Play all audios:
ABSTRACT There is limited data on whether diastolic dysfunction in patients with heart failure (HF) and recovered ejection fraction (HFrecEF) is associated with worse prognosis. We
retrospectively assessed 96 patients diagnosed with HFrecEF and created ROC curve of their diastolic function at the 1-year follow-up for the composite endpoint of cardiovascular death and
HF readmission after the follow-up. Eligible patients were divided into two groups according to the cutoff value of E/e′ ratio (12.1) with the highest AUC (0.70). Kaplan–Meier analysis
showed that HFrecEF with high E/e′ group had a significantly poorer prognosis than the low E/e′ group (log-rank, _p_ = 0.01). Multivariate Cox regression analysis revealed that the high E/e′
group was significantly related to the composite endpoint (hazard ratio 5.45, 95% confidence interval [CI] 1.23–24.1). The independent predictors at discharge for high E/e′ ratio at the
1-year follow-up were older age and female sex after adjustment for covariates (odds ratio [OR] 1.07, 95% CI 1.01–1.13 and OR 4.70, 95% CI 1.08–20.5). In conclusion, HFrecEF with high E/e′
ratio might be associated with a poor prognosis. Older age and female sex were independent predictors for a sustained high E/e′ ratio in patients with HFrecEF. SIMILAR CONTENT BEING VIEWED
BY OTHERS LEFT ATRIAL REVERSE REMODELING IMPROVES RISK STRATIFICATION IN PATIENTS WITH HEART FAILURE WITH RECOVERED EJECTION FRACTION Article Open access 16 March 2022 HEART FAILURE WITH
MID-RANGE OR MILDLY REDUCED EJECTION FRACTION Article 06 September 2021 HEART FAILURE WITH PRESERVED EJECTION FRACTION Article 14 August 2024 INTRODUCTION Improvement in systolic
function—such as left ventricular ejection fraction (LVEF)—is sometimes experienced in patients with heart failure (HF) and reduced ejection fraction (HFrEF)1. Patients with HF and recovered
ejection fraction (HFrecEF) demonstrate relatively better clinical outcomes than patients with persistent HFrEF2,3,4,5,6. It was previously reported that the independent predictors for
improving LVEF were young age, female sex, and an etiology of non-ischemic heart disease3,6,7. Meanwhile, the withdrawal of guideline-directed medical therapy (GDMT) for HF in patients with
dilated cardiomyopathy and recovered LVEF led to relapse of cardiomyopathy8. These results suggest that improvements in LVEF and recovery or remission of the injured myocardium may be
different; therefore, patients with HFrecEF may be at risk of future cardiovascular (CV) events. It is, thus, important to clarify the subset of individuals with HFrecEF that may have a poor
prognosis, despite exhibiting an improvement in systolic function. Although, there is limited data regarding appropriate risk stratification and management in patients with HFrecEF, we
hypothesized that diastolic function would be the prognostic indicator among HFrecEF patients as it is based on findings regarding the functional phenotypes of bioengineered cardiac tissue
in hypoxia and reoxygenation. Both the systolic and relaxation functions of the cardiac tissue had deteriorated in the hypoxic condition, while in reoxygenation, the relaxation dysfunction
remained, even after systolic function had fully recovered9. These results suggest that, in hypoxia, the relaxation dysfunction might be prolonged and the recovery or remission difficult to
achieve when compared with systolic dysfunction. Relaxation dysfunction is one of the component of diastolic dysfunction10. Peak velocity of the early wave (E) to early diastole (e′) (E/e′)
ratio and left atrial volume index (LAVI) assessed by echocardiography were correlated with LV filling pressure as well as the indexes for diagnosis of LV diastolic dysfunction10,11. The
aims of our study were to elucidate the predictors for sustained diastolic dysfunction at, as well as the long-term prognoses after, the 1-year follow-up. METHODS STUDY POPULATION AND
ENDPOINTS We retrospectively assessed consecutive patients who were hospitalized for HFrEF and discharge alive at the Tokyo Women’s Medical University Hospital between July 2013 and October
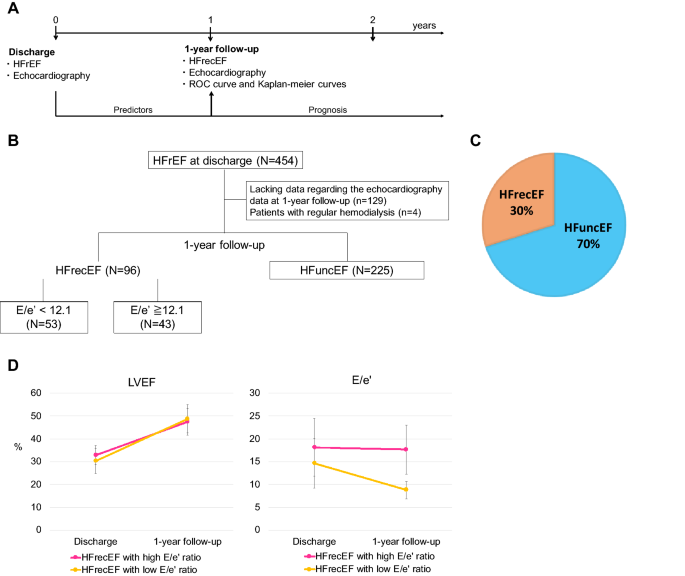
2018. We followed-up and reassessed them via echocardiography at the 1-year follow-up and we confirmed that the patients were either diagnosed with HFrecEF or not. Inclusion criteria were
follows: a diagnosis of HFrecEF at the 1-year follow-up; more than 1 year of follow-up; and obtaining the data describing the LVEF and E/e′ (septal e′) ratio (Fig. 1A,B). Patients were
diagnosed with HF using the Framingham HF diagnostic criteria12: HFrEF was defined as HF and an LVEF < 40% at discharge13,14; HFrecEF was defined as an LVEF < 40% at discharge that
improved to ≥ 40% at the 1-year follow-up, and this was based on the results of previous studies3,7,14. Exclusion criteria were as follows: a diagnosis of heart failure with unchanged
ejection fraction (HFuncEF: LVEF < 40% at discharge and 1-year follow-up)7; receipt of regular hemodialysis; and missing data describing the LVEF or peak velocity of the E/e′ ratio at the
1-year follow-up. Next, we created the receiver operating characteristic (ROC) curve of their diastolic function at the 1-year follow-up for the composite endpoint of CV death and HF
readmission after the follow-up. Eligible patients were divided into two groups according to the calculated cut-off value with highest area under the curve (AUC) among the four parameters
related to the diastolic function: E/e′ ratio, LAVI, maximal tricuspid regurgitation velocity (TR Vmax), and e′9. CV death included death caused by acute myocardial infarction, sudden
cardiac death, HF, stroke, CV procedures, CV hemorrhage, and other CV events15. During the study period, 454 patients with HFrEF who were discharged alive, were followed up. Of those, four
patients with regular hemodialysis and 129 patients with lacking data regarding the echocardiography data at 1-year follow-up were excluded. Of the remaining 321 patients, 225 patients (70%)
did not exhibit improved LVEF at the 1-year follow-up and were diagnosed with HFuncEF. Finally, 96 patients (30%) with HFrecEF were analyzed (Fig. 1B,C). The study population was divided
into two groups according to the cutoff value of the E/e′ ratio, as this exhibited the highest AUC among the four parameters. Fifty-three patients (55%) were classified into the low E/e′
ratio group, while 43 patients (45%) were classified into the high E/e′ ratio group (Fig. 1B). The present study was approved by the Institutional Review Board of the Tokyo Women’s Medical
University Ethical Committee (Approval Number: 2020–0028), conformed to the principles of the Declaration of Helsinki, and exempted from informed consent requirements owing to its
retrospective design. DATA COLLECTION Patients’ clinical data at discharge were recorded, including vital signs, past medical history, oral medications, echocardiographic parameters, and
laboratory data (complete blood count, estimated glomerular filtration rate [eGFR], and hemoglobin, total bilirubin, electrolyte, C-reactive protein, brain natriuretic peptide [BNP], and
total cholesterol levels). Echocardiographic parameters—including the heart rhythm, left atrial diameter (LAD), LAVI, left ventricular end-diastolic diameter (LVDd), left ventricular
end-systolic diameters (LVDs), LVEF, E/e′, e′, deceleration time, TR Vmax, estimated right ventricular systolic pressure (RVSP), maximum and minimum inferior vena cava (IVC) diameter, left
ventricular mass index (LVMI), and the presence of atrial arrhythmias at echocardiography—were evaluated at discharge and the 1-year follow-up. Transthoracic echocardiography was performed
using a Vivid 7 (GE Healthcare, Horten, Norway) or iE33 (Philips Healthcare, Bothell, WA, USA) ultrasound system. LVDd, LVDs, and LAD were recorded in the parasternal long-axis view, and the
LVEF and LAVI were calculated using the modified Simpson method. The estimated RVSP was calculated from the TR Vmax: estimated RVSP = 4 × (TR Vmax)2 + right atrial pressure. Atrial
arrhythmias were defined as the heart rhythm of atrial fibrillation, atrial flutter, or atrial tachycardia16,17. The eGFR was calculated using previously published equations: eGFR
(mL/min/1.73 m2) = 194 × serum creatinine(−1.094) × age(−0.287) (× 0.739, if female)18. Anemia was defined as hemoglobin levels < 12.0 g/dL in women and < 13.0 g/dL in men13. The
definition of ischemic cardiomyopathy was that left ventricular (LV) dysfunction due to severe ischemic heart disease including the myocardial infarction and angina pectoris according to
Japanese Circulation Society 2018 guideline on diagnosis and treatment of cardiomyopathies. STATISTICAL ANALYSES The data are expressed as means and standard deviations, median values and
interquartile range (IQR), or percentages, as appropriate. The Student’s t-test was used to compare normally distributed continuous variables between the groups, while the Mann–Whitney U
test was used for skewed continuous variables; Fischer’s exact test was used to evaluate the categorical variables. A paired t-test was used to compare the LVEF or E/e′ ratio at discharge
and the 1-year follow-up. Pearson product-moment correlation coefficient was used to identify the correlation between E/e′ ratio at the 1-year follow-up and LVEF changes from the discharge
to the 1-year follow-up. We plotted ROC curves for the composite of CV death and readmission for HF using the E/e′, LAVI, TR Vmax, and e′ at the 1-year follow-up; additionally, we estimated
the optimal cutoff value based on the Youden index. BNP levels were log-transformed. Logistic regression analysis was performed to identify independent factors at discharge related to high
E/e′ ratio at the 1-year follow-up. Variables were considered clinically significant if they reached a significance level of _p_ < 0.05 and were subsequently included in the multivariable
logistic regression model. The Kaplan–Meier method and log-rank tests were used to compare the event-free survival ratios between the two groups during the follow-up period after the 1-year
follow-up. Multivariable Cox regression analysis was performed to assess whether high E/e′ ratio at the 1-year follow-up was associated with the primary endpoint after adjusting for
covariates. Due to small number of patients who experienced the composite endpoint, the multivariate analysis was performed by adjusting for age and sex only. Two-sided significance was set
at _p_ < 0.05. Furthermore, sensitivity analysis was performed to remove the influence of different definition of HFrecEF on the results. We applied another definition of HFrecEF for the
sensitivity analysis. The definition was (1) decreased LVEF < 40% at baseline; (2) ≥ 10% absolute improvement in LVEF; and (3) a second measurement of LVEF ≥ 40%, according to the
previous reports19. All statistical analyses were performed using R software (version 1.41.1; R Foundation for Statistical Computing, Vienna, Austria)20. RESULTS The median follow-up
duration was 537 (IQR 309–923) days after the 1-year follow-up. During the study period, 14 patients (15%) were readmitted for HF or died as a consequence of CV events; only one patient died
from CV disease. The ROC curve for a composite of CV death and readmission of HF using the E/e′ ratio, LAVI, TR Vmax, and e′ at 1-year follow-up revealed cutoff values of 12.1, 49.4 mL/m2,
2.40 m/s, and 6.7 cm/s, respectively (AUC 0.70, 95% confidence interval [CI] 0.55–0.84; AUC 0.67, 95% CI 0.50–0.83; AUC 0.59, 95% CI 0.41–0.77; AUC 0.63, 95% CI 0.48–0.79, respectively; Fig.
2). There were no statistically significant differences regarding the AUC between the E/e′ ratio and LAVI at the 1-year follow-up (_p_ = 0.76). PATIENT CHARACTERISTICS AT DISCHARGE Table 1
shows the patient characteristics of the study population and comorbidities at discharge. Significant differences were evident between the two groups concerning age, sex, principal cause of
HF due to ischemic cardiomyopathy, and sodium, hemoglobin, and BNP levels. Regarding echocardiography during the hospitalization, LVEF and E/e′ were higher in the HFrecEF with high E/e′
group than the HFrecEF with low E/e′ group. The estimated RVSP, E-wave deceleration time, e′, LAVI, LVMI, IVC diameter, and presence of atrial arrhythmias at the echocardiography were
comparable between the two groups. Angiotensin-converting enzyme inhibitors or angiotensin receptor blocker inhibitors (ACEi/ARB) and beta-blockers were prescribed in approximately 90% of
patients at discharge; statin was more often prescribed in the high E/e′ than low E/e′ group. PATIENT CHARACTERISTICS AND ECHOCARDIOGRAPHY DATA AT THE 1-YEAR FOLLOW-UP The median follow-up
period between discharge and the 1-year follow-up was 365 (IQR 332–398) days. Although LVEF in both HFrecEF with low and high E/e′ ratio groups significantly improved from 30 ± 5.5 and 33 ±
4.1% at discharge to 49 ± 6.1% and 47 ± 5.8% at the 1-year (paired-t test: _p_ < 0.001, respectively, Fig. 1D), E/e′ ratio in HFrecEF with high E/e′ group were unchanged from 18 ± 6.3 at
discharge to 18 ± 5.4 at the 1-year follow-up (paired-t test: _p_ = 0.68), while E/e′ ratio in HFrecEF with low E/e′ group improved from 15 ± 5.4 at discharge to 8.8 ± 1.9 at the 1-year
follow-up (paired-t test: _p_ < 0.001, Fig. 1D). Interestingly, there was inversely mild correlation between the E/e′ ratio at the 1-year follow-up and LVEF changes (%) from the discharge
to the 1-year follow-up (_r_ = − 0.25,_ p_ = 0.02, Supplemental Fig. 1). There were no significant differences in systolic blood pressure, diastolic blood pressure, heart rate, atrial
arrhythmias at the 1-year follow-up, LVEF, LVDd, LVDs, LVMI, E-wave deceleration time, IVC diameter, the presence of atrial arrhythmias at the echocardiography, and the prescription rates of
GDMT for HF between the two groups at the 1-year follow-up. Changes in heart rate from discharge to the 1-year follow-up did not differ between the two groups (low E/e′ group: − 2.4 ± 14
bpm vs. high E/e′ group: 0.8 ± 18 bpm; _p_ = 0.33). Patients in the high E/e′ group had larger LADs and LAVIs, as well as a tendency to exhibit a higher RVSP and TR Vmax, compared with those
in the low E/e′ group. Then, e′ was lower and BNP level was higher in HFrecEF with high E/e′ group compared with HFrecEF with low E/e′ group. There was statistically significant difference
in the furosemide dose between the two groups. No patient had moderate to severe mitral valve regurgitation. The prescription rates for ACEi/ARB and β-blockers were compatible between
discharge and the 1-year follow-up; however, that of aldosterone antagonists decreased from 72% at discharge to 58% at the 1-year follow-up (Table 2). PROGNOSIS The Kaplan–Meier curve
revealed that the rate of the composite endpoint was significantly higher in the high E/e′ group than the low E/e′ group (log-rank test, _p_ = 0.01; Fig. 3). Furthermore, multivariate Cox
regression analysis revealed that an E/e′ ratio ≥ 12.1 at the 1-year follow-up was associated with the composite endpoint after the 1-year follow-up, after adjusting for age and sex (hazard
ratio [HR] 5.45, 95% CI 1.23–24.1; Table 3). This result was consistent with sensitivity analysis with other definition of HFrecEF (Supplemental Fig. 2). Meanwhile, when the study population
was divided into two groups according to a cutoff of 49.4 mL/m2 for LAVI, the Kaplan–Meier curve demonstrated that patients in the high LAVI group exhibited higher rate of the composite
endpoint, compared with those in the low LAVI group (log-rank test, _p_ _= _0.02; Supplemental Fig. 3). However, the age and sex adjusted HR for the composite endpoint did not significantly
differ between the two groups (HR 1.86, 95% CI 0.60–5.73). PREDICTING HIGH E/E′ RATIO AT THE 1-YEAR FOLLOW-UP Discharge parameters including age, female sex, ischemic cardiomyopathy, high
BNP level, low sodium level, high LVEF, high E/e′ ratio, and a prescription for statins correlated with a high E/e′ ratio at the 1-year follow-up in the univariate logistic regression
analyses. After adjusting for these parameters, older age and female sex remained independent predictors for high E/e′ ratio at the 1-year follow-up (odds ratio [OR] 1.07, 95% CI 1.01–1.13
and OR 4.70, 95% CI 1.08–20.5, respectively; Table 4). DISCUSSION This study investigated the predictors at discharge for high E/e′ ratio at the 1-year follow-up in patients with HFrecEF, as
well as their prognosis. The principal findings were as follows: (1) patients with HFrecEF and high E/e′ ratio at the 1-year follow-up had a poor prognosis after the 1-year follow-up, and
(2) older age and female sex at the hospitalization were associated with a high E/e′ ratio at the 1-year follow-up. Several studies have been conducted regarding the recovery of LVEF during
the follow-up period2,3,4,5,6. The current study reveals that the proportion of HFrecEF at the 1-year follow-up in patients with HFrEF is consistent with previous reports3,4. Additionally,
Bermejo et al. reported that the percentage of the implantable cardioverter defibrillator (ICD) implantation, ischemic etiology of HF, ACEi/ARB, and beta blocker in patients with HFrecEF as
the predictors of LVEF improvement were 3%, 20%, 90%, and 78%, respectively21. Those percentages were similar to those of the current study; ICD implantation: 2%, ischemic etiology of HF:
19%, ACEi/ARB: 89%, and beta blocker: 96% (Table 1). However, despite the LVEF improving in patients with HFrEF, the predictors of remaining diastolic dysfunction and the relationship
between prognosis and diastolic function at follow-up remain unknown. We thus focused on patients with HFrecEF and their diastolic function at follow-up. We selected the E/e′ ratio at the
1-year follow-up to classify the study population as the diastolic dysfunction parameter because this parameter demonstrated the highest AUC for the composite endpoint when compared with
LAVI, e′, or TR Vmax, which were also related to the diastolic dysfunction. The E/e′ ratio is one of the parameters assessing left ventricular filling pressures, which can be assessed by
combining the effects of the transmitral driving pressure and myocardial relaxation22. Patients in HFrecEF with high E/e′ ratio group had lower e′ (< 7 cm/s) compared with patients in
HFrecEF with low E/e′ group, suggesting that patients in HFrecEF with high E/e′ ratio group had the relaxation dysfunction at the 1-year follow-up10,11. Furthermore, patients in the high
E/e′ group had a larger LAVI (> 34 mL/mm2) and higher TR Vmax than those in the low E/e′ group at the 1-year follow-up. It suggested that patients in the high E/e′ group exhibited
diastolic dysfunction regardless of the improvement in LVEF, in reference to previous reports10,23. Table 1 showed that HFrecEF with high E/e′ group had higher LVEF and smaller LV size than
HFrecEF with low E/e′ group. HFrecEF with high E/e′ group had higher proportion of female patients compared with low E/e′ group, which might influence the LV size. Further, sex-specific
differences in the distribution of LVEF was reported in nationwide register (N = 499,153), which demonstrated that mean LVEF was higher in women than men24. We speculated that sex
differences between the two groups might affect the LVEF and LV size at the discharge. In addition, because Zhang et al. reported that the degree of LVEF improvement was associated with the
prognosis25, we created ROC curve and compared the AUC of E/e′ ratio at the 1-year follow-up and LVEF changes from the discharge to the 1-year follow-up for the composite of CV death and
rehospitalization for HF. Although there was no significant difference between them, AUC was higher in E/e′ ratio than LVEF change (E/e′ ratio: 0.70 vs. LVEF change: 0.61, _p_ = 0.48,
Supplemental Fig. 4). In previous studies, patients with HFrecEF were shown to have a lower risk of mortality or hospitalization for HF3,4,5. One of the predictors of increased EF was female
sex3,6,7. Interestingly, elderly women in the current study tended to exhibit diastolic dysfunction at the 1-year follow-up, regardless of recovered LVEF. This can be explained by the
effect of sex hormones as postmenopausal women with low levels of estrogen are prone to cardiac diastolic dysfunction through the suppression of sarcoplasmic reticulum Ca2+-ATPase (SERCA)
activity26,27. Furthermore, menopause is associated with a reduction in cGMP-protein kinase G signaling by decreasing plasma natriuretic peptide levels28,29,30. These mechanisms may lead to
CV events. Although Ghimire et al. reported that female patients exhibited lower mortality risk than men among HFrecEF patients31, the impact of sex differences on the prognosis was not
evaluated in the current study because of small sample size. HFrecEF with high E/e′ ratio could indicate a more severe comorbidity, potentially causing the poor prognosis. Regarding the
possible reason for persistent diastolic dysfunction, the fabricated human cardiac tissue revealed systolic and relaxation dysfunction in a hypoxic environment9. After reoxidation, systolic
function had completely improved; however, the relaxation dysfunction remained, with the mRNA expression of phospholamban upregulated. This suggests that compared with systolic dysfunction,
it might be difficult to achieve normalization of the relaxation dysfunction in hypoxia. The myocardium in heart failure is constantly exposed to the hypoxic and normoxemic environment. In
cases of acute decompensated HF, the myocardium may be exposed to the hypoxic environment as a result of decreasing oxygen supply due to pulmonary congestion—as well as increasing oxygen
demand due to volume or pressure overload—regardless of the etiology of HF32,33. The microvascular density decreased in patients with chronic HF compared with healthy subjects32,34,35.
Arnold et al. reported that the microvascular dysfunction in HF with preserved ejection fraction (HFpEF) was correlated with E/e′ ratio, which was related to the long-term prognosis36. On
the other hand, the compensatory mechanisms in HF help to supply the oxygen and to suppress the oxygen demand as well as GDMT can improve the hypoxic condition in the myocardium by
supporting the decrease of oxygen demand and suppression of sympathetic activity, thus possibly contributing to the improvement of systolic dysfunction33,37. Meanwhile, diastolic function
was affected by the relaxation function, left atrial size, and fibrosis in the cardiac tissue, in addition to oxygen demand38. Therefore, as shown in a previous report9, recovery of
diastolic dysfunction may be difficult in patients with HFrecEF who exhibit cardiac fibrosis due to aging, despite the condition being improved by GDMT. As the clinical implication, patients
with HFrecEF and high E/e′ ratio exhibited unfavorable clinical outcomes in this study. It was reported that the changes in heart rate during the follow-up periods were associated with
relapse in patients with dilated cardiomyopathy and recovered LVEF39. However, heart rate at discharge and at the 1-year follow-up, as well as changes in heart rate, were compatible between
the two groups in the current study; therefore, monitoring of the E/e′ ratio may be a useful risk stratification tool for future CV events in patients with HFrecEF. Meanwhile, Pritchett et
al. reported that LAVI was also correlated with the diastolic dysfunction as well as long-term prognosis in a cross-sectional sample with > 45 years of age40. In the current study, AUC of
E/e′ ratio at the 1-year follow-up for the composite endpoint was higher than that of LAVI (0.70 vs. 0.67), but not statistically significance between them (_p_ = 0.76). It is still unclear
which parameters are better for assessing the prognosis in patients with HFrecEF. The combination of the parameters might predict the prognosis accurately. Although appropriate strategies
for improving a patient’s diastolic dysfunction remain unestablished, and careful observation and management are needed for these patients, it was reported that the angiotensin receptor
neprilysin inhibitor (ARNI) might be effective in female patients with HFpEF, those who are older (> 65 years; postmenopausal women), or those with relatively lower LVEF (< 57%)31,41.
Furthermore, ARNI altered the biomarker of abnormal extracellular matrix (ECM) homeostasis and improved clinical outcomes in patients with HFpEF, likely through antifibrotic effects42. This
may indicate that ARNI had favorable effects on diastolic dysfunction. In this study population, ARNI might be effective in patients with HFrecEF and high E/e′ ratio because of the mean age
of 68 years, high rate of females, and mean LVEF of 47% at the 1-year follow-up. Therefore, we considered that clinicians should assess the implications of ARNI for patients with HFrecEF and
diastolic dysfunction, which may lead to better clinical outcomes. There were several limitations in this study. This was a retrospective study performed at a single center with a small
sample size. There may be a selection bias as some patients were excluded from this study due to missing echocardiographic data. Additionally, patients who underwent mitral valve plasty or
replacement were excluded as their E/e′ ratio could not be accurately measured. Although we grouped patients according to E/e′ ratio using a cutoff value of 12.1 (Youden index), other cutoff
values—such as an E/e′ ratio of 14 or 15—were not assessed, since the number of patients with an E/e′ ratio greater than 14 was very small. Although we assessed the septal e′, the
diagnostic accuracy of tissue Doppler for evaluating LV filling pressure and diastolic dysfunction have been still discussed43. We did not show the NYHA classification at the discharge and
the 1-year follow-up because there was not enough amount of data on it. No patients were prescribed ARNI and ivabradine at both discharge and the 1-year follow-up as these drugs were not
approved in Japan at the time, and sodium-glucose cotransporter 2 inhibitors were only approved for patients with diabetes during the study period; these differences may have influenced the
results. The multivariate analysis might be overfitting. Further large-scale, prospective investigations are needed to determine the best management for patients with HFrecEF and high E/e′
ratio. CONCLUSIONS Elderly and female patients hospitalized for HFrEF may exhibit diastolic dysfunction at the 1-year follow-up even if their LVEF had improved; additionally, patients with
HFrecEF and high E/e′ ratio at the 1-year follow-up had a poor prognosis. Close observation and novel strategies are, thus, needed for this population. DATA AVAILABILITY Data are available
from the corresponding authors upon reasonable request. ABBREVIATIONS * BNP: Brain natriuretic peptide * CI: Confidence interval * CV: Cardiovascular * E/e′: Peak velocity of the early wave
(E) to early diastole (e′) * HF: Heart failure * HFrecEF: Heart failure with recovered ejection fraction * HFrEF: Heart failure with reduced ejection fraction * LAVI: Left atrial volume
index * LVEF: Left ventricular ejection fraction * TR Vmax: Maximal tricuspid regurgitation velocity REFERENCES * DeVore, A. D. _et al._ Improvement in left ventricular ejection fraction in
outpatients with heart failure with reduced ejection fraction: Data from CHAMP-HF. _Circ. Heart Fail._ 13, e006833. https://doi.org/10.1161/CIRCHEARTFAILURE.119.006833 (2020). Article CAS
PubMed Google Scholar * Park, J. J. _et al._ Phenotyping heart failure according to the longitudinal ejection fraction change: Myocardial strain, predictors, and outcomes. _J. Am. Heart
Assoc._ 9, e015009. https://doi.org/10.1161/JAHA.119.015009 (2020). Article PubMed PubMed Central Google Scholar * Savarese, G. _et al._ Prevalence and prognostic implications of
longitudinal ejection fraction change in heart failure. _JACC Heart Fail._ 7, 306–317. https://doi.org/10.1016/j.jchf.2018.11.019 (2019). Article PubMed Google Scholar * Tsuji, K. _et
al._ Characterization of heart failure patients with mid-range left ventricular ejection fraction—a report from the CHART-2 Study. _Eur J. Heart Fail._ 19, 1258–1269.
https://doi.org/10.1002/ejhf.807 (2017). Article PubMed Google Scholar * Kalogeropoulos, A. P. _et al._ Characteristics and outcomes of adult outpatients with heart failure and improved
or recovered ejection fraction. _JAMA Cardiol._ 1, 510–518. https://doi.org/10.1001/jamacardio.2016.1325 (2016). Article PubMed Google Scholar * Park, C. S. _et al._ Characteristics,
outcomes, and treatment of heart failure with improved ejection fraction. _J. Am. Heart Assoc._ 8, e011077. https://doi.org/10.1161/JAHA.118.011077 (2019). Article PubMed PubMed Central
Google Scholar * Tsutsui, H. _et al._ JCS/JHFS 2021 guideline focused update on diagnosis and treatment of acute and chronic heart failure. _Circ. J._ 85, 2252–2291.
https://doi.org/10.1253/circj.CJ-21-0431 (2021). Article CAS PubMed Google Scholar * Halliday, B. P. _et al._ Withdrawal of pharmacological treatment for heart failure in patients with
recovered dilated cardiomyopathy (TRED-HF): An open-label, pilot, randomised trial. _Lancet_ 393, 61–73. https://doi.org/10.1016/S0140-6736(18)32484-X (2019). Article PubMed PubMed Central
Google Scholar * Yamasaki, Y., Matsuura, K., Sasaki, D. & Shimizu, T. Assessment of human bioengineered cardiac tissue function in hypoxic and re-oxygenized environments to understand
functional recovery in heart failure. _Regen. Ther._ 18, 66–75. https://doi.org/10.1016/j.reth.2021.03.007 (2021). Article PubMed PubMed Central Google Scholar * Nagueh, S. F. _et al._
Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the American Society of Echocardiography and the European Association of
Cardiovascular Imaging. _J. Am. Soc. Echocardiogr._ 29, 277–314. https://doi.org/10.1016/j.echo.2016.01.011 (2016). Article PubMed Google Scholar * Mitter, S. S., Shah, S. J. &
Thomas, J. D. A test in context: E/A and E/e’ to assess diastolic dysfunction and LV filling pressure. _J. Am. Coll. Cardiol._ 69, 1451–1464. https://doi.org/10.1016/j.jacc.2016.12.037
(2017). Article PubMed Google Scholar * McKee, P. A., Castelli, W. P., McNamara, P. M. & Kannel, W. B. The natural history of congestive heart failure: The Framingham study. _N. Engl.
J. Med._ 285, 1441–1446. https://doi.org/10.1056/NEJM197112232852601 (1971). Article CAS PubMed Google Scholar * Ponikowski, P. _et al._ 2016 ESC Guidelines for the diagnosis and
treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with
the special contribution of the Heart Failure Association (HFA) of the ESC. _Eur. J. Heart Fail._ 18, 891–975. https://doi.org/10.1002/ejhf.592 (2016). Article PubMed Google Scholar *
Tsutsui, H. _et al._ JCS 2017/JHFS 2017 guideline on diagnosis and treatment of acute and chronic heart failure- digest version. _Circ. J._ 83, 2084–2184.
https://doi.org/10.1253/circj.CJ-19-0342 (2019). Article PubMed Google Scholar * Garcia-Garcia, H. M. _et al._ Standardized end point definitions for coronary intervention trials: the
academic research consortium-2 consensus document. _Circulation_ 137, 2635–2650. https://doi.org/10.1161/CIRCULATIONAHA.117.029289 (2018). Article PubMed Google Scholar * Andrade, J. G.
_et al._ Cryoablation or drug therapy for initial treatment of atrial fibrillation. _N. Engl. J. Med._ 384, 305–315. https://doi.org/10.1056/NEJMoa2029980 (2021). Article CAS PubMed
Google Scholar * Verma, A. _et al._ Discerning the incidence of symptomatic and asymptomatic episodes of atrial fibrillation before and after catheter ablation (DISCERN AF): A prospective,
multicenter study. _JAMA Intern Med._ 173, 149–156. https://doi.org/10.1001/jamainternmed.2013.1561 (2013). Article PubMed Google Scholar * Matsuo, S. _et al._ Revised equations for
estimated GFR from serum creatinine in Japan. _Am. J. Kidney Dis._ 53, 982–992. https://doi.org/10.1053/j.ajkd.2008.12.034 (2009). Article CAS PubMed Google Scholar * Wilcox, J. E.,
Fang, J. C., Margulies, K. B. & Mann, D. L. Heart failure with recovered left ventricular ejection fraction: JACC scientific expert panel. _J. Am. Coll. Cardiol._ 76, 719–734.
https://doi.org/10.1016/j.jacc.2020.05.075 (2020). Article PubMed Google Scholar * Kanda, Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. _Bone
Marrow Transplant_ 48, 452–458. https://doi.org/10.1038/bmt.2012.244 (2013). Article CAS PubMed Google Scholar * AgraBermejo, R. _et al._ Heart failure with recovered ejection fraction:
Clinical characteristics, determinants and prognosis CARDIOCHUS-CHOP registry. _Cardiol. J._ 25, 353–362. https://doi.org/10.5603/CJ.a2017.0103 (2018). Article Google Scholar * Ommen, S.
R. _et al._ Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous
Doppler-catheterization study. _Circulation_ 102, 1788–1794. https://doi.org/10.1161/01.cir.102.15.1788 (2000). Article CAS PubMed Google Scholar * Andersen, O. S. _et al._ Estimating
left ventricular filling pressure by echocardiography. _J. Am. Coll. Cardiol._ 69, 1937–1948. https://doi.org/10.1016/j.jacc.2017.01.058 (2017). Article PubMed Google Scholar * Stewart,
S. _et al._ Ejection fraction and mortality: A nationwide register-based cohort study of 499 153 women and men. _Eur. J. Heart Fail._ 23, 406–416. https://doi.org/10.1002/ejhf.2047 (2021).
Article CAS PubMed Google Scholar * Zhang, X. _et al._ Characteristics and outcomes of heart failure with recovered left ventricular ejection fraction. _ESC Heart Fail._ 8, 5383–5391.
https://doi.org/10.1002/ehf2.13630 (2021). Article PubMed PubMed Central Google Scholar * Li, S. & Gupte, A. A. The role of estrogen in cardiac metabolism and diastolic function.
_Methodist Debakey Cardiovasc. J._ 13, 4–8. https://doi.org/10.14797/mdcj-13-1-4 (2017). Article PubMed PubMed Central Google Scholar * Jiao, L. _et al._ Estrogen and calcium handling
proteins: New discoveries and mechanisms in cardiovascular diseases. _Am. J. Physiol. Heart Circ. Physiol._ 318, H820–H829. https://doi.org/10.1152/ajpheart.00734.2019 (2020). Article CAS
PubMed Google Scholar * Salhotra, S., Arora, S., Anubhuti, X., Trivedi, S. S. & Bhattacharjee, J. Influence of menopause on biochemical markers of endothelial dysfunction—a
case–control pilot study in North Indian population. _Maturitas_ 62, 166–170. https://doi.org/10.1016/j.maturitas.2008.11.024 (2009). Article CAS PubMed Google Scholar * Lam, C. S. _et
al._ Influence of sex and hormone status on circulating natriuretic peptides. _J. Am. Coll. Cardiol._ 58, 618–626. https://doi.org/10.1016/j.jacc.2011.03.042 (2011). Article CAS PubMed
PubMed Central Google Scholar * McMurray, J. J. V. _et al._ Effects of sacubitril-valsartan versus valsartan in women compared with men with heart failure and preserved ejection fraction:
Insights from PARAGON-HF. _Circulation_ 141, 338–351. https://doi.org/10.1161/CIRCULATIONAHA.119.044491 (2020). Article PubMed Google Scholar * Ghimire, A. _et al._ Frequency, predictors,
and prognosis of ejection fraction improvement in heart failure: An echocardiogram-based registry study. _Eur. Heart J._ 40, 2110–2117. https://doi.org/10.1093/eurheartj/ehz233 (2019).
Article CAS PubMed Google Scholar * De Boer, R. A., Pinto, Y. M. & Van Veldhuisen, D. J. The imbalance between oxygen demand and supply as a potential mechanism in the
pathophysiology of heart failure: The role of microvascular growth and abnormalities. _Microcirculation_ 10, 113–126. https://doi.org/10.1038/sj.mn.7800188 (2003). Article PubMed Google
Scholar * Zhou, B. & Tian, R. Mitochondrial dysfunction in pathophysiology of heart failure. _J. Clin. Invest._ 128, 3716–3726. https://doi.org/10.1172/JCI120849 (2018). Article PubMed
PubMed Central Google Scholar * Hoffman, J. I. & Buckberg, G. D. The myocardial oxygen supply:demand index revisited. _J. Am. Heart Assoc._ 3, e000285.
https://doi.org/10.1161/JAHA.113.000285 (2014). Article PubMed PubMed Central Google Scholar * Mohammed, S. F. _et al._ Coronary microvascular rarefaction and myocardial fibrosis in
heart failure with preserved ejection fraction. _Circulation_ 131, 550–559. https://doi.org/10.1161/CIRCULATIONAHA.114.009625 (2015). Article PubMed Google Scholar * Arnold, J. R. _et
al._ Prevalence and prognostic significance of microvascular dysfunction in heart failure with preserved ejection fraction. _JACC Cardiovasc. Imaging_
https://doi.org/10.1016/j.jcmg.2021.11.022 (2022). Article PubMed Google Scholar * Mann, D. L. & Bristow, M. R. Mechanisms and models in heart failure: The biomechanical model and
beyond. _Circulation_ 111, 2837–2849. https://doi.org/10.1161/CIRCULATIONAHA.104.500546 (2005). Article PubMed Google Scholar * Nagueh, S. F. Left ventricular diastolic function:
Understanding pathophysiology, diagnosis, and prognosis with echocardiography. _JACC Cardiovasc. Imaging_ 13, 228–244. https://doi.org/10.1016/j.jcmg.2018.10.038 (2020). Article PubMed
Google Scholar * Halliday, B. P. _et al._ Heart rate as a marker of relapse during withdrawal of therapy in recovered dilated cardiomyopathy. _JACC Heart Fail._ 9, 509–517.
https://doi.org/10.1016/j.jchf.2021.03.010 (2021). Article PubMed PubMed Central Google Scholar * Pritchett, A. M. _et al._ Diastolic dysfunction and left atrial volume: A
population-based study. _J. Am. Coll. Cardiol._ 45, 87–92. https://doi.org/10.1016/j.jacc.2004.09.054 (2005). Article PubMed Google Scholar * Solomon, S. D. _et al._
Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. _N. Engl. J. Med._ 381, 1609–1620. https://doi.org/10.1056/NEJMoa1908655 (2019). Article CAS PubMed
Google Scholar * Cunningham, J. W. _et al._ Effect of Sacubitril/Valsartan on biomarkers of extracellular matrix regulation in patients with HFpEF. _J. Am. Coll. Cardiol._ 76, 503–514.
https://doi.org/10.1016/j.jacc.2020.05.072 (2020). Article CAS PubMed Google Scholar * Sharifov, O. F., Schiros, C. G., Aban, I., Denney, T. S. & Gupta, H. Diagnostic accuracy of
tissue Doppler index E/e’ for evaluating left ventricular filling pressure and diastolic dysfunction/heart failure with preserved ejection fraction: A systematic review and meta-analysis.
_J. Am. Heart Assoc._ https://doi.org/10.1161/JAHA.115.002530 (2016). Article PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS We thank Yuki Iijima for support
and assistance. This study was supported by a research grant from the Mitsukoshi Health and Welfare Foundation, and The Cardiovascular Research Fund, Tokyo, Japan. We would like to thank
Editage (www.editage.jp) for English language editing. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Cardiology, Tokyo Women’s Medical University, 8-1 Kawadacho, Shinjuku-ku,
Tokyo, 162-8666, Japan Takuma Takada, Katsuhisa Matsuura, Yuichiro Minami, Takuro Abe, Ayano Yoshida, Makoto Kishihara, Shonosuke Watanabe, Shota Shirotani, Kentaro Jujo & Nobuhisa
Hagiwara * Institute of Advanced Biomedical Engineering and Science, Tokyo Women’s Medical University, Tokyo, Japan Takuma Takada & Katsuhisa Matsuura Authors * Takuma Takada View author
publications You can also search for this author inPubMed Google Scholar * Katsuhisa Matsuura View author publications You can also search for this author inPubMed Google Scholar * Yuichiro
Minami View author publications You can also search for this author inPubMed Google Scholar * Takuro Abe View author publications You can also search for this author inPubMed Google Scholar
* Ayano Yoshida View author publications You can also search for this author inPubMed Google Scholar * Makoto Kishihara View author publications You can also search for this author inPubMed
Google Scholar * Shonosuke Watanabe View author publications You can also search for this author inPubMed Google Scholar * Shota Shirotani View author publications You can also search for
this author inPubMed Google Scholar * Kentaro Jujo View author publications You can also search for this author inPubMed Google Scholar * Nobuhisa Hagiwara View author publications You can
also search for this author inPubMed Google Scholar CONTRIBUTIONS T.T.: conceptualization, methodology, investigation, data curation, formal analysis, writing—original draft. K.M.:
conceptualization, methodology, project administration, writing—reviewing and editing, funding acquisition. Y.M.: validation, methodology, visualization. M.K.: data curation, validation;
visualization. W.S.: data curation, validation, visualization. S.S.: data curation, validation, visualization. T.A.: data curation, validation, visualization. A.Y.: data curation,
validation, visualization. K.J.: data curation, validation, visualization. N.H.: supervision. All authors reviewed the manuscript. CORRESPONDING AUTHOR Correspondence to Katsuhisa Matsuura.
ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional
claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative
Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the
original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in
the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your
intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence,
visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Takada, T., Matsuura, K., Minami, Y. _et al._ Prognosis and diastolic
dysfunction predictors in patients with heart failure and recovered ejection fraction. _Sci Rep_ 12, 8768 (2022). https://doi.org/10.1038/s41598-022-12823-z Download citation * Received: 05
January 2022 * Accepted: 16 May 2022 * Published: 24 May 2022 * DOI: https://doi.org/10.1038/s41598-022-12823-z SHARE THIS ARTICLE Anyone you share the following link with will be able to
read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative