Play all audios:
ABSTRACT Gallate material, a luminescent matrix with excellent performance is normally prepared by vapor deposition or solid phase sintering method at high temperature. However, it has not
been solved to prepare gallate-based fluorescent materials with full-color luminescent properties at low temperature. In this paper, ZnGa2O4 undoped or doped with Cr or Mn nanoflowers
composed of nanosheet-level structure were prepared by hydrothermal method at low temperature. Under ultraviolet light irradiation, ZnGa2O4, ZnGa2O4:Mn2+ and ZnGa2O4:Cr3+ display three
primary colors of blue, green and red luminescence through self-excitation, Mn2+ and Cr3+ excitation respectively. The solid fluorescence yields of blue, green, and red colors are 32.3,
36.5, and 40.7%, respectively. It is highly expected to be applied to color display, biological imaging, white light devices. SIMILAR CONTENT BEING VIEWED BY OTHERS UNDERSTANDING THE EFFECT
OF MN2+ ON YB3+/ER3+ CO-DOPED NAYF4 UPCONVERSION AND OBTAINING THE OPTIMAL COMBINATION OF THESE TRIDOPING Article Open access 16 October 2023 ASSESSING THE INFLUENCE OF MICROWAVE-ASSISTED
SYNTHESIS PARAMETERS AND STABILIZING LIGANDS ON THE OPTICAL PROPERTIES OF AIS/ZNS QUANTUM DOTS Article Open access 20 December 2022 ZINC OXIDE NANOSTRUCTURES ENHANCED PHOTOLUMINESCENCE BY
CARBON-BLACK NANOPARTICLES IN MOIRÉ HETEROSTRUCTURES Article Open access 15 June 2023 INTRODUCTION In recent years, inorganic luminescent materials have attracted people's attention
because of their wide applications in fluorescence imaging, color display, white Light Emitting Diodes (LEDs) and so on1,2,3. Therefore, constructing and designing efficient inorganic
luminescent materials has become a hot topic for material scientists. Zinc gallate (ZnGa2O4), a ternary spinel material with a band gap of 4.4–4.7 eV, has exhibited excellent potential in
future display system due to its prominent blue emission, high chemical and thermal stability, and good cathodoluminescence characteristics at low-voltage4,5. Compared with other luminescent
materials, zinc gallate can be self-excited by Ga-O group, and has blue light emission6,7. ZnGa2O4 can also be used as the matrix of fluorescent materials, which has high luminous
efficiency and narrow spectral band. The luminescent color can be changed by adjusting the surface properties4,8 and composition of fluorescent materials9,10, or by doping the dopant
activators11,12. Most fluorescent materials use rare earth metals as activators, such as Eu3+, Tb3+, Y3+, etc.13,14,15, but rare earth metals are expensive and lack of resources. Previous
studies have shown that transition metal ions Mn2+ and Cr3+ can be used as activators of fluorescent materials, such as ZnGa2O4:Mn2+ emitting green fluorescence and ZnGa2O4:Cr3+ emitting red
fluorescence11,16,17. Various synthetic methods have been adopted to synthesize these fluorescent materials, such as thermal evaporation18, solid-state reaction method19, Chemical Vapor
Deposition (CVD), Atomic Layer Deposition (ALD) and so on20. These synthetic methods usually require high reaction temperature, which is over 900 °C, so the energy consumption is high, which
brings hidden dangers to environmental pollution control. Therefore, it is an urgent problem to find a low-temperature synthesis method for energy saving and emission reduction in the field
of synthesis of full-color luminescent materials. In this paper, undoped (ZnGa2O4), Mn2+-doped (ZnGa2O4:Mn2+) and Cr3+-doped (ZnGa2O4:Cr3+) nano-luminescent materials have been synthesized
at low temperature by one step hydrothermal method under the template action of ethylenediamine. These luminescent materials are composed of 5 μm-sized nano-flowers, each of which is
composed of 6–10 nm nanoflake hierarchical structure. Under ultraviolet irradiation, ZnGa2O4, ZnGa2O4:Mn2+ and ZnGa2O4:Cr3+ display three primary colors of blue, green and red through
self-excitation, Mn2+ excitation and Cr3+ ion excitation, respectively. METHODS RAW MATERIALS AND REAGENTS Gallium nitrate hydrate (Ga(NO3)3·xH2O), Zinc acetate dihydrate (Zn(CH3COO)2·2H2O),
Manganese acetate tetrahydrate (Mn(CH3COO)2·4H2O), Chromium nitrate nonahydrate (Cr(NO3)3·9H2O), and Anhydrous ethylenediamine (NH2(CH2)2NH2) are analytically pure and boughten from
Sigma-Aldrich Shanghai trading Co., Ltd.. Anhydrous ethanol (CH3CH2OH), ≥ 99.9% are boughten from Shanghai Sinopharm Chemical Co., Ltd. All reagents were not further treated before use.
Water used in the experiment is Milli-Q ultrapure water. SAMPLE PREPARATION SYNTHESIS OF ZNGA2O4 Ga(NO3)3·xH2O 0.512 g (2 mmol) and Zn(CH3COO)2·2H2O 0.220 g (1 mmol) are added into 20 mL
deionized water, magnetically stirred for 20 min at room temperature. And then 10 mL anhydrous ethylenediamine is added into the above solution, with continue stirring for 20 min. The mixed
solution is transferred to a 50 mL reaction kettle and placed into an oven, and stirred at 220 °C for 12 h. The white precipitate was obtained by washed with water and absolute ethanol
several times, and dried at 60 °C for 12 h. SYNTHESIS OF ZNGA2O4:MN2+ Ga(NO3)3·xH2O 0.512 g (2 mmol) and Zn(CH3COO)2·2H2O 0.220 g (1 mmol) are added into 17.5 mL deionized water,
magnetically stirred for 20 min at room temperature. And then 2.5 mL Mn(CH3COO)2 solution with a concentration of 4 mmol L−1 is added to above solution so that the concentration of Mn2+ is
1% of that of Zn2+, and magnetically stirred at room temperature for 20 min. Next, 10 mL anhydrous ethylenediamine is added into the above solution, with continue stirring for 20 min. The
mixed solution is transferred to a 50 mL reaction kettle and placed into an oven, and stirred at 220 °C for 12 h. The white precipitate was obtained by washed with water and absolute ethanol
several times, and dried at 60 °C for 12 h. SYNTHESIS OF ZNGA2O4:CR3+ The method is as same as the preparation of ZnGa2O4:Mn2+, and finally the concentration of Cr3+ is 0.5% of that of
Zn2+. SAMPLE CHARACTERIZATION The phase structure of the sample was determined at room temperature by X-ray powder diffraction analyzer (Rigaku D/Max 2200PC, graphite monochromator filter,
Cu Kα radiation, λ = 0.1542 nm) with the condition of tube voltage 40 kV, the tube current 20 mA, the scanning range 10°–80° (2θ) and the scanning speed 10 min−1. The morphology and
microstructure of the product were characterized by transmission electron microscope (JEM-100CXII, accelerating voltage 80 kV), high resolution transmission electron microscope (Philips
Tecnai 20U-TWIN, accelerating voltage 200 kV) and scanning electron microscope (FE-SEM, S-4800, Hitachi, accelerating voltage 5 kV). X-ray photoelectron spectrometer (PHI-5300 ESCA
spectrometer, Perkin Elmer, Al Kα as excitation light source) was used to analyze the surface properties of the samples. Before the spectrogram analysis, the electron binding energies of all
elements were corrected with the C_1s_ peak at 284.6 eV as reference. Photoluminescence (PL) and fluorescence lifetime of samples were measured by Agilent Cary Eclipse Fluorescence
Spectrometer. The UV–Vis absorption spectrum of the sample at room temperature was tested by Agilent Cary Series UV-VIS spectrometer, and BaSO4 was used as baseline correction before the
test. The infrared spectrum of the sample was tested by NICOLET FT-IR spectrometer, and the KBr was used as the background. RESULTS AND DISCUSSION PHASE STRUCTURE AND MORPHOLOGY
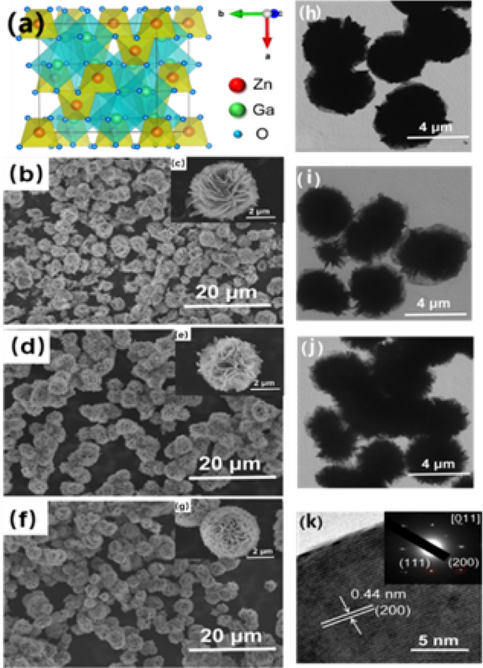
CHARACTERISTICS ZnGa2O4 is a bimetallic oxide composed of ZnO and Ga2O3, with _Fd-3m_ space group symmetry, a = b = c = 8.335, and spinel structure with chemical formula AB2O4, in which Zn2+
occupying tetrahedral center, Ga3+ occupying octahedral center21, as shown in Fig. 1a. Usually, the charge imbalance caused by the introduction of impurity ions is unfavorable to the
luminous intensity of luminophores, so higher energy is needed to eliminate the charge imbalance, such as calcination at high temperature for a long time22. The effective compensation factor
φ when ions are substituted was calculated according to the formula _φ_ = _Z/r_, in which _Z_ is the charge number of ions and _r_ is the effective radius of ions23. The greater the
difference of _φ_, the more difficult it is to substitute ions. As shown in Table S1, for hexa-coordinate substitution of Mn2+, the effective compensation factor_ φ_ is 2.41, which is much
lower than that of Ga3+ 4.83, so it is difficult for Mn2+ to replace Ga3+, while easy to replace Zn2+ due to the small difference of effective compensation factor11,17. Furthermore, in the
substitution reaction of ZnGa2O4, ions with similar effective radii are easy to be substituted with each other, that is, Mn2+ replaces Zn2+ to produce four coordinate substitutions, and Cr3+
replaces Ga3+ to produce six coordinate substitution17,24. The SEM photos of the three nanomaterials (ZnGa2O4, ZnGa2O4:Mn2+ and ZnGa2O4:Cr3+) with different magnification is shown in Fig.
1b–g. They all present a flower-like nanostructure of about 5 μm, which is composed of multiple nanosheet-level substructures with a thickness of 6–10 nm interspersed together. The
difference between the three samples is that the nanosheets composed of ZnGa2O4 are thicker and the degree of curling of the nanosheets is smaller, while the nanosheets doped with Mn2+ and
Cr3+ are thinner, and the nanosheets are freely curled to form spherical nanoflowers. As shown in Fig. S1. Zn, Ga, O and the corresponding doped elements Mn and Cr are uniformly distributed
in the flower-like nanostructures confirmed by the element distribution surface scans. The flower-like structure of ZnGa2O4, ZnGa2O4:Mn2+ and ZnGa2O4:Cr3+ can also be seen from the TEM
photos in Fig. 1h–j. Each flower is composed of the sub-structure of nano-flakes, and the size of nano-flowers is about 5 μm. From the contrast of the electron microscope photos, it can be
clearly seen that the nano-flakes constituting the flower-like structure become thinner in turn, which is similar to that of Scanning electron microscope (SEM). The phenomenon may be due to
the impurity ions adsorbed on the initial grain surface during hydrothermal process, which inhibiting the crystallization of the material to some extent, preventing the grain growth in some
directions, and resulting in the formation of thinner nanosheets. High-resolution photos and corresponding selective electron diffraction photos of ZnGa2O4 are shown in Fig. 1k. The lattice
spacing 0.44 nm is corresponding to the (200) crystal plane of ZnGa2O4, and the selective electron diffraction photo clearly shows single crystal structure of a ZnGa2O4 nanoflake. In the
single crystal structure of the sheet, the diffraction points correspond to (200) and (111) crystal plane. The direction of the crystal zone axis is confirmed to [011] by calculating,
therefore the exposed surface of the nanoplate is (110) plane. As shown in Fig. 2a the diffraction peaks of ZnGa2O4, ZnGa2O4:Mn2+ and ZnGa2O4:Cr3+ all correspond to the diffraction peaks of
standard card JCPDS38-1240 ZnGa2O4, which are cubic spinel structure. After doping Mn2+ and Cr3+, the intensity of the diffraction peak decreases, and the corresponding FWHM (full width at
half maximum) increases in sequence. The results of X-ray diffractometer (XRD) analysis also indicate that the secondary structure nanosheets that make up the nanoflowers become thinner in
turn after doping Mn2+ and Cr3+, which consistent precisely with the observation results of SEM and transmission electron microscopy (TEM). As shown in Fig. 2b–e the electron binding
energies of 2p1/2 orbitals and 2p3/2 orbitals of Ga are located at 1143.6 eV and 1116.8 eV, respectively. And the electron binding energies of 2p1/2 orbitals and 2p3/2 orbitals of Zn are
located at 1043.8 eV and 1021.1 eV, respectively. The characteristic peaks of electron binding energies located at 654.4 eV and 645.5 eV belong to 2p1/2 orbitals and 2p3/2 orbitals of Mn,
respectively and manganese ions show +2 valences. The characteristic peaks of electron binding energy at 586.5 eV and 576.5 eV respectively belong to 2p1/2 orbitals and 2p3/2 orbitals of Cr
and chromium ions show +3 valences. The intensity of these characteristic peaks is small due to the low contents of Mn and Cr. INFRARED AND ULTRAVIOLET SPECTRAL CHARACTERISTICS In order to
understand the chemical composition of the sample, the Fourier transform infrared spectrum is conducted. The Fourier Transform infrared spectroscopy (FT-IR) of ZnGa2O4, ZnGa2O4:Mn2+ and
ZnGa2O4:Cr3+ in Fig. S2 also indicate the samples were binary metal oxides consisting of Zn-O and Ga-O groups. As showed in Fig. S2, the broad absorption peak at the 3445 cm−1 wavelength
belongs to the stretching vibration of O-H and N-H. The stretching vibration of N-H may come from the residual ethylenediamine in the sample, but there is no obvious stretching vibration
peak of it near 1190 cm−1. Therefore, the residual ethylenediamine may be very small and can be ignored after repeated cleaning of water and anhydrous ethanol. In the fingerprint region at
low wavelength, the larger peaks of 585 cm−1 and 425 cm−1 are attributed to the vibration absorption of Zn-O and Ga-O, respectively. Through the infrared spectrum analysis, no other
vibration peaks are observed except Zn-O and Ga-O in the sample, so it is determined that the sample is a binary metal oxide composed of Zn-O group and Ga-O group. The UV-vis absorption
spectra of ZnGa2O4, ZnGa2O4:Mn2+ and ZnGa2O4:Cr3+ samples are shown in Fig. S3. It can be seen from the figure that the absorption regions of the three samples are basically the same, and
there is only absorption in the region smaller than 350 nm. It is also confirmed that ZnGa2O4 can only be excited at wavelengths less than 350 nm. However, in the 300–350 nm wavelength
region, compared with the absorption peak of ZnGa2O4, the absorption of ZnGa2O4:Mn2+ and ZnGa2O4:Cr3+ increases slightly, which may be due to the absorption of a small amount of Mn and Cr
itself, because the amount of Mn2+ and Cr3+ is very small, only 0.4% and 1%, so the absorption of these two elements is also very weak. LUMINESCENT PROPERTIES The excitation and emission
spectra of ZnGa2O4, ZnGa2O4:Mn2+ and ZnGa2O4:Cr3+ are shown in Fig. 3a–c. For undoped ZnGa2O4, three peaks can be seen in the excitation spectrum, which are located at 226 nm 239 nm and 257
nm, respectively. These excitation peaks are caused by the charge transfer from O2− to octahedral center Ga3+ and the ultraviolet absorption of ZnGa2O4 itself25. The emission spectrum
obtained using 226 nm as the excitation wavelength is a broad peak with the highest peak of 456 nm in the range of 340–750 nm. This broad emission peak is in all probability caused by the
self-excitation of Ga-O hexahedron in the spinel structure. The luminescent properties of fluorescent host materials are usually changed by introducing impurity ions26, that is also
applicable to ZnGa2O4 host materials. Mn2+-doped ZnGa2O4 has green fluorescence emission, as shown in Fig. 3b. Except for the charge transfer from O2- to Ga3+ in the octahedral center and
the ultraviolet absorption of ZnGa2O4 itself, the absorption of Mn2+ excites a red shift of 32 near 300 nm, which is consistent with the analysis of ultraviolet-visible absorption spectrum
in Fig. S3. Due to the activation of Mn2+, the emission spectrum with the highest emission peak of 505 nm is located in the range of 470–600 nm with the excitation wavelength of 226 nm.
After enlarged locally as shown in Fig. S4, the five smaller excitation peaks located between 351–443 nm and centered at 351 nm, 379 nm, 410 nm, 422 nm and 443 nm respectively correspond to
5A1–4E, 6A1–4T2, 6A1–4A1, 4E1 and 6A1–4T of Mn2+.27 When excited at 226 nm, ZnGa2O4:Mn2+ has green emission at 505 nm, which belongs to the 4T1–6A1 d orbital electron forbidden transition of
Mn2+11,17. This is the process of energy transfer from ZnGa2O4 matrix to Mn2+27. The transition process of 4T1–6A1 of Mn2+ is accompanied by strong 3d shell lattice vibration coupling, and
is affected by crystal field and symmetric sites. If Mn2+ is in a weak crystal field, i.e. tetrahedron, the splitting of excitation energy will be weak, which will be accompanied by high
energy emission, that is, green light and if Mn2+ is in a strong crystal field, i.e., octahedron, it will emit yellow or red light17,27, which is consistent with our previous analysis of
crystal structure. In our investigation, Mn2+ replaces Zn2+ with similar ionic radius in cubic ZnGa2O4 matrix to generate tetrahedral coordination and emit green light, which is completely
consistent with the test results of fluorescence spectrum. The excitation and emission spectra of ZnGa2O4:Cr3+ are shown in Fig. 3c. There is a wide excitation peak between 200–350 nm,
including four intensity excitation peaks (226 nm, 240 nm, 257 nm, 266 nm), which related to the charge transfer transition of O2− to the octahedral center Ga3+ and the absorption transition
with the belt, and the excitation spectrum of 300–350 nm is caused by the absorption of Cr3+. A red emission peak at 696 nm was obtained by excitation at 226 nm, which was attributed to the
2E–4A2 characteristic transformation of Cr3+28. Meanwhile, a similar red emission peak at 696 nm was obtained by excitation at 416 nm and 572 nm. These two excitation peaks at 416 nm and
572 nm are caused by d–d electron-electron transitions of Cr3+24,29, corresponding to the 4A2–4T1 and 4A2–4T2 characteristic transitions of Cr3+, respectively30. Undoped, Mn2+ doped and Cr3+
doped ZnGa2O4 have blue, green and red emission properties under ultraviolet (UV) excitation, respectively. The excitation wavelengths used in the emission spectra of ZnGa2O4, ZnGa2O4:Mn2+
and ZnGa2O4:Cr3+ are all 226 nm, indicating that the hetero ions in ZnGa2O4:Mn2+ and ZnGa2O4:Cr3+ can effectively enter the lattice of ZnGa2O4 under hydrothermal conditions to replace Zn2+
and Ga3+ to form tetrahedral and octahedral coordination, respectively. This is related to the addition of an appropriate amount of ethylenediamine during hydrothermal. Ethylenediamine
aqueous solution is a strongly alkaline solution, which plays an effective role in promoting the crystallization of materials and the entry of hetero ions into the crystal lattice of the
matrix under the condition of hydrothermal high temperature and high pressure. We also conduct experiment keeping other conditions remaining the same without ethylenediamine in the
synthesis. The obtained ZnGa2O4 doped with Mn2+ and Cr3+ does not show green and red emission properties after UV excitation, indicating that it is difficult for hydrothermal hetero ions to
enter into the lattice of ZnGa2O4 matrix under the condition of non-strong alkaline solvent. The fluorescence attenuation curves of ZnGa2O4, ZnGa2O4:Mn2+ and ZnGa2O4:Cr3+ are fitted
exponentially as shown in Fig. 3d. The attenuation curves of the three samples are all fitted by double exponents, which are in accordance with the formula. $$I = I_{1} {\text{exp}}\left( {
- t/\tau_{1} } \right) + I_{2} {\text{exp}}\left( { - t/\tau_{2} } \right)$$ (1) where _I_ is the fluorescence intensity when the time is _t_, _I__1_ and _I__2_ are fitting constants, and
_τ__1_ and _τ__2_ are fluorescence lifetime. After fitting, each sample corresponds to two millisecond lifetimes, a shorter lifetime _τ__1_ and a relatively longer life _τ__2_. The specific
fitting parameters are shown in Table S2. The longer lifetimes of each sample of ZnGa2O4, ZnGa2O4:Mn2+ and ZnGa2O4:Cr3+ correspond to the self-excitation of Ga-O in the bulk phase of
luminescent materials, the 4T1-6A1 transition of Mn2+ and the 2E–4A2 transition of Cr3+, respectively. The short lifetime of the three samples is due to the fact that the surface effect of
the materials has a great influence on the luminescence lifetime. These materials are all composed of ultra-thin 6–10 nm nanosheets with large surface area, and the increase of surface atoms
leads to the appearance of more activated ions on the surface of the nanosheets. However, the impurities, unsaturated bonds, vacancies and other surface defects on the surface of the
nanoparticles will quenched the activated ions and lead to radiation-free transition, thus shortening the life of the activated ions.8 Excited by 254 nm's handheld UV lamp, ZnGa2O4,
ZnGa2O4:Mn2+ and ZnGa2O4:Cr3+ appear bright blue, green and red, respectively. Their optical photos are shown in Fig. 4a. The solid fluorescence yields of blue, green, and red colors are
32.3, 36.5, and 40.7%, respectively. Under the light excitation of 226 nm wavelength, the Normalized fluorescence emission spectra of the three materials are shown in Fig. 4b. The maximum
fluorescence emission spectra of the three materials are located in 456 nm, 505 nm and 696 nm, respectively, which basically correspond to the central regions of blue, green and red. Their
emission spectra are imported into the CIE color coordinate software, respectively, and the color coordinate diagram shown in Fig. 4c is obtained. The color coordinates are located at (0.19,
0.23), (0.10, 0.65) and (0.66, 0.34), respectively, which indicates that any color including white in the triangular area connected by the three points can be obtained by changing the ratio
of the three luminescent materials. In order to demonstrate whether there is white luminescence phenomenon when mixing the three samples, we conducted packaged white LED luminescence
testing on their mixture. As shown in Fig. 5a, the encapsulated white LED device and the emitting color are shown. The encapsulated white LED prepared has a correlated colour temperature
(CCT) of 26702K and a color rendering index (CRI) of 55.1. Within the constant voltage range of 20 mA to 120 mA and 3 V, the relationship between the luminous intensity of the encapsulated
white LED and the input forward current is shown in Fig. 5b. From the color of the encapsulated white LED device and the CIE chromaticity diagram, we can see that the light emitted by this
mixture is not pure white light, but a cyanish white-like light. According to the emission spectrum of the mixture, we speculate that the reason is that the emission wavelength of red light
is mostly in the invisible near-infrared region after 650 nm, and there is an empty window in the wavelength range of 600–650 nm, resulting in the emission color of encapsulated white LED
being a cyan white light, rather than a pure white light like the results made by other phosphors31,32. CONCLUSIONS ZnGa2O4 nanoflowers with single size composed of ZnGa2O4 flake
substructures with a thickness of 6-10 nm were synthesized by a simple hydrothermal method under the action of ethylenediamine template. The luminescence color of ZnGa2O4 is controlled by
doping Mn2+ and Cr3+. Under UV excitation, undoped ZnGa2O4, ZnGa2O4 doped with Mn2+ and Cr3+ have blue, green and red emission properties, respectively. The luminescence properties of the
same matrix material with three primary colors are obtained. The blue, green and red fluorescence comes from the self-excited electron transfer of Ga-O itself and the 3d electron energy
transfer of Mn2+ and Cr3+. The three samples are mixed to do the encapsulated white LED luminescence test, which has the phenomenon of cyan white luminescence. These ZnGa2O4-based
fluorescent nanomaterials are expected to be used in color display, biological imaging and white light devices. DATA AVAILABILITY All data generated or analyzed during this study are
included in this published article (and its Supplementary Information files). REFERENCES * Nie, J., Li, Y., Han, G. & Qiu, J. In vivo clearable inorganic nanophotonic materials: Designs,
materials and applications. _Nanoscale_ 11, 12742–12754. https://doi.org/10.1039/c9nr02083g (2019). Article CAS PubMed Google Scholar * Shi, C. _et al._ Facile synthesis of a
color-tunable microcrystal phosphor for anti-counterfeit applications. _ACS Omega_ 5, 32420–32425. https://doi.org/10.1021/acsomega.0c04516 (2020). Article CAS PubMed PubMed Central
Google Scholar * Menendez-Velazquez, A., Morales, D. & Garcia-Delgado, A. B. Sunlike white light-emitting diodes based on rare-earth-free luminescent materials. _Materials (Basel)_
https://doi.org/10.3390/ma15051680 (2022). Article PubMed Google Scholar * Yang, W. _et al._ Multi-wavelength tailoring of a ZnGa2O4 nanosheet phosphor via defect engineering. _Nanoscale_
10, 19039–19045. https://doi.org/10.1039/c8nr05072d (2018). Article CAS PubMed Google Scholar * Liu, N. _et al._ In vivo repeatedly activated persistent luminescence nanoparticles by
radiopharmaceuticals for long-lasting tumor optical imaging. _Small_ 16, e2001494. https://doi.org/10.1002/smll.202001494 (2020). Article CAS PubMed Google Scholar * Moon, J. W., Kim, J.
S., Park, J. H., Ivanov, I. N. & Phelps, T. J. Synthesis of zinc-gallate phosphors by biomineralization and their emission properties. _Acta Biomater._ 97, 557–564.
https://doi.org/10.1016/j.actbio.2019.07.052 (2019). Article CAS PubMed Google Scholar * Hinuma, Y., Mine, S., Toyao, T., Kamachi, T. & Shimizu, K. I. Factors determining surface
oxygen vacancy formation energy in ternary spinel structure oxides with zinc. _Phys. Chem. Chem. Phys._ 23, 23768–23777. https://doi.org/10.1039/d1cp03657b (2021). Article CAS PubMed
Google Scholar * Wang, T., Layek, A., Hosein, I. D., Chirmanov, V. & Radovanovic, P. V. Correlation between native defects and dopants in colloidal lanthanide-doped Ga2O3 nanocrystals:
A path to enhance functionality and control optical properties. _J. Mater. Chem. C_ 2, 3212–3222. https://doi.org/10.1039/c3tc31823k (2014). Article CAS Google Scholar * Farvid, S. S.,
Wang, T. & Radovanovic, P. V. Colloidal gallium indium oxide nanocrystals: A multifunctional light-emitting phosphor broadly tunable by alloy composition. _J. Am. Chem. Soc._ 133,
6711–6719. https://doi.org/10.1021/ja111514u (2011). Article CAS PubMed Google Scholar * Wang, Z., Teramura, K., Hosokawa, S. & Tanaka, T. Highly efficient photocatalytic conversion
of CO2 into solid CO using H2O as a reductant over Ag-modified ZnGa2O4. _J. Mater. Chem. A_ 3, 11313–11319. https://doi.org/10.1039/c5ta01697e (2015). Article CAS Google Scholar * Hwang,
J. Y., Bark, C. W. & Choi, H. W. Application of ZnGa2O4: Mn down-conversion layer to increase the energy-conversion efficiency of perovskite solar cells. _J. Nanosci. Nanotechnol._ 21,
4362–4366. https://doi.org/10.1166/jnn.2021.19407 (2021). Article CAS PubMed Google Scholar * Monika, Yadav, R. S., Rai, A. & Rai, S. B. NIR light guided enhanced photoluminescence
and temperature sensing in Ho(3+)/Yb(3+)/Bi(3+) co-doped ZnGa2O4 phosphor. _Sci. Rep._ 11, 4148. https://doi.org/10.1038/s41598-021-83644-9 (2021). Article ADS CAS PubMed PubMed Central
Google Scholar * Lee, H. & Choi, H. W. Characteristics of perovskite solar cells according to thickness of doped ZnGa2O4 down-converting thin film. _J. Nanosci. Nanotechnol._ 20,
7081–7086. https://doi.org/10.1166/jnn.2020.18849 (2020). Article CAS PubMed Google Scholar * Safeera, T. A. & Anila, E. I. Wet chemical approach for the low temperature synthesis of
ZnGa2O4:Tb3+ quantum dots with tunable blue-green emission. _J. Alloys Compd._ 764, 142–146. https://doi.org/10.1016/j.jallcom.2018.06.048 (2018). Article CAS Google Scholar * Noto, L.
L. _et al._ Structure, photoluminescence and thermoluminescence study of a composite ZnTa2O6/ZnGa2O4 compound doped with Pr3+. _Opt. Mater._ 55, 68–72.
https://doi.org/10.1016/j.optmat.2016.03.029 (2016). Article ADS CAS Google Scholar * De Vos, A. _et al._ First-principles study of antisite defect configurations in ZnGa2O4: Cr
persistent phosphors. _Inorg. Chem._ 55, 2402–2412. https://doi.org/10.1021/acs.inorgchem.5b02805 (2016). Article CAS PubMed Google Scholar * Dazai, T., Yasui, S., Taniyama, T. &
Itoh, M. Cation-deficiency-induced crystal-site engineering for ZnGa2O4:Mn(2+) thin film. _Inorg. Chem._ 59, 8744–8748. https://doi.org/10.1021/acs.inorgchem.0c00359 (2020). Article CAS
PubMed Google Scholar * Lu, M. Y., Zhou, X., Chiu, C. Y., Crawford, S. & Gradecak, S. From GaN to ZnGa2O4 through a low-temperature process: Nanotube and heterostructure arrays. _ACS
Appl. Mater. Interfaces_ 6, 882–887. https://doi.org/10.1021/am404158f (2014). Article CAS PubMed Google Scholar * Lou, Z., Li, L. & Shen, G. High-performance rigid and flexible
ultraviolet photodetectors with single-crystalline ZnGa2O4 nanowires. _Nano Res._ 8, 2162–2169. https://doi.org/10.1007/s12274-015-0723-0 (2015). Article CAS Google Scholar * Tien, L.-C.,
Tseng, C.-C., Chen, Y.-L. & Ho, C.-H. Direct vapor transport synthesis of ZnGa2O4 nanowires with superior photocatalytic activity. _J. Alloys Compd._ 555, 325–329.
https://doi.org/10.1016/j.jallcom.2012.12.029 (2013). Article CAS Google Scholar * Zerarga, F., Bouhemadou, A., Khenata, R. & Bin-Omran, S. Structural, electronic and optical
properties of spinel oxides ZnAl2O4, ZnGa2O4 and ZnIn2O4. _Solid State Sci._ 13, 1638–1648. https://doi.org/10.1016/j.solidstatesciences.2011.06.016 (2011). Article ADS CAS Google Scholar
* Park, S., An, S., Mun, Y. & Lee, C. UV-enhanced room-temperature gas sensing of ZnGa2O4 nanowires functionalized with Au catalyst nanoparticles. _Appl. Phys. A_ 114, 903–910.
https://doi.org/10.1007/s00339-013-7745-9 (2013). Article ADS CAS Google Scholar * Lin, L., Sun, X., Jiang, Y. & He, Y. Sol-hydrothermal synthesis and optical properties of Eu3+,
Tb(3+)-codoped one-dimensional strontium germanate full color nano-phosphors. _Nanoscale_ 5, 12518–12531. https://doi.org/10.1039/c3nr04185a (2013). Article ADS CAS PubMed Google Scholar
* Viana, B. _et al._ Long term in vivo imaging with Cr3+ doped spinel nanoparticles exhibiting persistent luminescence. _J. Lumin._ 170, 879–887.
https://doi.org/10.1016/j.jlumin.2015.09.014 (2016). Article CAS Google Scholar * Zhou, W. _et al._ Synthesis of porous zinc gallate prisms composed of highly oriented nanoparticles by an
in situ conversion reaction. _Chemistry_ 18, 5367–5373. https://doi.org/10.1002/chem.201102673 (2012). Article CAS PubMed Google Scholar * Fu, Z., Xia, W., Li, Q., Cui, X. & Li, W.
Highly uniform NaLa(MoO4)2:Ln3+ (Ln = Eu, Dy) microspheres: Template-free hydrothermal synthesis, growing mechanism, and luminescent properties. _CrystEngComm_
https://doi.org/10.1039/c2ce06682c (2012). Article Google Scholar * Luchechko, A. _et al._ Afterglow, TL and OSL properties of Mn(2+)-doped ZnGa2O4 phosphor. _Sci. Rep._ 9, 9544.
https://doi.org/10.1038/s41598-019-45869-7 (2019). Article ADS CAS PubMed PubMed Central Google Scholar * Su, J. _et al._ Influence of oxygen vacancy on persistent luminescence in
ZnGa_2O_4:Cr3+ and identification of electron carriers. _Opt. Mater. Express._ https://doi.org/10.1364/ome.7.000734 (2017). Article Google Scholar * Li, L., Wang, Y., Huang, H., Li, H.
& Zhao, H. Long-lasting luminescence in ZnGa2O4: Cr3+ through persistent energy transfer. _Mod. Phys. Lett. B._ https://doi.org/10.1142/s0217984916500196 (2016). Article Google Scholar
* Zhang, Y. _et al._ Full color emission in ZnGa2O4: Simultaneous control of the spherical morphology, luminescent, and electric properties via hydrothermal approach. _Adv. Func. Mater._
24, 6581–6593. https://doi.org/10.1002/adfm.201402092 (2014). Article CAS Google Scholar * Kwon, K. _et al._ Luminescence properties and energy transfer of site-sensitive
Ca(6–x-y)Mg(x-z)(PO(4))(4):Eu(y)(2+), Mn(z)(2+) phosphors and their application to near-UV LED-based white LEDs. _Inorg. Chem._ 48, 11525–11532. https://doi.org/10.1021/ic900809b (2009).
Article CAS PubMed Google Scholar * Xie, Y. _et al._ Synthesis of quantum dot-ZnS nanosheet inorganic assembly with low thermal fluorescent quenching for LED application. _Materials
(Basel)_ https://doi.org/10.3390/ma10111242 (2017). Article PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS Thanks for the support of the Experimental Center of
Shandong University of Traditional Chinese Medicine, the Natural Science Foundation of Shandong Province (Grant No.: ZR2020 MB108), the Health Commission of Shandong Province (Grant No.:
202004010938 and Q-2023016) and the National Natural Science Foundation of China (Grant No.: 82304431). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Pharmacy, Shandong
University of Traditional Chinese Medicine, Jinan, 250355, Shandong, China Yan Liu, Tingting Zheng & Xiuyun Zhang * Key Laboratory of New Material Research Institute, Department of
Acupuncture-Moxibustion and Tuina, Shandong University of Traditional Chinese Medicine, Jinan, 250355, China Chen Chen Authors * Yan Liu View author publications You can also search for this
author inPubMed Google Scholar * Tingting Zheng View author publications You can also search for this author inPubMed Google Scholar * Xiuyun Zhang View author publications You can also
search for this author inPubMed Google Scholar * Chen Chen View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Y.L.: Conceptualization,
methodology, formal analysis, investigation, writing—original draft, writing—review and editing, T.Z.: conceptualization, data curation, formal analysis, funding acquisition, methodology,
supervision, writing-original draft, writing-review & editing. X.Z.: supervision, conceptualization, methodology, invisualization, writing-review & editing. C.C.: supervision,
conceptualization, methodology, invisualization, writing-review & editing. CORRESPONDING AUTHORS Correspondence to Tingting Zheng, Xiuyun Zhang or Chen Chen. ETHICS DECLARATIONS
COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published
maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution
4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and
the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's
Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not
permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Liu, Y., Zheng, T., Zhang, X. _et al._ Preparation of ZnGa2O4 nanoflowers and their
full-color luminescence properties. _Sci Rep_ 13, 14430 (2023). https://doi.org/10.1038/s41598-023-41658-5 Download citation * Received: 06 April 2023 * Accepted: 29 August 2023 *
Published: 02 September 2023 * DOI: https://doi.org/10.1038/s41598-023-41658-5 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable
link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative