Play all audios:
ABSTRACT To analyze the relationship between the composition of urinary stones and various influencing factors in the Enshi region. We used FT-IR to examine the composition of 1092 stone
samples. Combined with the relevant clinical materials, the data were analyzed using both one-dimensional statistical methods and multivariate statistical methods. The study included 1092
stone samples, classified as follows: 457 (41.8%) with a single component, 453 (41.5%) with two components, 149 (13.6%) with three components, and 33 (3.0%) with four components. Stones were
categorized into five types: Calcium Oxalate (CaOx) (76.4%), carbapatite (CaP) (9.3%), Struvite (ST) (8.3%), Uric Acid (UA) (4.9%), and Others (1.0%). Age, gender, urinary tract infection
(UTI), family history of urinary stones (FH), hyperuricemia (HUA) and stone location were significantly associated with stone type. Logistic regression revealed that females and UTI were
relative risk factors for predicting CaP and ST, while FH and HUA were relative risk factors for predicting UA. Our study indicates that the overall composition of urinary tract stones in
the Enshi region is consistent with that of the entire China. Additionally, the predisposing factors for stone formation vary in terms of gender, age, FH, UTI, hyperuricemia HUA, and stone
location. SIMILAR CONTENT BEING VIEWED BY OTHERS URINARY STONE COMPOSITION ANALYSIS AND CLINICAL CHARACTERIZATION OF 1520 PATIENTS IN CENTRAL CHINA Article Open access 19 March 2021 ANALYSIS
OF COMPONENTS AND RELATED RISK FACTORS OF URINARY STONES: A RETROSPECTIVE STUDY OF 1055 PATIENTS IN SOUTHERN CHINA Article Open access 16 November 2024 URINARY STONE ANALYSIS AND CLINICAL
CHARACTERISTICS OF 496 PATIENTS IN TAIWAN Article Open access 19 June 2024 INTRODUCTION In the past few decades, urolithiasis has become an increasingly common disease, with a rising global
incidence, prevalence, and recurrence rates1. The causes of urolithiasis are complex and closely related to various factors, such as race, gender, age, genetics, environment, and diet2.
Despite the common features of urolithiasis worldwide, unique variants exist in certain countries and regions. For instance, the incidence of urologic stones globally ranges from 1 to 5%3,
while in the southern region of China, it is notably higher at 5–10%, with recurrence rates of 50% within five years and 75% within twenty years4. In 2019, the Urolithiasis Group of the
Chinese Urological Association (CUA) conducted a prospective nationwide multicenter survey, investigating the impact of gender, age, body mass index (BMI), stone location, and geographical
region on the diversity of urinary stone composition5. In Enshi Prefecture, Southwest China, urinary stone disease poses a significant health concern. Due to its status as a
minority-inhabited area, the region's unique geographical environment, lifestyle habits, and climatic conditions may lead to distinct characteristics in the development of urinary
stones. Accurate analysis of urinary stone composition is crucial for identifying stone etiology, prevention, and treatment6. However, there is currently no research conducted to determine
the specific features of urinary stone composition in this region. Fourier transform infrared spectroscopy (FT-IR) has emerged as a sensitive, reliable, and accurate method for detecting
urinary stones, and it has become the standard diagnosis process7,8. FT-IR utilizes distinctive infrared absorption patterns exhibited by different chemical compounds to precisely identify
and characterize stone components7,9. Its high sensitivity allows for the detection of trace elements, and its rapid analysis capabilities require minimal sample preparation10. By comparing
the spectra of unknown stones to reference spectra, FT-IR enables accurate determination of stone composition and type11. The application of FT-IR in urinary stone analysis has significantly
advanced our understanding of stone formation, composition, and recurrence patterns. To better comprehend the composition of urinary tract stones and their association with various
influencing factors in the Enshi region, we conducted a retrospective analysis of 1092 stone specimens using FT-IR. These stone specimens were collected from the Department of Urology at the
Central Hospital of Enshi Tujia and Miao Autonomous Prefecture from January 2019 to August 2022. In this study, we explore whether differences in the stone composition may be explained by
differences in gender, age, diabetes mellitus (DM), hypertension (HTN), urinary tract infections (UTI), family history of urinary stones (FH), hyperuricemia (HUA), and anatomical locations
using univariate statistical analysis (The abbreviation table at the end of the document presents common abbreviations and their full forms). Additionally, we established an unordered
multi-categorical logistic statistical model to discern the relative risk factors associated with less common stone types. Ultimately, this study seeks to offer more scientifically accurate
urinary stone prevention strategies tailored to the specific population in the Enshi region. PATIENTS AND METHODS STONE COMPOSITION ANALYSES AND CLASSIFICATION The study received approval
from the Ethics Committee of the Central Hospital of Enshi Tujia and Miao Autonomous Prefecture (NO. 2022-013-01). Between January 2019 and August 2022, a total of 1092 urinary stone samples
and associated clinical information were included in the study. The patients were long-term residents (≥ 5 years) in the Enshi area, experiencing urinary stones symptoms for the first time
and undergoing surgical treatment. Stone fragments were collected intraoperatively during percutaneous nephrolithotomy (PCNL), ureteroscopic lithotripsy, and cystolithotripsy. Clinical and
demographic data, including age, gender, stone anatomical localization, geographical region, and underlying disease, were obtained. For stone sample analysis, FT-IR (Dingshun, SUN-3G, Jinan,
China) was employed, following standard operating procedures established by manufacturers and hospitals. Stone samples were split, washed, and dried in a 100 °C oven for 2–3 min. The stone
powder (1.5 mg) was then mixed with potassium bromide (200 mg) and ground to the micrometer level using an agate mortar and pestle. The mixed powder was pressed into a uniform and
translucent sheet using a tablet press mold and tablet press (16 Mpa, stop 5 s) as per the manufacturer's instructions. In addition, a potassium bromide tablet (without stones) was
prepared as a control. The assay parameters were set at a sampling resolution of 2 cm−1, a spectral range of 4000–400 cm−1, and a total of 32 scans. Before sample analysis, the instrument
background was measured using potassium bromide tablets (without stones). Subsequently, the stone samples were inserted into the sample compartment, and the FT-IR instrument recorded the
transmittance spectrum, generated the infrared spectrum, automatically analyzed the composition of the stones, and generated a corresponding test report. The stone composition was classified
following to the European Association of Urology stone classification practices6. The most common form of stone is calcium oxalate (CaOx), with two types: monohydrate (COM) and dihydrate
(COD)12. Stones containing over 50% calcium oxalate were classified as CaOx stones. Uric acid stones (UA) included stones with more than 50% uric acid and uric acid dihydrate. Stones with
over 50% carbapatite were classified as carbapatite (CaP) stones, while those with more than 50% struvite were labeled as struvite uroliths (ST). Cystine stones encompassed stones containing
cystine. For statistical analysis, rare stones such as cystine stones and drug-related stones were grouped into the "Other" category. ETHICS STATEMENT The experiments and
procedures were conducted following the relevant guidelines and regulations. Ethical approval was obtained from The Central Hospital of Enshi Tujia and Miao Autonomous Prefecture (Approval
No. 2022-013-01). All patients provided informed consent to participate in the study. CONSENT FOR PUBLICATION Informed consent was obtained from all participants involved in the study. For
participants under the age of 16 years, informed consent was obtained from their parents and/or legal guardians. STATISTICAL ANALYSES Quantitative data were presented as mean ± standard
deviation. Qualitative data were expressed as n (%). The chi-squared test was employed to evaluate the impact of gender, DM, HTN, and UTI on different types of urinary stones. In cases where
the chi-squared test was not applicable, Fisher's exact test was utilized for variables FH and HUA. Cramer's V coefficient was employed to assess the strength of correlation
between categorical variables, with values between 0.1 and 0.3 indicating weak correlation, between 0.3 and 0.5 indicating moderate correlation, and greater than 0.5 indicating strong
correlation. Adjusted standardized residuals (Adj.R) were used to assess the association between two categorical variables, with values greater than 3 indicating significant deviations
between observed and expected values, signifying a notable correlation between the two variables. The Kruskal–Wallis’s test was conducted for unidirectional ordered data (age groups),
followed by Z-tests for multiple comparisons, with p-values adjusted using Bonferroni correction. Unordered multi-categorical logistic regression was performed to evaluate the influence of
gender, age, DM, HTN, UTI, FH, HUA, and stone anatomical location on stone composition. Statistical analyses were conducted using SPSS software (version 23, IBM Corp., Armonk, NY, USA), and
a p-value (two-tailed) < 0.05 was considered statistically significant. Data visualization and graph plotting were performed using ORIGINE software (version 2022 Pro, OriginLab
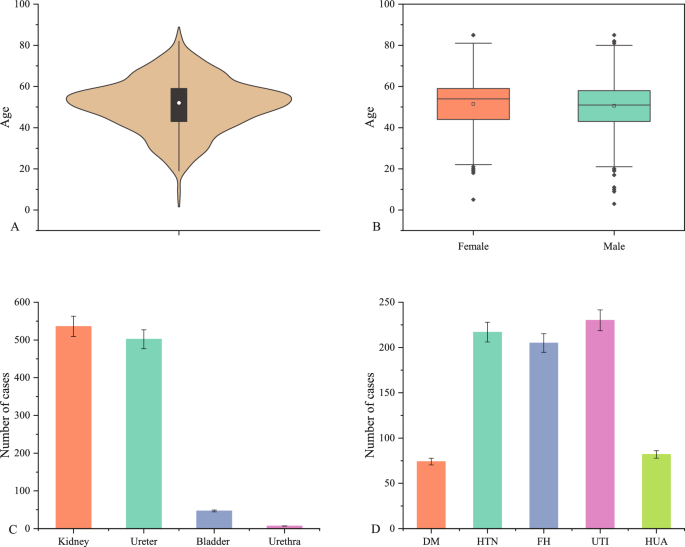
Corporation, Northampton, MA, USA). RESULTS INDIVIDUAL AND CLINICAL CHARACTERISTICS OF THE PATIENTS A total of 1092 patients were included in the study, with 745 (68.2%) men and 347 (31.8%)
women, resulting in a male-to-female ratio of 2.1:1. The age of the patients ranged from 3 to 85 years, with a mean age of 50.9 ± 12.8 years. There were no significant differences in age
between males (50.6 ± 12.9 years) and females (51.4 ± 12.8 years) (t = − 1.027, v = 1090, p > 0.05). The distribution of urinary stone locations among the patients was as follows: 536
(49.1%) cases of renal calculi, 502 (46.0%) cases of ureteral calculi, 47 (4.3%) cases of bladder calculi, and 7 (0.6%) cases of urethral calculi. Moreover, 74 (6.8%) patients had diabetes
mellitus (DM), 217 (19.9%) patients had hypertension (HTN), 205 (19.8%) patients had a family history (FH) of urinary stones, 230 (21.0%) patients had urinary infections (UTI), and 82 (7.5%)
patients had hyperuricemia (HUA) (Fig. 1). GENERAL INFORMATION ABOUT THE STONE COMPOSITION Infrared spectra of substances are unique, akin to human fingerprints13,14,15. Consequently,
distinct components of urinary tract stones exhibit characteristic infrared spectra, indicating their specific compositions. By utilizing these characteristic spectra (Fig. 2), we conducted
qualitative and quantitative analyses of different stone compositions. A total of 1092 stone samples underwent FT-IR analysis for stone composition. Quantitative analysis revealed that
single-component samples constituted 457 (41.8%) cases, including 367 (33.6%) cases of COM, 66 (6.0%) cases of COD, 13(1.2%) cases of UA, and 11(1.0%) cases of Others (rare components:
L-cystine, drug-induced stones). Stones composed of two components accounted for 453 (41.5%) cases, comprising 278 (25.4%) cases of COM + CaP, 47 (4.3%) cases of COM + COD, 54 (4.9%) cases
of Struvite + CaP, 41(3.8%) cases of UA + COM, and 33(3.0%) cases of COD + CaP. Stones with three components were observed in 149 (13.6%) cases, including 92 (8.4%) cases of COM + COD + CaP,
46 (4.2%) cases of CaP + COM + COD, 7(0.6%) cases of CaP + COM + COD, and 4 (0.4%) cases of Struvite + CaP + COM. Additionally, stones with four components were found in 33(3.0%) cases,
comprising 24 (2.2%) cases of Struvite + CaP + COM + COD and 9 (0.8%) cases of Struvite + COM + COD + CaP (Fig. 3). Based on the stone classification principle described above, we
categorized all stones into five types: CaOx, CaP, ST, UA, and Others (Fig. 4A). CaOx was the most prevalent stone type, with a total of 834(76.4%) cases, including 623(57.1%) in males and
211 (19.3%) in females. It was followed by 102 (9.3%) cases of CaP, including 61(5.6%) cases in males and 41(3.7%) cases in females. A total of 91(8.3%) cases of ST were detected, comprising
27 (5.8%) cases in males and 64(2.5%) cases in females. Among the 54 (4.9%) cases of UA, 46 (4.2%) were male and 8 (0.7%) were female. Additionally, there were 11(1.0%) other rare stone
types, including 8(0.07%) in males and 3(0.03%) in females, notably 3 of which were drug-induced stones in children. THE RELATIONSHIP BETWEEN GENDER AND FOUR TYPES OF URINARY STONES Because
the “Other” stone types are rare and inclusion in statistical models can produce extreme values that affect the accuracy of statistical results, we do not include this type in some
statistical models for analysis. A total of 1081 cases were included in the statistical model, which analyzed the relationship between sex and four main types of stones (CaOx, CaP, ST, UA).
The results show that any of the expected frequencies are greater than 5, and the chi-square test can be used, χ2 = 122.5, _p_ < 0.001, suggesting that the four main types of stones
differ significantly from gender type. There is a moderately strong correlation between four different stone types and gender (Cramer's V = 0.337, _p_ < 0.05) (Fig. 4B). We conducted
a more in-depth analysis of the above results using post hoc testing and assessed the relationship between the two categorical variables based on the adjusted standardized residuals
(Adj.R). In the CaOx group, 623 (57.6%) were males and the Adj. R value of 8.5 indicated a significant association between CaOx stones and male gender, suggesting a higher likelihood of CaOx
stones in male patients compared to females. Similarly, in the CaP group, there were 61 (5.6%) females, and the Adj.R value of 6.4 revealed a significant association between CaP stones and
the female gender. Likewise, in the ST group, there were 64 (5.9%) females, and the Adj.R value of 8.2 also indicated a significant association between ST stones and female gender. These
results consistently suggest a higher likelihood of CaP and ST stones in female patients compared to males (Fig. 5A). THE RELATIONSHIP BETWEEN DM, HTN, FH, UTI, HUA AND URINARY STONE TYPES A
total of 1081 cases were included in the statistical model, which examined the difference between DM, HTN, FH, UTI, HUA, and four main types of stones (CaOx, CaP, ST, UA). The results
showed that for DM, HTN, and UTI, all expected frequencies were greater than 5, allowing the use of the chi-square test. From the perspective of DM (χ2 = 2.8, _p_ = 0.418; Cramer's V =
0.051, _p_ > 0.05) and HTN (χ2 = 4.6, _p_ = 0.202; Cramer's V = 0.065,_ p_ > 0.05), there was no statistically significant difference, indicating that the presence or absence of
DM/HTN did not significantly affect the type of stone (Fig. 5B,C). However, among the 1081 study participants, 230 had UTI, and the data (χ2 = 161.7, _p_ < 0.001) indicated a significant
difference in the distribution of the four different stone types based on the presence or absence of UTI. There was a moderately strong correlation between four different stone types and
UTI, with Cramer's V = 0.387, _P_ < 0.001 (Fig. 5D). Further post hoc testing revealed that patients with UTI were more likely to develop CaP (Adj.R = 9.0) and ST (Adj.R = 8.0)
stones (Fig. 5A). Among the 1081 study participants, 205 had FH, and 82 had HUA. Due to expected frequencies of less than 5, Fisher's exact test was chosen. The results indicated a
significant difference between FH and the four different stone types (_p_ < 0.05), but there was no strong correlation between them (Cramer's V = 0.092, _p_ > 0.05) (Fig. 5E).
Furthermore, the test results showed a clear difference between HUA and different types of stones (_p_ < 0.001), and there was a weak correlation between four different stone types and
HUA, with Cramer's V = 0.26, _p_ < 0.001 (Fig. 5F). Post hoc testing revealed that patients with Hyperuricemia were more likely to develop UA stones (Adj.R = 8.4) (Fig. 5A). THE
RELATIONSHIP BETWEEN AGE AND URINARY STONE TYPES The relationship between age and urinary stone types was examined by dividing all study participants into 9 age strata, each representing a
10-year age group. This division allowed for a comprehensive analysis of the association between age and different stone compositions. Using Kruskal–Wallis tests, we found a statistically
significant difference between the various age groups and stone types (H = 78.388, p < 0.001) (Fig. 6A). Further multiple comparisons were performed using the Z test with
Bonferroni-adjusted p-values, revealing specific patterns. The proportion of CaOx cases was higher in the age groups of 21–30 years (compared to age groups 1–10, 51–60, 61–70, 71–80, 81–90
years, p < 0.05), 31–40 years (compared to age groups 1–10, 51–60, 61–70 years, p < 0.05), and 41–50 years (compared to age groups 1–10, 51–60, 61–70 years, p < 0.05) compared to
other types of stones. This suggests that individuals in these age groups are more likely to develop CaOx stones. In the 51–60 and 61–70 age groups, the proportion of ST cases (compared to
age group 41–50 years, p < 0.05; 61–70 years compared to age group 41–50 years, p < 0.05) and UA cases (compared to age groups 31–40, 41–50 years, p < 0.05; 61–70 years compared to
age group 41–50 years) was higher than other stone types. This indicates a higher likelihood of developing ST and UA stones in these age groups. Furthermore, we observed that rare stones
were more likely to affect children aged 1–10 (compared to age groups 31–40, 41–50, 51–60, p < 0.05). Additionally, individuals aged 81–90 (compared to age group 31–40, p < 0.05) were
most likely to develop CaP stones (Table 1).In summary, age appears to play a significant role in the distribution of different stone types, with distinct patterns observed in various age
groups. THE RELATIONSHIP BETWEEN STONE LOCATION AND URINARY STONE TYPES In the analysis of 1092 stone samples, due to the expected frequencies being less than 5, we opted for Fisher's
exact test. The results revealed a significant difference (_p_ < 0.001) in the distribution of stone types based on their locations. Furthermore, there was a weak correlation
(Cramer's V = 0.108, _p_ < 0.001) between different stone types and their locations (Fig. 6B). Subsequent post hoc testing indicated that ST tended to occur more frequently in the
kidneys (Adj.R = 3.1), UA tended to occur more in the bladder (Adj.R = 3.2), and rare stones tended to occur in the urethra (Adj.R = 3.5) (Fig. 6C). RELATIVE RISK ANALYSIS FOR NON-CAOX STONE
TYPES USING UNORDERED MULTI-CLASSIFICATION LOGISTIC REGRESSION In this study, we employed an unordered multi-classification Logistic regression model with four different stone types (CaOx,
CaP, ST, UA) as the unordered multi-class response variables. Simultaneously, we considered six statistically significant factors (gender, age, family history, urinary tract infection,
hyperuricemia, and stone location) from the previous research as independent variables. As CaOx had the highest number of cases, it served as the reference category. The Logistic regression
model was utilized to predict and assess the relative risk factors for stone types other than CaOx in specific populations. Compared to CaOx, we observed that female gender (RR= 3.087, 95%
CI 1.944–4.903) and urinary tract infection (RR= 5.272, 95% CI 3.219–8.635) were relative risk factors for predicting CaP. Similarly, female gender (RR= 4.871, 95% CI 2.944–8.060) and
urinary tract infection (RR= 4.921, 95% CI 2.910–8.323) were relative risk factors for predicting ST. Additionally, family history (RR= 2.539, 95% CI 1.286–5.012) and hyperuricemia (RR=
7.729, 95% CI 4.010–14.898) were relative risk factors for predicting UA (Tables 2, 3).This model enables us to understand the relative risk factors associated with specific factors and
different stone types, thus providing better support for predicting and implementing personalized treatment approaches for individuals with specific urinary stone types. DISCUSSION In this
study, we aimed to investigate the relationship between disease-related factors and urinary stone composition or types in the Enshi region. Urinary stones' composition and types vary in
different countries and regions due to multiple etiological factors16,17. Currently, FT-IR is the most widely used method for detecting urinary stone components18,19. Therefore, FT-IR was
employed to analyze 1092 urinary stone samples, revealing 457 single-component stones and the remainder being mixed stones (Fig. 3). The most frequent component was CaOx, accounting for
76.4% of cases, followed by CaP (9.3%), ST (8.3%), UA (4.9%), and Others (1.0%) (Fig. 4A). The study included 745 males and 347 females, with an approximate male to female ratio of 2.1:1,
which is similar to previous studies in other regions of Korea20, India21, the United Kingdom16, and Brazil17. Our results showed statistically significant differences (_p_ < 0.001) and
moderate correlations (Cramer's V = 0.337, _p_ < 0.05) between gender and the four main stone types. CaOx stones are more prevalent in men, whereas ST and CaP are more common in
women (Figs. 4B and 5A). Similar gender differences in stone types were observed in a multicenter study across China5. The male predisposition to CaOx may be attributed to the following
factors: the influence of testosterone and dihydrotestosterone on CaOx crystal formation in rat models22, the promotion of CaOx crystal formation by enhanced androgen receptor (AR) signaling
in hepatocytes23,24, as well as factors such as heavy physical work, excessive sweating, inadequate fluid intake, and concentrated urine volume25. Furthermore, the incidence of UA was
higher in men compared to women, with a male-to-female ratio of 5.75:1, which is consistent with previous studies26,27. Additionally, our study aligns with previous research, indicating a
higher incidence of ST and CaP in women17. The highest prevalence of urinary stones was observed in the age group of 13–60 years, with a distinct peak distribution (Fig. 6A). Our analysis
revealed significant differences in the distribution of five stone types across nine age groups (p < 0.001). Individuals aged 31–60, who constitute the main productive force in society,
showed higher susceptibility to CaOx stones, likely due to factors such as heavy physical labor leading to increased sweating and inadequate water intake, particularly in economically
disadvantaged rural areas5,28. Additionally, the risk of UTI tends to increase in women during menopause after the age of 50, when estrogen levels significantly decline, which may contribute
to the development of infectious stones in older women29. Our study observed similar trends to previous research conducted in China5,18 and other countries26,30, we further employed the
Kruskal–Wallis test and multiple comparison tests. This approach allowed us to offer a more detailed and comprehensive statistical analysis of the variations in stone occurrence among
different age groups (Fig. 6A and Table 1). Indeed, urolithiasis has been associated with metabolic syndrome, suggesting it may result from interactions between various metabolic risk
factors31. We observed that patients with DM and HTN did not exhibit a specific predisposition to any particular type of stone (_p_ > 0.05), while HUA was significantly associated with an
increased risk of UA formation (_p_ < 0.05) (Fig. 5). UA stones are strongly associated with causative factors such as dietary habits (red meat, seafood, beer), hyperuricemia, urine pH,
hypercholesterolemia, obesity and insulin resistance34,35. Studies in China have demonstrated that patients with metabolic syndrome have a significantly increased risk of developing
urolithiasis32,33. The complex interplay between metabolic factors and stone formation requires further exploration to better understand the underlying mechanisms. Furthermore, our study
showed significant variability (p < 0.001) and correlation (Cramer's V = 0.108) between stone locations and types. ST stones were more likely to occur in the kidney (Adj. R = 3.1),
UA stones in the bladder (Adj. R = 3.2), and rare stones in the urethra (Adj. R = 3.5) compared to the other anatomical locations (Fig. 6B,C). Consistent with previous studies in
China5,18,34, the most common stones in the kidney (36.0%) and ureter (36.9%) from a composition ratio standpoint were CaOx stones. The relationship between stone location and stone
composition can reflect part of the causes of stone formation: bladder stones are significantly associated with urinary tract obstruction, nutritional deficiencies, and uric acid excretion,
and therefore uric acid stones tend to form there35. It is widely known that CaOx stones constitute the largest proportion of patients and that patients have the highest probability of
getting CaOx stones30,36. However, predicting the type of stone with a lower composition ratio or probability of occurrence in patients with urinary stones has greater challenges and
implications. We performed a multifactorial logistic regression analysis with CaOx as the reference group, identifying female gender and urinary tract infection as relative risk factors for
CaP and ST, and FH and HUA as relative risk factors for UA stones (Tables 2, 3). Our study has some limitations. First, we only focused on the disease characteristics in the local area of
Enshi, which is not widely representative. Second, our data did not study the urine biochemical characteristics and some serum characteristics of patients. Third, information bias inevitably
causes some errors, such as possible recall of information provided by patients when collecting relevant disease information. We provide valuable insights into the characteristics of
urinary stones in the Enshi region. The consistency of our findings with studies from other regions in China highlights the universality of certain patterns of urinary stone prevalence
nationwide. However, it is important to recognize that regional differences and local risk factors may still influence the patterns of stone formation. Regional variations and local risk
factors require longer-term and larger cohort observations for further investigation. In summary, our study comprehensively investigated the relationships between individual characteristics,
disease history, stone location, and stone composition. Based on our findings, targeted management and prevention strategies can be developed for specific populations in the region. In
future research, exploring stone composition differences among different regions and populations to understand regional and individual influences on stone formation is crucial. Additionally,
delving into the association between metabolic factors and stone formation, considering lifestyle, dietary habits, and genetic factors, is important. Improved study designs and methods,
such as larger cohort observations, multicenter studies, and molecular biology techniques, will further enhance stone research and provide more effective prevention and treatment strategies
for patients. DATA AVAILABILITY On reasonable request, the corresponding author will provide the datasets used and analyzed in this study. ABBREVIATIONS * FT-IR: Fourier transform infrared
spectroscopy * CaOx: Calcium oxalate stone * COM: Calcium oxalate monohydrate * COD: Calcium oxalate dihydrate * CaP: Carbapatite * ST: Struvite * UA: Uric acid stones * Other: Rare stones
such as cystine * DM: Diabetes mellitus * HTN: Hypertension * UTI: Urinary tract infection * HUA: Hyperuricemia * FH: Family history of urinary stones * Adj.R: Adjusted standardized
residuals REFERENCES * Ordonez, M., Borofsky, M., Bakker, C. J. & Dahm, P. Ureteral stent versus no ureteral stent for ureteroscopy in the management of renal and ureteral calculi.
_Cochrane Database Syst. Rev._ https://doi.org/10.1002/14651858.Cd012703 (2017). Article PubMed Central Google Scholar * Jing, Z. _et al._ Analysis of urinary calculi composition by
infrared spectroscopy: A prospective study of 625 patients in eastern China. _Urol. Res._ 38, 111–115. https://doi.org/10.1007/s00240-010-0253-x (2010). Article CAS ADS PubMed Google
Scholar * Chen, G. _et al._ Relationship between the ApaI (rs7975232), BsmI (rs1544410), FokI (rs2228570), and TaqI (rs731236) variants in the vitamin D receptor gene and urolithiasis
susceptibility: An updated meta-analysis and trial sequential analysis. _Front. Genet._ 11, 234. https://doi.org/10.3389/fgene.2020.00234 (2020). Article CAS PubMed PubMed Central Google
Scholar * Zeng, G. _et al._ Prevalence of kidney stones in China: an ultrasonography based cross-sectional study. _BJU Int._ 120, 109–116. https://doi.org/10.1111/bju.13828 (2017). Article
PubMed Google Scholar * Ye, Z. _et al._ The status and characteristics of urinary stone composition in China. _BJU Int._ 125, 801–809. https://doi.org/10.1111/bju.14765 (2020). Article
CAS PubMed Google Scholar * Skolarikos, A. _et al._ Metabolic evaluation and recurrence prevention for urinary stone patients: EAU guidelines. _Eur. Urol._ 67, 750–763.
https://doi.org/10.1016/j.eururo.2014.10.029 (2015). Article PubMed Google Scholar * He, Z., Jing, Z., Jing-Cun, Z., Chuan-Yi, H. & Fei, G. Compositional analysis of various layers of
upper urinary tract stones by infrared spectroscopy. _Exp. Ther. Med._ 14, 3165–3169. https://doi.org/10.3892/etm.2017.4864 (2017). Article CAS PubMed PubMed Central Google Scholar *
Primiano, A. _et al._ FT-IR analysis of urinary stones: A helpful tool for clinician comparison with the chemical spot test. _Dis. Mark._ 2014, 176165. https://doi.org/10.1155/2014/176165
(2014). Article CAS Google Scholar * Zhao, Z. _et al._ Formation of curcumin nanoparticles via solution-enhanced dispersion by supercritical CO2. _Int. J. Nanomed._ 10, 3171–3181.
https://doi.org/10.2147/IJN.S80434 (2015). Article CAS Google Scholar * Sundaram, J. _et al._ Classification and structural analysis of live and dead Salmonella cells using Fourier
transform infrared spectroscopy and principal component analysis. _J. Agric. Food Chem._ 60, 991–1004. https://doi.org/10.1021/jf204081g (2012). Article CAS PubMed Google Scholar *
Manissorn, J., Fong-Ngern, K., Peerapen, P. & Thongboonkerd, V. Systematic evaluation for effects of urine pH on calcium oxalate crystallization, crystal-cell adhesion and
internalization into renal tubular cells. _Sci. Rep._ 7, 1798. https://doi.org/10.1038/s41598-017-01953-4 (2017). Article CAS ADS PubMed PubMed Central Google Scholar * Türk, C. _et
al._ EAU guidelines on diagnosis and conservative management of urolithiasis. _Eur. Urol._ 69, 468–474. https://doi.org/10.1016/j.eururo.2015.07.040 (2016). Article PubMed Google Scholar
* Kitazaki, T. _et al._ Parametric standing wave generation of a shallow reflection plane in a nonrigid sample for use in a noninvasive blood glucose monitor. _J. Biomed. Opt._ 24, 1–7.
https://doi.org/10.1117/1.JBO.24.3.036003 (2019). Article PubMed Google Scholar * Balan, V. _et al._ Vibrational spectroscopy fingerprinting in medicine: From molecular to clinical
practice. _Materials_ 12, 2884. https://doi.org/10.3390/ma12182884 (2019). Article CAS ADS PubMed PubMed Central Google Scholar * Sinanoglou, V. J. _et al._ On the characterization and
correlation of compositional, antioxidant and colour profile of common and balsamic vinegars. _Antioxidants_ 7, 139. https://doi.org/10.3390/antiox7100139 (2018). Article PubMed PubMed
Central Google Scholar * Kum, F. _et al._ Do stones still kill? An analysis of death from stone disease 1999–2013 in England and Wales. _BJU Int._ 118, 140–144.
https://doi.org/10.1111/bju.13409 (2016). Article PubMed Google Scholar * da Silva, S. F. R. _et al._ Determination of urinary stone composition based on stone morphology: A prospective
study of 325 consecutive patients in an emerging country. _Clin. Chem. Lab. Med._ 47, 124. https://doi.org/10.1515/cclm.2009.124 (2009). Article Google Scholar * Zhang, D. _et al._ Urinary
stone composition analysis and clinical characterization of 1520 patients in central China. _Sci. Rep._ 11, 6467. https://doi.org/10.1038/s41598-021-85723-3 (2021). Article CAS ADS
PubMed PubMed Central Google Scholar * Volmer, M. et al. Partial least-squares regression for routine analysis of urinary calculus composition with Fourier transform infrared analysis.
_Clin. Chem._ 39, 948–954. https://doi.org/10.1093/clinchem/39.6.948 (1993). * Cho, S. T., Jung, S. I., Myung, S. C. & Kim, T. H. Correlation of metabolic syndrome with urinary stone
composition. _Int. J. Urol._ 20, 208–213. https://doi.org/10.1111/j.1442-2042.2012.03131.x (2013). Article CAS PubMed Google Scholar * Bhat, A., Singh, V., Bhat, M., Kumar, V. &
Bhat, A. Spectrum of urinary stone composition in Northwestern Rajasthan using Fourier transform infrared spectroscopy. _Indian J. Urol._ 34, 144. https://doi.org/10.4103/iju.IJU_363_16
(2018). Article PubMed PubMed Central Google Scholar * Yoshioka, I. _et al._ Effect of sex hormones on crystal formation in a stone-forming rat model. _Urology_ 75, 907–913.
https://doi.org/10.1016/j.urology.2009.09.094 (2010). Article PubMed Google Scholar * Ou, S. M., Chen, Y. T., Shih, C. J. & Tarng, D. C. Increased risk of bone fracture among patients
with urinary calculi: A nationwide longitudinal population-based study. _Osteoporos. Int._ 26, 1261–1269. https://doi.org/10.1007/s00198-014-2998-5 (2015). Article PubMed Google Scholar
* Peng, Y. _et al._ Testosterone induces renal tubular epithelial cell death through the HIF-1alpha/BNIP3 pathway. _J. Transl. Med._ 17, 62. https://doi.org/10.1186/s12967-019-1821-7 (2019).
Article CAS PubMed PubMed Central Google Scholar * Jalal, S. M., Alsultan, A. A., Alotaibi, H. H., Mary, E. & Alabdullatif, A. A. I. Effect of phaseolus vulgaris on urinary
biochemical parameters among patients with kidney stones in Saudi Arabia. _Nutrients_ 12, 3346. https://doi.org/10.3390/nu12113346 (2020). Article CAS PubMed PubMed Central Google
Scholar * Deffert, C., StoermannChopard, C. & Lambeng, N. 10,000 urinary stones for 10 years of analysis: A retrospective study in western Switzerland. _Comptes Rendus Chimie_ 25,
235–245. https://doi.org/10.5802/crchim.140 (2022). Article CAS Google Scholar * Knoll, T. _et al._ Urolithiasis through the ages: data on more than 200,000 urinary stone analyses. _J.
Urol._ 185, 1304–1311. https://doi.org/10.1016/j.juro.2010.11.073 (2011). Article PubMed Google Scholar * Abeywickarama, B., Ralapanawa, U. & Chandrajith, R. Geoenvironmental factors
related to high incidence of human urinary calculi (kidney stones) in Central Highlands of Sri Lanka. _Environ. Geochem. Health_ 38, 1203–1214. https://doi.org/10.1007/s10653-015-9785-x
(2016). Article CAS PubMed Google Scholar * Maalouf, Naim M et al. Postmenopausal hormone use and the risk of nephrolithiasis: Results from the Women's Health Initiative hormone
therapy trials. _Archives of internal medicine _ 170(18), 1678–85. https://doi.org/10.1001/archinternmed.2010.342 (2010). Article CAS PubMed PubMed Central Google Scholar * Siener, R.
_et al._ Urinary stone composition in Germany: Results from 45,783 stone analyses. _World J. Urol._ 40, 1813–1820. https://doi.org/10.1007/s00345-022-04060-w (2022). Article CAS PubMed
PubMed Central Google Scholar * Frochot, V. & Daudon, M. Clinical value of crystalluria and quantitative morphoconstitutional analysis of urinary calculi. _Int. J. Surg._ 36, 624–632.
https://doi.org/10.1016/j.ijsu.2016.11.023 (2016). Article PubMed Google Scholar * Chen, W. _et al._ Association between metabolically healthy obesity and kidney stones: Results from the
2011–2018 National Health and Nutrition Examination Survey. _Front. Public Health_ 11, 1103393. https://doi.org/10.3389/fpubh.2023.1103393 (2023). Article PubMed PubMed Central Google
Scholar * Ye, Z. X. _et al._ Establishment and primary clinical application of metabolic evaluation database of urolithiasis. _Zhonghua Yi Xue Za Zhi_ 100, 2036–2039.
https://doi.org/10.3760/cma.j.cn112137-20191026-02321 (2020). Article CAS PubMed Google Scholar * Wang, P. _et al._ Study of risk factor of urinary calculi according to the association
between stone composition with urine component. _Sci. Rep._ 11, 8723. https://doi.org/10.1038/s41598-021-87733-7 (2021). Article CAS ADS PubMed PubMed Central Google Scholar *
Philippou, P., Moraitis, K., Masood, J., Junaid, I. & Buchholz, N. The management of bladder lithiasis in the modern era of endourology. _Urology_ 79, 980–986.
https://doi.org/10.1016/j.urology.2011.09.014 (2012). Article PubMed Google Scholar * Gavin, C. T. _et al._ Novel methods of determining urinary calculi composition: Petrographic thin
sectioning of calculi and nanoscale flow cytometry urinalysis. _Sci. Rep._ 6, 19328. https://doi.org/10.1038/srep19328 (2016). Article CAS ADS PubMed PubMed Central Google Scholar
Download references FUNDING The National Natural Science Foundation of China funded this study (No. 82260791). AUTHOR INFORMATION Author notes * These authors contributed equally: Junfeng
Zhang and Kailing Li. AUTHORS AND AFFILIATIONS * Department of Urology, Affiliated Tumor Hospital of Xinjiang Medical University, Urumqi, China Junfeng Zhang & Peng Chen * Department of
Urology, The Central Hospital of Enshi Tujia and Miao Autonomous Prefecture, No. 158 Wuyang Avenue, Enshi City, 445000, Hubei, China Junfeng Zhang, Kailing Li, Hongbo Chen, Xiaohui Hu,
Zicheng Guo, Su Chen, Fu Zheng, Wusong Cheng, Qian Mu & Yong Lan Authors * Junfeng Zhang View author publications You can also search for this author inPubMed Google Scholar * Kailing Li
View author publications You can also search for this author inPubMed Google Scholar * Hongbo Chen View author publications You can also search for this author inPubMed Google Scholar *
Xiaohui Hu View author publications You can also search for this author inPubMed Google Scholar * Zicheng Guo View author publications You can also search for this author inPubMed Google
Scholar * Su Chen View author publications You can also search for this author inPubMed Google Scholar * Fu Zheng View author publications You can also search for this author inPubMed Google
Scholar * Wusong Cheng View author publications You can also search for this author inPubMed Google Scholar * Qian Mu View author publications You can also search for this author inPubMed
Google Scholar * Yong Lan View author publications You can also search for this author inPubMed Google Scholar * Peng Chen View author publications You can also search for this author
inPubMed Google Scholar CONTRIBUTIONS J.Z. and K.L. designed the research and wrote the paper; H.C., X.H., Z.G., S.C. and F. Z. collected data on stone composition; W.C. and Q.M. performed
the test for stone composition; Y.L. and P.C. guided the statistical analysis and the design of the research. All authors read and approved the final manuscript. CORRESPONDING AUTHORS
Correspondence to Yong Lan or Peng Chen. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature
remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons
Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original
author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the
article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your
intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence,
visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Zhang, J., Li, K., Chen, H. _et al._ Retrospective analysis of urinary tract
stone composition in a Chinese ethnic minority colony based on Fourier transform infrared spectroscopy. _Sci Rep_ 13, 13453 (2023). https://doi.org/10.1038/s41598-023-40603-w Download
citation * Received: 05 April 2023 * Accepted: 14 August 2023 * Published: 18 August 2023 * DOI: https://doi.org/10.1038/s41598-023-40603-w SHARE THIS ARTICLE Anyone you share the following
link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature
SharedIt content-sharing initiative