Play all audios:
Ectomycorrhizal (ECM) fungi are functionally important in biogeochemical cycles in tropical ecosystems. Extracellular enzymatic activity of ECM on a ground-area basis is the product of two
attributes; exploration capacity (ECM surface-area) and specific enzymatic activity. Here, we elucidated which attribute better explained the ECM enzymatic activity in response to different
levels of soil phosphorus (P) and Nitrogen (N) availability in five Bornean tropical rainforests. We determined the surface area of ECM root tips as well as the enzymatic activities per ECM
surface area for carbon (C), N and P degrading enzymes in each site. We evaluated the relationship of ECM enzyme activities with the resource availabilities of C (Above-ground net primary
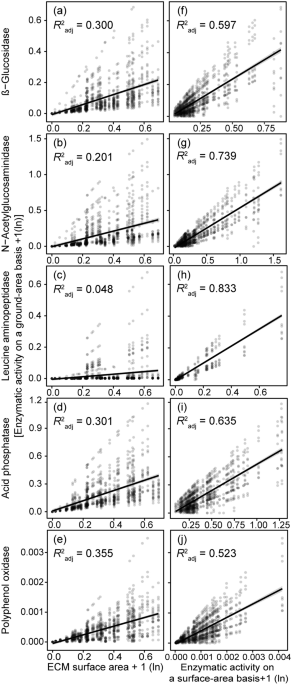
production; ANPP), N, and P of ECM by a generalized linear mixed model. The ECM enzymatic activities on a ground-area basis were more significantly determined by specific enzymatic activity
than by the exploration capacity. Specific enzymatic activities were generally negatively affected by C (ANPP) and soil P availability. ECM fungi enhance the specific enzyme activity rather
than the exploration capacity to maintain the capacity of nutrient acquisition. The less dependence of ECM fungi on the exploration capacity in these forests may be related to the limitation
of C supply from host trees. We highlighted the adaptive mechanisms of ECM fungi on nutrient acquisition in tropical ecosystems through the response of enzymatic activity to nutrient
availability across the elements.
Tropical ecosystems exhibit the highest net primary productivity of all terrestrial ecosystems, which plays a crucial role in the global carbon (C) cycle. At the same time, primary
productivity in tropical ecosystems is often limited by insufficient soil phosphorus (P) availability because of the high weathering rates of the soil minerals and an associated geochemical
transformation of soil P into unavailable forms1,2,3,4. In order to address the mechanisms of how tropical trees maintain primary productivity on such soils with reduced P availability, many
studies have focussed on the ecophysiology of the efficient P-use in photosynthetic C assimilation1,5,6,7, and on the nutrient acquisition strategy of plant root systems8,9. However, the
roles of root-associated microorganisms on biogeochemical P cycles and the plant P acquisition strategy have not fully been understood yet.
Most terrestrial plants rely on mycorrhizal fungi associated with plant roots for mineral nutrient acquisition. Mycorrhizal fungi can take up both mineral and organic forms (amino acid) of
nutrients from soils by expanding mycorrhizal root tips and extraradical hyphae, both of which function to enhance exploration capacity10,11. More specifically, ECM have a larger nutrient
foraging area, which benefits the host plant. Because ECM fungal sheath can often enclose the colonized root surface12, the direct contact of the fungal sheath with the soil act as a
substitute for the enclosed root surface. ECM fungi have a significant physiological capacity to access nutrients bound with complex organic compounds including cellulose, protein, chitin,
and phytate12,13,14,15. Some specialized species can also access recalcitrant organic matters such as lignin and phenol-complexes, and they have specific forms of extracellular enzymes to
degrade such recalcitrant matter16. Extracellular enzymatic activities associated with the degradation and nutrient release from soil organic matter reflect the functional diversity of ECM
fungal community in situ17,18. Substantial differences in enzymatic activities have been reported among ECM fungal species in a given site or across sites along environmental
gradients17,19,20,21,22, This suggests that changes in ECM fungal communities and their host trees can modify ECM enzyme activities, adapting to nutrient availability variations across
sites23,24,25. Despite these significant potential of ECM fungi on biogeochemical cycles, only a few measurements of extracellular enzymatic activities of ECM have been reported from
tropical soils26. Moreover, the adaptive response of ECM fungi under nutrient deficiency has been poorly elucidated so far. This oversight is particularly critical when considering the vital
role of ECM fungi in facilitating tree adaptation and survival in low-phosphorus (P) soils.
Soil microbes including ECM fungi secrete extracellular enzymes including those that mineralize C, N and P to access each nutrient. The relative activity of those enzymes is suggested to be
controlled by the availability of reactive substrates and/or soil mineral nutrients (i.e., the product of the enzyme reaction of each nutrient element)27. For example, phosphatase activity
of soil microbes and its ratio to the C mineralizing enzyme activity in tropical ecosystems were greater where soil organic-P fraction was relatively small28,29. Thus, the stoichiometry of
given two enzymes (enzymatic stoichiometry; i.e. the ratio of the activities of two enzymes) can vary with relative substrate abundances and/or nutrient availability and reflects the enzyme
allocation of soil microbes28.
In Southeast Asia, diverse ECM host trees occur as dominant taxa such as the families Dipterocarpaceae, Fagaceae and Myrtaceae30,31,32,33. The abundance of these taxa could be related to
soil nutrient availability, depending on the nutrient acquisition strategy of each taxa through ECM symbioses. Mount Kinabalu, Borneo, is one of the hotspots of world floristic biodiversity
and diverse species of ECM host trees occur within/across sites34. Soil nutrient availability of P (and N) is highly variable due to complex geology and a wide altitudinal gradient on this
mountain5,35,36,37,38. ECM fungal communities also remarkably changed along these gradients39. This natural setting on Mount Kinabalu is ideal for investigating the adaptive responses of ECM
fungi in terms of nutrient acquisition capacity.
Here, we aimed to elucidate the adaptive strategy of ECM symbiosis in the tropical ecosystems by investigating the response of the extracellular enzymatic activity of ECM fungi to
contrasting soil P and N availability in five tropical rain forests on Mount Kinabalu. We evaluated the enzymatic activity of ECM from two aspects, contact area with soil as an exploration
capacity (biomass and surface area of ECM on a ground-area basis) and specific enzymatic activity (enzymatic activity on an ECM surface-area basis). We also characterized the stoichiometric
relationships among enzymatic activities degrading different organic elements (C, N, and P). Subsequently, we evaluated the realized ECM enzymatic activity on a ground-area basis by
integrating the two aspects of ECM (exploration capacity and specific enzymatic activity) to clarify the overall performance of ECM symbiosis in response to P availability. We hypothesized
that ECM fungi, as an adaptive response in P-deficient forests with limited productivity, enhance P acquisition by increasing both exploratory capacity and specific enzymatic activity,
especially for the P-specific enzyme.
Five tropical rain forests on Mount Kinabalu (4095 m a.s.l.; 6°5′ N, 116° 33′ E), Borneo, were selected for this study. These include two lowland forests and three montane forests based on
the vegetation classification by Kitayama35. These study sites were part of the study plots for the ecosystem dynamics project designed by Aiba and Kitayama34 and Kitayama et al.38. The
climate of the study sites is humid equatorial with little seasonality in air temperature and precipitation. The mountain is non-volcanic and largely consists of Tertiary sedimentary rocks
of sandstone and/or mudstone and ultrabasic rocks that protruded the sedimentary rocks as mosaics. Two geological substrates (sedimentary and ultrabasic soils) were selected in each of the
lowland zone (700 m a.s.l.) and lower montane zone (1700 m a.s.l.), yielding a total of four forests in a matrix manner of altitude and substrate. The fifth forest is located in the lower
montane zone at 1700 m on Quaternary tilloid deposits mostly of sedimentary rocks (hereafter Quaternary substrate)38. All sites are on gentle slopes to avoid the effects of topography. The
ecosystem properties of the sites can be referred to (Table 1)5,34,38.
For soil P availability, the size of soluble inorganic P pools, extracted with hydrochloric-ammonium fluoride solution, varied substantially among the five forests due to differences in
geology and weathering as a function of altitude40. It was always greater on sedimentary than on ultrabasic substrate at the same altitude (Table 2)5,37. Among the three forests at 1700 m,
the pool size of soluble inorganic P was the greatest on the Quaternary substrate of the relatively young age reflecting the lesser weathering of soil minerals (Table 2)38.
The tree-species composition of the five forests was investigated by Aiba et al.41 and Aiba and Kitayama34. Three families of ECM host trees (Dipterocarpaceae, Fagaceae, and Myrtaceae) were
distributed in all study sites. Only the genus Tristaniposis was regarded as an ectomycorrhizal host within the family Myrtaceae31. Indeed, we confirmed the ECM formation of Tristaniopsis by
observing its ECM tips collected from seedlings in the study sites in our preliminary survey (Figure S1). No ECM tips were observed on the seedlings of Leptospermum or Syzygium (both
Myrtaceae), which were also abundant in some of our sites. The relative basal area (RBA) of ECM host trees in each site was sorted by family based on the dataset (Table S1) 34. RBA of
ectomycorrhizal host trees ranged from 21.4% at the Quarternary montane forest (17Q) to 38.5% at the ultrabasic lowland forest (07U).
ECM tips were collected twice for two measurements separately; biomass and enzyme activity assay of ECM tips. To measure the ECM biomass on a ground-area basis, ten soil cores (2 cm
diameter, 15-cm depth) were collected at random positions (at least 10 m apart) from the surface layer including the A0, A, and E soil horizons in each study site in September 2012. Each
soil core was kept in a plastic bag and stored at 4ºC for up to 2 weeks until further processing. Each soil sample was sieved (500 µm mesh) and rinsed with tap water to remove soil
particles. The remaining fine roots (diameter