Play all audios:
ABSTRACT Processing of spodumene ores requires calcination as a compulsory pre-treatment to convert α-spodumene to a more reactive β-spodumene phase. This transformation takes place at an
elevated temperature of above 900 °C and results in a 30% volumetric expansion of the mineral and the product having highly altered physical properties. This work examines these induced
properties and the effect of calcination on lithium grade deportment with particle size. XRD analysis showed a significant amount of β-spodumene in the calcined finest fraction (i.e. the
particles less than 0.6 mm). A marked reduction in the bond ball mill work index of the calcined lithium samples (i.e. 42.3%) was recorded supporting the observed fracturing and friable
appearance of the sample following α to β-spodumene conversion. The deportment of lithium to finer fractions was significantly increased when the sample was calcined, indicating selective
breakage of the spodumene over gangue minerals. SIMILAR CONTENT BEING VIEWED BY OTHERS LITHIUM DEPORTMENT BY SIZE OF A CALCINED SPODUMENE ORE Article Open access 31 October 2022 OPTIMIZATION
OF THE SYNTHESIS OF HIGHLY PURE CALCIUM SULPHATE FROM DOLOMITE ORE Article Open access 21 April 2025 ALTERATION IN MOLECULAR STRUCTURE OF ALKALI ACTIVATED SLAG WITH VARIOUS WATER TO BINDER
RATIOS UNDER ACCELERATED CARBONATION Article Open access 01 April 2022 INTRODUCTION The application of lithium compounds in the battery industry has increased worldwide lithium demand. Of
the two important resources of lithium, extraction from brines is commercially more viable as compared with hard rock mining. However, attention has shifted to an extent towards lithium
extraction from more evenly distributed hard rock ores because of two reasons. Firstly, this commodity is monopolised due to its presence in some specific regions. Secondly, the compound
annual growth rate of lithium is expected to be 25.5% that is a rise from 47.3 to 117.4 kt of lithium over 4 years between 2020 to 2024 forcing the expansion of production into other
feedstocks1. This growth is mainly the result of increased sales of electric vehicles which are projected to rise from 3.4 million vehicles in 2020 to 12.7 million by 20241. Spodumene
(LiAlSi2O6) is the most economically exploitable lithium-bearing mineral and is widely used in lithium extraction2. It is found in pegmatites and may occur with other lithium-bearing
compounds such as petalite (LiAlSi4O10) and lepidolite (K(Li,Al,Rb)3 (Al,Si)4O10 (F,OH)2). The processing of lithium ores starts with beneficiation such as gravity separation/dense media
separation, magnetic separation and/or flotation3. Beneficiation of lithium from spodumene is not a simple process because of similar properties of lithium-bearing minerals (i.e. spodumene)
and their associated gangue minerals i.e. quartz (SiO2), albite (NaAlSi3O8), microcline (KAlSi3O8) and muscovite (KAl2(Si3Al)O10(OH,F)2). A spodumene concentrate containing more than 6% Li2O
is considered high-grade4 corresponding to at least 75% spodumene. This concentrate is suitable to feed the next processing stages: heat treatment and lithium extraction2. Heat treatment is
required due to the natural existence of spodumene in a less reactive α-phase5. Heat treatment at elevated temperatures (above 950 °C) is an important step in lithium production because
this step transforms less reactive α-spodumene into more reactive β-spodumene6. This phenomenon of phase transformation is called calcination or decrepitation which is an endothermic
reaction7. α-Spodumene is the most dominant naturally occurring of the three possible phases of spodumene, namely: α, β, γ. α-Spodumene (monoclinic crystal structure) is a denser and less
reactive phase found at ambient temperature. However, β-spodumene (tetragonal crystal structure) is 30% less densely packed than α-spodumene. Thus, β-spodumene has lower specific gravity
than α-spodumene (2.4 and 3.15 g/cm3 respectively). Hexagonal γ-spodumene is recently discovered and is metastable because it is formed during the transition from α to β phase8. α-Spodumene
presents as a competent rock, while β-spodumene is much more brittle than primary gangue minerals in the ore (eg, quartz)7. A microscopic study reveals α-spodumene as a compact material
composed of multiple layers stacked over each other. Conversely, in β-spodumene samples, many cracks can be observed on particles leading to a more random crystal structure. Based on the
significant and potentially selective change in physical properties of spodumene the objective of this work is to investigate the implications of calcination on behaviour of the samples
during comminution and grade deportment by size (coarse gangue rejection). In this work, different comminution techniques were used for calcined spodumene samples: crushing, semi-autogenous
grinding, and autogenous grinding. The reason is that different comminution techniques result in varied particle size distributions and hence different grade deportment by size. In other
words, crushing produces the coarsest fractions while semi-autogenous grinding generates the finest fractions. It should be noted that although it is understood that calcination of the whole
ore would involve a marked increase in energy usage, the potential for separation and upgrading for challenging ores or mineralised waste streams is of interest. MATERIALS AND METHODS
SAMPLES Spodumene ores were provided from a mine in the Eastern Goldfields, Western Australia. The received ores had particle size −7 mm i.e. the particles less than 7 mm. The ore sample was
collected by belt cut from the final crusher product and is designated as the plant feed (PF). The other sample was collected by strafing a bucket through the tailings stream of the
secondary (cleaner) dense media separation (TDMS). Table 1 shows the mineralogy of PF and TDMS samples. The lithium contents in the TDMS and PF samples were 0.36% and 0.20%, respectively.
The lithium content was determined by forming a borate glass fusion bead, digestion in 10% citric acid, and subsequent ICP-OES (Agilent Technologies, Inc., USA), analysis. The solution was
transported by a peristaltic pump in the nebulizer to convert the solution into a fine aerosol spray. Finer droplets enter the hot plasma, leading to evaporation of the sample. As a result,
atoms and ions are excited causing the characteristic wavelengths emission which is quantified by the ICP-OES system; the wavelength used for ICP-OES analysis for lithium9 was 610.4 nm.
CALCINATION The ore samples were calcined for 1 h at 1100 °C in a muffle furnace (Cupellation furnace, Carbolite Sheffield England). The holding time of one hour allowed complete conversion
of spodumene from α-phase to β-phase for accurate results. The calcined and non-calcined samples were used to understand the influence of calcination on comminution operations (crushing,
autogenous milling or semi-autogenous milling). The mill had a low-ball loading (10%) in contrast to standard ball milling (50%) and thus the mill was used to simulate a semi-autogenous
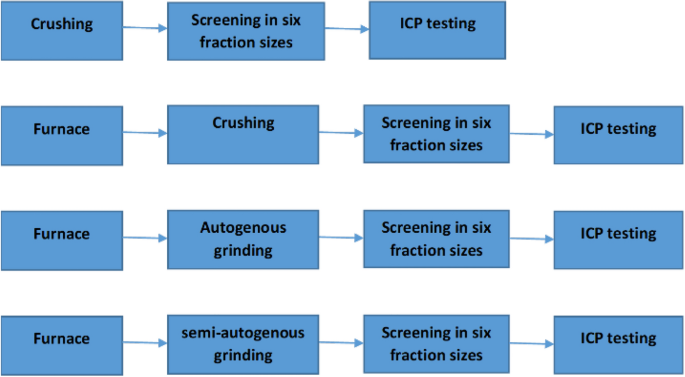
grinding mill. COMMINUTION Figure 1 shows the flowcharts used in this work. As seen in Fig. 1, underwent a range of comminution processes to provide information under a range of breakage
processes (semi-autogenous or autogenous mill), screened in six different size fractions (+ 3.35 mm, −3.35 + 2.36 mm, −2.36 + 1.7 mm, −1.7 + 1.18 mm, −1.18 + 0.6 mm, −0.6 mm) and then the
lithium grades were determined using ICP-OES (Agilent Technologies, US) in each size fraction; the standard deviation for three repeats of the lithium grade did not exceed 3%. The lithium
recoveries (R) were determined using Eq. (1): $$R=\frac{{m}_{p}\times {g}_{p}}{{m}_{f}\times {g}_{f}}$$ (1) where \({m}_{p} \, \mathrm{and } \, {m}_{f}\) are the mass of the product and the
feed, respectively; \({g}_{p}\) and \({g}_{f}\) are the lithium grade in the product and the feed. Crushing was performed using a cone crusher (Wescone, Australia) with a motor power of 9.2
kW; the closed side setting of the crusher is 3 mm. Semiautogenous and autogenous grinding were performed using a mill (the motor power of 1 kW) for 20 min. Semiautogenous grinding was
conducted using 12 grinding balls (each grinding ball had 27.3 mm in diameter) that had a total mass of 1060 g; the rotational speed of the mill was 70 rpm. The calcined ores were
investigated using crushing, semi-autogenous or autogenous mill. BOND BALL MILL WORK INDEX The Bond Ball Mill Work Index (BBMWI) is defined as the resistance offered by a material to the
grinding during ball milling10. The purpose is to find out the grinding power required for a certain throughput of material under ball mill grinding circumstances. Different steel balls were
used for grinding in each test as seen in Table 2. BBMWI was determined for both non-calcined and calcined samples. The standard bond ball mill procedure was followed10. The power required
for grinding, Wi, was determined using Eq. (2)10. $${W}_{i}=\frac{44.5\times 1.102}{{S}^{0.23}\times {G}^{0.82}\left(\frac{10}{\sqrt{{P}_{80}}}-\frac{10}{\sqrt{{F}_{80}}}\right)}$$ (2) where
the screen size required for 80% of a product or a feed to pass through the screen are P80 and F80, respectively; F80 and P80 were 1700 µm and 53 µm, respectively. G is the ore grindability
and S is the sieve size through which ore passes. X-RAY DIFFRACTION Mineralogical analyses of the lithium ore samples were conducted using an Olympus BTX™ II Benchtop (Co-Kα) X-ray
diffractometer (XRD). The XRD experiments were performed using two calcined finest fractions (−0.6 mm) and two non-calcined coarsest fractions (+ 3.35 mm) considering that these samples had
the maximum lithium content. This is very important to identify changes in the crystalline structure of spodumene before and after calcination. RESULTS AND DISCUSSIONS EFFECT OF CALCINATION
AND COMMINUTION METHOD ON MASS RETENTION Figure 2 shows the influence of calcination and comminution method on mass retention on different sieve sizes for the TDMS and the PF sample. As seen
in Fig. 2a., in the case of the TDMS sample, the highest percentage of ore was found on the largest size fraction when the ore was treated using the crushing only. It means that ore
particles have high hardness considering that spodumene in the ore is in α-phase at 25 °C and has a compact crystal structure2,7. However, when calcination was performed before any
comminution methods, the mass retention of the largest size fraction decreased significantly inducing fracturing before crushing. The difference between the non-calcined and calcined
material is greater with more communition. Figure 2 also shows that the lowest mass retention for the largest size fraction was when the ore was calcined followed by cone crushing. Similar
trends were also obtained in the case of the PF sample. However, in the case of the PF (Fig. 2b), calcination followed by semi-autogenous grinding had more mass retention of the largest size
fraction than calcination followed by autogenous grinding. The smallest mass in the largest size fraction was obtained after calcination and cone crushing; the reason could be that the
calcined ore was more brittle and thus more easily crushed than treated by autogenous grinding or semi-autogenous grinding. EFFECT OF CALCINATION AND COMMINUTION METHOD ON LITHIUM GRADE AND
LITHIUM RECOVERY Figure 3 shows the influence of the calcination and comminution method on the lithium grade for various size fractions for the TDMS and the PF sample. In the case of the
TDMS sample (Fig. 3a), the cone crushing method without calcination resulted in the maximum lithium grade of the largest size fraction while the lithium grade was lowest for the smallest
size fraction. However, the opposite trend was observed when calcination was used before comminution. This is particularly true when autogenous grinding or semi-autogenous grinding was
conducted after calcination i.e. the lithium grade for the finest size fraction was the highest, displaying the highest potential gangue rejection. The calcination impact on coarse gangue
rejection in the case of the PF sample (Fig. 3b) was similar to that in the case of the TDMS (Fig. 3a). However, the lithium grade in the finest fraction was significantly higher when
semi-autogenous grinding was conducted after calcination than that when cone crushing and autogenous grinding were performed after calcination. The influence of calcination on lithium
recovery can be seen in Fig. 4. As seen in Fig. 4, the lithium recovery after cone crushing and without calcination was the lowest at the finest screen size. However, when calcination was
performed before crushing or grinding operations, the lithium recovery improved in the finest size fractions, leading to coarse gangue rejection. The highest lithium recovery was achieved
when calcination was performed before semi-autogenous grinding. Figure 5 shows the relationships between the cumulative grade and the cumulative recovery. The higher the lithium recovery the
lower the lithium grade, which is true when calcination was used for both the TDSM (Fig. 5a) and the PF sample (Fig. 5b). It means that calcination may be effective in the rejection of
coarse gangue. The effect of calcination was more pronounced when the samples were ground after calcination. However, in the case of the non-calcined sample, there was a preferential
department of lithium to the largest size fraction because spodumene was present as a competent α phase. In the case of the TDMS sample, there are similarities between the grade recovery
curves when calcination was performed before autogenous or semi-autogenous grinding; it may suggest that the TDMS sample was more amenable to autogenous grinding than the PF sample. In the
case of the PF sample, the similarity between these curves was observed for the samples treated by cone crushing only, calcination and cone crushing, and calcination and autogenous grinding.
The differences between the TDMS and the PF sample were probably due to their differences in gangue mineralogy (see Fig. 6). Even though the lithium content was low in both TDMS and PF,
significant coarse gangue rejections were achieved only when calcination was performed before semi-autogenous grinding (i.e. the most efficient comminution method). X-ray diffraction was
used to investigate the impact on the mineral components of the samples through the various treatment schemes; Fig. 6 shows the diffraction patterns of the size fraction with the highest Li
content from four sample sets. As anticipated β-spodumene replaces α-spodumene with calcination and is more prominent for both TDMS and PF samples as it is concentrated in the finer
fraction11,12. For both TDMS and PF samples, the amount of albite also increased in the finest fraction of the calcined sample due to the albite transformation from the triclinic to
monoclinic crystal structure13 and thus degradation in strength. Figure 6 also shows that the amount of quartz was higher in the non-calcined TDSM sample than that in the calcined TDMS
sample showing that quartz was retained in the highest sieve size as it remained competent through calcination. EFFECT OF CALCINATION ON LITHIUM GRADE AND RECOVERY FOR VERY FINE SIZES The
influence of calcination on lithium deportment to very fine sizes was investigated using the sieves in the range of 150 and 53 µm. Both feed and products from the ball mill were analysed to
address this matter as seen in Figs. 7 and 8; the ball mill product was collected after conducting the BBMWI test. It was found that the calcination was also very beneficial for lithium
grade by sieve size even at very fine sizes (150 and 53 µm). The increase in sieve size reduced the amount of lithium grade for the sample retained on the sieve but increased the cumulative
lithium recovery. These trends were also observed in the case of the largest sieve sizes (see Figs. 3, 4, 5). However, Figs. 7 and 8 showed that the cumulative recovery increased more
dramatically with increasing in sieve size in the case of the ball mill product than that of the feed. EFFECT OF CALCINATION ON ENERGY CONSUMPTION DURING GRINDING The results showed that the
non-calcined ore required 1.73 times more energy for grinding than the calcined ore i.e. Wi (i.e. BBMWI) for the non-calcined ore was 44.9 kWh/t and for the calcined ore was 25.9 kWh/t. It
should be noted that the non-calcined ore had significantly higher BBMWI than α-spodumene14 i.e. 13.70 kWh/t; the reason is due to the presence of different gangue minerals such as mica,
quartz, albite and other silicates; the BBMWI for mica14 is 134.5 kWh/t, quartz14 is 32.2 kWh/t and albite14 is 34.9 kWh/t. The energy consumed, _Q_, during calcinations of spodumene is
obtained using the energy balance i.e. Equation (3): $$\mathrm{Q}=\frac{m}{M}\underset{298}{\overset{1373}{\int }}{C}_{p}(T)dT+{\mathrm{Q}}_{\alpha \beta }$$ (3) where \({C}_{p}(T)=\)
354.7–3375.7 T−0.5 J mol−1 K−1 as reported by Dessmond and colleagues7; _T_ is the temperature; _m_ is the mass of the sample; _M_ is the molecular mass of spodumene (186 g/mol). It should
be noted that the energy transformation from α to β spodumene, \({\mathrm{Q}}_{\alpha \beta },\) was 116.1 kJ/kg as reported by Dessmond and colleagues7. The energy consumed during
calcination was 582 kWh/t or 2096 kJ/kg. Therefore, calcination before grinding resulted in increased overall energy consumption. The summary of energy consumption for all unit operations is
given in Table 3. The energy for crushing, autogenous grinding and semi-autogenous grinding were determined by considering the duration of each operation, motor power and sample mass. As
seen in Table 3, the furnace consumes vastly more energy than the comminution circuit. Table 4 compares energy consumption and lithium grade for the finest size fractions (−0.6 mm) for four
different processing options used in this work. As seen in Table 4, although the combination of the furnace with the crusher or the mill (autogenous grinding or semi-autogenous grinding)
increased the energy consumption of the process, using the furnace increased the lithium grade by the screening of hard rock lithium ores. It is also very important to highlight that if
calcination is not used before grinding, calcination is used always after grinding and before leaching since leaching of spodumene is not possible without calcination. When ore calcination
is conducted before leaching or after flotation, the amount of energy consumed for calcination is approximately 1257.6 kJ/kg considering that flotation concentrates have typically 60% of
spodumene3 (i.e. 2096 × 60/100 = 1257.6 kJ/kg; 2096 kJ/kg is the energy consumption of calcination for pure spodumene as seen in Table 3). It is important to highlight that the main
objective of this paper is not to develop a new flowsheet but to investigate the implications of calcination on behaviour of the samples during comminution and grade deportment by size
(coarse gangue rejection). CONCLUSIONS This paper studies the influence of calcination of spodumene ore and comminution circuits on coarse gangue rejections by screening. The results showed
that the calcination made spodumene brittle, having a positive effect on coarse gangue rejection by increasing lithium grade and recovery in the finest fraction. This effect was observed
when the sieve size was in the range between 0.6 and 5 mm as well as 0.063 and 1 mm. The results of this work display the significantly altered properties of calcined material that promote
preferential breakage of the spodumene over other components. Semi-autogenous grinding after calcination generated significantly more fines than autogenous grinding or crushing after
calcination in the case of the PF sample. The energy consumed during the bond ball mill work test of the calcined ores was 42% less than that of the non-calcined ores. It must be noted that
the reduction in comminution energy does not account for the additional energy consumption in calcining feed streams rather than concentrates. REFERENCES * Fawthrop, A. _Global Lithium
Demand to More Than Double by 2024, Say Analysts_. https://www.nsenergybusiness.com/news/industry-news/global-lithium-demand-2024/. Accessed 16 Dec 2021 (2020). * Peltosaari, O., Tanskanen,
P. A., Heikkinen, E. P. & Fabritius, T. α→γ→β-phase transformation of spodumene with hybrid microwave and conventional furnaces. _Miner. Eng._ 82, 54–60 (2015). Article CAS Google
Scholar * Tadesse, B., Makuei, F., Albijanic, B. & Dyer, L. The beneficiation of lithium minerals from hard rock ores: A review. _Miner. Eng._ 131, 170–184 (2019). Article CAS Google
Scholar * Peltosaari, O., Tanskanen, P. A., Heikkinen, E. P. & Fabritius, T. Mechanical enrichment of converted spodumene by selective sieving. _Miner. Eng._ 98, 30–39 (2016). Article
CAS Google Scholar * Rosales, G. D., Ruiz, M. D. C. & Rodriguez, M. H. Novel process for the extraction of lithium from b-spodumene by leaching with HF. _Hydrometallurgy_ 147–148, 1–6
(2014). Article Google Scholar * Salakjani, N. K., Singh, P. & Nikoloski, A. N. Mineralogical transformations of spodumene concentrate from Greenbushes, Western Australia. Part 1:
Conventional heating. _Miner. Eng._ 98, 71–79 (2016). Article CAS Google Scholar * Dessemond, C., Lajoie-Leroux, F., Soucy, G., Laroche, N. & Magnan, J.-F. _Spodumene: The Lithium
Market, Resources and Processes, Minerals_. Accessed 12 Mar 2021. (2019). * Li, C. T. & Peacor, D. R. The crystal structure of LiAlSi2O6-II (β-spodumene). _Z. Kristallogr._ 126(1–3),
46–65 (1968). Google Scholar * Bach, T. C. _et al._ Nonlinear aging of cylindrical lithium-ion cells linked to heterogeneous compression. _J. Energy Storage_ 5, 212–223 (2016). Article
Google Scholar * Lynch, A., Mainza, A. & Morell, S., Ore comminution measurements techniques. in: _The AusIMM Comminution Handbook Carlton: AusIMM_. (Lynch, A.J. ed.). 43–60. (2015). *
Ellestad, R.B. & Leute, K.M. _Method of Extracting Lithium Values from Spodumene Ores. U.S. Patent 2516109_. (1950). * Abdullah, A. A. _et al._ Phase transformation mechanism of
spodumene during its calcination. _Miner. Eng._ 140, 105883 (2019). Article CAS Google Scholar * Shaocheng, J. S. & Mainprice, D. Experimental deformation of sintered albite above and
below the order-disorder transition. _Geodin. Acta_ 1(2), 113–124 (1987). Article Google Scholar * Perry, R. O. & Chilton, C. H. _Mineral Processing Handbook_ (Int Student's ed.,
McGraw Hill. 8–11/SME, Weiss ed. 3A-27, 1985). Download references ACKNOWLEDGEMENTS The collaboration between the authors would not have been possible without the financial support from CRC
ORE. CRC ORE is part of the Australian Government’s CRC Program, which is made possible through the investment and ongoing support of the Australian Government. The CRC Program supports
industry-led collaborations between industry, researchers and the community. The Bald Hill Mine (Alliance Mineral Assets Limited, Western Australia) is acknowledged for the provision of
samples for all the experiments. Financial support from Curtin University for this research work is appreciated. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Western Australia School of
Mines, Curtin University, Kalgoorlie, WA, 6430, Australia Muhammad Kashif Nazir, Laurence Dyer, Bogale Tadesse, Boris Albijanic & Nadia Kashif Authors * Muhammad Kashif Nazir View author
publications You can also search for this author inPubMed Google Scholar * Laurence Dyer View author publications You can also search for this author inPubMed Google Scholar * Bogale
Tadesse View author publications You can also search for this author inPubMed Google Scholar * Boris Albijanic View author publications You can also search for this author inPubMed Google
Scholar * Nadia Kashif View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS M.N. conducted the experimental work and wrote a draft manuscript.
N.K. prepared all Figures. L.D., B.T. and B.A. supervised. M.N., L.D., B.A. and M.N. prepared the final manuscript. CORRESPONDING AUTHOR Correspondence to Boris Albijanic. ETHICS
DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional claims
in published maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits
use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the
Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless
indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory
regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Nazir, M.K., Dyer, L., Tadesse, B. _et al._ Effect of calcination on coarse gangue
rejection of hard rock lithium ores. _Sci Rep_ 12, 12963 (2022). https://doi.org/10.1038/s41598-022-17277-x Download citation * Received: 11 April 2022 * Accepted: 22 July 2022 * Published:
28 July 2022 * DOI: https://doi.org/10.1038/s41598-022-17277-x SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative