Play all audios:
The potential germline effects of radiation exposure to military veterans present at British nuclear tests in Australia and the South Pacific is of considerable interest. We analyzed
germline mutations in 60 families of UK military personnel comprising 30 control and 30 nuclear test veterans (NTV). Using whole-genome sequencing we studied the frequency and spectra of de
novo mutations to investigate the transgenerational effect of veterans’ (potential) exposure to radiation at nuclear bomb test sites. We find no elevation in total de novo single nucleotide
variants, small insertion-deletions, structural variants or clustered mutations among the offspring of nuclear test veterans compared to those of control personnel. We did observe an
elevated occurrence of single base substitution mutations within mutation signature SBS16, due to a subset of NTV offspring. The relevance of this elevation to potential exposure of veteran
fathers and, future health risks, require further investigation. Overall, we find no evidence of increased mutations in the germline of a group of British nuclear test veterans. ISRCTN
Registry 17461668.
Fundamental gaps remain in our understanding of the pattern of mutation induction in humans1,2,3,4. The limited sensitivity of traditional approaches for monitoring newly arising (de novo)
mutations (DNMs) in the human germline and their application to the study of the hereditary effects of radiation in humans have often provided conflicting and inconclusive evidence toward
the consequences for the children of exposed parents5,6,7,8. Continued advances in the throughput and accuracy of whole-genome sequencing (WGS) tools and technologies are providing new
insights to the type and distribution, consequences and contributors of de novo germline mutation in humans9,10,11,12,13,14. Current estimates predict between 50 and 100 new mutations per
individual per generation which corresponds to a background rate per generation of 1 × 10–8 single nucleotide variants (SNVs), the dominant subtype of DNMs11,15,16. Exposure to ionizing
radiation is known to increase the mutation burden with evidence from animal studies showing elevated DNMs following parental exposure to acute high doses of radiation3. For instance, highly
significant increases in the incidence of structural variants (SVs), indels and clustered DNMs (1–4 SNVs or clusters of 1–2 SNVs and indels within a few base-pairs of each other) have been
reported in mice exposed to 3 Gy17. In humans, the frequency of minisatellite mutation has been shown to be significantly elevated for families inhabiting the heavily polluted rural areas of
Belarus and Ukraine following the Chernobyl accident18,19. Similarly, groups of exposed families living in the vicinity of the Semipalatinsk nuclear test site in Kazakhstan (exposed to
nuclear fallout; effective dose > 1 Sv), and Techa River residents (exposed to multiple discharges of radioactive waste), also found significantly elevated mutation rates in the germline of
irradiated parents6,20.
Radiation exposure to military veterans who participated in the UK’s atmospheric nuclear weapon test and experimental programmes in Australia and the South Pacific in the 1950s and 60 s and,
the potential for resulting germline effects, are of considerable interest. In total, ~ 22,000 British troops took part, of which ~ 7000 were alive in 2017. Approximately one quarter of all
participants were monitored for external radiation exposure, the majority of these are reported as being negligible and 8% as receiving a ‘non-zero’ dose. Of this 8%, 44 were categorized as
receiving between 50 and 100 mSv and 36 of receiving a dose of > 100 mSv. Overall, 759 test veterans were categorized by the Ministry of Defense into ‘special groups’, based upon their role
and their potential to receive a higher dose, such as those involved in air plume sampling21. Many present at test sites were involved in support roles, such as construction, transport or
catering, however additionally, were directly involved with the actual tests, including working in contaminated areas in the days, weeks and months following each test22. Such roles may not
have been accounted for by the formal categorization into a special group. Additionally, no record of internalized exposure was made.
Epidemiological studies carried out up to 1998 show no evidence of a detectable effect on overall life expectancy or risk of cancer22, however this has recently been revised whereby a small
excess in mortality (RR = 1.02, 90% CI 1.00–1.05, p = 0.04) associated with similar increased risks for both cancer and non-cancer diseases, compared to non-test veteran controls, is
reported23. Throughout the intervening decades, questions as to whether veterans could have received sufficient radiation exposure to cause harm and, worry about potential genetic risk of
any historical radiation exposure, persist24,25. We show elsewhere an over-representation of nuclear test families self-reporting congenital anomalies among their children or grandchildren
compared to control families26.
The current estimate of human hereditary risk of adverse effect from parental exposure to radiation is 0.2% per Gy. This is based upon extrapolations from animal studies and not through
observed increases in human heredity disease27. Estimates of the genetic risk for the families of British veterans are unknown, principally due to uncertainties in exposed human populations
in general and the uncertainties in dose received, as noted above. To this end, we employed whole genome sequencing tools to examine for any difference in the frequency or spectrum of
germline mutations in 30 families of British veterans of nuclear tests (NTV cohort) and 30 families of British military personnel not present at nuclear tests (control cohort).
Blood samples were obtained as part of the Genetic and Cytogenetic Family Trio (GCFT) study from the NTV-control family trios of military men (veteran father, mother, child) who were
enrolled in the ‘UK nuclear test veterans’ cohort21. The sample size of 60 participants was considered sufficient to identify a 1.5-fold increase in the mutation rate as statistically
significant (α = 5%, 80% power). The paternal medical diagnostics, parental age at conception, sex ratio among children, number of smokers and levels of alcohol consumption did not differ
significantly between the two cohorts (Table S1). To investigate the frequency and spectra of DNMs we performed Illumina paired-end WGS to > 35X coverage to identify de novo SNVs, indels and
SVs. The frequency of DNMs and clustered DNMs were investigated and compared between NTV and control families. Details of potential exposure and demographics for sampled family trios,
bioinformatic analysis and statistical approach appear in “Materials and methods”.
We first compared our data with those of other larger studies investigating the frequency of DNMs in the general population, our data are in line with these and confirm the effects of
parental age on the incidence of DNMs in control families (Fig S1)13. We next examined the incidence of transitions and transversions, these are in line with the observations of previous
studies and do not significantly differ between the two cohorts (control: 1208 transitions and 643 transversions; NTV: 1193 transitions and 675 transversions; χ2 = 0.793; p = 0.37).
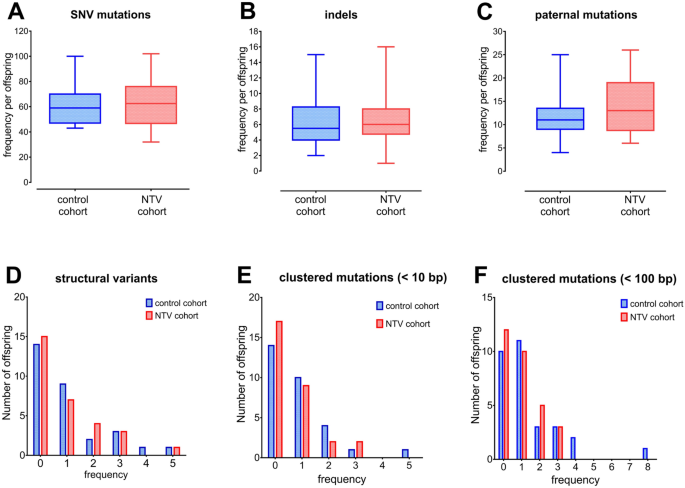
We next compared the frequency and distribution of DNMs between the NTV and control cohorts, the results of our analysis show no significant differences, and we found no statistically
significant differences in the spectra of small indels ( T, T > A, T > C, and T > G, as well as the nucleotides immediately 5′ and 3′ to the mutation28. Two statistical approaches were
performed. First, the total number of mutations identified (1851 SNVs in the control cohort and 1868 in the NT cohort) was considered (Fig. 4A). Differences in single base substitution (SBS)
mutations from 8 signatures were identified as being statistically significant between the two populations, with signature 16 showing the largest difference (controls: 432, NTV: 569, p