Play all audios:
ABSTRACT Proton therapy of prostate cancer (PCPT) was linked with increased levels of gastrointestinal toxicity in its early use compared to intensity-modulated radiation therapy (IMRT). The
higher radiation dose to the rectum by proton beams is mainly due to anatomical variations. Here, we demonstrate an approach to monitor rectal radiation exposure in PCPT based on prompt
gamma spectroscopy (PGS). Endorectal balloons (ERBs) are used to stabilize prostate movement during radiotherapy. These ERBs are usually filled with water. However, other water solutions
containing elements with higher atomic numbers, such as silicon, may enable the use of PGS to monitor the radiation exposure of the rectum. Protons hitting silicon atoms emit prompt gamma
rays with a specific energy of 1.78 MeV, which can be used to monitor whether the ERB is being hit. In a binary approach, we search the silicon energy peaks for every irradiated prostate
region. We demonstrate this technique for both single-spot irradiation and real treatment plans. Real-time feedback based on the ERB being hit column-wise is feasible and would allow
clinicians to decide whether to adapt or continue treatment. This technique may be extended to other cancer types and organs at risk, such as the oesophagus. SIMILAR CONTENT BEING VIEWED BY
OTHERS DATASET FOR PREDICTING SINGLE-SPOT PROTON RANGES IN PROTON THERAPY OF PROSTATE CANCER Article Open access 29 September 2021 ABSOLUTE DOSIMETRY FOR FLASH PROTON PENCIL BEAM SCANNING
RADIOTHERAPY Article Open access 04 February 2023 REAL-TIME TRACKING OF THE BRAGG PEAK DURING PROTON THERAPY VIA 3D PROTOACOUSTIC IMAGING IN A CLINICAL SCENARIO Article Open access 17
September 2024 INTRODUCTION Range verification is one of the most important problems to be solved in particle therapy1,2. Offline positron emission tomography (offline PET) has verified
range uncertainties of approximately 6 mm3. Offline PET scans are performed after irradiation, and the activated tissue is imaged. However, this technique suffers from low signal and
biological washout over time. More recent results with in-beam PET have demonstrated the online capabilities of this technique4. Prompt gamma imaging (PGI) has emerged as an alternative that
relies on the prompt nature of the gamma radiation emitted during particle therapy. Range verification can be accomplished in real time during treatment, thus providing a means to avoid
unwanted irradiation of healthy tissues. Since 2006, several concepts based on imaging and non-imaging systems have been developed5,6,7,8,9,10,11. Eventually, two of them—the knife-edge slit
camera and prompt gamma spectroscopy—reached the clinical phase12,13 and are currently being used at proton facilities. Proton therapy for prostate cancer (PCPT) has been a reality since
the 1990s14,15. Several clinical studies have estimated the toxicity of prostate cancer therapy with photons16,17, protons14,18,19,20,21,22,23, and carbon ions24,25. At the outset, PCPT was
considered to deliver less dose than photon radiation to normal tissues surrounding the prostate, such as the rectum and bladder26,27,28. PCPT had, however, a major setback, with two
clinical studies reporting higher toxicity than conventional photon therapy19,21. Sheets _et al._ showed that although intensity-modulated radiation therapy (IMRT) delivered three times more
radiation to the body, it presented 50% less gastrointestinal morbidity. Proton therapy-treated patients were more likely to receive a diagnosis of gastrointestinal morbidity and undergo
gastrointestinal procedures. There were, however, no significant differences in urinary nonincontinence or incontinence diagnoses or procedures, erectile dysfunction, or hip fractures21. Kim
_et al._ also showed that proton therapy had the highest rate of grade 3/4 toxicity among radiotherapy modalities (20.1 per 1000 person-years)19. However, the authors pointed out that the
sample size for the proton cohort was quite small because the study included patients diagnosed from 1992 to 2005, a period when proton therapy was in its relative infancy and only passively
scattered proton therapy (PSPT) was available. In the meantime, intensity-modulated proton therapy (IMPT) was developed both for protons29,30 and carbon ions31. More recent studies have
demonstrated more favourable toxicity outcomes with proton therapy20,22,23. Another drawback of PCPT is the irradiation of the femoral heads, which lay in the path of the beam to the
prostate. These structures influence the stopping power due to their high density. This effect is even more pronounced in the presence of prosthetic hips, where proton therapy is not
recommended at all. Anterior-oblique (AO) fields have been proposed to circumvent this problem with proton32,33 and carbon beams34. However, the target coverage is substantially reduced
after considering interfractional variations in AO plans, thus increasing the susceptibility to underdosing33. Adaptive range verification could play a decisive role in recovering the target
coverage. In these cases, superior sparing of the rectum was demonstrated. For this purpose, an in vivo diode-based range verification system was developed and commissioned at the
Massachusetts General Hospital (MGH)35,36. This system was designed for passive-scattering proton delivery and relies on a \(3 \times 4\) matrix of 1 mm diodes mounted in a water balloon. An
accuracy on the order of 1 mm for the water equivalent path length (WEPL) measurements was demonstrated. The rectal wall was shown to receive doses of 1.6% for anterior fields and 0.4% for
AO fields36. Another technique to improve gastrointestinal toxicity and mitigate the uncertainties in proton relative biological effectiveness (RBE) is the use of rectal hydrogel spacers
located between the prostate and the rectum37,38,39. This is, however, an invasive technique that demands surgery, as the spacers are later absorbed. Endorectal balloons (ERBs) have long
been used to stabilize the prostate location40,41, especially during radiotherapy with photons, such as three-dimensional conformal radiotherapy (3D CRT)40,42,43 and intensity-modulated
radiotherapy (IMRT)44,45,46,47. Clinical studies using ERBs in IMRT evaluated acute toxicity48,49 and rectal wall sparing50. Reduction in the dose to the rectal wall by means of an ERB has
been observed by several authors40,51,52. Among the range verification techniques, prompt gamma spectroscopy (PGS) has demonstrated the ability to measure absolute range deviations during
the course of treatment. This technique relies on the analysis of the prompt gamma energy spectrum, which is characterized by specific energy lines corresponding to the reaction channels of
the irradiated protons with the elements of the human body. The most common reactions are those with oxygen and carbon atoms, which become excited and eventually emit prompt gamma rays up to
10 MeV6,53. However, other reactions emitting low-energy prompt gammas following the irradiation of metals by protons54 and helium ions55 have been shown. We describe the implementation of
an ERB inflated with a mixture of water and silicon dioxide (SiO\(_2\)) to wirelessly monitor the proton range in PCPT via prompt gamma rays. The presence of silicon in the mixture allows
the differentiation of the characteristic gamma emission lines resulting from the bombardment of the \(^{28}\)Si atoms by protons. The cross-sections associated with such reactions have been
investigated56,57,58,59. A gamma line emitted at 1.78 MeV provides a unique signature for differentiation. Instantaneous feedback from the ERB being hit by protons allows for prompt binary
output regarding the irradiation of the rectum. We irradiated single spots with beams, hitting only water or other water mixtures and solutions inside the ERB. We evaluated a worst-case
scenario of an ERB surrounded by a phantom filled with water that emits prompt gamma rays under irradiation, with energy lines strongly competing with those of interest in the ERB. The water
flasks in the beam path played a stronger role in signal deterioration. The prompt gamma attenuation within the water flasks placed in the path from the interaction point to the detectors
was less pronounced. Finally, we evaluated two real treatment plans with a single 2 Gy anterior field and a 1 Gy AO field. A realistic phantom with an inserted ERB filled with the SiO\(_2\)
water mixture was irradiated with active pencil beams. A scenario with the plan overlapping the ERB and another one with the real plan were considered. The iso-energy layers (IELs) crossing
the balloon were identified, as well as the columns parallel to the ERB within each IEL. RESULTS We started by irradiating different water solutions and mixtures with single-spot proton
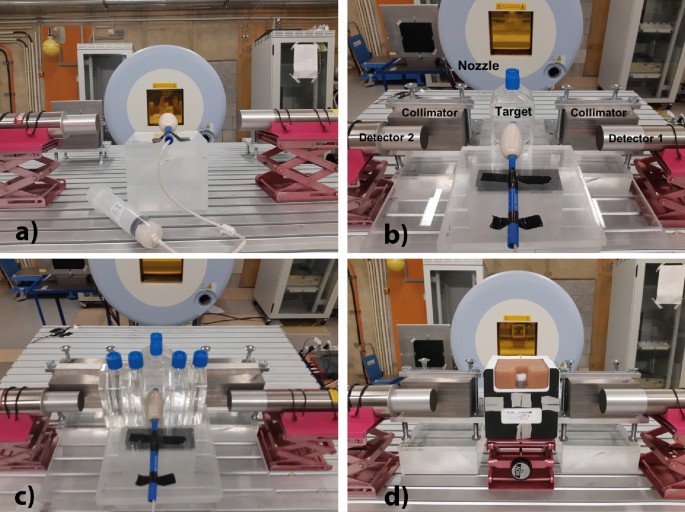
beams. Figure 1 shows the detectors, the targets, and the beam nozzle. Figure 1a shows an ERB filled with a water mixture to be irradiated with the lowest energy available (48 MeV).
Afterwards, we increased the energy of our proton beam to an energy applicable in PCPT. Figure 1b shows two flasks of water in front of our target. To evaluate the prompt gamma attenuation
in the patient, we placed two water flasks on each side of the target, i.e., in the path from the target to the detectors, as shown in Fig. 1c. Figure 1d shows a prostate phantom with a
custom-made insert filled with a commercial silicone sealant. Two tungsten collimators were placed in a semi-collimation configuration in front of each detector in the beam direction to
prevent scattered particles in the nozzle from hitting the detectors and to collimate the prompt gammas only from the most downstream region. These collimators had a strong impact in
reducing the detector count rate, thus allowing higher beam intensities. In Fig. 2a, we show the energy spectra of several water solutions and mixtures irradiated by single-spot proton beams
at the lowest energy. The mixture with silicon dioxide (SiO\(_2\)) exhibits several differences from the other solutions. The solution of heptahydrate magnesium sulphate (MgSO\(_4\cdot
\)7H\(_2\)O), also known as Epsom salt, responds to higher temperatures with higher solubility. This is not observed in the SiO\(_2\) mixture. The addition of sodium hydroxide (NaOH) to the
SiO\(_2\) mixture creates a solution of sodium metasilicate (Na\(_2\)SiO\(_3\)), but the quantity in grams of dissolved solute remains the same as that in the mixture. The limit for
SiO\(_2\), either mixed or dissolved in 60 mL of water, is 40 g. Above that quantity, the viscosity increases, and the mixture or solution cannot flow inside the small diameter tube between
the syringe and the balloon. A commercial silicone sealant was also irradiated for the sake of comparison with the expected silicon gamma lines. Figure 2b shows the spectra of these targets
with two water flasks placed in front of them. Due to the increased lateral spread and range straggling, the 1.78 MeV silicon gamma line is smeared out, and the nearby 1.635 MeV energy line
resulting from oxygen irradiation becomes more prominent. The addition of NaOH creates a sodium line at 1.278 MeV, increases the oxygen and sodium lines at 1.635 MeV, and decreases the
silicon line at 1.78 MeV. In view of these results and due to the simplicity of operation and its harmlessness (lack of toxic effects), we decided to continue our studies with a mixture of
water and SiO\(_2\). Figure 2c shows the spectra obtained with two water flasks placed on each side of the target. The prompt gamma attenuation effect is hardly visible. All sequential
effects were combined, thus mimicking a worst-case scenario of a target mostly made of water. In this case, the prompt gamma water lines compete strongly with the prompt gamma silicon lines.
Figure 2d compares the energy spectra from the proton irradiation of a prostate phantom with either a silicone insert or an ERB filled with a water mixture of SiO\(_2\). The differences in
the prominences of the peaks of interest are negligible. We then aimed to evaluate the cumulative effects of range straggling and prompt gamma attenuation in a prostate phantom with an
inserted ERB filled with a mixture of water and SiO\(_2\). Therefore, we irradiated the prostate and the ERB with single-spot proton beams at different phantom positions. To reproduce a real
treatment scenario within a rotating gantry, the phantom was rotated by 90\(^\circ \) in the transaxial direction and irradiated by a horizontal beam. Figure 3a–c shows the phantom at three
gantry angles: 0\(^\circ \), 90\(^\circ \), and 270\(^\circ \). Figure 3d–f shows the spectra resulting from the irradiation of the prostate and the ERB with single spot beams in the three
positions. A 1.78 MeV silicon line is present in the ERB irradiation and absent in the prostate irradiation. For the lateral beams, the closer the ERB is to the detector, the better the
signal from the 1.78 MeV prompt gammas. Detector 1 collects a higher signal for the 90\(^\circ \) angle, while detector 2 collects a higher signal for the 270\(^\circ \) angle. To increase
the signal at a 0\(^\circ \) angle, we used the timing information of the arrival time of the protons provided by the scintillating fibres placed between the nozzle and the target60. The
trigger was not further used in the treatment plans due to the strong impact in the statistics and due to the intensity constraints (increasing pile-up above \(8\times 10^7\) p/s). In the
last setup, we considered real treatment-like plans. Figure 4 shows the computed tomography (CT) and the plans of an anterior beam irradiating the prostate either conformally or overlapping
with the ERB. In Fig. 4a–c, the sagittal views through the prostate and the ERB clearly show their structure and the spacing between the ERB and the prostate. The CT also shows the seminal
vesicles, the bladder, and the small tube inside a larger tube that transports the solution or mixture from the syringe to the ERB. Figure 4d shows a coronal plan where the IELs as well as
the spots overlapping the prostate and the ERB are visible. While IEL 17 has all spots overlapping within the ERB, IEL 12 only has six central overlapping spots. Our goal was to determine at
which IEL the protons hit the ERB with the overlapping anterior beam. However, since not every spot within each IEL overlapped with the ERB, we sorted the irradiation within each IEL by
columns parallel to the ERB and attributed time stamps to each column. Figure 5 shows the prompt gamma spectra from the irradiation of the phantom at IELs 12, 13, and 14. IEL 12 is at the
interface between the prostate and the ERB. Columns were detected from the first to the last starting in beam-eye view (BEV) on the left for detector 1 and on the right for detector 2. While
detector 1 detects the columns to the left in BEV with higher sensitivity, detector 2 has a higher count rate for columns to the right in BEV. For detector 1, we observe a 1.78 MeV silicon
line emerging from column 8 to column 11 at IEL 12 (103.22 MeV). At IEL 14 (107.51 MeV), a 1.78 MeV silicon energy line emerges from column 4. For detector 2, this energy line emerges from
column 4 for IEL 13 (105.42 MeV) and column 3 for IEL 14. The number of protons per column is on the order of 1–3.7 \(\times \) 10\(^8\), and the detected prompt gammas per column are on the
order of 25-85 kcts. In the AO plan, we also reordered each IEL of the plan in such way that they were irradiated in columns parallel to the ERB from left to right in the BEV. Figure 6a and
b shows a photo of the prostate phantom at an angle of 279\(^\circ \) and schematics of the irradiation of IEL 12 from column 1 to column 15. The plans with and without overlap with the ERB
are shown in Fig. 6c and d. In Fig. 7a, we observe that the columns to the right overlapping with the ERB produce a 1.78 MeV prompt gamma line, while those to the left irradiate the
prostate and therefore present no such line. Such tracking is possible with columns comprising less than 108 protons. For IEL 12, the protons start hitting the ERB at column 6 with \(8.4
\times 10^7\) particles. In Fig. 7b, we confirm that the real plan without overlap with the ERB does not yield a 1.78 MeV energy line for the last columns to the right. For the sake of
irradiation speed, the first depicted column aggregates several columns to the left in the prostate region. An independent measurement undertaken after one month with the same gantry angle
demonstrates the existence of 1.78 MeV energy lines for the columns overlapping with the ERB (Fig. 7c). An additional measurement at a symmetric position of 81\(^\circ \) shows 1.78 MeV
energy lines for the columns to the left closer to detector 1 (Fig. 7d). A peak analysis within the region of interest for the spectra presented in Fig. 7 is depicted in Fig. 8. The
prominence and the width at half prominence are shown for the peaks of interest. The top four peaks that result from the irradiation of the ERB are indicative of the prompt gamma lines
associated with the reaction between the protons and the silicon atoms. DISCUSSION Prompt gamma spectroscopy (PGS) is currently one of the most promising techniques for particle range
monitoring and measurements of the elemental composition of irradiated targets in particle therapy6,13,55,61. This technique facilitates absolute range measurements with millimetre precision
due to accurate knowledge of the nuclear reaction cross-sections between the irradiated particles and the types of atoms in the patient. Two PGI modalities, PGS and the knife-edge slit
camera, have now reached the level of clinical prototypes12,13. The combination of in vivo range monitoring and adaptation methods has been proposed for the treatment of prostate cancer with
either anterior beams35 or anterior oblique (AO) beams33. An in vivo range verification system has already been commissioned36. This system is composed of a 4 by 3 array matrix of silicon
diodes attached by a self-adhesive surface to an ERB and presents a WEPL measurement accuracy on the order of 1 mm. In this paper, we propose a wireless solution that uses prompt gamma rays
to monitor the interaction of protons within an ERB filled with a silicon dioxide water mixture and inserted in a prostate phantom. This concept aims to monitor the proton range in PCPT in
real time. The irradiation of atomic nuclei within the human body by protons emits prompt gamma rays with characteristic energy lines6,56. The irradiation of carbon and oxygen atoms is
followed by the emission of prompt gamma radiation with low and high energies (0.511 MeV, 0.718 MeV, 1.022 MeV, 1.635 MeV, 2.31 MeV, 2.8 MeV, 4.4 MeV, 5.2 MeV, and 6.1 MeV)6,54. Conversely,
during the irradiation of metals, prompt gamma rays are emitted with lower energy (below 3 MeV)54,55. This radiation exits the patient under proton bombardment and may be detected by
scintillating crystals, e.g., CeBr\(_3\). The signals are digitally converted and processed to extract energy and time information. Metals usually not present within the human body are good
candidates for ranging probes. Although not a metal, silicon dioxide has been shown to be a good choice due to the unique signature provided by the emission of a prompt gamma energy line at
1.78 MeV. This line is distinguishable from the remaining spectrum and can therefore provide binary information about the elemental composition of the material being hit. However, even with
good dose confinement to the target, the patient is still exposed to a dose in the organ at risk (OAR) and very likely prompt gammas emitted from the ERB. Therefore, a possible solution
would be to set a threshold on the 1.78 MeV prompt gammas detected at a certain IEL and neighbouring IELs. This binary output might trigger a decision on whether to continue or stop/adapt
the treatment since an organ at risk may be endangered. Proton beam delivery with spot- or raster-pencil-beam scanning (PBS) is particularly suitable for such an approach. A synchronization
between beam delivery and prompt gamma detection may allow real-time monitoring of the voxels being hit and simultaneous comparison to the prediction. A standard 2 Gy prostate treatment
provides sufficient statistics for such monitoring. Due to the round shape of the rectum, an anterior beam requires column-wise delivery parallel to the rectum so that which IEL column the
nuclear reactions with the silicon take place in can be inferred. The range monitoring also requires detectors closer to the irradiated column. Therefore, the right columns in the beam-eye
view require detectors on the right side, and the left columns are better detected by detectors on the left side. The AO beams present an even more preferable solution, as the geometry
allows the detectors to be placed closer to the ranging probe. All columns within IELs overlapping with the range probe are prone to be detected with higher sensitivity. In the case of a
range probe located in the rectum or the oesophagus, the AO beams are especially suitable, as the detector may be positioned at right angles with the patient and close to the probe. Range
monitoring by means of PGS is feasible in PCPT. Once the proton range is under control, one may use fields other than the commonly used bilateral opposing fields that are more robust to
range uncertainties. The two AO beams may assume variable angles due to the flexibility provided by the method presented in this paper. Therefore, SiO\(_2\)-filled ERB combined with PBS
delivery and PGS monitoring may allow AO beams to be sensitive against rectal changes within and between treatment fractions. METHODS PROSTATE PHANTOM The phantom was a prostate training
phantom, CIRS model 070L (CIRS Inc., Norfolk, USA). This phantom is commonly used for ultrasound images and biopsy through the ZSkin rectal wall or perineal membrane. The main inner
composition is Zerdine. It includes a urethra with a diameter of 0.7 cm, seminal vesicles with a diameter of 0.7 cm and length of 10 cm, and two lesions. The container has a volume of 9 cm
\(\times \) 10 cm \(\times \) 10 cm and a probe opening of 1.2 cm. ENDORECTAL BALLOON The endorectal balloon (ERB) was a QLRAD Rectal Pro75 (QLRAD International, Larnaca, Cyprus), which is
commonly used to stabilize prostate movement in radiotherapy. It is coupled to a syringe via a smaller tube, and a latch closes the liquid flow. Each ERB was filled with 50 mL. Two ERBs
operated over six shifts of six hours with different water solutions. WATER SOLUTIONS AND MIXTURES The mixture of water and silicon dioxide (SiO\(_2\)) consisted of 90 mL of deionized water
and 60 g of diatomaceous earth (_Kieselgur_) from Health Leeds (Health Leeds UK Ltd, Horeb, UK). The solution of sodium metasilicate (Na\(_2\)SiO\(_3\)) consisted of 60 mL of water, 27 g of
sodium hydroxide, and 40 g of diatomaceous earth. The magnesium sulphate solution consisted of 90 mL of water and 100 g of MgSO\(_4\cdot \)7H\(_2\)O (_Epsom salts_) from Health Leeds
previously heated to 40 \(^{\circ }\)C. THE HIT FACILITY The Heidelberg Ion-Beam Therapy Center (HIT)62 accelerates proton, helium, carbon, and oxygen ions from 48 MeV/u to 430 MeV/u. While
protons and carbon ions are routinely implemented in the clinical setting, helium ions are currently being commissioned63,64, and oxygen ions remain a research beam species. The intensities
in clinical practice range from 2 \(\times \) 10\(^6\) p/s for carbon ions to 3.2 \(\times \) 10\(^{9}\) p/s for protons. There are two horizontal beam rooms and a 360\(^{\circ }\) gantry
for therapy. There is a horizontal experimental room where all the experiments referred to in this paper were performed. COMPUTED TOMOGRAPHY The computed tomography (CT) followed the routine
CT protocol for ion beam therapy planning at HIT with the Siemens SOMATOM Confidence RT Pro (Siemens Healthineers, Erlangen, Germany). The phantom and the inserted ERB were scanned with a
tube voltage of 120 kV, and the image was reconstructed for a field of view (FOV) of 50 cm with a convolution kernel B40s and a spacing between slices of 3 mm. PLANS The treatment-like plans
were optimized using a RayStation 10A (RaySearch Laboratories, Stockholm, Sweden) and calculated with a Monte Carlo algorithm. The plans conforming to the prostate (either with 2 oblique
fields mimicking AO 279 and 81 degree beams or a single AP beam at 0 degrees) were designed to prevent any proton Bragg peak localization within the ERB and for a maximum rectum dose below
0.3 Gy (RBE) per fraction. The plans overlapping with the ERB for the same beam configurations covered an extended target including the prostate and 1.5 cm extension in the posterior
direction (towards the ERB). The dose prescriptions to the targets were a median dose of 2 Gy (RBE) per fraction. EXPERIMENTAL SETUP The main components of the experimental setup were the
nozzle, the target, and the CeBr\(_3\) detectors. These detectors are scintillation detectors with very good time and energy resolution. They feature a measured energy resolution of 3.49%55
and a measured time resolution of 0.85 ns60. They are mainly used for range verification of the proton and ion beams in a patient. The CeBr\(_3\) detectors were aligned with the isocentre
and positioned at a distance of 15 cm from the beam axis. The CeBr\(_3\) crystals are identical in size (diameter d = 3.81 cm and length l = 7.62 cm). One crystal was coupled to a Hamamatsu
R13089 photomultiplier tube (PMT), and the other crystal was coupled to a Hamamatsu R9420-100 PMT. Both detectors were plugged to a voltage divider. The anode output fed the data acquisition
system (DAQ)65. This is a module of a FlashCam FADC system, originally designed for the Cherenkov Telescope Array (CTA)66. INTENSITIES, ACQUISITION TIMES, AND COUNTS The results shown in
Figs. 2 and 3 were obtained with an intensity of 2 \(\times \) 10\(^8\) p/s and the spill time lasted 1:07 min (14 spills). A total of 1.36 \(\times \) 10\(^{10}\) protons were delivered
and the counts were variable. The results shown in Fig. 5 were obtained with an intensity of 2 \(\times \) 10\(^8\) p/s and the spill time lasted 2:59 min. A total of 3.43 \(\times \)
10\(^{10}\) protons were delivered and a total of 7.8 Mcts were recorded. The results shown in Fig. 7a were obtained with an intensity of 2 \(\times \) 10\(^8\) p/s and the spill time lasted
1:33 min. A total of 1.79 \(\times \) 10\(^{10}\) protons were delivered and a total of 5.1 Mcts were recorded. The results shown in Fig. 7b were obtained with an intensity of 2 \(\times \)
10\(^8\) p/s and the spill time lasted 1:11 min. A total of 1.36 \(\times \) 10\(^{10}\) protons were delivered and a total of 3.8 Mcts were recorded. The results shown in Fig. 7d were
obtained with an intensity of 2 \(\times \) 10\(^8\) p/s and the spill time lasted 1:35 min. A total of 1.83 \(\times \) 10\(^{10}\) protons were delivered and a total of 5.7 Mcts were
recorded. PEAK ANALYSIS The presence or absence of the silicon line could not be visually verified. Therefore, a simple method was developed to identify the presence of 1.635 MeV and 1.78
MeV peaks within a region of interest. We subtracted the background from the peaks by fitting a straight line through their high and low energy values. The MATLAB function _findpeaks_ was
adapted to identify the peaks within a certain energy interval and to meet certain criteria. The parameters, such as the minimum peak height or prominence, the minimum peak width at half
prominence, and the maximum and minimum distances between energy peaks, were adjusted after the spectra were properly calibrated. Other methods, such as that presented by Dal Bello _et
al._55, could also have been used. REFERENCES * Paganetti, H. Range uncertainties in proton therapy and the role of Monte Carlo simulations. _Phys. Med. Biol._ 57, R99–R117 (2012). Article
CAS PubMed PubMed Central ADS Google Scholar * Parodi, K. & Polf, J. In vivo range verification in particle therapy. _Med. Phys._ 45, e1036–e1050 (2018). Article PubMed CAS
Google Scholar * Moteabbed, M., España, S. & Paganetti, H. Monte Carlo patient study on the comparison of prompt gamma and PET imaging for range verification in proton therapy. _Phys.
Med. Biol._ 56, 1063–1082 (2011). Article CAS PubMed Google Scholar * Ferrero, V. _et al._ Online proton therapy monitoring: Clinical test of a Silicon-photodetector-based in-beam PET.
_Sci. Rep._ 8, 4100 (2018). Article PubMed PubMed Central ADS CAS Google Scholar * Smeets, J. _et al._ Prompt gamma imaging with a slit camera for real-time range control in proton
therapy. _Phys. Med. Biol._ 57, 3371–3405 (2012). Article CAS PubMed Google Scholar * Verburg, J. & Seco, J. Proton range verification through prompt gamma-ray spectroscopy. _Phys.
Med. Biol._ 59, 7089–7106 (2014). Article PubMed CAS Google Scholar * Golnik, C. _et al._ Range assessment in particle therapy based on prompt \(\gamma \)-ray timing measurements. _Phys.
Med. Biol._ 59, 5399–5422 (2014). Article CAS PubMed Google Scholar * Krimmer, J., Dauvergne, D., Létang, J. & Testa, É. Prompt-gamma monitoring in hadrontherapy: A review. _Nucl.
Instrum. Methods A_ 878, 58–73 (2018). * Draeger, E. _et al._ 3D prompt gamma imaging for proton beam range verification. _Phys. Med. Biol._ 63, 035019 (2018). Article CAS PubMed PubMed
Central Google Scholar * Wronska, A. & Dauvergne, D. Range verification by means of prompt-gamma detection in particle therapy. _Radiation Detection Systems_, In press. hal-03085504
(2021). * Magalhaes Martins, P., Dal Bello, R. & Seco, J. in _Radiation Therapy Dosimetry: A Practical Handbook (1st ed.)_ (ed. Darafsheh, A.) Ch. 27 (CRC Press, 2021). * Richter, C. _et
al._ First clinical application of a prompt gamma based in vivo proton range verification system. _Radiother. Oncol._ 118, 232–237 (2016). Article PubMed Google Scholar * Hueso-González,
F., Rabe, M., Ruggieri, T. A., Bortfeld, T. & Verburg, J. A full-scale clinical prototype for proton range verification using prompt gamma-ray spectroscopy. _Phys. Med. Biol._ 63,
185019 (2018). Article PubMed PubMed Central CAS Google Scholar * Slater, J. D. _et al._ Proton therapy for prostate cancer: The initial Loma Linda University experience. _Int. J.
Radiat. Oncol. Biol. Phys._ 59, 348–352 (2004). Article PubMed Google Scholar * Ishikawa, H. _et al._ Particle therapy for prostate cancer: The past, present and future. _Int. J. Urol._
26, 971–979 (2019). Article PubMed PubMed Central Google Scholar * Storey, M. R. _et al._ Complications from radiotherapy dose escalation in prostate cancer: Preliminary results of a
randomized trial. _Int. J. Radiat. Oncol. Biol. Phys._ 48, 635–642 (2000). Article CAS PubMed Google Scholar * Pollack, A. _et al._ Prostate cancer radiation dose response: Results of
the M.D. Anderson phase III randomized trial. _Int. J. Radiat. Oncol. Biol. Phys._ 53, 1097–1105 (2002). Article PubMed Google Scholar * Mock, U., Bogner, J., Georg, D., Auberger, T.
& Pötter, R. Comparative treatment planning on localized prostate carcinoma. _Strahlenther. Onkol._ 181, 448–455 (2005). * Kim, S. _et al._ Late gastrointestinal toxicities following
radiation therapy for prostate cancer. _Eur. Urol._ 60, 908–916 (2011). Article PubMed PubMed Central Google Scholar * Nihei, K. _et al._ Multi-institutional phase II study of proton
beam therapy for organ-confined prostate cancer focusing on the incidence of late rectal toxicities. _Int. J. Radiat. Oncol. Biol. Phys._ 81, 390–396 (2011). Article PubMed Google Scholar
* Sheets, N. C. _et al._ Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. _JAMA_ 307,
1611–1620 (2012). Article CAS PubMed PubMed Central Google Scholar * Mendenhall, N. P. _et al._ Five-year outcomes from 3 prospective trials of image-guided proton therapy for prostate
cancer. _Int. J. Radiat. Oncol. Biol. Phys._ 88, 596–602 (2014). Article PubMed Google Scholar * Bryant, C. _et al._ Five-year biochemical results, toxicity, and patient-reported quality
of life after delivery of dose-escalated image guided proton therapy for prostate cancer. _Int. J. Radiat. Oncol. Biol. Phys._ 95, 422–434 (2016). Article PubMed Google Scholar * Akakura,
K. _et al._ Phase I/II clinical trials of carbon ion therapy for prostate cancer. _The Prostate_ 58, 252–258 (2004). Article CAS PubMed Google Scholar * Fukahori, M. _et al._ Estimation
of late rectal normal tissue complication probability parameters in carbon ion therapy for prostate cancer. _Radiother. Oncol._ 118, 136–140 (2016). Article PubMed Google Scholar *
Vargas, C. _et al._ Dose-volume comparison of proton therapy and intensity-modulated radiotherapy for prostate cancer. _Int. J. Radiat. Oncol. Biol. Phys._ 70, 744–751 (2008). Article
PubMed Google Scholar * Trofimov, A. _et al._ Radiotherapy treatment of early-stage prostate cancer with IMRT and protons: A treatment planning comparison. _Int. J. Radiat. Oncol. Biol.
Phys._ 69, 444–453 (2007). Article PubMed PubMed Central Google Scholar * Chera, B. S. _et al._ Dosimetric study of pelvic proton radiotherapy for high-risk prostate cancer. _Int. J.
Radiat. Oncol. Biol. Phys._ 75, 994–1002 (2009). Article PubMed Google Scholar * Lomax, A. Intensity modulation methods for proton radiotherapy. _Phys. Med. Biol._ 44, 185–205 (1999).
Article CAS PubMed Google Scholar * Mohan, R. & Grosshans, D. Proton therapy - Present and future. _Adv. Drug Deliv. Rev._ 109, 26–44 (2017). Article PubMed Google Scholar *
Debus, J. _et al._ Bestrahlung von Schädelbasistumoren mit Kohlenstoffionen bei der GSI Erste klinische Ergebnisse und zukünftige Perspektiven. _Strahlenther. Onkol._ 176, 211–216 (2000).
Article CAS PubMed Google Scholar * Cuaron, J. J. _et al._ Anterior-oriented proton beams for prostate cancer: A multi-institutional experience. _Acta Oncol._ 54, 868–874 (2015). Article
PubMed Google Scholar * Moteabbed, M. _et al._ Proton therapy of prostate cancer by anterior-oblique beams: Implications of setup and anatomy variations. _Phys. Med. Biol._ 62, 1644–1660
(2017). Article CAS PubMed Google Scholar * Kubota, Y. _et al._ Changes in rectal dose due to alterations in beam angles for setup uncertainty and range uncertainty in carbon-ion
radiotherapy for prostate cancer. _PLOS ONE_ 11, 1–11 (2016). Google Scholar * Bentefour, E. H. _et al._ Validation of an in-vivo proton beam range check method in an anthropomorphic pelvic
phantom using dose measurements. _Med. Phys._ 42, 1936–1947 (2015). Article CAS PubMed Central Google Scholar * Hoesl, M. _et al._ Clinical commissioning of an in vivo range
verification system for prostate cancer treatment with anterior and anterior oblique proton beams. _Phys. Med. Biol._ 61, 3049–3062 (2016). Article CAS PubMed Google Scholar * Rucinski,
A. _et al._ Ion therapy of prostate cancer: Daily rectal dose reduction by application of spacer gel. _Radiat. Oncol._ 10, 56 (2015). Article PubMed PubMed Central CAS Google Scholar *
Underwood, T. S. A. _et al._ Hydrogel rectum-prostate spacers mitigate the uncertainties in proton relative biological effectiveness associated with anterior-oblique beams. _Acta Oncol._ 56,
575–581 (2017). Article PubMed Google Scholar * Dinh, T.-K.T. _et al._ Rectal hydrogel spacer improves late gastrointestinal toxicity compared to rectal balloon immobilization after
proton beam radiation therapy for localized prostate cancer: A retrospective observational study. _Int. J. Radiat. Oncol. Biol. Phys._ 108, 635–643 (2020). Article PubMed Google Scholar *
D’Amico, A. V. _et al._ A practical method to achieve prostate gland immobilization and target verification for daily treatment. _Int. J. Radiat. Oncol. Biol. Phys._ 51, 1431–1436 (2001).
Article PubMed Google Scholar * McGary, J. E., Teh, B. S., Butler, E. B. & Grant, W. Prostate immobilization using a rectal balloon. _J. Appl. Clin. Med. Phys._ 3, 6–11 (2002).
Article PubMed PubMed Central Google Scholar * van Lin, E. N. T., Hoffmann, A. L., van Kollenburg, P., Leer, J. W. & Visser, A. G. Rectal wall sparing effect of three different
endorectal balloons in 3D conformal and IMRT prostate radiotherapy. _Int. J. Radiat. Oncol. Biol. Phys._ 63, 565–576 (2005). Article PubMed Google Scholar * van Lin, E. N. _et al._
Reduced late rectal mucosal changes after prostate three-dimensional conformal radiotherapy with endorectal balloon as observed in repeated endoscopy. _Int. J. Radiat. Oncol. Biol. Phys._
67, 799–811 (2007). Article PubMed Google Scholar * Bastasch, M. _et al._ Tolerance of endorectal immobilization balloon in 396 patients treated with intensity-modulated radiation therapy
(IMRT) for prostate cancer. _Int. J. Radiat. Oncol. Biol. Phys._ 54, 270 (2002). Article Google Scholar * Hardcastle, N., Metcalfe, P. E., Rosenfeld, A. B. & Tomé, W. A. Endo-rectal
balloon cavity dosimetry in a phantom: Performance under IMRT and helical tomotherapy. _Radiother. Oncol._ 92, 48–56 (2009). Article PubMed PubMed Central Google Scholar * Smeenk, R. J.,
Teh, B. S., Butler, E. B., van Lin, E. N. & Kaanders, J. H. Is there a role for endorectal balloons in prostate radiotherapy? A systematic review. _Radiother. Oncol._ 95, 277–282
(2010). Article PubMed Google Scholar * Smeenk, R. J., Hopman, W. P., Hoffmann, A. L., van Lin, E. N. & Kaanders, J. H. Differences in radiation dosimetry and anorectal function
testing imply that anorectal symptoms may arise from different anatomic substrates. _Int. J. Radiat. Oncol. Biol. Phys._ 82, 145–152 (2012). Article PubMed Google Scholar * Teh, B. S. _et
al._ Intensity-modulated radiation therapy (IMRT) for prostate cancer with the use of a rectal balloon for prostate immobilization: acute toxicity and dose-volume analysis. _Int. J. Radiat.
Oncol. Biol. Phys._ 49, 705–712 (2001). Article CAS PubMed Google Scholar * Teh, B. S. _et al._ Clinical experience with intensity-modulated radiation therapy (IMRT) for prostate cancer
with the use of rectal balloon for prostate immobilization. _Med. Dosim._ 27, 105–113 (2002). * Teh, B. S. _et al._ Rectal wall sparing by dosimetric effect of rectal balloon used during
Intensity-Modulated Radiation Therapy (IMRT) for prostate cancer. _Med. Dosim._ 30, 25–30 (2005). Article PubMed Google Scholar * Patel, R. R., Orton, N., Tomé, W. A., Chappell, R. &
Ritter, M. A. Rectal dose sparing with a balloon catheter and ultrasound localization in conformal radiation therapy for prostate cancer. _Radiother. Oncol._ 67, 285–294 (2003). Article
PubMed Google Scholar * Dubouloz, A. _et al._ Urethra-sparing stereotactic body radiotherapy for prostate cancer: how much can the rectal wall dose be reduced with or without an endorectal
balloon?. _Radiat. Oncol._ 13, 114 (2018). Article PubMed PubMed Central CAS Google Scholar * Kelleter, L. _et al._ Spectroscopic study of prompt-gamma emission for range verification
in proton therapy. _Phys. Med._ 34, 7–17 (2017). Article PubMed Google Scholar * Martins, P. M. _et al._ Prompt gamma spectroscopy for range control with CeBr\(_3\). _CDBME_ 3, 113–117
(2017). Google Scholar * Dal Bello, R. _et al._ Results from the experimental evaluation of CeBr\(_3\) scintillators for \(^4\)He prompt gamma spectroscopy. _Med. Phys._ 46, 3615–3626
(2019). Article CAS Google Scholar * Foley, K., Clegg, A. & Salmon, G. Gamma-radiation from the medium energy proton borbardment of sodium, magnesium, aluminium, silicon phosphorus
and sulphur. _Nucl. Phys._ 37, 23–44 (1962). Article CAS Google Scholar * Kozlovsky, B., Murphy, R. J. & Ramaty, R. Nuclear deexcitation gamma-ray lines from accelerated particle
interactions. _ApJS_ 141, 523–541 (2002). Article CAS ADS Google Scholar * Boromiza, M. _et al._ Nucleon inelastic scattering cross sections on \(^{16}\)O and \(^{28}\)Si. _Phys. Rev. C_
101, 024604 (2020). Article CAS ADS Google Scholar * Kiener, J. _et al._ Gamma-ray emission in alpha-particle reactions with C, Mg, Si, Fe. _J. Phys. Conf. Ser._ 1555, 012011 (2020).
Article CAS Google Scholar * Magalhaes Martins, P. _et al._ A single-particle trigger for time-of-flight measurements in prompt-gamma imaging. _Front. Phys._ 8, 169 (2020). Article
Google Scholar * Magalhaes Martins, P. _et al._ PIBS: Proton and ion beam spectroscopy for in vivo measurements of oxygen, carbon, and calcium concentrations in the human body. _Sci. Rep._
10, 7007 (2020). Article CAS PubMed PubMed Central ADS Google Scholar * Haberer, T. _et al._ The Heidelberg ion therapy center. _Radiother. Oncol._ 73, S186–S190 (2004). Article
PubMed Google Scholar * Tessonnier, T. _et al._ Proton and helium ion radiotherapy for meningioma tumors: A Monte Carlo-based treatment planning comparison. _Radiat. Oncol._ 13, 2 (2018).
Article PubMed PubMed Central CAS Google Scholar * Mein, S. _et al._ Biophysical modeling and experimental validation of relative biological effectiveness (RBE) for \(^4\)He ion beam
therapy. _Radiat. Oncol._ 14, 123 (2019). Article PubMed PubMed Central CAS Google Scholar * Werner, F. _et al._ Performance verification of the FlashCam prototype camera for the
Cherenkov Telescope Array. _Nucl. Instrum. Methods A_ 876, 31–34 (2017). Article CAS ADS Google Scholar * The CTA Consortium _et al._ Design concepts for the Cherenkov Telescope Array
CTA: An advanced facility for ground-based high-energy gamma-ray astronomy. _Exp. Astron._ 32, 193–316 (2011). Download references ACKNOWLEDGEMENTS P.M.M. is supported by a research
fellowship for postdoctoral researchers from the Alexander von Humboldt Foundation, Bonn, Germany. H.F. is supported by Erasmus+ Programme of the European Union. P.M.M. would like to thank
M.C.F. Magalhães for fruitful discussions and support with the preparation of the water solutions. The authors would like to thank German Hermann and Thomas Kihm for providing the data
acquisition system. The authors would like to thank Semi Harrabi for suggesting the use of the ERB and to Nami Saito for proposing the use of silicon as a ranging probe. The authors thank
Arjen Winkel for providing the ERBs. The authors thank the staff of the DKFZ, in particular Gernot G. Echner, Armin Runz, and Ruediger Schmidt for the technical support. The authors also
thank the radiation protection department of the DKFZ, in particular Mechthild Kaemmer, for the support with calibration sources. FUNDING Open Access funding enabled and organized by Projekt
DEAL. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * German Cancer Research Center - DKFZ, Heidelberg, Germany Paulo Magalhaes Martins, Hugo Freitas & Joao Seco * Instituto de Biofísica
e Engenharia Biomédica, Faculdade de Ciências da Universidade de Lisboa, Lisbon, Portugal Paulo Magalhaes Martins * Departamento de Física e Astronomia, Faculdade de Ciências da Universidade
do Porto, Porto, Portugal Hugo Freitas * Heidelberg Ion-Beam Therapy Center (HIT), Department of Radiation Oncology, Heidelberg University Hospital, Heidelberg, Germany Thomas Tessonnier,
Benjamin Ackermann & Stephan Brons * Department of Physics and Astronomy, University of Heidelberg, Heidelberg, Germany Joao Seco Authors * Paulo Magalhaes Martins View author
publications You can also search for this author inPubMed Google Scholar * Hugo Freitas View author publications You can also search for this author inPubMed Google Scholar * Thomas
Tessonnier View author publications You can also search for this author inPubMed Google Scholar * Benjamin Ackermann View author publications You can also search for this author inPubMed
Google Scholar * Stephan Brons View author publications You can also search for this author inPubMed Google Scholar * Joao Seco View author publications You can also search for this author
inPubMed Google Scholar CONTRIBUTIONS P.M.M. and J.S. conceptualized the project. P.M.M. and H.F. performed experiments and acquired data. B.A. performed the computed tomography and
supported the experiments. T.T. prepared the treatment plans. S.B. provided continuous support throughout all experiments. P.M.M., H.F., T.T., B.A., S.B. and J.S. discussed content and
researched data. P.M.M processed and analysed the data. P.M.M. and T.T. prepared figures. J.S. acquired funding and supervised the project. CORRESPONDING AUTHORS Correspondence to Paulo
Magalhaes Martins or Joao Seco. ETHICS DECLARATIONS COMPETING INTERESTS The international patent application number PCT/EP2020/070128 for a PGS system including the features presented in
this work has been filed with J.S. and P.M.M. as inventors. H.F., T.T., B.A. and S.B. declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains
neutral with regard to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons
Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original
author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the
article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your
intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence,
visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Magalhaes Martins, P., Freitas, H., Tessonnier, T. _et al._ Towards real-time
PGS range monitoring in proton therapy of prostate cancer. _Sci Rep_ 11, 15331 (2021). https://doi.org/10.1038/s41598-021-93612-y Download citation * Received: 12 November 2020 * Accepted:
24 June 2021 * Published: 28 July 2021 * DOI: https://doi.org/10.1038/s41598-021-93612-y SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get
shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative