Play all audios:
ABSTRACT Consumption of animal-sourced food is an important factor in broadening the diet of early hominins, promoting brain and body growth, and increasing behavioural complexity. However,
whether early hominins obtained animal food by scavenging or hunting large mammals remains debated. Sabre-toothed felids have been proposed to facilitate the expansion of early _Homo_ out of
Africa into Europe 1.4–0.8 Ma by creating a niche for scavengers in Eurasia as the carcasses abandoned by these felids still contained abundant edible resources. In contrast, it has been
argued that the niche for a large scavenger was already occupied in Eurasia by the giant hyena, preventing hominins from utilising this resource. This study shows that sabre-toothed felids
generated carcasses rich in edible resources and that hominins were capable of competing with giant hyenas for this resource. The simulation experiments showed that maintaining an optimum
group size is essential for the success of the hominin scavenging strategy. Early hominins could outcompete giant hyenas only if they could successfully dispute carcasses with them. Thus, in
the presence of a strong competitor, passive scavenging is essentially the same as confrontational scavenging. SIMILAR CONTENT BEING VIEWED BY OTHERS FACTORS INFLUENCING SCAVENGER GUILDS
AND SCAVENGING EFFICIENCY IN SOUTHWESTERN MONTANA Article Open access 19 February 2021 INFLUENCE OF HETEROSPECIFICS ON MESOCARNIVORE BEHAVIOUR AT SHARED SCAVENGING OPPORTUNITIES IN THE
CANADIAN ROCKY MOUNTAINS Article Open access 07 July 2023 GROUP DIFFERENCES IN FEEDING AND DIET COMPOSITION OF WILD WESTERN GORILLAS Article Open access 10 June 2022 INTRODUCTION Hominins
arrived to southern Europe at least 1.4 Ma ago1,2,3,4 and were settled there during the Epivillafranchian (approximately 1.2–0.8 Ma)5. However, the roles of changing climate,
palaeogeography, faunal assemblages, and other environmental drivers in their dispersion into Europe are under debate6,7,8,9. A key question is how the large European mammalian fauna,
especially the composition of the carnivore guild, influenced the accessibility of the early hominins to animal food resources10,11,12,13. Although the dichotomy of hunting vs. scavenging as
the main foraging strategies of early _Homo_ is still unresolved14,15,16,17,18,19,20,21,22,23, scavenging has been a common adaptive behaviour in the genus _Homo_ since its origins24,25.
Thus, despite the fact that the first hominins in Europe were likely omnivores8, it may be assumed that scavenging was part of their behavioural repertoire (Supplementary Note S1). The
scavenging opportunities for a hominin species in a particular ecological scenario can be determined by the complex interaction of several factors, such as the density, size, and quality of
carcasses dispersed around the landscape, which can further be determined by the abundance, behavioural, and morphofunctional characteristics of the predators and by the ecological
characteristics and abundance of their potential prey8,26. The other main factors to be considered are the presence of competitors and their ecological characteristics and behaviours. It has
been suggested that sabre-toothed felids generated many large carcasses because of their inability to entirely consume their kills27,28, facilitating the survival of early _Homo_ during the
Epivillafranchian (Supplementary Note S1). This argument is usually applied to species of the genus _Megantereon_, but may also be applied to _Homotherium_ if solitary behaviour is assumed.
Nevertheless, quantitative estimates of the rate of carcass production by these predators and of the amount of nutrients in the abandoned carcasses are currently lacking. In this scenario,
an opposite but equally important role, was played by the giant hyena (_Pachycrocuta brevirostris_)12,29,30, frequently regarded as a “hyperscavenger” and direct competitor of hominins31.
Indeed, it has been claimed that the Fuente Nueva-3 site provides evidence of the direct competition between hominins and giant hyenas for an elephant carcass32. Moreover, it has been
suggested that the giant hyena was dependent on the partially consumed carcasses produced by sabre-toothed cats, such that the decline of _Pachycrocuta_ in Europe was linked to the
extinction of sabre-toothed cats, particularly _Megantereon whitei_29,30. If hominins practised a flexible strategy of carrion acquisition, several foraging scenarios are
possible15,16,18,19,20,33. It has been proposed that groups of hominins were capable of stealing the kills of large predators (confrontational scavenging or kleptoparasitism)17. Moreover,
endurance running was suggested to be an advantage for hominins when competing with giant hyenas for carrion, as discussed in4. However, the real advantages provided by endurance running is
a controversial topic34. An alternative strategy would be the passive scavenging of partially or completely defleshed carcasses and interference competition for carrion with giant
hyena12,30,35. Group size was likely a key factor for both strategies, as a group of hominins was large enough to defend a carcass from any direct competitor. In this study, quantitative
estimates of the nutrients contained in the carcasses abandoned by the main Epivillafranchian large predators are provided. Moreover, the competition for carrion between hominins and giant
hyenas is approached here through the energetic costs and returns that this interaction represents for both species. Computer-based simulation experiments were performed to simulate the
competition between hominins and giant hyenas, evaluate the feasibility of passive scavenging by hominins, and determine the factors that influenced scavenging in the Epivillafranchian
ecosystems of the Iberian Peninsula. We aimed to evaluate the effect of ecosystem carrying capacity on the feasibility of passive scavenging and how the size of the hominin group affected
the efficiency of this strategy. Although the simulations are an oversimplification of the trophic niche of hominins, this is a necessary methodology for understanding and examining the
competition among hominins and hyenas in a tractable and efficient way36. RESULTS The effects of hominin group size and predator density on the competition for carrion between hominins and
giant hyenas in two different ecological scenarios were evaluated using six simulation experiments (Table 1). Each experiment was replicated 70 times (see Methods and Supplementary Methods
S1), and all the outputs of the 420 replicates (70 runs × six experiments) are shown in Supplementary Dataset 1. For the experiments, it was assumed that giant hyenas were strict solitary
scavengers and hominins competed with them for the carcasses produced by large felids (_Homotherium latidens_, _Megantereon whitei,_ and _Panthera gombaszoegensis_) in a trophic strategy of
passive scavenging. CARRION PRODUCTION IN THE EPIVILLAFRANCHIAN Estimated amounts of energy available in the carcasses abandoned by the three large predators were obtained based on the body
weight of their preferred prey, the estimated hunting frequency, and the daily intake rate of the predators, as detailed in the “Methods” section. The values included in the experiments are
listed in Table 2. These estimates are in general agreement with the values observed in recent ecosystems (Supplementary Methods S2) and confirm the assertion that the two sabre-toothed
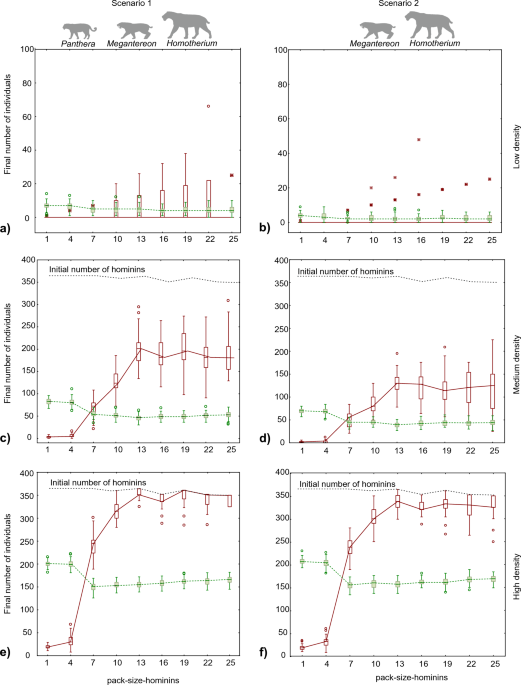
species generated far more scavengeable resources than recent and fossil pantherines. The results for the two scenarios were similar. As expected, the final populations of both hyenas and
hominins were larger in experiments with higher predator densities (Fig. 1). Indeed, the main difference between the results of both scenarios was that the available resources were
insufficient to sustain a hominin population when the predator density was low and only two sabre-toothed cats were present (Fig. 1b). In the scenario of low resources, only giant hyenas
survived, although at a low population density (Fig. 1b). In contrast, the presence of a third predator (_P. gombaszoegensis_) increased the available carrion enough to sustain the
populations of both scavengers in most runs, even at a low predator density (Fig. 1a). COMPETITION BETWEEN HOMININS AND GIANT HYENAS Hominin group size (hominin-pack-size) can predict the
competition between hominins and giant hyenas. The final number of hyenas exceeded that of hominins when the size of the hominin group was less than five (Fig. 1), which was arbitrarily set
as the threshold necessary for a hominin group to chase away a single hyena. Moreover, hominins could not survive until the end of the simulations when their group size was less than five
and the population density of predators was low or medium. When the hominin groups were larger than five, the final population of hominins was larger than that of giant hyenas; however,
giant hyenas subsisted under all the conditions tested. The positive effect of increasing hominin group size on the final population of hominins continued until a group size of 13
individuals was reached and levelled off beyond this point (Fig. 1). This pattern can be explained by the fact that a group of more than 10 hominins was necessary to chase away any predator.
The differences in the final number of hominins for groups of 13 or more individuals were due to variations in the initial number of groups at the start of the simulation (Fig. 1). This
variation was caused by the rounding down of the number of groups to a closer integer after dividing the initial population by the established hominin-pack-size. Energy expenditure is
simulated in SCAVCOMP-ABM by letting agents to expend energy at their basal metabolic rate when quiet and at a higher rate when moving (see Methods). Individual hominin energy expenditure
decreased with hominin group size, whereas hyena energy expenditure was mostly unaffected by hominin group size (Fig. 2). The effect was difficult to detect at a low predator density in
Scenario 2 (Fig. 2c) because the hominins survived to the end of the simulation in very few runs, but it was clearer in Scenario 1 (Fig. 2a). Nevertheless, the pattern appeared to be the
same at all three levels of predator density assessed in the experiments. Energy expenditure per hominin decreased steeply with group size until the group size was 13 and increased gently
beyond this point. This suggests the existence of an optimal group size that reduces the energy investment required for an activity. The optimum group size was found to be related to the
strength necessary to chase away predators and competitors. The decrease in energy expenditure with respect to group size was also influenced by predator density. The higher the predator
density, the greater the difference in energy expenditure between individuals in large and small groups of hominins. Similarly, the energy expenditure of hyenas was higher when the predator
density was high, since the number of interactions with predators increased (Fig. 2b,d). DISCUSSION Scavenging is a fundamental factor in the structure of carnivorous communities in
terrestrial ecosystems37. Moreover, the role of scavenging in the evolution and expansion of early hominins is a frequently debated and controversial issue19,20,22. The simulation
experiments suggest that passive scavenging could be a very successful strategy for late-early Pleistocene hominins in Europe, even in competition with giant hyenas. Only when hominins
foraged in very small groups, the ecosystem productivity was low, and the population densities of _Megantereon_ and _Homotherium_ were low or moderate_,_ giant hyenas displaced the hominins.
However, our simulations considered scavenging a unique procurement strategy for hominins, and this is an entirely unrealistic assumption. The hominins may be assumed to exhibit flexible
omnivorous behaviour and are capable of adopting their diet by exploiting different plant and animal resources, including carrion, according to their availability6,8,38,39. A requisite for
the coexistence of two large scavengers, hyenas and hominins, is the availability of sufficient carcasses containing large amounts of edible resources. It has been suggested that these
resources existed in Early Pleistocene Europe, owing to the presence of two sabre-tooths, especially _Megantereon_29,35. The estimates of the number of edible resources on the carcasses of
large ungulates abandoned by the two sabre-toothed species support the interpretation of sabre-tooths as significant carrion providers. If _Megantereon_ killed one prey every week, based on
a conservative estimate, only one-third of the edible energy in the carcass would be consumed before killing a new prey (Table 2). This estimate supports the claims linking the extinction of
the giant hyena in Europe to the extinction of _Megantereon_29,30. Interestingly, although this argument is usually applied only to _Megantereon,_ our estimations suggest that _Homotherium_
also produces carcasses with similar amounts of edible resources. In contrast, the resources contained in the carcasses of prey killed by the European jaguar would be markedly lower (Table
2) and similar to the average caloric content of the carcasses abandoned by recent large predators40. However, it should be acknowledged that the role of _Homotherium_ as a producer of
carcasses with high nutrient content relies on the assumption that it was a solitary species. If _Homotherium_ was a social felid, as sometimes suggested41, a pack would be able to consume a
large proportion of the carcass before abandoning it. Indeed, according to the estimates in Table 2, a pack of five _Homotherium_ individuals would require approximately 45,000 kcal/day and
consume the entire carcass of a 400 kg ungulate every 3–4 days. In such cases, only some portions, such as the brain and bone marrow, remain in the carcass because of the sabre-tooth’s
inability to break bones41. Our simulations modelled _Pachycrocuta brevirostris_ as a solitary passive scavenger; however, this decision may be controversial because it has been proposed
that _P. brevirostris_ occupied a niche similar to that of extant spotted hyenas (_Crocuta crocuta_), which are highly active hunters and kleptoparasites29. However, the taphonomic study of
the bone assemblage preserved at Venta Micena27,30,42 together with the morphofunctional analyses of the mandibles and teeth of _Pachycrocuta_ from several European localities30, strongly
suggests that the giant hyena was a dedicated strict scavenger or specialised kleptoparasite that stole the prey of sabre-tooths and other large carnivores. A possible argument against
considering _Pachycrocuta_ a passive scavenger30 is that carrion eaters must range over large areas in search of food, a task to which the large and non-cursorial giant hyena is not
especially adapted. Based on this argument, giant hyenas did not prospect the environment in search of carrion but pursued other predators and stole their preys30. Thus, giant hyenas were
kleptoparasites rather than passive scavengers. In this regard, their behaviour would be similar to that of recent spotted hyenas, as suggested by Turner and Antón29. Nevertheless, the
simulation experiments suggest that if the carrion is sufficiently abundant, high mobility is not required for a passive scavenger. The walking speed of _P. brevirostris_ was set at 5 km/h
(the same as that of the hominins), and it was assigned a high energy expenditure during movement. Moreover, if giant hyenas were kleptoparasites, they would have frequent primary access to
carcasses, and the bone accumulation generated by giant hyenas would be difficult to differentiate from that generated by hunting carnivores. The social behaviour of giant hyenas is another
potentially controversial topic. Turner and Antón29 suggested that giant hyenas were social, which allowed them to confront large predators and steal their prey. In contrast, the Venta
Micena assemblage showed that the giant hyenas selectively transported certain parts of the carcass to their dens42. This behaviour supports the interpretation of solitary social behaviour
because recent spotted hyenas transport all anatomical elements of the carcass to their den when scavenging in groups, but only selected parts when scavenging alone42. Moreover, the social
behaviour of recent spotted hyenas is related to the expansion of the frontal region of the brain, a trait recently acquired in the _Crocuta_ lineage43_._ Therefore, sociality may be a
unique and recent acquisition in spotted hyenas. Scavenging is a widespread behaviour among medium-sized carnivores in recent terrestrial ecosystems40, which is also practised by
contemporary hunter-gatherers. Hadza obtained 20% of their meat through confrontational scavenging17,23. However, the consumption of carrion represents a “windfall” resource for Hadza
foragers and not a regular activity due to some shortcomings, such as seasonal variations in encounter rates and the size and completeness of carcasses44. Wild chimpanzees also scavenge, but
rarely. Anecdotal evidence of scavenging by chimpanzees has been reported from Gombe, Mahale, Taï, and Ngogo21,45. Confronting large carnivores is risky, but chimpanzees reduce this risk by
increasing the number of participants and shouting and throwing stones to intimidate leopards. Scavenging, even passive scavenging, is risky. Indeed, the “fatal attraction” hypothesis46
proposes that carcass sites amplify the suppression effect of large carnivores on medium-sized carnivores. Despite being a widespread behaviour, scavenging has only been presented as a
successful strategy for early hominins in the short term33. In contrast, the simulations show that scavenging could be an efficient and adaptive behaviour for the Epivillafranchian under
certain conditions. The results of the simulation experiments highlight the importance of group size for the viability of scavenging when competition is considered. Indeed, it can be argued
that defending or stealing a carcass from other scavengers, as simulated in our experiments, does not differ from stealing a carcass from a predator. Interestingly, our results showed that
when the group size of hominins was not sufficient to chase away their competitors, the hominins survived until the end of the simulation only when carcasses were abundant because of the
high density of predators in a highly productive ecosystem (Fig. 1). This suggests that a fully passive scavenging strategy without direct confrontation with competitors would be
energetically inefficient as a regular strategy (Fig. 2), although it could still be viable on an opportunistic basis. Hominins foraging alone or in very small groups could not rely on the
active search for carrion as the main food resource, although they could feed on an abandoned carcass, which was found as a stroke of luck when foraging on other resources, until competitors
appeared. In contrast, roaming around the landscape in search of carcasses would be an efficient behaviour for a group of hominins that was large enough to chase away other scavengers.
Another important issue demonstrated in the experiments was the existence of an optimal group size for the foraging band (13 individuals in our simulations). The energetic cost of the
scavenging activity increases with group size when the group is larger than the minimum size necessary to chase away all competitors and predators. This is because a group that is too large
is not satiated by a single carcass and should expend energy in search of additional resources. Thus, the less productive the ecosystem and the scarcer the carcasses, the more
energy-intensive this strategy is for a large group. However, it is important to note that the results of our simulations should not be interpreted as estimates of the viable population
density of hominins or the optimum group size. The values obtained for these response variables are dependent on the values arbitrarily assigned to parameters such as the group size
necessary to chase away giant hyenas and predators or the carrion waste rate. The results suggest the existence of an optimum group size but do not provide an estimate of it. In the real
world, this optimum would be determined by the strength necessary to chase away competitors and by the size and nutrient content of the carcasses. The positive effect of foraging in a group
with size close to the optimum is larger, and the encounter rate with competitors and predators is higher. Thus, foraging in a group of size close to the optimum is more beneficial in highly
productive ecosystems, where the density of carnivores and the encounter rate are higher. Moreover, scavenging large carcasses in competition with other carrion eaters may have led hominins
to coordinate their movements, group cohesion, defence, cooperation, and communication. A relationship between scavenging and language emergence was proposed47. It has been suggested that
cooperative behaviour also allowed rapid processing and disarticulation of large carcasses with stone tools to minimise the time spent at the kill site and reduce the encounter rate with
carnivores19,48, but this behaviour was not included in our simulations. Direct competition between scavengers, in our case _Homo_ and _Pachycrocuta brevirostris_, could favour grouping. A
certain number of hominins banding together, even brandishing sticks or stones, and shouting could chase out larger predators from their preys17,49. Indeed, archaeological evidence from
Fuente Nueva-350 and Dmanisi51 suggests that cobbles and limestone blocks could be used as throwing stones to drive away predators and competitors, reducing the risk of the confrontation52.
The results showed that maintaining an optimum group size can be an important factor for success in the competition for carrion in the form of interference competition53. Therefore, an
optimum group size protects against predation 45 and improves scavenging efficiency. SCAVCOMP-ABM54 simulated the trophic behaviour of hominins without using a central place-foraging
model55,56,57,58. This is an important difference from other computer simulations of hominin foraging strategies, including simulations of scavenging activities59,60. In the HOMINIDS (Hungry
Omnivores Moving, Interacting, and Nesting in Independent Decision-making Simulations) model59, hominin agents leave their nests in the morning and roam individually to search for food. If
an agent finds an abandoned carcass, it feeds on it; however, if there are other scavengers on the spot, the hominin calls for help and waits until more hominins arrive to chase away
competitors and feed on the spot. Szilágyi et al.60 developed an ELBA model and simulated confrontational scavenging to test a hypothesis regarding the emergence of language. Since
carnivores are not included in the ELBA model60, it closely simulates passive scavenging rather than confrontational scavenging. In the ELBA model, hominins forage during the day and return
to a campsite at night, where they share food and information regarding the location of the carcasses. Moreover, in the ELBA model, group size had no influence on the ability of hominins to
access a carcass but did influence their capability to transport the carcass to the campsite. In contrast, in SCAVCOMP-ABM54, hominins live in small bands that move from one resource patch
to another and remain in the patch until the resources (carrion) are depleted; this type of mobility is better described as an optimal patch-use strategy61. Similarly, a strategy without a
central place or reference site and provisioning in a fission–fusion social model is common among non-human primates, such as chimpanzees and baboons62,63,64,65. Although a central place
strategy is usually assumed for coeval hominin populations in Africa56, assuming a different behaviour for the European hominins during the Epivillafranchian does not conflict with the
archaeological records. Most early Pleistocene sites from Iberia are interpreted as marginal occupations by hominins, as Fuente Nueva-332,39 Barranco León D2, Vallparadís EVT766,67, or
Barranc de la Boella sites68,69, or as low intensity occupations as Sima del Elefante TE970. Most of these sites are open-air localities usually associated with floodplains or riparian
environments and are interpreted as foraging sites. Only the Atapuerca TD6.2 assemblage has been interpreted as a home base intensively occupied by hominins over long periods of time71,72
and might thus be evidence of a central place foraging strategy. However, TD6.2, which has been dated to approximately 0.8 Ma73, has a faunal assemblage characterised by the absence of both
_P. brevirostris_ and _Megantereon_ and the presence of _Crocuta crocuta_74. Thus, Atapuerca TD6.2, which corresponds to the time after the extinction of _Megantereon_ and the replacement of
_Pachycrocuta brevirostris_ by _C. crocuta_, is younger than that considered here and with an entirely different ecological scenario. The quantitative estimates of carrion production
support that sabre-toothed felids created a niche for scavengers by abandoning carcasses with a high nutrient content. In this scenario, scavenging was a reliable food procurement strategy
for early hominins in southern Europe, as they foraged in groups strong enough to chase giant hyenas away from the carcasses. This suggests that the differentiation between passive
scavenging and kleptoparasitism is limited in the presence of a strong competitor. However, group size had to be moderate in order to maximize the energetic efficiency of the activity.
Scavenging does not require advanced technology only group cohesion and cooperation and was likely an important source of meat and fat for _Homo_ sp. in Europe, especially in winter when
plant resources were scarce. METHODS SCAVCOMP-ABM Several simulation experiments were performed using SCAVCOMP-ABM v.40.254, an agent-based model (ABM) developed in Net Logo 6.2.275 that
could simulate the competition for carrion among hominins and carnivores in an early Pleistocene European ecosystem. An ABM is a computational modelling paradigm that simulates complex
systems by encoding the behaviour of simulated entities (agents) in simple rules to observe the results of these agents’ interactions36. In this study, an overview of the SCAVCOMP-ABM is
provided; however, a full description of the model following the ODD (Overview, Design concepts, and Details) protocol76 is provided elsewhere54. The terminology of Montgomery77 was followed
in this study, where the parameters that may be changed between simulation experiments are called “factors”, the factor values are called “levels”, parameters that are fixed are called
“constants”, and the output variables are called “responses”. Therefore, each experiment was defined by a combination of the initial levels of the factors. By comparing the results of the
different experiments, the effects of these factors on the response variables were observed. SCAVCOMP-ABM was designed to evaluate the viability of scavenging as a foraging strategy for
hominins under different carnivore guild compositions and different group sizes. The environment of the model consisted of a grid of 51 × 51 cells (patches) representing an area of 2601 km2
in a homogeneous landscape. Each patch had an area of 1 km2. The agents of the model were groups of hominins and packs or individuals belonging to the large carnivore species present in the
late Villafranchian and Epivillafranchian8,78. These agents were classified as predators (_Homotherium latidens_, _Meganthereon_ sp., and _Panthera gombaszoegensis_) or scavengers (_Homo_
sp. and _Pachycrocuta brevirostris_). SCAVCOMP-ABM allows the inclusion of a third type of agent called a hybrid, which shares characteristics with both predators and scavengers; however,
this type was excluded in these simulations. The composition of the carnivore guild changed among experiments by excluding some of the species listed above and modifying the initial
densities of the species included. The agents in each group were defined by a separate set of state variables, and each species was defined by a distinct set of values for the state
variables. The behaviour of the agents was determined by a set of rules, which were different for predators and scavengers. All agents of the same species were identical; that is, they were
defined by the same values for all their state variables. This model simulated the dynamics of a community over a year or a few years. Each tick represents an hour and agents did not
reproduce. Predators moved randomly around the environment and stochastically produced carcasses. The frequency of production and the amount of nutrients in the carcass (energy) differed for
each predator species, and hunting was not simulated using this model. The energy contained in the carcass decreased with time at a rate determined by the carrion-wastage-rate (kcal/day).
The carrion-wastage-rate simulated the “natural decay” of the energetic content of a carcass abandoned in the landscape. In the real world, decay occurs through the action of microorganisms,
and because carcasses were consumed by other species (birds, invertebrates, or other carnivores), this was not simulated. Scavengers aimed to collect carrion and obtain energy to compensate
for their energy losses. Scavengers spent energy continuously at a basal rate and at a higher rate when moving. The energy expenditure rate differed for each species. If the energy of the
scavenger agent declines to zero, the agent dies. Thus, the objective of scavengers was to balance energy expenditure and gain energy for survival. Survival success was measured based on the
total population of the scavenger species at the end of the simulation. Direct competition, or confrontation, is simulated in the model by allowing only one agent to stay in a patch. If two
or more agents coincide in the same patch, only one of the agents with the highest _rank_ stays in the patch and the rest of agents move away. The _rank_ is an attribute of the species. All
the agents of the same species have the same _rank_. All predators have a _rank_ value of 5, but the _rank_ of the scavengers depends on the size of the pack as follows:
$$Pachycrocuta\;brevirostris\;{\text{rank }} = {\text{ pack}} - {\text{size}} \times {2}.{5}$$ $${\text{Hominins}}\;{\text{rank = pack - size/2}}$$ An agent representing a solitary _P.
brevirostris_ had a lower rank than any predator but a higher rank than a group of hominins with less than five individuals. Groups of six or more hominins had a rank higher than any other
agent. These values were selected arbitrarily; however, they reflected the effect of group size on confrontations between hominins and carnivores. Ethnographical observations indicate that
less than five adult Hadza are able to chase away any large felid23, and a group of ten chimpanzees has been reported to confront a leopard at Mahale (Tanzania)45. Direct competition for a
single carcass between two agents was simulated by allowing only one of them to stay in the patch containing carrion, even if they were agents of the same species, for example, two different
groups of hominins. Moreover, it was assumed that scavengers avoided direct interactions with predators unless they could chase them away (because the predator had a lower rank). However,
notably, this model did not intend to simulate kleptoparasitism or confrontational scavenging17,18,19,79. PARAMETERIZATION The default levels of the SCAVCOMP-ABM parameters are listed in
Supplementary Table S2. The levels of many parameters were derived based on theoretical or real-world observations. However, several parameters were assigned values after observing the
effect of their variation on the behaviour and results of the model. The levels of initial-nutrition-state_,_ range-of-view, and daily-carrion-wastage-rate could not be determined from
theory or derived from the real world, and therefore were based on the sensitivity analysis results, as shown in Supplementary Methods S3. PREDATORS AND CARRION AVAILABILITY The number of
energetic resources available in the environment in the form of scavengeable carcasses depends on the frequency of carcass production by predators, the average size of the carcasses produced
by each predator, the population density of the predator23, and their food intake rate. Carcass size was determined based on the size of the prey preferred by each predator (Table 3). A
discussion on the feeding preferences of large Epivillafranchian carnivores was provided by Rodríguez et al.26. In this study, the body weight of the preferred prey was obtained as the
average body weight of the two prey species representing more than 80% of the predator’s diet, as indicated by isotopic analyses of the fossils of these species80. The estimations of carcass
production rates were based on recent reports of the feeding behaviour of large carnivores (Supplementary Methods S2). In the simulations, the hunting frequencies were assumed to be 1 kill
per 8 days for _Homotherium_ and 1 kill per 7 days for _Megantereon_ sp. and _Panthera gombaszoegensis_. The equation provided by Farlow81 was used to estimate the daily energy requirements
of the predators based on their estimated average body masses13. The amount of energy consumed by the predator before abandoning the carcass was obtained by multiplying the daily energy
requirements by the average interval between kills (in days). A kill should fulfil the energetic requirements of the predator until the next kill. Thus, assuming low killing ratios, we
obtained conservative estimates of carcass production rates and carcass energy content for these predators. The edible mass of a complete carcass was obtained by multiplying the average prey
body weight by the wastage factor given by Viljoen82. This wastage factor is dependent on body size, as follows: < 50 kg, 80% edible; 50–150 kg, 75% edible; 151–250 kg, 70% edible;
251–500 kg, 65% edible; 500–1000 kg, 60% edible; > 1000 kg, 55% edible. The amount of energy that could be obtained from an entire carcass was then computed by multiplying the edible mass
by a conversion factor of 1300 kcal/kg. This conversion factor was based on the caloric content of a kilogramme of the edible mass of different ungulates83,84. Finally, the energy content
of the carcass produced by a predator was obtained by subtracting the energy consumed by the predator from the edible mass of the entire carcass. The published estimates of sustainable
population densities of large carnivores in Mediterranean ecosystems during the late Early Pleistocene are reviewed in Table 385,86. All these estimations are roughly coincident, although
they are based on different methodologies. In our simulations, the estimates of the lowest, maximum, and average population densities8 were used to represent the three different conditions.
These conditions can be interpreted as three different ecosystems with a gradient of increasing primary production, with the most productive ecosystems sustaining higher population
densities. SCAVENGERS AND ENERGY REQUIREMENTS The energy requirements of any mammal are largely determined by its body size87. Therefore, the well-known relationship between body size and
metabolic rate was used in this simulation to estimate the basal metabolic rates of humans88 and giant hyenas89; the details of which are provided in Table 4. The body weight of
_Pachycrocuta brevirostris_ was obtained from Rodríguez-Gómez et al.85. The body weight of the hominins was assumed to be 62 kg, which is the estimated body weight of _Homo ergaster_ and
_Homo erectus_90. The daily energy requirements of hominins (DER), which were used in the model to estimate the energetic cost of movement, were set to 3000 kcal/day. This energy expenditure
was similar to the estimated cost of walking for _H. ergaster_91. Locomotion is energetically costly for giant hyenas because of their shortened distal limbs29. Thus, the DER of the giant
hyena was set at three times the estimated BMR (basal metabolic rate). In the simulations, the giant hyenas were assumed to be solitary scavengers10,30,92. Therefore, the pack-size was set
to one. However, it has also been proposed that the giant hyena would require group action to defend the carcasses from other predators and practise confrontational scavenging29. According
to this alternative interpretation, social behaviour would allow giant hyenas to complete their diet by hunting their own prey; however, this was not addressed in this study. The velocity
parameter determines the speed at which agents move when searching for food or avoiding an enemy. In the simulation, hominins moved at 5 km/h, corresponding to the medium walking speed of
humans currently93, and the same velocity was used for giant hyenas because of the lack of an estimate of the speed at which they moved, as it is generally accepted that they were not highly
cursorial carnivores29,30. Notably, the velocities represented in the model were the average speeds when moving long distances and not at a fast running speed. The initial population
densities of hominins (14 individuals/100 km2) and giant hyenas (12 individuals/100 km2) were based on the values observed for living species and published estimates. The ecological density
of hominins in Barranco León D and Fuente Nueva-3 was estimated to be in the range of 10–14 individuals/100 km2 if they follow a pure scavenging strategy, 9–10 individuals/100 km2 if they
are hunters, and 10–12 individuals if they adopt a hunting-scavenging strategy86. The densities of recent hunter-gatherer groups from mid-latitude areas (30–50°N) range from 0.4 to 300
individuals/100 km294. Estimated sustainable densities of 4.1–12.2 individuals/100 km2 were estimated for _P. brevirostris_ in the Iberian Peninsula during the late Early Pleistocene8.
Estimated sustainable densities for the giant hyena were 4–6 individuals/100 km2 at Barranco León D and Fuente Nueva-386, and 6–7 individuals/100 km2 at Venta Micena85. The initial densities
were set to the upper limit of the estimated intervals because the simulations proceeded by reducing the number of groups or packs in the scavenger guild until a stable configuration was
reached or, alternatively, until the system collapsed. EXPERIMENT DESIGN In this study, the competition between _P. brevirostris_ and hominins in the late Early Pleistocene of the Iberian
Peninsula and the role of sabre-tooths as carrion producers were focused. The large carnivorous guilds of Iberia during the Epivillafranchian included _Canis etruscus, Lycaon lycaonoides,
Homotherium latidens, Megantereon whitei, Panthera gombaszoegensis,_ and _Pachycrocuta brevirostris_8. Archaeological records show that the first European hominins coexisted in the same
community as two sabre-toothed felids (_H. latidens_ and _M. whitei_), Pleistocene wild dogs (_Lycaon lycaonoides_), small canid (_Canis etruscus/mosbachensis_), and giant hyena in
localities such as Fuente Nueva-3 and Barranco León D86. The European jaguar (_P. gombaszoegensis_) was recorded together with the two sabre-toothed felids and the giant hyena in several
palaeontological assemblages of the late Villafranchian or Epivillafranchian age, including Ceyssaguet-195, Monte Argentario96, Olivola97, Untermassfeld98, Venta Micena85, and Cueva
Victoria99. Therefore, the experiments were conducted under two different ecological scenarios. In Scenario 1, the predator guild was composed of _H. latidens, M. whitei,_ and _P.
gombaszoegensis,_ whereas in Scenario 2, only two sabre-toothed felids comprised the guild. Under both scenarios, the scavenger guild comprised _Homo sp._ and _P. brevirostris_. Furthermore,
_Canis etruscus_ was excluded from our simulation experiments because this small canid is unlikely to be a large carcass producer. _Lycaon lycaonoides_ was also excluded as a significant
carcass producer because the early Pleistocene wild dogs were assumed to possess a behaviour similar to that of the recent African wild dogs (_L. pictus_)_._ A pack of African wild dogs can
effectively eat an entire carcass in a short time, unless disturbed by hyenas or other carnivores attempting to steal their kill100. Thus, it was assumed that _L. lycaonoides_ did not
abandon carcasses containing a significant amount of nutrients and were not included in the experiments. Moreover, _C. etruscus_ probably consumed carrion opportunistically but was also
excluded from the experiments as an agent for the sake of simplicity. Instead, the effect of its scavenging activity on the availability of carcasses was summarised in the
carrion-wastage-rate parameter. The experiments evaluated the effects of two factors (size of the hominin group and population densities of predators and scavengers) on the performance of
hominins as passive scavengers in two ecological scenarios. The two tested factors were ultimately dependent on the primary production of the ecosystem8. The effect of these two factors was
evaluated on two response variables: the total population of hominins and hyenas at the end of the simulation experiment and the average daily energy expenditure by a single hominin and _P.
brevirostris_. The daily energy expenditure is a measure of the energy cost of a scavenging strategy. It was assumed in the framework of the Optimal Foraging Theory101,102 that the best
feeding strategy is that which allows an individual to survive at the lowest cost, and allocate more energy to other activities such as reproduction. We performed simulation experiments with
hominin group sizes of 1, 4, 7, 10, 13, 16, 19, 22, and 25 individuals at low, medium, and high densities of predators (see the Parameterization section). This yielded 27 simulations for
each scenario. All simulations were performed for 9000 ticks, representing 9000 h or 375 days. Since the SCAVCOMP-ABM includes a certain degree of stochasticity54, a previous analysis was
necessary to determine the number of runs required to obtain a valid estimate of the response variables. A replication assessment was conducted prior to the experiments103 performing 120
runs of the model with default parameter levels (Supplementary Methods S1). After the results of the replication assessment were obtained, all simulations were replicated 70 times. DATA AND
CODE AVAILABILITY All data generated or analysed during this study are included in this published article [and its supplementary information files]. The output of simulation experiments is
available as Supplementary Dataset 1. The SCAVCOMP-ABM v40.2 and ODD Protocol are available at 10.6084/m9.figshare.22716427. REFERENCES * Arzarello, M. _et al._ Evidence of earliest human
occurrence in Europe: The site of Pirro Nord (Southern Italy). _Naturwissenschaften_ 94, 107–112. https://doi.org/10.1007/s00114-006-0173-3 (2007). Article ADS CAS PubMed Google Scholar
* Toro-Moyano, I. _et al._ The oldest human fossil in Europe dated to ca. 1.4 Ma at Orce (Spain). _J. Hum. Evol._ 65, 1–9. https://doi.org/10.1016/j.jhevol.2013.01.012 (2013). Article
PubMed Google Scholar * Carbonell, E. _et al._ The first hominin of Europe. _Nature_ 452, 465–470. https://doi.org/10.1038/nature06815 (2008). Article ADS CAS PubMed Google Scholar *
Palmqvist, P. _et al._ Insights on the early pleistocene hominin population of the Guadix-Baza depression (SE Spain) and a review on the ecology of the first peopling of Europe. _Front.
Ecol. Evol._ 10, 881651. https://doi.org/10.3389/fevo.2022.881651 (2022). Article Google Scholar * Iannucci, A., Mecozzi, B., Sardella, R. & Iurino, D. A. The extinction of the giant
hyena _Pachycrocuta brevirostris_ and a reappraisal of the Epivillafranchian and Galerian Hyaenidae in Europe: Faunal turnover during the early-middle Pleistocene transition. _Quat. Sci.
Rev._ 272, 107240. https://doi.org/10.1016/j.quascirev.2021.107240 (2021). Article Google Scholar * Altolaguirre, Y. _et al._ An environmental scenario for the earliest hominins in the
Iberian Peninsula: Early Pleistocene palaeovegetation and palaeoclimate. _Rev. Palaeobot. Palynol._ 260, 51–64. https://doi.org/10.1016/j.revpalbo.2018.10.008 (2019). Article Google Scholar
* O’Regan, H. J., Turner, A., Bishop, L. C., Elton, S. & Lamb, A. L. Hominins without fellow travellers? First appearances and inferred dispersals of Afro-Eurasian large-mammals in the
Plio-Pleistocene. _Quat. Sci. Rev._ 11–12, 1343–1352. https://doi.org/10.1016/j.quascirev.2009.11.028 (2011). Article ADS Google Scholar * Rodríguez, J. & Mateos, A. Carrying
capacity, carnivoran richness and hominin survival in Europe. _J. Hum. Evol._ 118, 72–88. https://doi.org/10.1016/j.jhevol.2018.01.004 (2018). Article PubMed Google Scholar * Rolland, N.
The early Pleistocene human dispersals in the circum-mediterranean basin and initial peopling of Europe: Single or multiple pathways?. _Quat. Int._ 316, 59–72.
https://doi.org/10.1016/j.quaint.2013.06.028 (2013). Article Google Scholar * Croitor, R. & Brugal, J.-P. Ecological and evolutionary dynamics of the carnivore community in Europe
during the last 3 million years. _Quat. Int._ 212, 98–108. https://doi.org/10.1016/j.quaint.2009.06.001 (2010). Article Google Scholar * Raia, P., Meloro, C. & Barbera, C. Inconstancy
in predator/prey ratios in quaternary large mammal communities of Italy, with an appraisal of mechanisms. _Quat. Res._ 67, 255–263. https://doi.org/10.1016/j.yqres.2006.10.005 (2007).
Article Google Scholar * Turner, A. Large carnivores and earliest European hominids: changing determinants of resource availability during the lower and middle Pleistocene. _J. Hum. Evol._
22, 109–126. https://doi.org/10.1016/0047-2484(92)90033-6 (1992). Article Google Scholar * Rodríguez-Gómez, G., Rodríguez, J., Martín-González, J. A. & Mateos, A. Evaluating the
impact of Homo-carnivore competition in European human settlements during the early to middle Pleistocene. _Quat. Res._ 88, 129–151. https://doi.org/10.1017/qua.2017.20 (2017). Article
Google Scholar * Binford, L. R. _Bones. Ancient Men and Modern Myths_ (Academic Press, 1981). Google Scholar * Blumenschine, R. J. Characteristics of an early hominid scavenging niche.
_Curr. Anthropol._ 28, 383–407. https://doi.org/10.1086/203544 (1987). Article Google Scholar * Brantingham, P. J. Hominid-carnivore coevolution and invasion of the predatory guild. _J.
Anthropol. Archaeol._ 17, 327–353. https://doi.org/10.1006/jaar.1998.0326 (1998). Article Google Scholar * Bunn, H. T. in _Meat-eating and Human Evolution._ (eds C.B. Standford & H.T
Bunn) 199–218 (Oxford University Press, 2001). * Domínguez-Rodrigo, M. Hunting and scavenging by early humans: The state of the debate. _J. World Prehist._ 16, 1–54.
https://doi.org/10.1023/A:1014507129795 (2002). Article Google Scholar * Pobiner, B. L. The zooarchaeology and paleoecology of early hominin scavenging. _Evol. Anthropol. Issues News Rev._
29, 68–82. https://doi.org/10.1002/evan.21824 (2020). Article Google Scholar * Pobiner, B. L. New actualistic data on the ecology and energetics of hominin scavenging opportunities. _J.
Hum. Evol._ 80, 1–16. https://doi.org/10.1016/j.jhevol.2014.06.020 (2015). Article PubMed Google Scholar * Watts, D. P. Scavenging by chimpanzees at Ngogo and the relevance of chimpanzee
scavenging to early hominin behavioral ecology. _J. Hum. Evol._ 54, 125–133. https://doi.org/10.1016/j.jhevol.2007.07.008 (2008). Article PubMed Google Scholar * Speth, J. D. Early
hominid hunting and scavenging: The role of meat as an energy source. _J. Hum. Evol._ 18, 329–343. https://doi.org/10.1016/0047-2484(89)90035-3 (1989). Article Google Scholar * O’Connell,
J. F., Hawkes, K. & BlurtonJones, N. Hadza scavenging: Implications for Plio-Pleistocene hominid subsistence. _Curr. Anthropol._ 29, 356–363. https://doi.org/10.1086/203648 (1988).
Article Google Scholar * Ungar, P. S., Grine, F. E. & Teaford, M. F. Diet in early homo: A review of the evidence and a new model of adaptive versatility. _Annu. Rev. Anthropol._ 35,
209–228. https://doi.org/10.1146/annurev.anthro.35.081705.123153 (2006). Article Google Scholar * Wood, B. & Strait, D. Patterns of resource use in early Homo and Paranthropus. _J.
Hum. Evol._ 46, 119–162. https://doi.org/10.1016/j.jhevol.2003.11.004 (2004). Article PubMed Google Scholar * Rodríguez, J., Rodríguez-Gómez, G., Martín-González, J. A., Goikoetxea, I.
& Mateos, A. Predator–prey relationships and the role of homo in early Pleistocene food webs in Southern Europe. _Palaeogeogr. Palaeoclimatol. Palaeoecol._ 365–366, 99–114.
https://doi.org/10.1016/j.palaeo.2012.09.017 (2012). Article Google Scholar * Palmqvist, P., Martínez-Navarro, B. & Arribas, A. Prey selection by terrestrial carnivores in a lower
Pleistocene Paleocommunity. _Paleobiology_ 349, 514–534. https://doi.org/10.1017/S009483730001650X (1996). Article Google Scholar * Martínez-Navarro, B. & Palmqvist, P. Presence of the
African Machairodont _Megantereon whitei_ (Bromm, 1937) (Felidae, Carnívora, Mammalia) in the lower Pleistocene Site of Venta Micena (Orce, Granada, Spain), with some considerations on the
origin, evolution and dispersal of the genus. _J. Archaeol. Sci._ 22, 569–582. https://doi.org/10.1006/jasc.1994.0054 (1995). Article Google Scholar * Turner, A. & Antón, M. The giant
hyaena _Pachycrocuta brevirostris_ (Mammalia, Carnivora Hyaenidae). _Geobios_ 29, 455–468. https://doi.org/10.1016/S0016-6995(96)80005-2 (1996). Article Google Scholar * Palmqvist, P. _et
al._ The giant hyena Pachycrocuta brevirostris: Modelling the bone-cracking behavior of an extinct carnivore. _Quat. Int._ 243, 61–79. https://doi.org/10.1016/j.quaint.2010.12.035 (2011).
Article Google Scholar * Madurell-Malapeira, J. _et al._ Were large carnivorans and great climatic shifts limiting factors for hominin dispersals? evidence of the activity of _Pachycrocuta
brevirostris_ during the Mid-Pleistocene Revolution in the Vallparadís Section (Vallès-Penedès Basin, Iberian Peninsula). _Quat. Int._ 431, 42–52.
https://doi.org/10.1016/j.quaint.2015.07.040 (2017). Article Google Scholar * Palmqvist, P. _et al._ Déjà vu: on the use of meat resources by sabretooth cats, hominins, and hyaenas in the
early Pleistocene site of Fuente Nueva 3 (Guadix-Baza Depression, SE Spain). _Archaeol. Anthropol. Sci._ 15, 17. https://doi.org/10.1007/s12520-022-01712-1 (2023). Article Google Scholar *
Domínguez-Rodrigo, M., Egeland, C. P., Cobo-Sánchez, L., Baquedano, E. & Hulbert, R. C. Sabertooth carcass consumption behavior and the dynamics of Pleistocene large carnivoran guilds.
_Sci. Rep._ 12, 6045. https://doi.org/10.1038/s41598-022-09480-7 (2022). Article ADS CAS PubMed PubMed Central Google Scholar * Ruxton, G. D. & Wilkinson, D. M. Endurance running
and its relevance to scavenging by early hominins. _Evolution_ 67, 861–867. https://doi.org/10.1111/j.1558-5646.2012.01815.x (2013). Article PubMed Google Scholar * Arribas, A. &
Palmqvist, P. On the ecological connection between sabre-tooths and hominids; Faunal dispersal events in the lower pleistocene and a review of the evidence for the first human arrival in
Europe. _J. Archaeol. Sci._ 26, 571–585. https://doi.org/10.1006/jasc.1998.0346 (1999). Article Google Scholar * Wilensky, U. & Rand, W. _An Introduction to Agent-Based Modeling
Modeling Natural, Social, and Engineered Complex Systems with NetLogo_ (The MIT Press, 2015). Google Scholar * Prugh, L. R. & Sivy, K. J. Enemies with benefits: Integrating positive and
negative interactions among terrestrial carnivores. _Ecol. Lett._ 23(5), 902–918 (2020). Article PubMed Google Scholar * Blasco, R. _et al._ Earliest evidence for human consumption of
tortoises in the European early Pleistocene from Sima del Elefante, Sierra de Atapuerca. _Spain. J. Hum. Evol._ 61, 503–509. https://doi.org/10.1016/j.jhevol.2011.06.002 (2011). Article
PubMed Google Scholar * Espigares, M. P. _et al._ The earliest cut marks of Europe: A discussion on hominin subsistence patterns in the Orce sites (Baza basin, SE Spain). _Sci. Rep._ 9,
15408. https://doi.org/10.1038/s41598-019-51957-5 (2019). Article ADS CAS PubMed PubMed Central Google Scholar * Prugh, L. R. & Sivy, K. J. Enemies with benefits: integrating
positive and negative interactions among terrestrial carnivores. _Ecol. Lett._ 23, 902–918. https://doi.org/10.1111/ele.13489 (2020). Article PubMed Google Scholar * DeSantis, L. R. G.,
Feranec, R. S., Antón, M. & Lundelius, E. L. Dietary ecology of the scimitar-toothed cat _Homotherium serum_. _Curr. Biol._ 31, 2674-2681.e2673. https://doi.org/10.1016/j.cub.2021.03.061
(2021). Article CAS PubMed Google Scholar * Palmqvist, P. & Arribas, A. Taphonomic decoding of the paleobiological information locked in a lower Pleistocene assemblage of large
mammals. _Paleobiology_ 27, 512–530. https://doi.org/10.1666/0094-8373(2001)027%3c0512:TDOTPI%3e2.0.CO;2 (2001). Article Google Scholar * Vinuesa, V. _et al._ Inferences of social behavior
in bone-cracking hyaenids (Carnivora, Hyaenidae) based on digital paleoneurological techniques: Implications for human–carnivoran interactions in the Pleistocene. _Quatern. Int._ 413, 7–14.
https://doi.org/10.1016/j.quaint.2015.10.037 (2016). Article Google Scholar * Harrison, S. & Connel, J. H. Introduction: Merging evolutionary and ecologycal approaches to
understanding geographic gradients in species richness. _Am. Nat._ 170(Supplement), S1–S4. https://doi.org/10.1086/519011 (2007). Article PubMed Google Scholar * Nakamura, M. _et al._
Wild chimpanzees deprived a leopard of its kill: Implications for the origin of hominin confrontational scavenging. _J. Hum. Evol._ 131, 129–138. https://doi.org/10.1016/j.jhevol.2019.03.011
(2019). Article PubMed Google Scholar * Sivy, K. J., Pozzanghera, C. B., Grace, J. B. & Prugh, L. R. Fatal attraction? intraguild facilitation and suppression among predators. _Am.
Nat._ 190, 663–679. https://doi.org/10.1086/693996 (2017). Article PubMed Google Scholar * Wilson, B. J. & Harris, S. R. Language and cooperation in hominin scavenging. _Evol. Hum.
Behav._ 38, 376–396. https://doi.org/10.1016/j.evolhumbehav.2016.11.009 (2017). Article Google Scholar * Bickerton, D. & Szathmáry, E. Confrontational scavenging as a possible source
for language and cooperation. _BMC Evol. Biol._ 11, 261. https://doi.org/10.1186/1471-2148-11-261 (2011). Article PubMed PubMed Central Google Scholar * Treves, A. & Naughton-Treves,
L. Risk and opportunity for humans coexisting with large carnivores. _J. Hum. Evol._ 36, 275–282. https://doi.org/10.1006/jhev.1998.0268 (1999). Article CAS PubMed Google Scholar *
Espigares, M. _et al._ Sharing food with hyenas: a latrine of Pachycrocuta brevirostris in the early Pleistocene assemblage of Fuente Nueva-3 (Orce, Baza Basin, SE Spain). _Archaeol.
Anthropol. Sci._ 15, 1784. https://doi.org/10.1007/s12520-023-01784-7 (2023). Article Google Scholar * Coil, R. _et al._ Spatial patterning of the archaeological and paleontological
assemblage at Dmanisi, Georgia: An analysis of site formation and carnivore-hominin interaction in Block 2. _Journ. Hum. Evol._ 143, 102773. https://doi.org/10.1016/j.jhevol.2020.102773
(2020). Article Google Scholar * Treves, A. & Palmqvist, P. in _Primate anti-predator strategies, Developments in PrimatologySeries: Progress and Prospects_ (ed KAI N Gursky SL)
335–381 (Springer, 2007). * Eaton, R. L. Interference competition among carnivores a model for the evolution of social behavior. _Carnivore_ 2, 9–16 (1979). Google Scholar * Rodríguez, J.,
Hölzchen, E. & Mateos, A. SCAVCOMP-ABM. A Comput. Model Simul Compet. Scav. https://doi.org/10.6084/m9.figshare.22716427 (2023). * Kelly, R. L. Hunter-gatherer mobility strategies. _J.
Anthropol. Res._ 39, 277–306. https://doi.org/10.1086/jar.39.3.3629672 (1983). Article Google Scholar * Rose, L. & Marshall, F. Meat eating, hominid sociality, and home bases
revisited. _Curr. Anthropol._ 37, 307–338. https://doi.org/10.1086/204494 (1996). Article Google Scholar * Marlowe, F. W. Hunter-gatherers and human evolution. _Evolut. Anthropol. Issues
News Rev._ 14, 54–67. https://doi.org/10.1002/evan.20046 (2005). Article Google Scholar * Orians, G. H., & Pearson, N. E. . in _Analysis of ecological systems_ (eds G. D. J. Horn &
& R. D. Mitchell R. Stairs) 155–177 (Ohio State University Press, 1979). * Griffith, C. S., Long, B. L. & Sept, J. M. HOMINIDS: An agent-based spatial simulation model to evaluate
behavioral patterns of early Pleistocene hominids. _Ecol. Model._ 221, 738–760. https://doi.org/10.1016/j.ecolmodel.2009.11.009 (2010). Article Google Scholar * Szilágyi, A., Kovács, V.
P., Czárán, T. & Szathmáry, E. Evolutionary ecology of language origins through confrontational scavenging. _Philos. Trans. R. Soc. B Biol. Sci._ 378, 20210411.
https://doi.org/10.1098/rstb.2021.0411 (2023). Article Google Scholar * MacArthur, R. H. & Pianka, E. R. On optimal use of a patchy environment. _Am. Nat._ 100, 603–609.
https://doi.org/10.1086/282454 (1966). Article Google Scholar * Barton, R. A., Whiten, A., Strum, S. C., Byrne, R. W. & Simpson, A. J. Habitat use and resource availability in baboons.
_Anim. Behav._ 43, 831–844. https://doi.org/10.1016/S0003-3472(05)80206-4 (1992). Article Google Scholar * Bates, L. A. & Byrne, R. W. Sex differences in the movement patterns of
free-ranging chimpanzees (Pan troglodytes schweinfurthii): Foraging and border checking. _Behav. Ecol. Sociobiol._ 64, 247–255. https://doi.org/10.1007/s00265-009-0841-3 (2009). Article
Google Scholar * Cowlishaw, G. U. Y. Trade-offs between foraging and predation risk determine habitat use in a desert baboon population. _Anim. Behav._ 53, 667–686.
https://doi.org/10.1006/anbe.1996.0298 (1997). Article Google Scholar * Jang, H., Boesch, C., Mundry, R., Ban, S. D. & Janmaat, K. R. L. Travel linearity and speed of human foragers
and chimpanzees during their daily search for food in tropical rainforests. _Sci. Rep._ 9, 11066. https://doi.org/10.1038/s41598-019-47247-9 (2019). Article ADS CAS PubMed PubMed Central
Google Scholar * Madurell-Malapeira, J., Alba, D. M., Minwer-Barakat, R., Aurell-Garrido, J. & Moyà-Solà, S. Early human dispersals into the Iberian Peninsula: A comment on Martínez
et al. (2010) and García et al. (2011). _J. Hum. Evol._ 62, 169–173 (2012) doi:https://doi.org/10.1016/j.jhevol.2011.10.005. * Martínez, K. _et al._ A new lower Pleistocene archeological
site in Europe (Vallparadís, Barcelona, Spain). _Proc. Natl. Acad. Sci._ 107, 5762–5767. https://doi.org/10.1073/pnas.0913856107 (2010). Article ADS PubMed PubMed Central Google Scholar
* Pineda, A. _et al._ Characterizing hyena coprolites from two latrines of the Iberian Peninsula during the early Pleistocene: Gran Dolina (Sierra de Atapuerca, Burgos) and la Mina
(Barranc de la Boella, Tarragona). _Palaeogeogr. Palaeoclimatol. Palaeoecol._ 480, 1–17. https://doi.org/10.1016/j.palaeo.2017.04.021 (2017). Article Google Scholar * Vallverdú, J. _et
al._ Age and date for early arrival of the Acheulian in Europe (Barranc de la Boella, la Canonja, Spain). _PLoS ONE_ 9, e103634. https://doi.org/10.1371/journal.pone.0103634 (2014). Article
ADS CAS PubMed PubMed Central Google Scholar * Huguet, R. _et al._ Subsistence strategies of the first human settlements in Eurasia. _Quat. Int._ 295, 168–182.
https://doi.org/10.1016/j.quaint.2012.11.015 (2013). Article Google Scholar * Saladié, P. _et al._ Dragged, lagged, or undisturbed: reassessing the autochthony of the hominin-bearing
assemblages at Gran Dolina (Atapuerca, Spain). _Archaeol. Anthropol. Sci._ 13, 1303. https://doi.org/10.1007/s12520-021-01303-6 (2021). Article Google Scholar * Saladié, P. _et al._
Carcass transport decisions in _Homo antecessor_ subsistence strategies. _J. Hum. Evol._ 61, 425–446. https://doi.org/10.1016/j.jhevol.2011.05.012 (2011). Article PubMed Google Scholar *
Duval, M. _et al._ New chronological constraints for the lowermost stratigraphic unit of Atapuerca Gran Dolina (Burgos, N Spain). _Quat. Geochronol._ 71, 101292.
https://doi.org/10.1016/j.quageo.2022.101292 (2022). Article Google Scholar * Rodríguez, J. _et al._ One million years of cultural evolution in a stable environment at Atapuerca (Burgos,
Spain). _Quatern. Sci. Rev._ 30, 1396–1412. https://doi.org/10.1016/j.quascirev.2010.02.021 (2011). Article ADS Google Scholar * NetLogo v. 6.2.2 (Northwestern University, Evanston.
Center for Connected Learning and Computer-Based Modeling, Evaston, IL, 1999). * Grimm, V. _et al._ The ODD protocol: A review and first update. _Ecol. Model._ 221, 2760–2768.
https://doi.org/10.1016/j.ecolmodel.2010.08.019 (2010). Article Google Scholar * Montgomery, D. C. _Design and Analysis of Experiments_ (John Wiley & Sons Inc, 2013). Google Scholar *
Bellucci, L., Sardella, R. & Rook, L. Large mammal biochronology framework in Europe at Jaramillo: The Epivillafranchian as a formal biochron. _Quatern. Int._ 389, 84–89.
https://doi.org/10.1016/j.quaint.2014.11.012 (2015). Article Google Scholar * Bunn, H. T. & Gurtov, A. N. Prey mortality profiles indicate that early Pleistocene _Homo_ at Olduvai was
an ambush predator. _Quatern. Int._ 322–323, 44–53. https://doi.org/10.1016/j.quaint.2013.11.002 (2014). Article Google Scholar * Palmqvist, P., Pérez-Claros, J. A., Janis, C. M. &
Gröcke, D. R. Tracing the ecophysiology of ungulates and predator-prey relationships in an early Pleistocene large mammal community. _Palaeogeogr. Palaeoclim. Palaeoecol._ 266, 95–111.
https://doi.org/10.1016/j.palaeo.2008.03.015 (2008). Article ADS Google Scholar * Farlow, J. O. A consideration of the trophic dynamics of a late Cretaceous large dinosaur community
(Oldman formation). _Ecology_ 57, 841–857. https://doi.org/10.2307/1941052 (1976). Article Google Scholar * Viljoen, P. C. The effects of changes in prey availability on lion predation in
a large natural ecosystem in northern Botswana. _Symp. Zool. Soc. Lond._ 1993, 193–213 (1993). Google Scholar * Byers, D. A. & Ugan, A. Should we expect large game specialization in the
late Pleistocene? An optimal foraging perspective on early Paleoindian prey choice. _J. Archaeol. Sci._ 32, 1624–1640. https://doi.org/10.1016/j.jas.2005.05.003 (2005). Article Google
Scholar * Rodríguez, J., Zorrilla-Revilla, G. & Mateos, A. Does optimal foraging theory explain the behavior of the oldest human cannibals?. _Journ. Hum. Evol._ 13, 228–239.
https://doi.org/10.1016/j.jhevol.2019.03.010 (2019). Article Google Scholar * Rodríguez-Gómez, G., Palmqvist, P., Ros-Montoya, S., Espigares, M. P. & Martínez-Navarro, B. Resource
availability and competition intensity in the carnivore guild of the Early Pleistocene site of Venta Micena (Orce, Baza Basin, SE Spain). _Quatern. Sci. Rev._ 164, 154–167.
https://doi.org/10.1016/j.quascirev.2017.04.006 (2017). Article ADS Google Scholar * Rodríguez-Gómez, G. _et al._ On the ecological context of the earliest human settlements in Europe:
Resource availability and competition intensity in the carnivore guild of Barranco León-D and Fuente Nueva-3 (Orce, Baza Basin, SE Spain). _Quat. Sci. Rev._ 143, 69–83.
https://doi.org/10.1016/j.quascirev.2016.05.018 (2016). Article ADS Google Scholar * White, C. R. & Seymour, R. S. Mammalian basal metabolic rate is proportional to body mass2/3.
_Proc. Natl. Acad. Sci. U. S. A._ 100, 4046–4049. https://doi.org/10.1073/pnas.04364281 (2003). Article ADS CAS PubMed PubMed Central Google Scholar * Kleiber, M. Body size and
metabolic rate. _Physiol. Rev._ 27, 541. https://doi.org/10.1152/physrev.1947.27.4.511 (1947). Article ADS MATH Google Scholar * McNab, B. Complications inherent in scaling the basal
rate of metabolism in mammals. _Q. R. Biol._ 63, 25–54. https://doi.org/10.1086/415715 (1988). Article CAS Google Scholar * de Sousa, A. & Cunha, E. In _Progress in Brain Research_
Vol. 195 (eds Michel A. Hofman & Dean Falk) 293–322 (Elsevier, 2012). * Vidal-Cordasco, M., Mateos, A., Zorrilla-Revilla, G., Prado-Nóvoa, O. & Rodríguez, J. Energetic cost of
walking in fossil hominins. _Am. J. Phys. Anthropol._ 164, 609–622. https://doi.org/10.1002/ajpa.23301 (2017). Article CAS PubMed Google Scholar * Arribas, A. & Palmqvist, P.
Taphonomy and palaeoecology of an assemblage of large mammals: Hyaenid activity in the lower Pleistocene site at Venta Micena (Orce, Guadix-Baza Basin, Granada, Spain). _Geobios_ 31, 3–47.
https://doi.org/10.1016/S0016-6995(98)80056-9 (1998). Article Google Scholar * Murtagh, E. M., Mair, J. L., Aguiar, E., Tudor-Locke, C. & Murphy, M. H. Outdoor walking speeds of
apparently healthy adults: A systematic review and meta-analysis. _Sports Med._ 51, 125–141. https://doi.org/10.1007/s40279-020-01351-3 (2021). Article PubMed Google Scholar * Binford, L.
R. _Constructing Frames of Reference: An Analytical Method for Archaeological Theory Building Using Ethnographic and Environmental Data Set_ (University of California Press, 2001). Google
Scholar * Tsoukala, E. & Bonifay, M. F. The early Pleistocene carnivores (Mammalia) from Ceyssaguet (Haute-Loire). _Paléo_ 16, 193–242 (2004). Google Scholar * Siori, M. S. _et al._
New data on early pleistocene vertebrates from Monte Argentario (Central Italy) paleoecological and biochronological implication. _Geobios_ 47, 403–418.
https://doi.org/10.1016/j.geobios.2014.10.001 (2014). Article Google Scholar * Palombo, M. R., Azanza, B. & Alberdi, M. T. Italian Mammal biochronology from the Latest Miocene to the
Middle Pleistocene: A multivariate approach. _Geol Romana_ 36, 335–368 (2000–2002). * Koenigswald, W. V. & Heinrich, W.-D. Mittelpleistozäne Säugetierfaunen Mitteleuropa - der versuch
einer bioestratigraphischen Zuordnung. _Kaupia_ 9, 53–112 (1999). Google Scholar * Gibert, L. _et al._ Chronology for the cueva victoria fossil site (SE Spain): Evidence for early
pleistocene Afro-iberian dispersals. _J. Hum. Evol._ 90, 183–197. https://doi.org/10.1016/j.jhevol.2015.08.002 (2016). Article PubMed Google Scholar * Carbone, C. _et al._ Feeding success
of African wild dogs (_Lycaon pictus_) in the Serengeti: The effects of group size and kleptoparasitism. _J. Zool._ 266, 153–161. https://doi.org/10.1017/s0952836905006710 (2005). Article
Google Scholar * Pyke, G. H., Pulliam, H. R. & Charnov, E. L. Optimal foraging: A selective review of theory and tests. _Q. Rev. Biol._ 52, 137–154. https://doi.org/10.1086/409852
(1977). Article Google Scholar * Winterhalder, B. In _Hunter-Gatherer Foraging Strategies: Ethnographic and Archeological Analyses_ (ed Eric Alden Smith Bruce Winterhalder) 13–35
(University of Chicago Press, 1981). * Hoad, K., Robinson, S. & Davies, R. Automated selection of the number of replications for a discrete-event simulation. _JORS_ 61, 1632–1644.
https://doi.org/10.1057/jors.2009.121 (2010). Article Google Scholar Download references ACKNOWLEDGEMENTS This research was supported by TROPHIc Project (Grant I+D+i PID2019-105101GB-I00
funded by MCIN/AEI/) (A. Mateos, J. Rodríguez, E. Hoelzchen, and J.O. Berndt). C. Hertler was supported by the “The Role of Culture in Early Expansions of Humans” (ROCEEH Project). AUTHOR
INFORMATION AUTHORS AND AFFILIATIONS * National Research Center On Human Evolution (CENIEH), Paseo Sierra de Atapuerca 3, 09002, Burgos, Spain Jesús Rodríguez & Ana Mateos * Chair for
Business Informatics 1, Trier University, Behringstraße 21, 54296, Trier, Germany Ericson Hölzchen & Ingo J. Timm * German Research Center for Artificial Intelligence (DFKI). Smart Data
and Knowledge Services - Cognitive Social Simulation, Trier University, Behringstraße 21, 54296, Trier, Germany Ericson Hölzchen, Jan Ole Berndt & Ingo J. Timm * Facultad de Ciencias.
Edificio de Biología, Universidad Autónoma de Madrid. C/ Darwin, 2. Campus de Cantoblanco, 28049, Madrid, Spain Ana Isabel Caso-Alonso * The Role of Culture in Early Expansion of Humans
(ROCEEH), Senckenberg Research Institute, Senckenberganlage 25, 60325, Frankfurt Am Main, Germany Christine Hertler * The Role of Culture in Early Expansion of Humans (ROCEEH), Heidelberg
Academy of Sciences, Karlstraße 4, 69117, Heidelberg, Germany Christine Hertler Authors * Jesús Rodríguez View author publications You can also search for this author inPubMed Google Scholar
* Ericson Hölzchen View author publications You can also search for this author inPubMed Google Scholar * Ana Isabel Caso-Alonso View author publications You can also search for this author
inPubMed Google Scholar * Jan Ole Berndt View author publications You can also search for this author inPubMed Google Scholar * Christine Hertler View author publications You can also
search for this author inPubMed Google Scholar * Ingo J. Timm View author publications You can also search for this author inPubMed Google Scholar * Ana Mateos View author publications You
can also search for this author inPubMed Google Scholar CONTRIBUTIONS A.M. and J.R. designed the research. E.H., I.J.T., J.O.B., C.H., A.M., and J.R. established the methodology. E.H. and
J.R. wrote the code of SCAVCOMP-ABM. J.R., A.M., E.H., and A.I.C. validated the model. J.R., A.M., and A.I.C. run the experiments and analysed the output. The original draft was written by
J.R. and A.M, and all authors discussed the paper, review and edited the manuscript. CORRESPONDING AUTHOR Correspondence to Ana Mateos. ETHICS DECLARATIONS COMPETING INTERESTS The authors
declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional
affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION 1. SUPPLEMENTARY INFORMATION 2. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons
Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original
author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the
article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your
intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence,
visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Rodríguez, J., Hölzchen, E., Caso-Alonso, A.I. _et al._ Computer simulation
of scavenging by hominins and giant hyenas in the late Early Pleistocene. _Sci Rep_ 13, 14283 (2023). https://doi.org/10.1038/s41598-023-39776-1 Download citation * Received: 08 May 2023 *
Accepted: 31 July 2023 * Published: 28 September 2023 * DOI: https://doi.org/10.1038/s41598-023-39776-1 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative