Play all audios:
ABSTRACT Atmospheric deposition-related potentially toxic elements (PTEs) can contaminate mountain forest ecosystems. The influence of tree species is being increasingly recognised as an
important factor in the deposition loads in forest soils. However, relevant modelling studies about the forest pollution with PTEs, concerning the tree species composition, are lacking. The
aim of this study was to evaluate the effect of European beech (_Fagus sylvatica_ L.) and Norway spruce (_Picea abies_ (L.) H. Karst.) on soil and mushroom pollution and the associated
health risks to define their significance for pollution modelling. Therefore, topsoil samples and samples of eight edible mushroom species were taken from 51 mature beech- and
spruce-dominated stands. The results showed that forest composition had an indirect influence on the PTEs contents in the topsoil; it significantly differentiated the relationship between
PTEs and soil C as the beech stands showed significantly increasing PTEs content with increasing C content. Despite the absence of soil pollution, above-limit levels of Cd and Zn were found
in mushrooms. The total content of PTEs in mushrooms posed a potential health risk to consumers in 82% of the samples. The most Cd-contaminated and potentially the riskiest species for
consumption was _Xerocomellus pruinatus_ (Fr. and Hök) Šutara. The results suggest that the source of PTEs for mushrooms is not only the soil but probably also the current wet deposition.
The influence of the forest type on the accumulation of PTEs in mushrooms was confirmed mainly due to the strongly divergent behaviour of Zn in beech- vs. spruce-dominated stands. The
results point to the need to evaluate mushroom contamination even in the contamination-unburdened forest areas. For future modelling of PTEs pollution in forests, it is necessary to
differentiate the tree species composition. SIMILAR CONTENT BEING VIEWED BY OTHERS THE SPATIAL DISTRIBUTION OF POTENTIALLY TOXIC ELEMENTS IN THE MOUNTAIN FOREST TOPSOILS (THE SILESIAN
BESKIDS, SOUTHERN POLAND) Article Open access 03 January 2024 THE IMPACTS OF MINING ON SOIL POLLUTION WITH METAL(LOID)S IN RESOURCE-RICH MONGOLIA Article Open access 16 February 2023
SEASONAL VARIATIONS AND RISK ASSESSMENT OF MICROPLASTIC CONTAMINATION IN AGRICULTURAL SOIL AND ASSOCIATED MACROINVERTEBRATES IN EGYPT Article Open access 24 February 2025 INTRODUCTION
Environmental pollution with potentially toxic elements (PTEs) is a global environmental problem. Studies addressing this issue focus mainly on urban, mining, or agricultural areas due to
the higher risk of direct human exposure and potentially easier intoxication. Less attention is paid to forest ecosystems1, which are expected to be less polluted due to the predominant
absence of nearby contamination sources. However, recent results suggest that these assumptions may be wrong2,3. Intensive industrialisation began in Central Europe in the eighteenth century
and the resulting environmental pollution led to strong impacts on forest ecosystems, which are ongoing4,5,6. PTEs can be transported by air far from their source such as industrial areas.
The depositions are intercepted by forest vegetation, precipitated to the forest floor, and subsequently, infiltrated into the mineral soil with percolating water1,3. Due to the affinity of
PTEs to soil organic matter and their long-term persistence in soil7, the current inputs as well as the historical accumulation of PTEs are important when determining the critical loads8.
Regular high intake of PTEs poses a health risk to all living organisms7,9. Mushrooms are significant accumulators of PTEs from the surrounding environment and therefore pose a potential
risk to their consumers10,11,12,13. The accumulation of PTEs in fruiting bodies of wild ectomycorrhizal mushrooms with culinary uses depends on the ability of fungal hyphae to absorb them
from the soil and transport them through the mycelium14. These mushroom species preferentially absorb nutrients and other substances from the forest floor and mineral topsoil10,15, i.e. from
potentially the most contaminated soil layers16. Mushroom species are generally highly discriminating regarding the accumulation of PTEs17. However, Cd, Zn, and Cu can be effectively
accumulated in many mushroom species14,18. In some countries, mushroom picking is a kind of national hobby, involving up to 65% of households in the Czech Republic, for example19. Therefore,
the risk of large-scale intoxication by contaminated wild-growing edible mushrooms can be high. The tree species composition of a forest ecosystem can be significant for the overall
contamination load16,20. There is a difference in the throughfall deposition of elements in coniferous stands, which can capture more dust and gaseous pollution, compared to deciduous
species due to the higher total surface area of the needles and the evergreen nature of the conifers. The depositions are washed by precipitation into the soil, which significantly affects
topsoil properties20,21,22,23. However, there is also the view that evergreen conifers can store contaminated dust in their foliage for a few seasons, while deciduous trees transfer the
annual input of dust with PTEs into the soil as litterfall. In addition, the stem flow of deciduous trees generates higher solute fluxes to the soil in comparison to solute fluxes during
throughfall24. Norway spruce (_Picea abies_ (L.) H. Karst.) and European beech (_Fagus sylvatica_ L.) are given special attention as they are two of the most economically important European
tree species25. Spruce has a higher potential for capturing atmospheric pollutants than beech as it is associated with higher S and N depositions22. Despite ongoing research, there are
uncertainties and therefore, relevant studies and methodologies for modelling tree species-related forest pollution with the risk of picking contaminated mushrooms are lacking. The topic is
mostly studied separately with a strong local focus on one or two selected stands as a subtopic to evaluate the impact of tree species on soils6,24 or the risk of consuming mushrooms from
areas with proven heavily polluted soils14,26. Data from mountain areas without a significant local large-scale source of pollution are mostly lacking. The objectives of this study were (1)
to assess topsoil pollution with PTEs (Cd, Cu, Pb, and Zn as potentially serious contaminants related to atmospheric deposition) in the forests of the mountain region; (2) to assess the
contamination of mushrooms and the potential health risks associated with their consumption; (3) to evaluate the effect of beech and spruce on PTE contents and risks; and (4) to identify the
main factors for spatial modelling of PTE distribution in forest topsoil. Tree species (forest type) are assumed to be a significant factor in spatial modelling of PTE pollution in forest
areas. Particularly, we hypothesise that (H1) despite the absence of a significant local pollution source, the topsoil in the studied stands will be polluted due to atmospheric depositions
from remote industrial areas and (H2) due to the expected higher topsoil pH, there will be a higher topsoil accumulation of PTEs in beech stands. On the other hand, for spruce stands, we
hypothesise that (H3) due to the higher spruce acidy-related mobility of PTEs, there will be significantly higher contents of PTEs in mushrooms and related potential health risks. MATERIALS
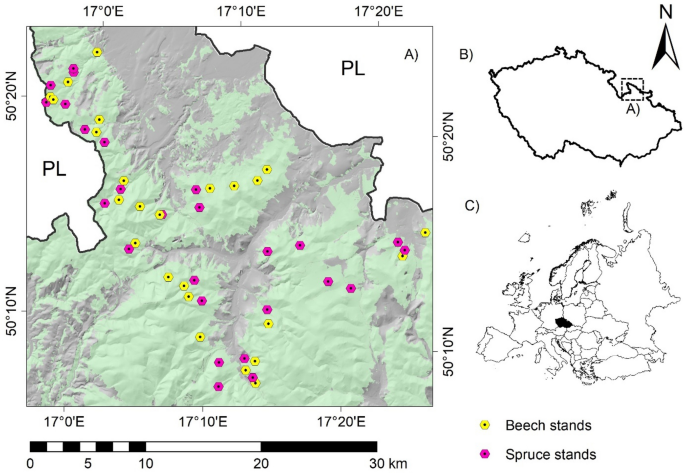
AND METHODS STUDY AREA The study was carried out in a highly tourism-exposed region of the Jeseníky Mountains, the Czech Republic (Fig. 1). The region is marginally affected by historical
and current contamination originating from atmospheric depositions, mostly from the industrial Upper Silesian Coal Basin27,28. The studied area was located at altitudes of 396–990 m, with an
average annual precipitation of 810 mm (predominance in the growing season) and an average annual temperature of 7.8 °C (considered from 1989 through 2019)29. Forest stands in the area are
predominantly even-aged Norway spruce (_Picea abies_ (L.) H. Karst.) and European beech (_Fagus sylvatica_ L.) monocultures growing on Cambisols and Podzols. The atmospheric deposition data
(in a square network of 1 × 1 km) are available only for Cd and Pb, with a range of 0.3–0.6 mg/l and 5.0–8.2 mg/l, respectively28. Further information on the study area is provided in
chapter 2.2. SOIL AND MUSHROOM SAMPLING Beech- and spruce-dominated (considered for > 80% basal area of the species) stands were selected for soil and mushroom sampling according to the
following parameters: (a) area > 1 ha; (b) age 80–100 years; (c) absence of a local source of pollution (e.g. built-up area, heaps, roads, and landfills); and (d) comparable bedrock
consisting of acid metamorphic rocks (mostly phyllites and gneiss). According to the parameters, 51 stands (26 × spruce and 25 × beech; Fig. 1) were selected after field verification. The
methodology partly followed previous studies16,30. A random plot was sampled within each of the stands, which included some previously published soil data (6 × spruce and 6 × beech
stands30). To avoid edge effects, the plots were placed at least 25 m apart from the stand edge. At each plot, a standard composite sample was collected from the 0–2 cm mineral soil using a
plastic shovel at three sampling points spaced 4–6 m apart, so the sampling was never performed twice under the canopy projection of a single tree. Mushroom samples of the species from the
_Boletaceae_ family commonly used for culinary purposes, were collected from each stand, depending on the current occurrence of the fruiting bodies of the species. The fruiting mushroom
bodies were sorted into paper bags as a mixed sample for each mushroom species. To minimise the bias due to the presence of other tree species in the stands, all mushroom samples were
collected only under the dominant tree species. A total of 96 mushroom samples of eight species were collected (Table S1). SAMPLE PREPARATION AND ANALYSIS The soil samples were dried at room
temperature, homogenised, and treated to fine soil following the standard ČSN ISO 11464. The pH was measured in a suspension of mineral and organic soil in water, with a soil-to-water
volume ratio of 1:5. Total C contents of oven-dry samples were determined using a catalytic tube combustion method with thermal conductivity detector on an elemental analyser VarioMAX CNS
(Elementar Analysensysteme GmbH, Germany) following ČSN ISO 10694. Total C content was analysed to express the organic matter content in soil as they are approximately equal. The fruiting
bodies of the mushrooms were manually cleaned of the coarse debris (parts of plants, forest floor, or mineral particles) with a ceramic knife. The samples were sliced and dried at room
temperature for several weeks in a dust-free environment. After drying, the mushroom samples were homogenised by grinding to powder in a stainless-steel mill for subsequent PTEs content
analysis. Pre-prepared soil and mushroom samples were digested in _aqua regia_ (mixture of HNO3 and HCl in a ratio of 1:3) to determine the content of Cd, Cu, Pb, and Zn. The digestion took
place in a microwave digestion platform ETHOS EASY (Milestone, Italy). After digestion, the samples were quantitatively transferred with Milli-Q water (Merck, Germany) to a volume of 25 ml.
The concentration of the elements in the digested samples was measured on an atomic absorption spectrophotometer ContrAA 800D (Analytik Jena, Germany) with a continuous radiation source (Xe
lamp), a high-resolution monochromator, and a dual flame system (FAAS) or electrothermal atomiser (ET-AAS). The optical system is based on a high-resolution Echelle monochromator and a
sensitive CCD detector. Cd, Cu, and Pb were determined using the ET-AAS method, while Zn was determined using the FAAS method. Blanks, triplicate measurements, and matrix reference material
measurements (METRANAL 31, METRANAL 33, and METRANAL 34; Analytika, Czech Republic) were performed for quality assurance and quality control (Table S2). The range of relative standard
deviations of the triplicate measurements was 0.5–6.8% for soils and 0.1–8.3% for mushrooms. Recoveries related to the reference materials ranged between 93.5 and 112.3% (METRANAL 31), 94.2
and 103.6% (METRANAL 33), and from 96.3 to 102.9% (METRANAL 34). The limits of detection for Cd, Cu, Pb, and Zn were 0.003, 0.012, 0.033, and 0.007 mg/kg, respectively. Mushroom results were
given for dry weight. SOIL POLLUTION ASSESSMENT The Integrated Nemerow Pollution Index (IPIN) was used for the assessment of the PTEs-related soil pollution level using two different
standards (see the following text). IPIN classes are safe (≤ 0.7), precaution (0.7–1), slight pollution (1–2), moderate pollution (2–3), and heavy pollution (≥ 3). IPIN was calculated as
follows31: $${\mathrm{PI}}_{\mathrm{i}}= \frac{{\mathrm{C}}_{\mathrm{i}}}{{\mathrm{L}}_{\mathrm{i}}}$$ $${\mathrm{IPI}}_{\mathrm{N}} = [(\mathrm{P}{{\mathrm{I}}^{2}}_{\mathrm{avg}} +
{{\mathrm{PI}}^{2}}_{\mathrm{max}})/2{]}^{1/2}$$ where PIi means single pollution index of individual PTE, Ci means the content of the PTE, Li means the limit value of the PTE—Dutch Target
Value (VROM32) or Czech legislation (Decree No. 153/2016 Coll.33), PIavg means the average value of all PIi of the PTEs, and PImax means the maximum PIi value of the PTEs. MUSHROOM
CONTAMINATION AND HEALTH RISK ASSESSMENT The contents of PTEs in mushrooms were compared with the Czech legislative limits from Decree No. 298/1997 Coll.34 for Cu and Zn and Decree No.
53/2002 Coll.35 for Cd and Pb (the last one available; currently, there is no legislative limit for wild-growing mushrooms in the Czech Republic) and the usually reported PTE contents for
species grown in unpolluted sites18 in the assessment of a contamination. To evaluate the relationship between the mushroom and soil PTE contents, the ability to accumulate a PTE was
measured using Bioconcentration Factor (BCF)—a simple method to quantitatively characterise the transfer of available PTEs from the soil to the mushroom. BCF is calculated as the ratio of
the mushroom PTE content to the total soil PTE content. The stand-specific soil values were used for the calculation, i.e., the PTE value in the mushroom sample was divided by the respective
PTE value in the soil from the stand where the mushroom was collected. Mushrooms with a BCF value of < 1 correspond to PTE excluders while a BCF value of > 1 to accumulators11,36. The
potential health risk of the mushrooms, expressed as the Health Risk Index (HRI), was calculated as the ratio of daily intake of metal (DIM) through the edible mushrooms to the oral
reference dose (RfDo)37,38. HRI values > 1 represent a potential health risk. RfDo values used for Cd, Cu, Pb, and Zn were 0.001, 0.04, 0.0035, and 0.3 mg/kg/day, respectively31,39. Daily
intake was calculated using the following equation26,37,38,40: $${\mathrm{DIM}}_{\mathrm{i}}=\frac{{\mathrm{C}}_{mushroom}\times {\mathrm{D}}_{IM}}{\mathrm{BW}}$$ where Cmushroom represents
the PTE content in the mushrooms (mg/kg, based on dry weight); DIM represents the daily intake of mushrooms (0.03 kg)13,38; and BW represents an average bodyweight of the consumer (74.6, an
average of men and women in the Czech Republic)41. STATISTICAL AND SPATIAL ANALYSIS Statistical analysis of data sets was performed in the Statistica 12 program, both for the whole sets and
subsets of spruce and beech stands separately. Due to the lack of mushroom biomass for analysis after drying from one spruce stand, only 50 stands were statistically analysed. In addition
to the standard descriptive statistics, normality tests (Shapiro–Wilk) were performed, and the basic graphical methods of exploratory analysis were used (box plot, QQ plot and histogram).
Since most of the data sets did not show a normal distribution and a larger number of outliers were found, subsequent comparisons of the beech and spruce stands were performed using a
nonparametric test (Mann–Whitney U test). Linear regression analysis was used to determine the relationship between PTEs’ contents and soil properties. The tests were evaluated at the level
of significance _p_ = 0.05. Spatial distribution maps of PTEs were created in the program ArcGIS 10.4. The position of the sampling sites and the values of Cd, Cu, Pb, and Zn contents in the
soil and mushrooms were converted to a geodatabase format. Spatial interpolation of the soil PTE contents was performed by the Geographically Weighted Regression (GWR) method. The
dependent, i.e. modelled variable of GWR, was the soil PTE content, and the independent variable was the total C content as a continuous variable significantly influencing the PTEs in the
soils. Due to the different density and irregular spatial distribution of sites with variable neighbour distance, the context of the positions was analysed using the adaptive kernel of
weighted regression. The scope of the kernel of weighted regression used Akaike's information criterion for small selections (AICc). The spatial output of the GWR was subsequently
processed by the method of maximum probability classification, where the rasters of beech and spruce representation in forest stands were used as independent variables. The weight of the
independent variable was the percentage share of beech or spruce in the stands compared to other tree species. Due to the high variability in mushroom contamination (both within and between
mushroom species), a point presentation of the results was performed with an indication of potential health risk (HRI). RESULTS AND DISCUSSION SOIL POLLUTION AND TREE SPECIES (FOREST TYPE)
INFLUENCE ASSESSMENT The overall IPIN assessment classified the soils as safe according to both calculation variants (Table S3). Despite long-term atmospheric deposition of PTEs28,
originating predominantly from the heavy-industry areas of Silesia27,42, the severity of the accumulated soil contamination does not pose any risk in the context of the applied
standards32,33. Although other studies3 have pointed to the potential risk of a serious forest soil enrichment with PTEs even in the case of remote protected areas, the hypothesis H1 was not
confirmed. Minor differences between beech and spruce stands were indicated in the IPIN values (Table S3). Similarly, there were no significant differences in the soil contents of Cu, Pb,
and Zn (Fig. 2). The cadmium content (Table S3, S4) was predominantly below the limit of detection; therefore, it was omitted from the statistical comparison and graphical presentation.
Consequently, at the larger scale, the hypothesis H2 should be rejected. The absence of differences between the species-related soil pollution is in contradiction to the observed increased
magnetic susceptibility of topsoil in the beech as compared to the spruce stands24. Similarly, Novotný et al.30 observed significantly higher topsoil (0–2 cm) contents of Pb and Zn in beech
as compared to spruce; the authors explained this phenomenon by the different effects of the tree species on soil chemistry and the presumed differences in PTEs’ mobility, including their
retention and redistribution within the soil profile30. A probable reason for the difference compared to the literature is that the results of both studies are based on a smaller number of
sites (2 and 12, respectively). Because a larger sampling range here (51 sites), specific site factors could outweigh species factors despite the precise methodology in the selection of the
stands and characteristically similar defined region. The assumption is that the predetermining site factor was the slightly different atmospheric depositions resulting from the spatial
distribution of individual forest stands (see chapter 2.1). Soil pH and the quantity and quality of soil organic matter are essential for PTEs cycling and stabilisation in soils3,9,43.
Spruce-related soil acidification20,22,44 resulted in a significantly lower topsoil pH in the spruce as compared to the beech stands (Fig. 2). This could initiate a higher mobility of PTEs
and their bioavailability for plants and other living organisms42,43. Although species effect alone seems to have a small to no relevance for topsoil enrichment with PTEs, an indirect
influence of the tree species on topsoil PTEs’ contents was demonstrated in the correlations of Cu, Pb, and Zn contents to the other soil properties (Table 1). The overall medium to strong
positive correlations of Cu, Pb, and Zn with C in the beech-dominated stands confirmed the important role of soil organic matter quantity and quality in the PTEs’ accumulation in soil. In
contrast, there were no significant relationships of Cu, Pb, or Zn with C and pH in the spruce-dominated stands. Therefore, it was necessary to reconsider the forest type factor for spatial
modelling of soil pollution with PTEs (Figure S1). MUSHROOM CONTAMINATION ASSESSMENT Despite the absence of soil pollution, the PTE contents in mushrooms (Table S5) exceeded the pollution
and hygienic limits (Table 2). Compared to the PTE values for unpolluted sites18, contamination of mushrooms is evident, especially by Cd and Zn. The Czech hygienic limits were exceeded in
the case of Pb, Cd, and Zn in 2%, 66%, and 100% of the samples, respectively. Furthermore, a report by the Institute of Agricultural Economics and Information45 indicated a serious risk of
mushroom contamination with Cd in forest ecosystems in the Czech Republic, where 52% of the samples (_n_ = 23) were above the hygienic limit of 2 mg/kg. In this study, Cd and Zn contents
often reached the values typical for heavily polluted areas10,13. This reveals that mushrooms can be heavily polluted even in forests of a mountain region with neither significant
close-source contamination nor recognisable soil pollution. The most Cd-contaminated species was _Xerocomellus pruinatus_, whose average contents exceeded the hygienic limit by more than 6
times (Table 2). In addition, _Boletus edulis_ and _Xerocomellus chrysenteron_ can be considered as heavily Cd-contaminated. In the case of Zn, _Porphyrellus porphyrosporus_, _Neoboletus
luridiformis_, _Boletus edulis,_ and _Imleria badia_ reached high over-limit values in particular (Table 2, Table S5). Pb and Cu are unlikely to be serious mushroom contaminants, as they had
predominantly below-limit values in all the species. The high variability of the contents of PTEs among the species and at the species level (Table 2 and S3) is common for mushrooms12,46
and can be attributed to environmental factors (influence of site and atmospheric depositions) and age and size of fruiting bodies in the sample or age of mycelium10,26,46,47. Due to the
common abundance of _I. badia_, _X. chrysenteron,_ and _B. edulis_, the greatest research focus is typically on these species. Kalač et al.13 found even 2 times higher Cd contents in _B.
edulis_ (15.2 mg/kg) at sites heavily polluted by the lead smelter, as compared to the other two species. Strong accumulation of Cd in _B. edulis_, as compared to the other species (Table
2), was also found by Cocchi et al.17 and Komárek et al.10. The results of this study were also consistent with the findings of Komárek et al.10 in the accumulation of Cu and Zn in _B.
edulis_, as compared to _X. chrysenterone_ (Table 2). Also, Alonso et al.47 found _B. edulis_ to be the most important Cu accumulator of these three species, while the strongest accumulation
of Zn found in _I. badia_. Other species usually receive little or no attention, which points to the need for more extensive research on this issue. The origin of PTEs in the studied
mushrooms is questionable. Results of the BCF assessment indicated that all the species are strong accumulators of Cd, Cu, and Zn from the soil (Table S5 and S6). However, the general
absence of stronger relationships between the soil and mushroom PTE contents (moderate correlation in the whole data set was found only for Pb; Table S7) suggests the possibility of another
source. Although BCF values > 1 are common for mushrooms, which can reach values in the order of tens18 to hundreds11, extreme values as in Cd (BCF > 1000) indicate the importance of
another accumulation mechanism than the uptake from bulk soil. The life cycle of mushrooms is more dynamic and more easily influenced by short-term (current) environmental conditions
compared to soil. Thus, it can be assumed that wet atmospheric deposition of PTEs associated with both vertical and horizontal precipitation, may also have an impact. Mushrooms'
accumulation potential12,26 enables them to rapidly and efficiently take up and store soil solution-bound atmospheric depositions, thus accelerating the Cd cycle in particular. Consequently,
Cd does not accumulate in the soil, as Cd contents were below the detection limit in 59% of the soil samples. On the other hand, in mushrooms, Cd was found in all samples, often at levels
typical for areas with serious soil pollution10,13. The soil contents of Cd in the studied area do not reach the values of contaminated areas even in the potentially most contaminated forest
floor30 from which mushrooms also take up nutrients. Without a direct site-related source of Cd, only precipitation-related atmospheric deposition can be a predominant source. Although
direct atmospheric deposition is typically considered to be of minor importance due to the short lifespan of fruiting bodies in most edible mushroom species46, the results of this study may
question this assumption as they suggest the opposite. Due to species-dependent variation in PTEs accumulation17,46,47 (Table 2 and S4), _B. edulis_, _X. chrysenteron,_ and _I. badia_ were
tested to verify the effect of beech and spruce on the accumulation of PTEs in mushrooms. The results mostly did not confirm any significant importance of the forest type for these mushroom
species contents of PTEs (Table 3). However, analysis of the whole dataset of mushrooms or separately for the two forest types revealed stronger correlations for particular forest types
(Table S7), especially in the case of Pb (_r_ = 0.4138 = > EB _r_ = 0.5408 and NS _r_ = 0.4390) and Zn (_r_ = 0.0226 = > EB _r_ = −0.3792 and NS _r_ = 0.4223). For Zn, this
differentiation led to a reverse relationship in beech as compared to the spruce stands, suggesting differences in the biogeochemical cycling of this element depending on the tree species.
These findings point to the need for further research on this issue. HEALTH RISK ASSESSMENT OF MUSHROOMS The total HRI for all PTEs (defined only for easier summarization and orientation)
exceeded the limit value, indicating a potential health risk in 82% of mushroom samples (Table S5). Due to its toxicity31,36,39, Cd had the largest contribution to this risk, exceeding the
limit value in 59% of the cases (Fig. 3). Spatial distribution of the mushrooms-related health risk assessment is presented in Figure S1A–D. The results showed _X. pruinatus_ as potentially
the riskiest species for consumption, in which the average HRI value reached 5.69, mainly due to the high levels of Cd (HRICd = 4.74). However, due to the limited number of samples and the
absence of studies on contamination and health risk of this species, further research is needed to verify this finding. The significance of Cd-related risk is also evidenced by the fact that
such a high HRI was not achieved for any PTE in the 19 mushroom species studied by Sarikurkcu et al.38. However, some species of the _Boletaceae_ family can reach even higher HRICd
values12. _Boletaceae edulis_ and _X. chrysenterone,_ with HRI values of 3.33 and 3.01, respectively, also pose a high risk. As these two species are among the most abundant from their
family in Central European forests, the risk of poisoning is worthy of interest. Furthermore, _B. edulis_ belongs among the widely consumed species18. _N. luridiformis_ (HRI = 1.98), _I.
badia_ (HRI = 1.66), and _X. subtomentosus_ (HRI = 1.11) pose a lower risk. Similarly, Komárek et al.10 report _B. edulis_, _I. badia,_ and _X. chrysenteron_ as the species whose consumption
may pose a significant toxicological risk. Their study, however, was focused on the area contaminated by the lead smelter. The influence of beech and spruce on the potential health risk of
mushrooms was significant (Fig. 3) only in the case of _I. badia_ (HRIPb) and _X. chrysenteron_ (HRICu, HRIZn). The visible potential influence of forest type on the other species cannot be
statistically verified due to the small sample size. In general, no significant differences were found between the forest types for any of the PTEs (Mann–Whitney U Test, _p_ > 0.05). The
mushroom species and related ability to accumulate a given element (Fig. 3) appear to be a more important factor in the overall health risk assessment. Nevertheless, based on the results
(Table 3 and S7; Fig. 3), the effect of forest type on PTEs levels in mushrooms and their health risks can be considered significant. Hypothesis H3, however, cannot be confirmed. CONCLUSIONS
A soil pollution assessment classified the studied forest soils as safe. Although there were no significant differences between the forest types in the average topsoil contents of PTEs, the
correlations of PTEs contents to soil C contents showed different results for particular forest types. Unlike the spruce-dominated stands, the PTEs contents in beech stands showed
significant positive correlations with soil C contents. Despite the absence of soil pollution, serious contamination was found in mushrooms as Cd and Zn exceeded the Czech hygienic limits in
66 and 100% of the samples, respectively, and the total PTEs content represented a potential health risk in 82% of the samples. Thus, even mushrooms in forests without a local source of
contamination and soil pollution can be hazardous. Other sources, such as direct and indirect uptake of PTEs from vertical and horizontal precipitation and throughfall (in addition to soil),
should be considered. The results on the forest type influence on the accumulation of PTEs in common mushrooms are inconclusive; however, in the general evaluation, they confirm the
significant influence of the tree species, especially in the case of Zn. These findings point to the need for further research on this issue. The results suggest that for spatial modelling
of PTEs pollution in forest soils, not only the interpolation of data based on random sampling in forest stands is sufficient, but it is also necessary to consider the factor of tree species
and its representation in forest stands due to their significant influence on other soil properties essential for the behaviour of PTEs. REFERENCES * Türtscher, S., Berger, P., Lindebner,
L. & Berger, T. W. Declining atmospheric deposition of heavy metals over the last three decades is reflected in soil and foliage of 97 beech (_Fagus sylvatica_) stands in the Vienna
Woods. _Environ. Pollut._ 230, 561–573 (2017). Article Google Scholar * Szopka, K., Karczewska, A., Jezierski, P. & Kabała, C. Spatial distribution of lead in the surface layers of
mountain forest soils, an example from the Karkonosze National Park, Poland. _Geoderma_ 192, 259–268 (2013). Article ADS CAS Google Scholar * Mazurek, R. _et al._ Assessment of heavy
metals contamination in surface layers of Roztocze National Park forest soils (SE Poland) by indices of pollution. _Chemosphere_ 168, 839–850 (2017). Article ADS CAS Google Scholar *
Suchara, I. & Sucharová, J. Distribution of sulphur and heavy metals in forest floor humus of the Czech Republic. _Water Air Soil Pollut._ 136, 289–316 (2002). Article ADS CAS Google
Scholar * de Vries, W., Dobbertin, M. H., Solberg, S., van Dobben, H. F. & Schaub, M. Impacts of acid deposition, ozone exposure and weather conditions on forest ecosystems in Europe:
an overview. _Plant Soil_ 380, 1–45 (2014). Article Google Scholar * Kochergina, Y. V., Udatný, M., Penížek, V. & Mihaljevič, M. Mobility of Pb, Zn, Cu and As in disturbed forest soils
affected by acid rain. _Environ. Monit. Assess._ 189, 570 (2017). Article Google Scholar * Sun, X., Zhang, L. & Lv, J. Spatial assessment models to evaluate human health risk
associated to soil potentially toxic elements. _Environ. Pollut._ 268, 115699 (2021). Article CAS Google Scholar * Helmisaari, H.-S. _et al._ Copper in scots pine forests around a
heavy-metal smelter in south-western Finland. _Water Air Soil Pollut._ 85, 1727–1732 (1995). Article ADS CAS Google Scholar * Rocha, L. _et al._ The water-soluble fraction of potentially
toxic elements in contaminated soils: relationships between ecotoxicity, solubility and geochemical reactivity. _Chemosphere_ 84, 1495–1505 (2011). Article ADS CAS Google Scholar *
Komárek, M., Chrastný, V. & Štíchová, J. Metal/metalloid contamination and isotopic composition of lead in edible mushrooms and forest soils originating from a smelting area. _Environ.
Int._ 33, 677–684 (2007). Article Google Scholar * Melgar, M. J., Alonso, J. & García, M. A. Mercury in edible mushrooms and underlying soil: bioconcentration factors and toxicological
risk. _Sci. Total Environ._ 407, 5328–5334 (2009). Article ADS CAS Google Scholar * Sun, L., Chang, W., Bao, C. & Zhuang, Y. Metal contents, bioaccumulation, and health risk
assessment in wild edible boletaceae mushrooms. _J. Food Sci._ 82, 1500–1508 (2017). Article CAS Google Scholar * Kalač, P., Burda, J. & Stakova, I. Concentrations of lead, cadmium,
mercury and copper in mushrooms in the vicinity of a lead smelter. _Sci. Total Environ._ 105, 109–119 (1991). Article ADS Google Scholar * Cejpková, J. _et al._ Bioaccumulation of heavy
metals, metalloids, and chlorine in ectomycorrhizae from smelter-polluted area. _Environ. Pollut._ 218, 176–185 (2016). Article Google Scholar * Nováčková, J., Fiala, P., Chrastný, V.,
Svoboda, L. & Kalač, P. Contents of mercury, cadmium and lead in edible mushrooms and in uderlying substrates from a rural area with an occurrence of serpentines and amphiboles. _Ekol.
(Bratislava)_ 26, 322–329 (2007). Google Scholar * Pecina, V. _et al._ Vertical distribution of mercury in forest soils and its transfer to edible mushrooms in relation to tree species.
_Forests_ 12, 539 (2021). Article Google Scholar * Cocchi, L., Vescovi, L., Petrini, L. E. & Petrini, O. Heavy metals in edible mushrooms in Italy. _Food Chem._ 98, 277–284 (2006).
Article CAS Google Scholar * Kalač, P. Trace element contents in European species of wild growing edible mushrooms: a review for the period 2000–2009. _Food Chem._ 122, 2–15 (2010).
Article Google Scholar * MZe. _Zpráva o stavu lesa a lesního hospodářství České republiky v roce 2019 (in Czech)_. (Ministerstvo zemědělství (MZe), 2020). * Augusto, L., Ranger, J.,
Binkley, D. & Rothe, A. Impact of several common tree species of European temperate forests on soil fertility. _Ann. For. Sci._ 59, 233–253 (2002). Article Google Scholar * Vannier,
C., Didon-Lescot, J.-F., Lelong, F. & Guillet, B. Distribution of sulphur forms in soils from beech and spruce forests of Mont Lozre (France). _Plant Soil_ 154, 197–209 (1993). Article
CAS Google Scholar * Rothe, A., Huber, C., Kreutzer, K. & Weis, W. Deposition and soil leaching in stands of Norway spruce and European beech: results from the Höglwald research in
comparison with other European case studies. _Plant Soil_ 240, 33–45 (2002). Article CAS Google Scholar * Berger, T. W., Untersteiner, H., Schume, H. & Jost, G. Throughfall fluxes in
a secondary spruce (_Picea abies_), a beech (_Fagus sylvatica_) and a mixed spruce-beech stand. _For. Ecol. Manag._ 255, 605–618 (2008). Article Google Scholar * Łukasik, A., Gruba, P.
& Magiera, T. Application of magnetometry to assess distribution of dust pollution in topsoil of under-crown area of Norway spruce (_Picea abies_ Karst.) and European beech (_Fagus
sylvatica_ L.). _CATENA_ 150, 246–255 (2017). * Vitasse, Y. _et al._ Contrasting resistance and resilience to extreme drought and late spring frost in five major European tree species.
_Glob. Change Biol._ 25, 3781–3792 (2019). Article ADS Google Scholar * Sithole, S. C., Mugivhisa, L. L., Amoo, S. O. & Olowoyo, J. O. Pattern and concentrations of trace metals in
mushrooms harvested from trace metal-polluted soils in Pretoria, South Africa. _South African J. Bot._ 108, 315–320 (2017). Article CAS Google Scholar * Novák, M. _et al._ Origin of lead
in eight central european peat bogs determined from isotope ratios, strengths, and operation times of regional pollution sources. _Environ. Sci. Technol._ 37, 437–445 (2003). Article ADS
Google Scholar * CHMI. Pětileté průměrné koncentrace. (2021). * CHMI. Denní data dle zákona 123/1998 Sb. _Czech Hydrometeorological Institute_ (2021). * Novotný, R., Pecina, V., Černý, J.,
Valtera, M. & Juřička, D. Influence of Norway spruce and European beech on Cd, Cu, Pb and Zn content in the surface horizons of forest soils in the area of the Jeseníky Mountains.
_Zprávy lesnického výzkumu_ 66, 86–94 (2021). Google Scholar * Pecina, V. _et al._ Human health and ecological risk assessment of trace elements in urban soils of 101 cities in China: a
meta-analysis. _Chemosphere_ 267, 129215 (2021). Article ADS CAS Google Scholar * VROM. _Circular on Target Values and Intervention Values for Soil Remediation_. (Dutch Ministry of
Housing Spatial Planning and Environment (VROM), 2013). * Decree No. 153/2016 Coll. _Vyhláška č. 153/2016 Sb. ze dne 9. května 2016 o stanovení podrobností ochrany kvality zemědělské půdy a
o změně vyhlášky č. 13/1994 Sb., kterou se upravují některé podrobnosti ochrany zemědělského půdního fondu (in Czech)_. * Decree No. 298/1997 Coll. _Vyhláška č. 298/1997 Sb. ze dne 28.
listopadu 1997 kterou se stanoví chemické požadavky na zdravotní nezávadnost jednotlivých druhů potravin a potravinových surovin, podmínky jejich použití, jejich označování na obalech,
požadavky na čistotu a identitu přídatných látek a potravních doplňků a mikrobiologické požadavky na potravní doplňky a látky přídatné (in Czech)_. * Decree No. 53/2002 Coll. _Vyhláška č.
53/2002 Sb. ze dne 29. ledna 2002 kterou se stanoví chemické požadavky na zdravotní nezávadnost jednotlivých druhů potravin a potravinových surovin, podmínky použití látek přídatných,
pomocných a potravních doplňků (in Czech)._ * Frankowska, A., Ziółkowska, J., Bielawski, L. & Falandysz, J. Profile and bioconcentration of minerals by King Bolete (_Boletus edulis_)
from the Płocka Dale in Poland. _Food Add. Contam. B_ 3, 1–6 (2010). Article CAS Google Scholar * Liu, B. _et al._ Study of heavy metal concentrations in wild edible mushrooms in Yunnan
Province, China. _Food Chem._ 188, 294–300 (2015). Article CAS Google Scholar * Sarikurkcu, C., Popović-Djordjević, J. & Solak, M. H. Wild edible mushrooms from Mediterranean region:
Metal concentrations and health risk assessment. _Ecotoxicol. Environ. Saf._ 190, 110058 (2020). Article CAS Google Scholar * USEPA. Regional Screening Level (RSL) Resident Soil Table
(TR=1E-06, HQ=1) May 2020 (corrected). _US Environmental Protection Agency_ (2020). * Koutrotsios, G., Danezis, G. P., Georgiou, C. A. & Zervakis, G. I. Rare earth elements concentration
in mushroom cultivation substrates affects the production process and fruit-bodies content of _Pleurotus ostreatus_ and _Cyclocybe cylindracea_. _J. Sci. Food Agric._ 98, 5418–5427 (2018).
Article CAS Google Scholar * IHIS CR. _European Health Interview Survey in CR - EHIS CR (Body Mass Index, physical activity, consumption of fruits and vegetables)._ (Institute of Health
Information and Statistics of the Czech Republic, 2010). * Magiera, T., Strzyszcz, Z. & Rachwal, M. Mapping particulate pollution loads using soil magnetometry in urban forests in the
Upper Silesia Industrial Region, Poland. _For. Ecol. Manag._ 248, 36–42 (2007). Article Google Scholar * Bravo, S. _et al._ Influence of the soil pH in the uptake and bioaccumulation of
heavy metals (Fe, Zn, Cu, Pb and Mn) and other elements (Ca, K, Al, Sr and Ba) in vine leaves, Castilla-La Mancha (Spain). _J. Geochem. Explor._ 174, 79–83 (2017). Article CAS Google
Scholar * Augusto, L., Bonnaud, P. & Ranger, J. Impact of tree species on forest soil acidification. _For. Ecol. Manag._ 105, 67–78 (1998). Article Google Scholar * IAEI. _Zpráva o
stavu zemědělství ČR za rok 2018. Ministerstvo zemědělství_. (2019). * Svoboda, L. & Chrastný, V. Levels of eight trace elements in edible mushrooms from a rural area. _Food Add. Contam.
A_ 25, 51–58 (2008). Article CAS Google Scholar * Alonso, J., Garcia, M. A., Pérez-López, M. & Melgar, M. J. The concentrations and bioconcentration factors of copper and zinc in
edible mushrooms. _Arch. Environ. Contam. Toxicol._ 44, 180–188 (2003). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS This research was financially supported by the
Technology Agency of the Czech Republic by project TJ02000128—Determination of the vertical mobility of heavy metals in forest soils as a basis for the optimization of tree species
composition to reduce the risk of their transfer to edible mushrooms and the Ministry of Education, Youth and Sports of the Czech Republic under the project FCH-S-21-7398 and Ministry of
Agriculture of the Czech Republic, institutional support MZE-RO0118. The authors are grateful to Dagmar Obsilova, Ivana Kusnierova, Petr Rolinc, Petr Skocdopole and Arcibiskupske lesy a
statky Olomouc s.r.o. for their information support and for enabling research in the area. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Institute of Chemistry and Technology of
Environmental Protection, Faculty of Chemistry, Brno University of Technology, Purkyňova 118, 61200, Brno, Czech Republic Václav Pecina, Renata Komendová & Martin Brtnický * Department
of Agrochemistry, Soil Science, Microbiology and Plant Nutrition, Faculty of AgriSciences, Mendel University in Brno, Zemědělská 1, 613 00, Brno, Czech Republic Václav Pecina & Martin
Brtnický * Department of Geology and Soil Science, Faculty of Forestry and Wood Technology, Mendel University in Brno, Zemědělská 3, 613 00, Brno, Czech Republic Martin Valtera & David
Juřička * Department of Forest Management and Applied Geoinformatics, Faculty of Forestry and Wood Technology, Mendel University in Brno, Zemědělská 3, 613 00, Brno, Czech Republic Karel
Drápela & Petr Vahalík * Forestry and Game Management Research Institute, Strnady 136, 252 02, Jíloviště, Czech Republic Radek Novotný Authors * Václav Pecina View author publications
You can also search for this author inPubMed Google Scholar * Martin Valtera View author publications You can also search for this author inPubMed Google Scholar * Karel Drápela View author
publications You can also search for this author inPubMed Google Scholar * Radek Novotný View author publications You can also search for this author inPubMed Google Scholar * Petr Vahalík
View author publications You can also search for this author inPubMed Google Scholar * Renata Komendová View author publications You can also search for this author inPubMed Google Scholar *
Martin Brtnický View author publications You can also search for this author inPubMed Google Scholar * David Juřička View author publications You can also search for this author inPubMed
Google Scholar CONTRIBUTIONS All authors contributed to the study conception and design. The first draft of the manuscript was written by Václav Pecina and all authors critically revising it
for important intellectual content. All authors read and approved the final manuscript. All authors agree with the sequence of authors listed in the article. All authors agree to designate
David Juřička as the corresponding author for the submission. Conceptualization and design of the study: VP; Methodology: VP, DJ; Formal analysis and investigation: MV, KD, PV, DJ; Writing
-original draft preparation: VP; Writing -review and editing: MV, DJ, RN, RK, MB; Visualization: DJ, KD, PV; Funding acquisition: VP, DJ, RN; Resources: VP, DJ, RN; Supervision: RN, RK, MB;
Project administration: DJ, VP, RN. CORRESPONDING AUTHOR Correspondence to David Juřička. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL
INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION
SUPPLEMENTARY INFORMATION. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation,
distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and
indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit
line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use,
you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS
ARTICLE CITE THIS ARTICLE Pecina, V., Valtera, M., Drápela, K. _et al._ Influence of beech and spruce on potentially toxic elements-related health risk of edible mushrooms growing on
unpolluted forest soils. _Sci Rep_ 12, 5407 (2022). https://doi.org/10.1038/s41598-022-09400-9 Download citation * Received: 26 November 2021 * Accepted: 08 March 2022 * Published: 30 March
2022 * DOI: https://doi.org/10.1038/s41598-022-09400-9 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable
link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative