamus volumes in very preterm children at 8
years: Neonatal pain and genetic variation. _Front. Behav. Neurosci._ https://doi.org/10.3389/fnbeh.2019.00051 (2019). Article PubMed PubMed Central Google Scholar * Kelly, C. E. _et
al._ Brain structural and microstructural alterations associated with cerebral palsy and motor impairments in adolescents born extremely preterm and/or extremely low birthweight. _Dev. Med.
Child Neurol._ 57, 1168–1175. https://doi.org/10.1111/dmcn.12854 (2015). Article PubMed Google Scholar * Bjuland, K. J., Løhaugen, G. C., Martinussen, M. & Skranes, J. Cortical
thickness and cognition in very-low-birth-weight late teenagers. _Early Hum. Dev._ 89, 371–380. https://doi.org/10.1016/j.earlhumdev.2012.12.003 (2013). Article PubMed Google Scholar *
Duerden, E. G. _et al._ Early procedural pain is associated with regionally-specific alterations in thalamic development in preterm neonates. _J. Neurosci._ 38, 878–886.
https://doi.org/10.1523/JNEUROSCI.0867-17.2017 (2018). Article CAS PubMed PubMed Central Google Scholar * Lax, I. D. _et al._ Neuroanatomical consequences of very preterm birth in
middle childhood. _Brain Struct. Funct._ 218, 575–585. https://doi.org/10.1007/s00429-012-0417-2 (2013). Article PubMed Google Scholar * Wehrle, F. M. _et al._ Altered resting-state
functional connectivity in children and adolescents born very preterm short title. _Neuroimage Clin._ 20, 1148–1156. https://doi.org/10.1016/j.nicl.2018.10.002 (2018). Article PubMed
PubMed Central Google Scholar * Schneider, J. _et al._ Procedural pain and oral glucose in preterm neonates: brain development and sex-specific effects. _Pain_ 159, 515–525.
https://doi.org/10.1097/j.pain.0000000000001123 (2018). Article CAS PubMed Google Scholar * Duerden, E. G. _et al._ White matter injury predicts disrupted functional connectivity and
microstructure in very preterm born neonates. _Neuroimage Clin._ 21, 101596. https://doi.org/10.1016/j.nicl.2018.11.006 (2019). Article PubMed Google Scholar * White, T. P. _et al._
Dysconnectivity of neurocognitive networks at rest in very-preterm born adults. _Neuroimage Clin._ 4, 352–365. https://doi.org/10.1016/j.nicl.2014.01.005 (2014). Article PubMed PubMed
Central Google Scholar * Cui, J. _et al._ Microstructure of the default mode network in preterm infants. _AJNR Am. J. Neuroradiol._ 38, 343–348. https://doi.org/10.3174/ajnr.A4997 (2017).
Article CAS PubMed Google Scholar * Arichi, T. _et al._ Somatosensory cortical activation identified by functional MRI in preterm and term infants. _Neuroimage_ 49, 2063–2071.
https://doi.org/10.1016/j.neuroimage.2009.10.038 (2010). Article CAS PubMed Google Scholar * Cao, M. _et al._ Early development of functional network segregation revealed by connectomic
analysis of the preterm human brain. _Cereb. Cortex_ 27, 1949–1963. https://doi.org/10.1093/cercor/bhw038 (2017). Article PubMed Google Scholar * Xu, Y. _et al._ Development and emergence
of individual variability in the functional connectivity architecture of the preterm human brain. _Cereb. Cortex_ 29, 4208–4222. https://doi.org/10.1093/cercor/bhy302 (2019). Article
PubMed Google Scholar * Damoiseaux, J. S. _et al._ Reduced resting-state brain activity in the “default network” in normal aging. _Cereb. Cortex_ 18, 1856–1864.
https://doi.org/10.1093/cercor/bhm207 (2008). Article CAS PubMed Google Scholar * Gozdas, E. _et al._ Altered functional network connectivity in preterm infants: Antecedents of cognitive
and motor impairments?. _Brain Struct. Funct._ 223, 3665–3680. https://doi.org/10.1007/s00429-018-1707-0 (2018). Article PubMed PubMed Central Google Scholar * Bäuml, J. G. _et al._ The
association of children’s mathematic abilities with both adults’ cognitive abilities and intrinsic fronto-parietal networks is altered in preterm-born individuals. _Brain Struct. Funct._
222, 799–812. https://doi.org/10.1007/s00429-016-1247-4 (2017). Article PubMed Google Scholar * Keunen, K., van Elburg, R. M., van Bel, F. & Benders, M. J. Impact of nutrition on
brain development and its neuroprotective implications following preterm birth. _Pediatr. Res._ 77, 148–155. https://doi.org/10.1038/pr.2014.171 (2015). Article CAS PubMed Google Scholar
* Coviello, C. _et al._ Effects of early nutrition and growth on brain volumes, white matter microstructure, and neurodevelopmental outcome in preterm newborns. _Pediatr. Res._ 83,
102–110. https://doi.org/10.1038/pr.2017.227 (2018). Article PubMed Google Scholar * Stephens, B. E. _et al._ First-week protein and energy intakes are associated with 18-month
developmental outcomes in extremely low birth weight infants. _Pediatrics_ 123, 1337–1343. https://doi.org/10.1542/peds.2008-0211 (2009). Article PubMed Google Scholar * Schneider, J. _et
al._ Nutrient intake in the first two weeks of life and brain growth in preterm neonates. _Pediatrics_ https://doi.org/10.1542/peds.2017-2169 (2018). Article PubMed Google Scholar *
Isaacs, E. B. _et al._ The effect of early human diet on caudate volumes and IQ. _Pediatr. Res._ 63, 308–314. https://doi.org/10.1203/PDR.0b013e318163a271 (2008). Article PubMed Google
Scholar * Tsang, R., Uauy, R., Koletzko, B. & Zlotkin, S.E. _Nutrition of the Preterm Infant. Scientific Basis and Practical Application, 2 edn._ (Digital Publishing Inc, 2005). *
Tottman, A. C. _et al._ Relationships between early neonatal nutrition and neurodevelopment at school age in children born very preterm. _J. Pediatr. Gastroenterol. Nutr._ 70, 72–78.
https://doi.org/10.1097/MPG.0000000000002471 (2020). Article PubMed Google Scholar * Moiseev, A., Doesburg, S. M., Herdman, A. T., Ribary, U. & Grunau, R. E. Altered network
oscillations and functional connectivity dynamics in children born very preterm. _Brain Topogr._ 28, 726–745. https://doi.org/10.1007/s10548-014-0416-0 (2015). Article PubMed Google
Scholar * Doesburg, S. M., Moiseev, A., Herdman, A. T., Ribary, U. & Grunau, R. E. Region-specific slowing of alpha oscillations is associated with visual-perceptual abilities in
children born very preterm. _Front. Hum. Neurosci._ 7, 791. https://doi.org/10.3389/fnhum.2013.00791 (2013). Article PubMed PubMed Central Google Scholar * Tottman, A. C. _et al._
Relationships between early neonatal nutrition and neurodevelopment at school age in children born very preterm. _J. Pediatr. Gastroenterol. Nutr._
https://doi.org/10.1097/MPG.0000000000002471 (2019). Article Google Scholar * Beery, K. E., Buktenica, N. A. & Beery, N. A. _The Beery–Buktenica Developmental Test of Visual–Motor
Integration: Administration, Scoring and Teaching Manual, 5th edn_. (NCS Pearson, 2004). * Power, V. A. _et al._ Nutrition, growth, brain volume, and neurodevelopment in very preterm
children. _J. Pediatr._ https://doi.org/10.1016/j.jpeds.2019.08.031 (2019). Article PubMed Google Scholar * Cormack, B. E., Bloomfield, F. H., Dezoete, A. & Kuschel, C. A. Does more
protein in the first week of life change outcomes for very low birthweight babies?. _J. Paediatr. Child Health_ 47, 898–903. https://doi.org/10.1111/j.1440-1754.2011.02106.x (2011). Article
PubMed Google Scholar * Barreault, S. _et al._ Impact of early protein and energy intakes on neurodevelopment at 2 years of corrected age in very low birth weight infants: A
single-center observational study. _PLoS ONE_ 14, e0218887. https://doi.org/10.1371/journal.pone.0218887 (2019). Article CAS PubMed PubMed Central Google Scholar * Sammallahti, S. _et
al._ Nutrition after preterm birth and adult neurocognitive outcomes. _PLoS ONE_ 12, e0185632. https://doi.org/10.1371/journal.pone.0185632 (2017). Article CAS PubMed PubMed Central
Google Scholar * Cusick, S. E. & Georgieff, M. K. The role of nutrition in brain development: The golden opportunity of the “first 1000 days”. _J. Pediatr._ 175, 16–21.
https://doi.org/10.1016/j.jpeds.2016.05.013 (2016). Article PubMed PubMed Central Google Scholar * Georgieff, M. K. Nutrition and the developing brain: Nutrient priorities and
measurement. _Am. J. Clin. Nutr._ 85, 614S-620S. https://doi.org/10.1093/ajcn/85.2.614S (2007). Article CAS PubMed Google Scholar * Lee, W., Morgan, B. R., Shroff, M. M., Sled, J. G.
& Taylor, M. J. The development of regional functional connectivity in preterm infants into early childhood. _Neuroradiology_ 55(Suppl 2), 105–111.
https://doi.org/10.1007/s00234-013-1232-z (2013). Article PubMed Google Scholar * Doria, V., Arichi, T. & Edwards, D. A. Magnetic resonance imaging of the preterm infant brain. _Curr.
Pediatr. Rev._ 10, 48–55 (2014). Article Google Scholar * Toulmin, H. _et al._ Specialization and integration of functional thalamocortical connectivity in the human infant. _Proc. Natl.
Acad. Sci. U S A_ 112, 6485–6490. https://doi.org/10.1073/pnas.1422638112 (2015). Article ADS CAS PubMed PubMed Central Google Scholar * Ball, G. _et al._ Thalamocortical connectivity
predicts cognition in children born preterm. _Cereb. Cortex_ 25, 4310–4318. https://doi.org/10.1093/cercor/bhu331 (2015). Article PubMed PubMed Central Google Scholar * Hunt, B. A. E.
_et al._ Disrupted visual cortex neurophysiology following very preterm birth. _Biol. Psychiatry Cognit. Neurosci. Neuroimaging_. https://doi.org/10.1016/j.bpsc.2019.08.012. Download
references ACKNOWLEDGEMENTS We would like to sincerely thank the families that participated in this research. We would also like to thank the nursing staff and the MRI technologists for
making this research possible. Funding for this research was provided by the Health Research Council of New Zealand, Gravida: National Centre for Growth and Development, and the University
of Auckland. AUTHOR INFORMATION Author notes * A list of authors and their affiliations appears at the end of the paper. AUTHORS AND AFFILIATIONS * Applied Psychology, Faculty of Education,
Western University, London, ON, Canada Emma G. Duerden * Department of Paediatrics, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada Emma G. Duerden & Steven P.
Miller * School of Optometry and Vision Science, University of Waterloo, Waterloo, Canada Benjamin Thompson & Benjamin S. Thompson * School of Optometry and Vision Science, University
of Auckland, Auckland, New Zealand Benjamin Thompson, Joanna M. Black, Myra Leung, Geraint Phillips, Tanya Poppe & Benjamin S. Thompson * Liggins Institute, University of Auckland,
Auckland, New Zealand Tanya Poppe, Greg Gamble, Yannan Jiang, Myra Leung, Anna C. Tottman, Jane E. Harding, Janene B. Biggs, Frank H. Bloomfield, Kelly Fredell, Greg D. Gamble, Jane E.
Harding, Sabine Huth, Yannan Jiang, Christine Kevan, Jennifer A. Rogers, Heather Stewart & Anna C. Tottman * Department of Paediatrics: Child and Youth Health, University of Auckland,
Auckland, New Zealand Tanya Poppe, Jane Alsweiler, Myra Leung, Jane M. Alsweiler, Coila Bevan & Kathryn A. Williamson * Department of Psychological Medicine, University of Auckland,
Auckland, New Zealand Trecia Wouldes & Trecia A. Wouldes * Centre for the Developing Brain, King’s College London, London, United Kingdom Tanya Poppe Authors * Emma G. Duerden View
author publications You can also search for this author inPubMed Google Scholar * Benjamin Thompson View author publications You can also search for this author inPubMed Google Scholar *
Tanya Poppe View author publications You can also search for this author inPubMed Google Scholar * Jane Alsweiler View author publications You can also search for this author inPubMed Google
Scholar * Greg Gamble View author publications You can also search for this author inPubMed Google Scholar * Yannan Jiang View author publications You can also search for this author
inPubMed Google Scholar * Myra Leung View author publications You can also search for this author inPubMed Google Scholar * Anna C. Tottman View author publications You can also search for
this author inPubMed Google Scholar * Trecia Wouldes View author publications You can also search for this author inPubMed Google Scholar * Steven P. Miller View author publications You can
also search for this author inPubMed Google Scholar * Jane E. Harding View author publications You can also search for this author inPubMed Google Scholar CONSORTIA PIANO STUDY GROUP * Jane
M. Alsweiler * , Janene B. Biggs * , Coila Bevan * , Joanna M. Black * , Frank H. Bloomfield * , Kelly Fredell * , Greg D. Gamble * , Jane E. Harding * , Sabine Huth * , Yannan Jiang * ,
Christine Kevan * , Myra Leung * , Geraint Phillips * , Tanya Poppe * , Jennifer A. Rogers * , Heather Stewart * , Benjamin S. Thompson * , Anna C. Tottman * , Kathryn A. Williamson * &
Trecia A. Wouldes CONTRIBUTIONS B.T., T.P., J.A., Y.J., M.L., A.T., T.W., J.E.H. and G.G. were involved in the study design, database variable creation, test material selection, and data
acquisition design and execution of the data analytic strategy, reviewed and/or revised the final version of the manuscript. S.P.M. conceptualized the execution of the data analytic
strategy, contributed to the data analysis and reviewed/revised the final draft of the manuscript. E.G.D. contributed to the execution of the data analytic strategy, analyzed the data,
prepared all figures and wrote the initial draft of the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
CORRESPONDING AUTHOR Correspondence to Emma G. Duerden. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a
Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit
to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are
included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons
licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of
this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Duerden, E.G., Thompson, B., Poppe, T. _et al._ Early protein
intake predicts functional connectivity and neurocognition in preterm born children. _Sci Rep_ 11, 4085 (2021). https://doi.org/10.1038/s41598-021-83125-z Download citation * Received: 22
March 2020 * Accepted: 25 January 2021 * Published: 18 February 2021 * DOI: https://doi.org/10.1038/s41598-021-83125-z SHARE THIS ARTICLE Anyone you share the following link with will be
able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative