Play all audios:
ABSTRACT The large Weilasituo Sn-polymetallic deposit is a recent exploration discovery in the southern Great Xing’an Range, northeast China. The ore cluster area shows horizontal
mineralization zoning, from the inner granite body outward, consisting of high-_T_ Sn–W–Li mineralization, middle-_T_ Cu–Zn mineralization and peripheral low-_T_ Pb–Zn–Ag mineralization.
However, the intrinsic genetic relationship between Sn-W-Li mineralization and peripheral vein-type Pb–Zn–Ag–Cu mineralization, the formation mechanism and the deep geological background are
still insufficiently understood. Here, we use fluid inclusions, trace elements concentrations in quartz and sphalerite, and H–O isotope studies to determine the genetic mechanism and
establish a metallogenic model. Fluid inclusion microthermometry and Laser Raman spectroscopic analysis results demonstrates that the aqueous ore-forming fluids evolved from low-medium
salinity, medium–high temperature to low salinity, low-medium temperature fluids. Laser Raman spectroscopic analysis shows that CH4 is ubiquitous in fluid inclusions of all ore stages. Early
ore fluids have δ18OH2O (v–SMOW) values from + 5.5 to + 6.2‰ and δD values of approximately − 67‰, concordant with a magmatic origin. However, the late ore fluids shifted toward lower
δ18OH2O (v–SMOW) (as low as 0.3‰) and δD values (~ − 136‰), suggesting mixing between external fluids derived from the wall rocks and a contribution from meteoric water. Ti-in-quartz
thermometry indicates a magmatic crystallization temperature of around 700 °C at a pressure of 1.5 kbar for the magmatic ore stage. Cathodoluminescence (CL) imaging and trace element
analysis of quartz from a hydrothermal vug highlight at least three growth episodes that relate to different fluid pulses; each episode begins with CL-bright, Al-Li-rich quartz, and ends
with CL-dark quartz with low Al and Li contents. Quartz from Episode 1 formed from early Sn-(Zn)-rich fluids which were likely derived from the quartz porphyry. Quartz from episodes 2 and 3
formed from Zn-(Sn)-Cu-rich fluid. The early magmatic fluid is characterized by low _f_S2. The SO2 produced by magma degassing reacted with heated water to form SO42−, causing the shift from
low _f_S2 to high _f_S2. The SO42− generated was converted to S2– by mixing with CH4-rich, Fe and Zn-bearing external fluid which led to late-stage alteration and dissolution of micas in
vein walls, thus promoting crystallization of pyrrhotite, Fe-rich sphalerite and chalcopyrite and inhibiting the precipitation of anhydrite. This study shows that ore formation encompassed
multiple episodes involving steadily evolved fluids, and that the addition of external fluids plays an important role in the formation of the later Cu–Zn and Ag–Pb–Zn mineralization in the
Weilasituo ore district. SIMILAR CONTENT BEING VIEWED BY OTHERS MINERALIZATION AND METALLOGENIC MODEL OF THE LAURANI HIGH-SULFIDATION EPITHERMAL DEPOSIT IN NORTHEASTERN BOLIVIAN ALTIPLANO
Article Open access 30 December 2024 COPPER SULFIDE DEPOSITION AND REMOBILISATION TRIGGERED BY NON-MAGMATIC FLUID INCURSION IN THE SINGLE-INTRUSION TONGCHANG PORPHYRY SYSTEM, SE CHINA
Article Open access 31 January 2024 FLUID INCLUSIONS IN MAGMATIC ILMENITE RECORD DEGASSING IN BASIC MAGMAS Article Open access 25 October 2024 INTRODUCTION In recent years, numerous examples
of tin-polymetallic mineralization have been discovered in the southern Great Xing’an Range (SGXR), making the SGXR the most important Sn belt in North China. Among these newly explored
deposits, the Weilasituo Sn-polymetallic deposit is the best suited for detailed investigation because of its large reserves and its large-scale metal zonation. The Weilasituo deposit was
discovered in 2013 and contains 11.2 Mt of ore at grades of 0.8% Sn, 0.42% WO3, 0.12% Mo, and 2.59% Zn1. Other nearby deposits include the Weilasituo Cu–Zn deposit (~ 2 km NW) and the
Bairendaba Ag–Pb–Zn deposit (~ 6 km NW). These three deposits have been studied previously, focusing on the geology of the deposit2, timing of ore formation1,2,3,4, character of ore-forming
fluids5,6,7, and ore source and precipitation mechanisms8,9,10,11,12. However, the genetic relationship between the Sn-polymetallic deposit and the other two deposits in the district, if any
exists, is poorly understood, thus hampering development of a holistic exploration model. If all mineralization types in the district are products of a single ore system, the observed
lateral zoning of mineralization across the district may reflect migration and cooling of ore-forming fluids derived from a single magmatic-hydrothermal system. Alternatively, if ore
formation was a multi-episode process, fluids and possibly also metals, may be derived from different sources. For polymetallic deposits related to granitic intrusions, the
magmatic-hydrothermal transition is a key process for the formation of hydrothermal ore deposits13,14. Consequently, constraining the physical and chemical evolution of ore-forming fluids
during this process is important. Rapid advances in microanalytical capability in recent years have led to an improved understanding of the evolution of ore-forming fluids and ore
precipitation mechanisms15,16,17. Given that quartz is mechanically and chemically resistant, quartz geochemistry is a robust tool to evaluate the fractionation mechanisms and
crystallization processes across the magmatic-hydrothermal transition18,19,20,21,22,23. The Ge/Ti and Al/Ti ratios of quartz can be used as an index of igneous differentiation20,24,25 and
the Ti content in quartz can indicate temperature, pressure, and TiO2 activity in magma26,27. In addition, experimental and microanalytical studies show that trace element contents and their
ratios in sphalerite can effectively reflect the physical and chemical conditions of the mineralization process28,29,30. In this contribution, we present new fluid inclusion, isotope
geochemistry to ascertain the source and evolution of ore-forming fluids. The abundances of trace elements in sphalerite and quartz in different stages are determined to clarify the
distribution and behavior of ore-forming elements during the magmatic-hydrothermal transitions and to trace ore-forming processes. These results enable us to establish a genetic model for
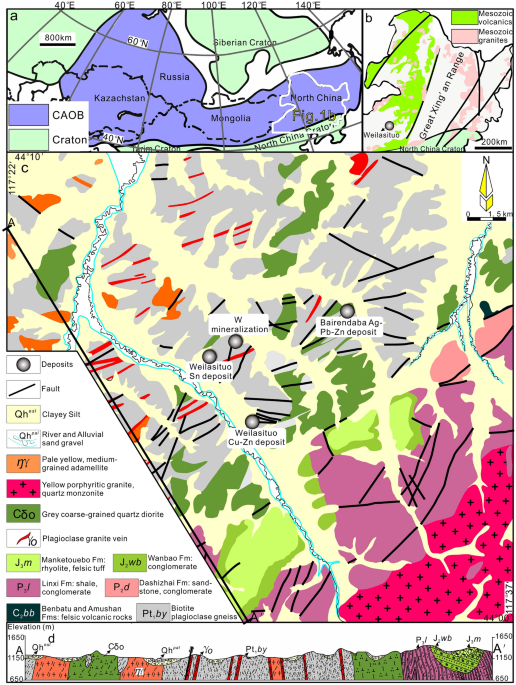
the Sn-polymetallic mineralization. REGIONAL GEOLOGICAL BACKGROUND AND GEOLOGY OF THE WEILASITUO ORE DISTRICT The SGXR is located in the southeastern part of Inner Mongolia, China, which
belongs to the eastern part of the Central Asian Orogenic Belt (CAOB) (Fig. 1a, b). Amphibole-plagioclase and biotite-plagioclase gneisses of the Late Paleozoic Xilin Gol Complex31 (397–294
Ma) are overlain by (Carboniferous)-Permian to Jurassic sedimentary units (Fig. 1c, d). The latter are dominated by sandstones, siltstones, and carbonaceous mudstones deposited on a shallow
shelf (Fig. 1d). There are intercalations of Upper Carboniferous and Jurassic felsic volcanic rocks32,33 and Permian andesite, as well as marine, typically bioclastic limestone (Fig. 1c).
The Weilasituo ore district, at the northern margin of Keshketen Banner in Inner Mongolia, includes the Weilasituo Sn-polymetallic and Weilasituo Cu–Zn deposits, within an exposed area of 8
km2. The Bairendaba Ag–Pb–Zn deposit is located ~ 4 km NE from the Weilasituo Cu–Zn deposit (Fig. 1c). These three deposits occur within the Xilin Gol Complex, which in this region is only
covered by Quaternary sediments (Fig. 2a). Biotite plagioclase gneiss of the Xilin Gol Complex is the dominant rock type and the main lithology hosting ore (Fig. 2c; Table 1). Intrusive
rocks include quartz diorite and quartz porphyry (in some publications also called fine-grained porphyritic alkali-feldspar granite), the latter associated with Sn-W-Li mineralization.
Quartz diorite is exposed in the central part of the area and hosts Cu–Zn mineralization (Fig. 2b), zircon ages indicate late Carboniferous crystallization with a weighted-mean SHRIMP U–Pb
age of 308.3 ± 4.2 Ma3. The quartz porphyry is much younger and has an Early Cretaceous U–Pb zircon age of 135 ± 2 Ma2, in agreement with U–Pb cassiterite ages of 138 ± 6 Ma to 135 ± 6 Ma1.
CONCEALED QUARTZ PORPHYRY INTRUSION WITH ASSOCIATED WEILASITUO SN-POLYMETALIC DEPOSIT The Weilasituo Sn-polymetallic deposit was discovered in 2013. Reserve estimates list 67 economic
orebodies, containing a total of 87,281 t Sn, 12,633 t WO3, 357,200 t Li2O, 16,987 t Zn, and 271 t Mo34. The concealed quartz porphyry body was identified in drill holes at depth > 400 m
below surface (Figs. 2c, 3a). The deepest drillhole intersecting the quartz porphyry reached a depth of ~ 1450 m. Evaluation of this drillhole indicates three facies of quartz porphyry. The
quartz porphyry in the deepest area is light sky-blue due to the presence of amazonite, has a massive structure and a porphyritic texture with dominant quartz phenocrysts (Fig. 3b). The
matrix is composed of fine-grained quartz and K-feldspar, the accessory mineral assemblage consists of biotite, topaz, and zircon (Fig. 3b). The central part of the intrusion, about ~ 800 m
below surface, is albitized and has a porphyritic texture with phenocrysts of quartz and albite occurring in a matrix of albite, quartz and some Fe-Li mica and accessory minerals including
zircon (Figs. 3c, 4a). At shallower levels (400–500 m below surface), the intensity of albitization decreases, whereas quartz and mica contents increase (i.e., greisenization), accessory
minerals include fluorite, topaz and zircon, Sn-W-Li and sulfide mineralization is present (Fig. 3d). The porphyry belongs to the high-K calc-alkaline series and is strongly enriched in Si,
Al, and depleted in Ca, Ti and P. The A/CNK value of quartz porphyry usually ranges from 1.0 to 1.2; most analyses yield a value < 1.1, which is typical for I-type granites. The low
values of K/Rb, Nb/Ta, and Zr/Hf of the porphyry reflect a high degree of magma differentiation1,4. At the top of the stock, the quartz porphyry is rimmed by a pegmatite-like stockscheider
zone with coarse K-feldspar crystals growing perpendicularly from the contact inside the stock. The stockscheider zone (Fig. 3e) occurs as a shell on top of the parent rock and has a maximum
thickness of ~ 4 m. Its upper part is dominated by amazonite and its lower part by quartz. A pipe-shaped cryptoexplosive breccia body extends from the uppermost part of the granite porphyry
body toward the surface (Fig. 2c). It is ~ 500 m in length with an elliptical outcrop of 30 m × 20 m at the present surface. The breccia comprises strongly altered clasts of
biotite-plagioclase gneiss and quartz diorite in a cement of quartz, mica, and felsic igneous minerals (ESM Fig. 1c). Three types of mineralization are associated with the quartz porphyry:
(1) shallow quartz-cassiterite-sphalerite veins; disseminated ore in (2) breccia pipe and in (3) the upper part of the porphyry intrusion. The deeper part of the deposit is a lenticular body
directly at the top of the porphyry stock (Fig. 2c). Eighteen disseminated mineralization zones (14 Sn and 4 Zn mineralization zones) have been delineated, including 7 zones that exceed the
cut-off grade (Sn: 0.2%, Zn: 1%). Among these, orebody #Sn200 hosts > 82.6% of the dissemination-type ore reserves. It is 337 m long with a thickness of 10 m in its central part, has an
overall strike of 25°, dip of 5° to E. The average Sn grade is 0.23%, with highest Zn and WO3 grades of 1.74% and 0.098%, respectively. Stannite, sphalerite, and molybdenite are the dominant
ore minerals in this type of mineralization and are associated with cassiterite and wolframite (Table 1). Breccia-type ore bodies occur in the cryptoexplosive breccia, which has the shape
of a steep pipe with an elliptical cross-section 140–300 m in diameter, a long axis strike of 30°, and 640 m vertical extent34. Breccia clasts are commonly rimmed by zinnwaldite rich in Li,
Nb, and Ta (ESM Fig. 1c). Lithium contents exceed cut-off grade (0.7% Li2O) with good prospects for exploitation34. Cassiterite, sphalerite and other ore minerals occur within the breccia
cement. The shallow quartz-cassiterite-sphalerite veins form inclined orebodies consisting of densely packed veins, with an overall strike of 25° and dip 11–54° SE (Table 1). A total of 231
quartz veins have been identified so far, 60 of these exceed cut-off grades (Sn: 0.2%, Zn: 1%) for resource/reserve estimation. The #Sn0 Sn-Zn-Mo-W vein contains ~ 66% of the total reserves,
and is located in the middle part of the exploration area. It is accessible 130 m below the present surface and is 900 m long with a maximum thickness of 9 m in its central part, with a
strike and dip of 13–45/23–25° SE1 (Fig. 2c). Cassiterite (ESM Fig. 1e; Fig. 4d), wolframite, and sphalerite (Fig. 4b, c) are the main ore minerals. Quartz, zinnwaldite (ESM Fig. 1a), and
smectite (ESM Fig. 1g) are the main gangue minerals. Montmorillonite is widespread in the surrounding rock (Fig. 3f), and abundant zinnwaldite and cassiterite are observed attached to the
montmorillonite. About ~ 1.0 km east to the Weilasituo Sn-polymetallic deposit (Fig. 2a), there is a small tungsten deposit with an average grade of 0.83% that contains 603 t WO3. The main
tungsten-polymetallic vein (#121–1) has a strike and dip of 35–41/38–52° SE. Wolframite, molybdenite and sphalerite are the main ore minerals (Table 1). The deposit has a molybdenite Re-Os
isochron age of 129.0 ± 4.6 Ma4, comparable with the crystallization and mineralization age of the Weilasituo Sn-polymetallic deposit and the mineralization age of the Weilasituo Cu–Zn
deposit (Table 1). WEILASITUO CU–ZN DEPOSIT The Weilasituo Cu–Zn deposit lies ~ 2 km SE of the Weilasituo Sn-polymetallic deposit and contains a 10 Mt ore reserves at grades of 5% Zn, 0.8%
Cu, and 75 g/t Ag (Table 1). The deposit is composed of 121 ore bodies, mostly simple quartz veins5, two of which outcrop at surface. The host rocks are mainly biotite plagioclase gneiss and
locally also quartz diorite (Table 1). Thirty-five ore veins, exceeding cut-off grade (Cu: 0.3%, Zn: 1%, Pb: 0.7%, Ag: 50 g/t), are included in the reserve estimate. Vein size varies from
tens of meters to > 1000 m in length and they extend from depths of tens of meters to more than 900 m; vein thickness locally reaches up to > 10 m. Except for some NNE-trending veins,
the veins trend nearly E-W and dip to the N with an inclination of 8–35° (Table 1). Among these, the #1 vein is the main orebody (Fig. 2b), accounting for over half the total resource. It
mainly contains Zn and Cu, accompanied by Ag and W. The sulfide ore is composed of sphalerite, chalcopyrite, pyrrhotite, arsenopyrite and galena (ESM Fig. 1d; Fig. 4f, g); gangue minerals
include quartz and mica, as well as calcite, fluorite, and illite (Fig. 4h–l). Alteration in the main mineralization stage includes silicification and sericitization, as well as the
formation of fluorite and topaz. Alteration during late mineralization stages includes carbonatization, chloritization (Fig. 3g) and kaolinization. Silicification and sericitization occur at
the contact between quartz veins and plagioclase gneiss, accompanied by flurite and topaz. Carbonation and kaolinization often occur at the contact between plagioclase gneiss and quartz
diorite, accompanied by chloritization. BAIRENDABA AG–PB–ZN DEPOSIT The deposit is divided into an eastern and a western domain. In the eastern domain, 54 veins were identified and 22 are
exploited for Ag, Pb, and Zn. The veins strike almost E–W and are inclined 10–50° to the north. The eastern domain has reserves of 0.9 Mt Zn, 0.45 Mt Pb and 3961 t Ag (Table 1). The #1 vein
accounts for nearly 80% of the total ore reserves in the eastern domain2. It is 2075 m long with an average thickness of 4.6 m and an average ore grade of ~ 250 g/t Ag, 4.8% Pb, and 6.02%
Zn. The dip of the vein gradually changes from 17° in the west to 20° in the deposit center (No. 0 exploration line), and 35° in the east. The western domain includes 167 veins, of which 47
are of exploitable grade: the main vein (No. 3) accounts for ~ 65% of the total reserves in the western domain (0.49 Mt Zn, 0.05 Mt Pb, and 276 t Ag). It is 1200 m long, extends to a depth
of ~ 700 m, and has an average thickness of almost 5 m. The veins generally strike nearly E–W, and dip 8–50° to the north. The ore is mostly composed of pyrrhotite, sphalerite, and
chalcopyrite, with an average Zn grade of about 5%. In the middle and lower parts of the veins, Cu-rich and/or Cu–Zn ores have average Cu grades of ~ 0.70%, whereas in the west, Ag-Pb–Zn
ores have an average grade of 286.6 g/t Ag and 4.11% Pb. Pre- and syn-ore wall rock alteration is represented by chloritization, silicification, and illite-fluorite alteration. Late-stage
carbonatization and fluoritization is recognised. The main sulfides are sphalerite, chalcopyrite, pyrrhotite and galena (Table 1). Gangue minerals are mainly quartz, calcite, and fluorite.
SAMPLES AND RESULTS A suite of 38 samples were collected from outcrops, tunnels and drillcores in the Weilasituo deposit, 3 samples were sourced from tunnels in the Bairendaba deposit.
Sample locations and characterization is presented in ESM Table 1. These samples originate from all four stages of ore formation1,4 described below. In addition to these rock samples, we
collected two quartz crystals from vugs adjacent to the Sn-bearing quartz veins of the Weilasituo Sn deposit near the quartz porphyry. These quartz crystals have inclusions of cassiterite
and opaque minerals with metallic appearance (samples WQ-5 and WQ-6; Fig. 5a–c). The applied analytical methods included cathodoluminescence imaging, fluid inclusion and Raman studies,
hydrogen and oxygen isotope analysis and LA-ICP-MS trace element analysis (see ESM for details). STAGES OF MINERALIZATION According to their different mineral assemblages combined with
information from prior studies1,4, four stages are identified in the Weilasituo Sn-polymetallic deposit: * 1. Magmatic stage (Stage I), including the evolution of the granitic magma,
undercooling, and crystallization of the stockscheider (Fig. 3e) followed by crystallization of the quartz porphyry, which contains abundant snowball-structured quartz phenocrysts (Fig. 4a).
Only minor amounts of ore minerals (zinnwaldite) precipitated at this stage, gangue minerals are mainly quartz, albite, amazonite, topaz and a small amount of fluorite and zircon. * 2.
Magmatic-hydrothermal transition stage (Stage II). This stage includes exsolution and accumulation of volatiles from the residual magma, followed by the formation of a cryptoexplosive
breccia pipe due to the rapid separation and crystallization of volatiles. The mineralization mainly occurs in the breccia pipe (ESM Fig. 1c), which contains abundant zinnwaldite with a Li2O
concentration of 4.7 wt%4. Zinnwaldite is the major ore minerals are mainly (ESM Fig. 1c) in a mineral paragenesis including quartz, albite, fluorite, topaz, and minor amazonite and zircon
(Table 1). * 3. High-temperature hydrothermal stage (Stage III), which includes early greisenization at the top of the quartz porphyry body and late quartz veins, is the main mineralization
stage in the Weilasituo Sn-polymetallic deposit. The greisenization is associated with zones of disseminated mineralization. Minor disseminations of stannite and sphalerite also formed
during this stage. Quartz + zinnwaldite + cassiterite + sphalerite veins were encountered in the 100–400 m interval (Fig. 2c; ESM Fig. 1a), where veins host abundant vugs containing crystals
of euhedral quartz, sphalerite, cassiterite and fluorite (ESM Fig. 1b; Fig. 4c–e). Ore minerals are mainly cassiterite, wolframite, sphalerite and minor molybdenite (Table 1). Cassiterite
is mainly associated with sphalerite (ESM Fig. 1e; Fig. 4c) and features a regular zonal structure with dark colored cores and brighter rims (Fig. 4d, e), while wolframite is associated with
molybdenite. Furthermore, the mineral assemblage includes quartz, fluorite, zinnwaldite, montmorillonite, löllingite, and arsenopyrite (ESM Fig. 1a, b; Table 1). Pyrite is minor in this
stage, and the main Fe-bearing minerals are löllingite, arsenopyrite, and zinnwaldite. Chalcopyrite is sometimes observed within quartz (Fig. 5e). * 4. Low-temperature hydrothermal stage
(Stage IV), representing both the main mineralization stage of the Weilasituo Cu–Zn deposit and a late overprint in the Sn-polymetallic deposit. Sulfide assemblages evolve from sphalerite +
löllingite + arsenopyrite in the Sn deposit to sphalerite + chalcopyrite + pyrrhotite + pyrite + arsenopyrite in the Cu–Zn deposit. The main ore minerals are sphalerite and chalcopyrite (ESM
Fig. 1d). Futhermore, there is quartz, mica, fluorite, pyrrhotite and arsenopyrite (Table 1). Mica, associated with fluorite in the wall rocks, displays obvious early-stage growth zoning
(Fig. 4h). FLUID INCLUSION MICROTHERMOMETRY Most of the fluid inclusion measurements were carried out on quartz phenocrysts from granite and quartz vein samples. The inclusions commonly show
negative crystal shapes and generally have a size between 10 and 35 μm (Fig. 6a–f). Although fluid inclusions are occasionally observed in sphalerite (Fig. 6g, h), most are too small
(typically 2–8 μm in diameter) to enable observation of phase changes during freezing-heating. It was, however, possible to obtain homogenization temperatures and salinity data from several
large fluid inclusions in Stage III and IV sphalerite (Fig. 6g, h). Two main types of fluid inclusions are distinguished based on phase relations at room temperature: Type I liquid–vapor
(LV) two-phase inclusions (Fig. 6d–g); and Type II liquid–vapor-solid (LVS) three-phase inclusions (Fig. 6a–c). Type I fluid inclusions are the dominant type in the Weilasituo ore district.
Sporadic minor solid phases occur in all types of fluid inclusions, except for those of the final stage. Type I fluid inclusions occur in three stages of ore formation (Stages I, III and
IV). Compositions change from aqueous two-phase inclusions with 25–50 vol% H2O–vapor at room temperature (Stage I) (Fig. 6a, b), through vapor-rich aqueous two-phase inclusions with 33–40
vol% gas phase at room temperature (Stage III) (Fig. 6c, d), to 25–33 vol% gas phase at room temperature (Stage IV) (Fig. 6e, f). Stage III and Stage IV fluid inclusions in quartz are
typically 10–30 μm in diameter, whereas in sphalerite, they may reach 40 μm in diameter and generally contain 25–33 vol% vapor (Fig. 6g, h). Abundant LV fluid inclusions with 30–35 vol%
vapor occur in sample WQ-5. Groups of small (15–25 μm) fluid inclusions occur in the crystal core while individual larger (25–45 μm) inclusions were found in the crystal rim (Fig. 7).
THLV–L, SALINITY AND RAMAN SPECTROSCOPY The salinities of primary inclusions were determined from the final ice melting temperatures35. Primary Stage I and Stage III inclusions in quartz
from the porphyry and from quartz veins containing W- and Sn-minerals have salinities of 2.7–13.7 wt% and 3.9–8.0 wt% NaClequiv, respectively, and respective homogenization temperatures of
226–379 °C and 208–367 °C (Table 2). Stage IV fluid inclusions in quartz show liquid homogenization temperatures of 176–317 °C (mean 240 °C) and salinities of 2.2–7.6 wt% NaClequiv (mean 3.9
wt% NaClequiv) (Fig. 8a, b). There are only few temperature and salinity determinations for Stage III and IV fluid inclusions in sphalerite. These data are shown for reference in Fig. 8a,
b. For sample WQ-5, the mean ThLV–L of the crystal core is 284 °C, consistent with the ThLV–L data of Stage III (Fig. 7), and 260 °C for the crystal rim. The corresponding salinities are 6.0
wt% and 5.5 wt% NaClequiv, respectively (Fig. 7). Raman spectra of five representative Type I and Type II fluid inclusions are shown in ESM Fig. 2. Most spectra show broad peaks for water
and host quartz (464 cm−1) (Fig. ESM Fig. 2a–d), and sharp peaks for CH4 at 2918 cm−1 (ESM Fig. 2a, Stage I) and 2915 cm−1 (ESM Fig. 2d, Stage III). Peaks at 3077 cm−1 (C6H6) in Stage III
inclusion (ESM Fig. 2e) indicate alkylation in the early and middle stages of fluid evolution. Sharp peaks at 1091 cm−1 in Stage I fluid inclusions (ESM Fig. 2b) are typical for carbonates.
Fluids from Stage I inclusions show broad water peaks. The sharp peak at 877 cm−1 (ESM Fig. 2c) may indicate that the liquid contains F-bearing phase. H–O ISOTOPES Oxygen isotope data for 15
quartz samples and hydrogen isotope data for 14 quartz samples are presented in ESM Table 2. Two Stage I samples show δ18OQ (v–SMOW) values of + 12.6 and + 12.8‰, corresponding to
calculated δ18OH2O (v–SMOW) values of + 5.5‰ and + 6.2‰. The δDv–SMOW value of a single Stage I sample is − 67‰. Seven Stage III samples were analyzed. The δ18OQ (v–SMOW) values fall within
the range + 9.1‰ to + 11.7‰ and the calculated δ18OH2O (v–SMOW) values lie between + 2.3‰ and + 4.0‰ (Fig. 9). The δDv–SMOW values are around − 90‰, except for sample WL-18 which has a
δDv–SMOW value of − 109‰. The δDv–SMOW and δ18OQ (v–SMOW) values decrease from Stage I to Stage III (Fig. 9). The δ18OQ (v–SMOW) the δDv–SMOW values of Stage IV samples fall in the range
from + 11.3‰ and + 12.0‰, whereas those from Stage IV samples are around − 130‰ (except for sample WL-24 with a δDv–SMOW value of − 93‰) (Fig. 9). SPHALERITE GEOCHEMISTRY Representative
sphalerite samples from the Weilasituo Sn and Cu–Zn deposits including quartz-vein type, porphyry-type ore, and quartz crystals from vugs, and three quartz-vein ore samples from the
Bairendaba Ag–Pb–Zn deposit were analyzed by LA-ICP-MS. Results (142 spot analyses) are presented in ESM Table 3. Sphalerite from all samples is enriched in Cu (117 ppm–1.87 wt.%), Fe
(0.91–14.4 wt%), Mn (182–1580 ppm), and Cd (893–4050 ppm). Sphalerite from the Weilasituo Cu–Zn and Bairendaba Ag–Pb–Zn deposits have higher Cu + Fe, Mn, Cd contents and higher Fe/Zn ratios
than sphalerites from the Weilasituo Sn deposit (Fig. 10d–f; ESM Fig. 3). In the latter, there is a systematic decrease of Cu and Fe content from porphyry-type sphalerite to quartz-vein-type
sphalerite (Fig. 10d). The concentrations of trace elements are highly variable, e.g. 0.08–312 ppm Sb, 0.44–5.18 ppm Ge, 1–8830 ppm Sn, and 1–1120 ppm In (Fig. 10a). Compared to the
Weilasituo Sn deposit, the sphalerite from the Weilasituo Cu–Zn and Bairendaba Ag–Pb–Zn deposits has higher Ge contents. Different types of sphalerite from the Weilasituo Sn deposit show
similar Ge content (Fig. 10b). Quartz-vein-type sphalerite has lower Ga contents than sphalerite from the quartz crystal and porphyry-type mineralization (Fig. 10b). Ga/Ge ratio of
sphalerite decreases from Weilasituo Sn deposit to Cu–Zn deposit and shows a positive correlation with the Cd/Fe ratio (limited to the hydrothermal system) (Fig. 10c). Sphalerite from the
quartz crystal has the highest Sn content, whereas quartz-vein-type sphalerite from the Weilasituo Sn deposit has the highest In contents (Fig. 10a). CATHODOLUMINESCENCE TEXTURES AND QUARTZ
CHEMISTRY CL images show growth zoning for the quartz crystals WQ-5 and WQ-6 from the vug (Fig. 5f, g). Inclusions show a regular pattern with cassiterite inclusions in the crystal core
(Fig. 5d), sphalerite, chalcopyrite and arsenopyrite inclusions in the transitional zone (Fig. 5e, g), and arsenopyrite in the crystal rim (Fig. 5f). A total of 136 spot analyses were
conducted, with 6–15 analyses of each quartz type in three representative rock samples (quartz porphyry, breccia pipe, quartz vein), and 86 analyses from two quartz crystals from vugs. Data
are presented in ESM Table 4. Titanium contents in quartz (2.3–17.8 ppm, Fig. 11a) decrease from breccia pipe (8.0–17.8 ppm Ti) to quartz vein (4.4–9.6 ppm), quartz crystals from vugs
(2.4–7.4 ppm Ti), and phenocrysts in quartz porphyry (2.3–3.2 ppm). Concentrations of Ge (Fig. 11e) show a similar trend with decreasing contents from breccia pipe (3.8–6.2 ppm) to quartz
vein (3.2–4.4 ppm), granite porphyry (2.6–3.5 ppm), and quartz crystal (1.2–5.5 ppm). The Ge and Ti contents of quartz broadly correlate positively and the compositional ranges covered by
the different types of quartz partially overlap (Fig. 11a, e). The Al content in quartz from quartz porphyry (165–695 ppm) is generally higher than in the other quartz types (mostly < 200
ppm and as low as 14.7 ppm in some cases; Fig. 11b). The Al contents of the quartz crystals from vugs range from 14.7 to 263 ppm, typically with higher concentrations in the core than the
rim (Fig. 11b). Lithium contents of our samples range from 16–35 ppm in magmatic quartz to 0.05–29 ppm in hydrothermal quartz (Fig. 11c). Note that the cores of quartz crystals have very
high Li contents (13.5–495 ppm Li). Our samples show Ge/Ti values in the range 0.86–1.16 for magmatic quartz (Fig. 11h), but only 0.19–1.0 for most hydrothermal quartz (Fig. 11b). The
contents of Sn, Sc, and B in quartz are low (Fig. 11d, f, g), typically < 1, < 3, and < 8 ppm, respectively. Higher contents of these elements are only observed in quartz crystals
from vugs, which display markedly higher Sn (up to 3.1 ppm) (Fig. 11f) and Sc (up to 4.9 ppm) (Fig. 11g) in the rim. Boron contents of quartz range from 0.4 to 11.7 ppm with no clear
distinction between core and rim (Fig. 11d). DISCUSSION PROPERTIES AND EVOLUTION OF ORE-FORMING FLUIDS From the magmatic to late hydrothermal stage, the homogenization temperatures of fluid
inclusions decreased from relatively high temperature (313 °C, Fig. 8a) to low temperature (240 °C, Fig. 8a) and from relatively high salinity (7.1 wt% NaClequiv, Fig. 8b) to low salinity
(3.9 wt% NaClequiv, Fig. 8b). Raman data show that the main ore-forming fluids in Stage I and III have H2O–NaCl–NaF ± CO2 ± CH4 compositions. The CH4 Raman signal is conspicuous, whereas the
CO2 signal is weak, reflecting the relatively reduced nature of the ore-forming fluids. Furthermore, CH4 was previously shown to be ubiquitous in fluid inclusions from the Weilasituo Cu–Zn
and Bairendaba Ag–Pb–Zn deposits5,6 (Table 1). The occurrence of falkmanite (Pb5Sb4S11) reported from the Bairendaba Ag–Pb–Zn deposit36 is further evidence for reducing conditions. The
origin of CH4 can be related to microbial activity, thermal decomposition of organic matter, or abiogenic sources, such as the mantle or Fischer–Tropsch-type (FTT) reactions (reaction of CO2
or CO with H2)37,38. The temperature of microbial activity39 (< 120 °C) is considerably lower than the mineralization temperature in the Weilasituo mining area, suggesting that the
effect of microbial activity on the formation of CH4 is negligible. It is hard to envisage how CH4 from deep mantle degassing might be incorporated into ore-forming fluid40 (CH4 reacts with
O2 leading to the formation of CO2), it cannot be retained because of oxidation by Fe3+41. Formation of large quantities of CH4 by FTT reactions requires large volumes of hydrogen as well as
catalysts like FeNi and FeCr oxides42, which may be present in mafic, but not in felsic rocks. Moreover, if all CH4 comes from the FTT reaction, higher hydrocarbons like C6H6 (ESM Fig. 2e)
and C2H6 should not occur in extracted gas37. To sum up, we believe that the source of CH4 is mainly organic, and only partly originates from FTT reactions. Carbonaceous slates of the Upper
Permian Linxi Formation (Fig. 1c), an important hydrocarbon source horizon in the SGXR, may represent one plausible CH4 source. The hydrogen and oxygen isotope data (Fig. 9) indicate that
samples from Stage I are within or near the field of magmatic fluids, indicating that the ore-forming fluid may be derived from magma. Whereas samples of Stage III and Stage IV fall below
the field of magmatic fluids, showing lower δD values than Stage I fluids. In addition to changes in _f_O2 of ore-forming fluid and the influence of CH4 (in natural hydrothermal systems this
effect is typically small43), there are several processes that may cause a decrease of δD values. These include addition of high-altitude meteoric water43,44, magma degassing45,46, boiling
with loss of the vapor phase45,47, and mixing with external fluids, including formation water from host-rocks48. The formation of a breccia pipe extending from the upper part of the granite
porphyry body supports the important role of magma degassing during the magmatic-hydrothermal transition (Stage II). Moreover, in addition to a decrease in δD values, δ18OH2O (v–SMOW) values
also decreased slightly from Stage I to Stage III. Our fluid inclusion study provide evidence for fluid boiling in Stage III (Fig. 6d). Thus, the depleted δD values of the Stage III fluid
can be attributed to magma degassing during Stage II and fluid boiling in Stage III. Magma degassing and fluid boiling cannot, however, explain the markedly depleted δD values (− 136‰) in
Stage IV fluid (Fig. 9). The high CH4 concentrations in the fluids indicate the addition of external fluid to ore-forming fluids, which is confirmed by previous He-Ar isotopic data of
sulfide minerals from Stage III and IV showing higher 40Ar/36Ar ratios than saturated rainwater but lower 40Ar*/4He ratios than crustal and mantle fluids4. The addition of external fluids
can lead to a decrease in both the δD and δ18O values of the fluid. However, δ18O values show small displacement from Stage III (mean δ18O value: + 3.4‰) to Stage IV (mean δ18O value: +
1.8‰). Therefore, the continuous decrease of δD values from Stage III to Stage IV fluids could be interpreted as resulting from mixing with small amount external fluids that could be derived
from the wall rocks and may have contributions of meteoric water. TRACING ORE-FORMING PROCESSES: INSIGHTS FROM SPHALERITE GEOCHEMISTRY AND _F_S2 EVOLUTION Ions that have a similar radius as
Zn2+ (0.60 Å) may easily substitute into sphalerite in tetrahedral coordination30. Cd2+ (0.78 Å), Mn2+ (0.66 Å), and Co2+ (0.58 Å) are all typically enriched in sphalerite relative to
coexisting minerals28. Cu+ (0.60 Å) is preferentially incorporated into sphalerite via the coupled substitution Cu+ + X3+ ↔ 2Zn2+49, where trivalent X is commonly In or Sb30. Differences in
concentrations of these substituting elements may reflect their variable temporal or spatial availability. Sphalerite from the central Sn-Zn veins in Weilasituo Sn deposit has higher In
contents than sphalerite from the Weilasituo Cu–Zn and Bairendaba Ag–Pb–Zn deposits (Fig. 10a), possibly indicating that the early Sn-Zn veins formed from hydrothermal fluids with higher In
content. Sphalerite from different mineralization types within the Weilasituo Sn deposit, a pyrrhotite-free sulfide system, show a systematic decrease in Fe and increase in Cd content from
porphyry-type to quartz vein-type mineralization and individual sphalerite inclusions in quartz (Fig. 10e). In contrast, sphalerite from the Weilasituo Cu–Zn and Bairendaba Ag-Pb–Zn
deposits, which both carry pyrrhotite-bearing sulfide assemblages, shows elevated Fe and Cd (Fig. 10e). Factors controlling the Fe content in sphalerite, in addition to Fe availability and
Py/Po buffering, include pressure and temperature. For instance, in pyrrhotite-free sulfide systems, the FeS content in sphalerite increases with fluid temperature29, whereas the Cd/Fe ratio
decreases50. Sphalerite from the Weilasituo Sn deposit show a systematic variation in Cd/Fe ratio from 0.02 (porphyry type), 0.05–0.12 (quartz-vein type), to 0.12–0.16 (inclusions in the
quartz crystal), suggesting a temperature decrease (Fig. 10c, e). The increase of Cd/Fe with decreasing temperature may also be affected by the precipitation of Fe-rich zinnwaldite (which
contains ~ 6.5 to 10.3 wt% FeO)4 during the magmatic-hydrothermal transition stage. Sphalerite from Sn-Zn veins in the central part of the Sn deposit contains 2.09 wt% Fe (average value) and
the fluid inclusions in sphalerite homogenize at 271 °C. However, sphalerites from Cu–Zn veins in the peripheral Cu–Zn deposit have much higher Fe contents (up to 12.15 wt%), while
displaying similar homogenization temperatures of ~ 250 °C (Fig. 10d, e). These major differences in Fe content are not explained by temperature differences, but may have two alternative
explanations. First, pyrrhotite-dominant sulfide systems buffered by Py/Po typically contain sphalerite with very high Fe content51,52. Second, availability of Fe may control the
distribution of Fe-sulfides, with Fe being mainly hosted in minerals such as löllingite, arsenopyrite and zinnwaldite in the Sn deposit (Table 1) and in pyrrhotite in the peripheral Cu–Zn
deposit (Table 1), where Fe is more available. The different Fe availability may be contributed by either of two processes. (i) Magma degassing and boiling during formation of the breccia
pipe released vapor-rich fluids with higher Fe content forming a distal Cu–Zn deposit, whereas the remaining liquid-rich fluid formed the Sn-Zn deposit. However, vapor-rich fluid inclusions
account for only a small proportion in the Weilasituo area5,6. Additionally, Fe and Zn preferentially partition into a Cl–-bearing liquid-rich fluid, whereas Cu is preferentially found in
the HS–-bearing vapor-rich fluid during phase separation53,54 (Cu concentrations remain higher in the coexisting liquid). Although part of metal endowment of the vapor-rich fluid could
migrate further, that amount was insufficient to form peripheral Cu–Zn and Ag–Pb–Zn deposits (Table 1). We can thus rule out this possibility as unlikely. (ii) Magmatic fluids had lower Fe
contents than late fluids that had mixed with an external Fe-rich source. Our hydrogen and oxygen isotope data indicate the contribution of external fluids derived from the wall rocks in
Stage IV. Stable (S-Pb) isotope data (Table 1) indicates that the source of ore-forming materials in the Weilasituo Cu–Zn and Bairendaba Ag–Pb–Zn deposits were mainly magmatic with some
contribution from wall rocks5,55. The biotite plagioclase gneiss, the main ore-host, contains 3–6 wt% TFeO, while the quartz porphyry is Fe-poor (~ 0.3 wt% TFeO)4. Petrographic evidence
shows that mica from the wall rock of Cu–Zn mining area underwent strong late-stage alteration, releasing a large amount of Fe (Fig. 4h–l). In addition, the mica accompanied by sphalerite or
galena from the Cu–Zn deposit contains 2169–3772 ppm Zn but extremely low Cu (< 1 ppm Cu)7. Therefore, external fluids that altered or dissolved mica from wall rocks (Fig. 4j) may be an
important source of Fe, potentially also Zn in the late stage of mineralization. The trace element content characteristics of sphalerite from the Bairendaba mining area are consistent with
those of the Weilasituo Cu–Zn deposit suggesting similar formation processes or the involvement of similar sources (Fig. 10a–e). EVOLUTION OF QUARTZ COMPOSITIONS AND TI-IN-QUARTZ
GEOTHERMOBAROMETRY Quartz has a highly variable trace element composition spanning the fields of magmatic quartz (quartz porphyry) and hydrothermal quartz (quartz veins) (Fig. 11b–e).
Magmatic quartz shows extremely low Ti contents (2–4 ppm) and higher Al and Li, whereas hydrothermal quartz has higher Sn and Sc contents. The Al and Li contents in quartz decrease gradually
from the quartz porphyry, through the breccia pipe, to the quartz veins (Fig. 11b, c), indicating that Al and Li availability diminished as the magma-hydrothermal system evolved. The quartz
phenocrysts from the porphyry have lower Ti contents than those from most rare-metal granites (mostly 20–110 ppm Ti)21. The quartz porphyry has low bulk-rock Ti contents (< 0.01 wt%) and
low K/Rb, Nb/Ta, and Zr/Hf values4. These very low Ti contents may reflect the higher differentiation of the quartz porphyry21, or alternatively, a slower rate of quartz precipitation27.
Lithium contents are strongly correlated with Al contents in all types of quartz (Fig. 11c). The Li/Al molar ratios are 0.4–0.6 in magmatic quartz but 0.6–0.8 for most hydrothermal types.
This is indicative of the coupled substitution shifting from Si4+ ↔ Al3+ + Li+0.4–0.6 (X+)0.4–0.6 to Si4+ ↔ Al3+ + Li+0.6–0.8 (X+)0.2–0.4 during magmatic-hydrothermal evolution, and that
the monovalent X+ was most likely H+56,57. Variation of Li and Al concentrations in quartz are most probably related to the medium from which it crystallized (either melt or fluid) as well
as pressure and temperature conditions. The Li concentration in the coexisting phases (melt, fluid and minerals) can be expected to be temperature (-pressure) dependent. Five calibrations
for the Ti-in-quartz thermobarometer have been published, one based on quartz coexisting with Ti-bearing silicate melts58, two for quartz coexisting with fluids (H2O ± NaCl fluids) and
rutile26,27, and the others synthesized quartz in the presence of rutile and either aqueous fluid or hydrous silicate melt59,60. Growth entrapment mechanisms and growth rate were considered
to cause deviations in calculation results27. Deviations of equilibrium concentration of Ti in quartz caused by growth entrapment is 10% in the worst-case scenario61. Osborne et al.60,
however, found that concentrations of Ti in individual quartz crystals from each experiment do not show evidence for growth rate-related phenomena. Given that the calibrations of Wark and
Watson59 and Thomas et al.26 were derived from high pressure data, we applied the Zhang et al.58 calibration to magmatic quartz and the latest calibration (by Osborne et al.60) to
hydrothermal quartz. Application of the Ti-in-quartz thermobarometer requires two conditions to be met: (1) the activity of rutile (aTiO2) in the system needs to be constrained; and (2)
either T or P needs to be constrained. The solubility of rutile is low in aqueous fluids, for instance, the calculated Ti solubility in H2O is ≤ 15 ppm at 800 °C, 1 kbar62. Thus, aTiO2 can
be assumed as 1 for hydrothermal veins in the present study. In contrast for rutile undersaturated quartz porphyry, \(\rm C_{\rm Ti}^{\rm Qz}\) (Ti concentrations of quartz), \(\rm C_{\rm
Ti}^{\rm liq}\) (Ti concentrations of coexisting silicate melt), and compositional parameters of the melt (FM values63) must be known, pressure or T can be constrained58. The estimated
formation pressure for quartz in breccia pipe, and quartz veins from microthermometric data of fluid inclusions based on the PVTX properties of H2O–NaCl64 was around 0.1 kbar. We therefore
assume a pressure of 0.1 kbar for the breccia pipe, the quartz veins, and the quartz crystals in vugs, and estimate the pressure of the quartz porphyry using the solidus curve of
water-saturated granite65 (ESM Fig. 5). For \(\rm C_{\rm Ti}^{\rm Qz}\) = 2.81 ppm (average value), \(\rm C_{\rm Ti}^{\rm liq}\) = 20.6 ppm, FM = 1.5264, and assuming water-saturated at the
granite solidus conditions65, crystallization conditions for the quartz in the porphyry can be constrained at around 700 °C for P = 1.5 kbar (ESM Fig. 5). For the breccia pipe and the
quartz veins, Ti-in-quartz thermometry gives 302–345 °C and 270–310 °C, respectively. ZONING IN QUARTZ: MULTI-EPISODIC FLUID AND IMPLICATIONS FOR SULFIDE PRECIPITATION Quartz crystals from
the vug in Weilasituo Sn deposit display a distinct CL response and chemical zoning (Fig. 5f, g) reflecting the involvement of at least three different fluids during crystal growth and,
implicitly, during deposit formation. Each episode begins with early CL-bright quartz and ends with CL-dark quartz. Solid inclusions in quartz are cassiterite for the first episode (Ep 1),
and sphalerite + chalcopyrite + arsenopyrite for the second and third episodes (Ep 2, Ep 3). Sulfides are mostly observed within CL-dark quartz (Fig. 5f, g). Trace element contents in quartz
crystals (from vug) display a continuous transition from magmatic signatures in the core (Ep 1core) to hydrothermal signatures in the rim (Ep 1rim) (Fig. 11b, c). This implies that early
fluids which separated from the magma inherited the characteristics of the quartz porphyry. The Ep 1 fluid is rich in Sn but depleted in Cu and Zn. Ep 2 and Ep 3 fluids are rich in Zn (Sn)
and Cu. The variation characteristics of element contents in Ep 2 fluid are consistent with those in Ep 1, for instance, the contents of Al and Li decrease from Ep 1core to Ep 1rim and
correspondingly from Ep 2core to Ep 2rim, although at a slightly lower overall level in the later fluids (Fig. 7). Sulfides only appear in the late stages of fluid evolution and are
concentrated within CL-dark quartz (Fig. 5f, g). Three possible processes may cause a decrease of Al and Li concentrations in quartz from core to rim. First, with ongoing zinnwaldite
precipitation, Li and Al concentrations in the fluid decrease. A second alternative involves temperature decrease. However, crystallization temperatures of quartz show broadly overlapping
ranges with 251–298 °C (Ep 1core), 261–290 °C (Ep 1rim), 253–289 °C (Ep 2core), and 248–290 °C (Ep 2rim)60. Thus, a decrease of temperature is unlikely to apply here. Thirdly, the addition
of heated external fluid or CH4-rich fluid from the Linxi Formation would dilute the Al and Li concentrations, resulting in transformation from a magmatic to a hydrothermal fluid (Fig. 11b,
c), and provide CH4 and possibly also Fe, Zn. Furthermore, SO2 produced by magma degassing (ESM Fig. 4) reacted with heated water to form SO42−, which was reduced by CH4 to S2−37. Thus,
addition of a reducing fluid promotes precipitation of chalcopyrite and sphalerite and inhibits precipitation of anhydrite and magnetite. On the larger scale, i.e. the Weilasituo Cu–Zn and
Bairendaba Ag-Pb–Zn deposits (Fig. 1c; Table 1), addition of externally sourced, CH4-rich fluids may have played an important role in formation of these deposits. METALLOGENIC MODEL FOR THE
WEILASITUO DISTRICT AND IMPLICATIONS Tin-(W)-Cu-Pb-Zn-Ag mineralization in the Weilasituo district is related to emplacement of Early Cretaceous Sn-rich quartz porphyry in an extensional
setting2,3. The quartz porphyry is highly differentiated and rich in F, Cl, S and H2O4. During magma ascent and crystallization, zinnwaldite crystallized at the top of the granite pluton,
consuming Fe2+ and Mg2+ from melt, reducing Cl solubility in the melt, and promoting exsolution of HCl ± HF-bearing fluids66,67. Reaction between magmatic HCl ± HF-bearing aqueous fluids and
feldspar resulted in greisen formation and precipitation of disseminated mineralization68,69. At the magmatic-hydrothermal transition stage, vapor was concentrated at the top of the
porphyry intrusion, eventually resulting in the formation of a cryptoexplosive breccia pipe that was flushed by upwelling volatile-rich fluids. During this process, halogen (Cl-F)-rich
fluids were likely to scavenge Sn, W, Zn, Li, and some Cu from highly evolved melts70. Formation of the breccia pipe resulted in a pressure drop that triggered fluid boiling, which in turn
led to precipitation of ore minerals. Part of the Cu, Fe, and Zn separated into a low-density, vapor-rich fluid and migrated away from the parent fluid to form fine Cu–Zn veins in distant
wall rocks. SO2 produced by magma degassing modified the ore-forming fluid from low _f_S2 to high _f_S2 (ESM Fig. 4). Most of the Nb and Ta was incorporated into zinnwaldite4. Tungsten
mineralization occurs in veins distal to the source of magmatic fluids, whereas Sn-Zn mineralization formed in veins proximal to the intrusion (Fig. 12a). Fluids were exsolved from the
intruding crystallizing granitic magma, leading to the formation of Ep 2 and Ep 3 fluids, which mobilized the remaining Zn, Sn and Cu from the residual melt. Copper and Zn probably migrated
far from the intrusion in the form of Cl–, HS– and SO42− complexes. Precipitation of chalcopyrite mainly took place via the reaction: CuCl − 2 + FeCl2 (aq) + H2S + ¼O2 = CuFeS2 + 4 Cl− +
3H+ + ½H2O71. In the late stage, Ep 2 and Ep 3 fluids mixed with external fluid, which include meteoric water and reducing basin water from the Late Permian Linxi Formation. In addition to
an increase in pH and decrease in temperature and Cl− concentration, the mixing also provided Fe and Zn to the fluid by altering and dissolving mica (Fig. 4h − l) on the vein walls. Addition
of Zn, Fe (an increase of FeCl2(aq)) and CH4 from external fluid facilitated reduction of SO42− to S2−, leading to precipitation of chalcopyrite and Fe-rich sphalerite, finally forming the
peripheral Weilasituo Cu–Zn and Bairendaba deposits. The addition of external fluid also led to a transition from high _f_S2 to low _f_S2 fluid conditions (ESM Fig. 4 and Fig. 12b). The SGXR
has emerged as an important Sn-W-Mo-Zn-Cu-Pb-Ag belt72,73,74,75. Deposits such as Weilasituo, Anle, Baiyinchagan, and Bianjiadayuan, define spatial zonation with respect to metals,
including Sn ± W (Mo) ± Zn mineralization proximal to, and Cu ± Pb ± Zn ± Ag mineralization distal from the intrusion. These mineralizations share similar metallogenic ages, although whether
these data represent a continuous cooling of a single fluid event, or alternatively, the metal zonation pattern derived from the superposition of multi-episodic fluid processes is a
question that remains controversial2,3,76. Our results suggest that the second alternative involving multi-episodic fluids with different ore-forming components is better suited to explain
the observed metal zonation. Additional metals may have been leached from the wall rocks and contributed to late ore-forming fluid. The Linxi Formation, as an important hydrocarbon source
horizon77, is a prominent unit of the SGXR. Our fluid inclusion analyses indicate that CH4-rich fluids from Linxi Formation may have played a key role in sulfide precipitation. CONCLUSIONS
The three deposits (Weilasituo Sn-polymetallic, Weilasituo Cu–Zn and Bairendaba Ag-Pb–Zn) are all related to emplacement of an Early Cretaceous, highly differentiated reduced quartz porphyry
in plagioclase gneiss and quartz diorite. The district shows Sn (W) -Li-Zn-Cu-Pb zoning from the center of metallogenic activity to the margins. Fluid inclusion and H–O isotopic data,
combined with chemical variations observed in the quartz and sphalerite from different stages of mineralization, allow us to draw the following conclusions. * 1. The Weilasituo
Sn-polymetallic deposit formed from low-medium temperature, low-salinity fluids. Magmatic fluids first evolved by magma degassing, fluid boiling, and eventually by addition of reducing basin
water from the Late Permian Linxi Formation. * 2. Magmatic quartz crystallized at around 700 °C, 1.5 kbar, and has higher contents of Al and Li, and lower Ge, Ti contents than hydrothermal
quartz that crystallized at lower temperatures. * 3. Sphalerite from the Weilasituo Cu–Zn and Bairendaba Ag–Pb–Zn deposits has much higher Cd, Fe + Cu, Fe/Zn, and Cd/Zn ratios than those in
the Sn deposit. * 4. Multi-episodic fluid processes and external fluid mixing contributed to the metal zonation in the Weilasituo and Bairendaba areas. Episode 1 fluids are characterized by
high Sn-(Zn), and possibly also W concentrations, whereas fluids from episodes 2 and 3 mainly carried Zn-(Sn) together with Cu. Mixing between external fluids and episodes 2 and 3 fluids
contributed to late-stage Cu, Zn mineralization, leading to precipitation of the peripheral Weilasituo Cu–Zn and Bairendaba deposits. The external fluids may have contributed to the metal
endowment of these deposits. DATA AVAILABILITY All data analysed during this study are included in the supplemental information files. REFERENCES * Wang, F. X., Bagas, L., Jiang, S. H. &
Liu, Y. F. Geological, geochemical, and geochronological characteristics of Weilasituo Sn-polymetal deposit, Inner Mongolia, China. _Ore Geol. Rev._ 80, 1206–1229 (2017). Article Google
Scholar * Liu, Y. F., Jiang, S. H. & Bagas, L. The genesis of metal zonation in the Weilasituo and Bairendaba Ag-Zn-Pb-Cu-(Sn-W) deposits in the shallow part of a porphyry Sn-polymetal
system, Inner Mongolia, China. _Ore Geol. Rev._ 75, 150–173 (2016). Article Google Scholar * Wang, X. Y. _et al._ SHRIMP Geochronology and Hf Isotope of Zircons from Granitoids of the
Weilasituo Deposit in Inner Mongolia. _Geoscience_ 27, 67–78 (2013) ((IN CHINESE WITH ENGLISH ABSTRACT)). Google Scholar * Gao, X., Zhou, Z. H., Breiter, K., Ouyang, H. & Liu, J.
Ore-formation mechanism of the Weilasituo tin-polymetallic deposit, NE China: Constraints from bulk-rock and mica chemistry, He-Ar isotopes, and Re-Os dating. _Ore Geol. Rev._ 109, 163–183
(2019). Article Google Scholar * Ouyang, H. G. _et al._ The Early Cretaceous Weilasituo Zn-Cu-Ag vein deposit in the SGXR, northeast China: Fluid inclusions, H, O, S, Pb isotope
geochemistry and genetic implications. _Ore Geol. Rev._ 56, 503–515 (2014). Article Google Scholar * Liu, R. L. _et al._ Characteristics of fluid inclusions and H-O-C-S-Pb isotopes of
Weilasituo Sn-polymetallic deposit in southern Da Hinggan Mountains. _Miner. Deposits_ 37, 199–224 (2018) ((IN CHINESE WITH ENGLISH ABSTRACT)). Google Scholar * Shi, R. Z. _et al._
Temporal-spatial variations in Li-Fe mica compositions from the Weilasituo Sn-polymetallic deposit (NE China): Implications for deposit-scale fluid evolution. _Ore Geol. Rev._ 134, 1–19
(2021). Article Google Scholar * Zhu, K. Y., Jiang, S. Y., Su, H. M. & Duan, Z. P. In situ geochemical analysis of multiple generations of sphalerite from the Weilasituo Sn-Li-Rb-Cu-Zn
ore field (Inner Mongolia, northeastern China): Implication for critical metal enrichment and ore-forming process. _Ore Geol. Rev._ 139, 1–19 (2021). Article Google Scholar * Zhou, Z. H.
_et al._ Tin isotopes as geochemical tracers of ore-forming processes with Sn mineralization. _Am Mineral._ 107, 2111–2127 (2022). Article ADS Google Scholar * Huang, T. C., Chen, C., Lv,
X. B., Wang, S. G. & Liu, H. Y. Evolution and origin of the Bairendaba Ag–Pb–Zn deposit in Inner Mongolia, China: Constraints from infrared micro-thermometry, mineral composition,
thermodynamic calculations, and in situ Pb isotope. _Ore Geol. Rev._ 154, 105316 (2023). Article Google Scholar * Han, L., Pan, J. Y., Ni, P. & Chen, H. Cassiterite deposition induced
by cooling of a single-phase magmatic fluid: Evidence from SEM-CL and fluid inclusion LA-ICP-MS analysis. _Geochim. Cosmochim. Acta_ 342, 108–127 (2023). Article ADS CAS Google Scholar *
Li, Y. _et al._ Transient tin mineralization from cooling of magmatic fluids in a long-lived system. _Geology_ 51, 305–309 (2023). Article ADS CAS Google Scholar * Hedenquist, J. W.
& Lowenstern, J. B. The role of magmas in the formation of hydrothermal ore deposits. _Nature_ 40, 519–527 (1994). Article ADS Google Scholar * Chang, Z. S. & Meinert, L. D. The
magmatic-hydrothermal transition-evidence from quartz phenocryst textures and endoskarn abundance in Cu-Zn skarns at the Empire Mine, Idaho, USA. _Chem. Geol._ 210, 149–171 (2004). Article
ADS CAS Google Scholar * Audétat, A. The metal content of magmatic-hydrothermal fluids and its relationship to mineralization potential. _Econ. Geol._ 114, 1033–1056 (2019). Article
Google Scholar * Peterková, T. & Dolejšc, D. Magmatic-hydrothermal transition of Mo-W-mineralized granite-pegmatite greisen system recorded by trace elements in quartz: Krupka district,
Eastern Krušné hory/Erzgebirge. _Chem. Geol._ 523, 179–202 (2019). Article ADS Google Scholar * Li, W. _et al._ Complementary textural, trace element, and isotopic analyses of sulfides
constrain ore-forming processes for the slate-hosted Yuhengtang Au deposit, South China. _Econ. Geol._ 116, 1825–1848 (2021). Article Google Scholar * Rusk, B. & Reed, M. Scanning
electron microscope–cathodoluminescence analysis of quartz reveals complex growth histories in veins from the Butte porphyry copper deposit, Montana. _Geology_ 30, 727–730 (2002). Article
ADS CAS Google Scholar * Götze, J., Plötze, M., Graupner, T., Hallbauer, D. K. & Bray, C. J. Trace element incorporation into quartz: A combined study by ICP-MS, electron spin
resonance, cathodoluminescence, capillary ion analysis, and gas chromatography. _Geochim. Cosmochim. Acta_ 68, 3741–3759 (2004). Article ADS Google Scholar * Breiter, K., Svojtka, M.,
Ackerman, L. & Švecová, K. Trace element composition of quartz from the Variscan Teplice caldera (Krušné hory/Erzgebirge Mts, Czech Republic/Germany): Insights into the volcano–plutonic
complex evolution. _Chem. Geol._ 326, 36–50 (2012). Article ADS Google Scholar * Breiter, K., Ďurišová, J. & Dosbaba, M. Chemical signature of quartz from S- and A-type rare-metal
granites—a summary. _Ore Geol. Rev._ 125, 25–35 (2020). Article Google Scholar * Audétat, A. _et al._ Characterization of a natural quartz crystal as a reference material for
microanalytical determination of Ti, Al, Li, Fe, Mn, Ga and Ge. _Geostand. Geoanal. Res._ 39, 171–184 (2015). Article Google Scholar * Michaud, J.A.-S. & Pichavant, M. Magmatic
fractionation and the magmatic-hydrothermal transition in rare metal granites: Evidence from Argemela (Central Portugal). _Geochim. Cosmochim. Acta_ 289, 130–157 (2020). Article ADS CAS
Google Scholar * Jacamon, F. & Larsen, R. B. Trace element evolution of quartz in the charnockitic Kleivan granite, SW-Norway: The Ge/Ti ratio of quartz as an index of igneous
differentiation. _Lithos_ 107, 281–291 (2009). Article ADS CAS Google Scholar * Breiter, K., Ďurišová, J. & Dosbaba, M. Quartz chemistry-A step to understanding magmatic-hydrothermal
processes in ore-bearing granites: Cínovec/Zinnwald Sn-W-Li deposit, Central Europe. _Ore Geol. Rev._ 90, 25–35 (2017). Article Google Scholar * Thomas, J. B. _et al._ TitaniQ under
pressure: The effect of pressure and temperature on the solubility of Ti in quartz. _Contrib. Miner. Petrol._ 160, 743–759 (2010). Article ADS CAS Google Scholar * Huang, R. &
Audétat, A. The titanium-in-quartz (TitaniQ) thermobarometer: A critical examination and recalibration. _Geochim. Cosmochim. Acta_ 84, 75–89 (2012). Article ADS CAS Google Scholar *
Cook, N. J. _et al._ Trace and minor elements in sphalerite: A LA–ICPMS study. _Geochim. Cosmochim. Acta_ 73, 4761–4791 (2009). Article ADS CAS Google Scholar * Keith, M. _et al._
Effects of temperature, sulfur, and oxygen fugacity on the composition of sphalerite from submarine hydrothermal vents. _Geology_ 42, 699–702 (2014). Article ADS CAS Google Scholar *
George, L. L., Cook, N. J. & Ciobanu, C. L. Partitioning of trace elements in co-crystallized sphalerite-galena-chalcopyrite hydrothermal ores. _Ore Geol. Rev._ 77, 97–116 (2016).
Article Google Scholar * Cao, D. D., Wang, Y. C., Yin, L. & Xu, B. Metamorphic patterns and zircon U-Pb dating of the Xilingol complex in Inner Mongolia, China: Implications for
rifting metamorphism and tectonic evolution in eastern Central Asian Orogenic Belt. _Lithos_ 428, 106826 (2022). Article Google Scholar * Zhou, Z. H., Mao, J. W. & Lyckberg, P.
Geochronology and isotopic geochemistry of the A-type granites from the Huanggang Sn-Fe deposit, southern Great Hinggan Range, NE China: Implication for their origin and tectonic setting.
_J. Asian Earth Sci._ 49, 272–286 (2012). Article ADS Google Scholar * Ouyang, H. G., Mao, J. W., Zhou, Z. H. & Su, H. M. Late Mesozoic metallogeny and intracontinental magmatism,
SGXR, northeastern China. _Gondwana Res._ 27, 1153–1172 (2015). Article ADS CAS Google Scholar * Li, B. Y. _et al._ Geological characteristics and prospecting significance of Weilasituo
Li polymetallic deposit, Inner Mongolia. _Miner. Explor._ 6, 1185–1191 (2018) ((IN CHINESE WITH ENGLISH ABSTRACT)). Google Scholar * Bodnar, R. J. Revised equation and table for determining
the freezing point depression of H2O–NaCl solutions. _Geochim. Cosmochim. Acta_ 57, 683–684 (1993). Article ADS CAS Google Scholar * Liu, J. J. _et al._ Discovery of falkmanite from the
Bairendaba superlarge Ag-Pb-Zn polymetallic deposit, Inner Mongolia and its origin significance. _J. Jilin Univ. (Earth Sci. Ed.)_ 40, 565–572 (2010) ((IN CHINESE WITH ENGLISH ABSTRACT)).
CAS Google Scholar * Shen, P. & Pan, H. D. Methane origin and oxygen-fugacity evolution of the Baogutu reduced porphyry Cu deposit in the West Junggar terrain, China. _Miner. Deposita_
50, 967–986 (2015). Article ADS CAS Google Scholar * Li, C. H., Shen, P., Pan, H. D. & Cao, C. Formation mechanism of reducing gas in ore-forming fluid of West Junggar, Xinjiang.
_J. Earth Sci. Env._ 39, 386–396 (2017) ((IN CHINESE WITH ENGLISH ABSTRACT)). CAS Google Scholar * Shen, P. _et al._ Methane-rich fluid evolution of the Baogutu porphyry Cu-Mo-Au deposit,
Xinjiang, NW China. _Chem. Geol._ 275, 78–98 (2010). Article ADS CAS Google Scholar * Simakov, S. K. Redox state of Earth’s upper mantle peridotites under the ancient cratons and its
connection with diamond genesis. _Geochim. Cosmochim. Acta_ 62, 1811–1820 (1998). Article ADS CAS Google Scholar * Sun, W. D. _et al._ Porphyry deposits and oxidized magmas. _Ore Geol.
Rev._ 65, 97–131 (2015). Article Google Scholar * Horita, J. & Berndt, M. E. Abiogenic methane formation and isotopic fractionation under hydrothermal conditions. _Science_ 285,
1055–1057 (1999). Article CAS PubMed Google Scholar * Taylor, J. H. P. The application of oxygen and hydrogen isotope studies to problems of hydrothermal alteration and ore deposition.
_Econ. Geol._ 40, 843–883 (1974). Article Google Scholar * Wilkinson, J. J., Jenkin, G. R. T., Fallick, A. E. & Foster, R. P. Oxygen and hydrogen isotope evolution of crustal fluids,
south Cornwall, UK. _Chem. Geol._ 40, 239–254 (1995). Article ADS Google Scholar * Taylor, B. E., Eichelberger, J. & Westrich, H. Hydrogen isotopic evidence of rhyolitic magma
degassing during shallow intrusion and eruption. _Nature_ 306, 541–545 (1983). Article ADS CAS Google Scholar * Rye, R. O. The evolution of magmatic fluids in the epithermal environment:
The stable isotope perspective. _Econ. Geol._ 40, 733–753 (1993). Article Google Scholar * Koděra, P., Lexa, J., Rankin, A. H. & Fallick, A. E. Epithermal gold veins in a caldera
setting: Banská Hodruša, Slovakia. _Miner. Deposita_ 39, 921–943 (2005). Article ADS Google Scholar * Barnes, H. L. Solubilities of ore minerals. In _Geochemistry of Hydrothermal Ore
Deposits_ 2nd edn (ed. Barnes, H. L.) 404–460 (Wiley, 1979). Google Scholar * Goh, S. W., Buckley, A. N., Lamb, R. N., Rosenberg, R. A. & Moran, D. The oxidation states of copper and
iron in mineral sulphides, and the oxides formed on initial exposure of chalcopyrite and bornite to air. _Geochim. Cosmochim. Acta_ 70, 2210–2228 (2006). Article ADS CAS Google Scholar *
Ye, L., Gao, W., Yang, Y. L., Liu, T. G. & Peng, S. S. Trace elements in sphalerite in Laochang Pb-Zn polymetallic deposit, Lancang, Yunnan Province. _Acta Petrol. Sin._ 28, 1362–1372
(2012) ((IN CHINESE WITH ENGLISH ABSTRACT)). CAS Google Scholar * Craig, J. R., Ljekjell, P. & Vokes, F. M. Sphalerite compositional variations in sulfide ores of the Norwegian
Caledonides. _Econ. Geol._ 79, 1727–1735 (1984). Article Google Scholar * Cook, N. J., Klemd, R. & Okrusch, M. Sulphide mineralogy, metamorphism and deformation in the Matchless
massive sulphide deposit, Namibia. _Miner. Deposita_ 29, 1–15 (1994). Article ADS CAS Google Scholar * Heinrich, C. A., Gunther, D., Audétat, A., Ulrich, T. & Frischknecht, R. Metal
fractionation between magmatic brine and vapor, determined by microanalysis of fluid inclusions. _Geology_ 27, 755–758 (1999). Article ADS CAS Google Scholar * Rempel, K. U., Liebscher,
A., Meixner, A., Romer, R. L. & Heinrich, W. An experimental study of the elemental and isotopic fractionation of copper between aqueous vapour and liquid to 450°C and 400bar in the
CuCl–NaCl–H2O and CuCl–NaHS–NaCl–H2O systems. _Geochim. Cosmochim. Acta_ 94, 199–216 (2012). Article ADS CAS Google Scholar * Jiang, S. H., Nie, F. J., Liu, Y. F. & Yun, F. Sulfur
and lead isotopic compositions of Bairendaba and Weilasituo silver-polymetallic deposits, Inner Mongolia. _Miner. Deposits_ 28, 101–112 (2010) ((IN CHINESE WITH ENGLISH ABSTRACT)). Google
Scholar * Müller, A. & Koch-Müller, M. Hydrogen speciation and trace element contents of igneous, hydrothermal and metamorphic quartz from Norway. _Miner. Mag._ 73, 569–583 (2009).
Article Google Scholar * Breiter, K., Gardenová, N., Kanický, V. & Vaculovič, T. Galium and germanium geochemistry during magmatic fractionation and post-magmatic alteration in
different types of granitoids: A case study from the Bohemian Massif, Czech Republic. _Geol. Carpathica_ 64, 171–180 (2013). Article ADS CAS Google Scholar * Zhang, C. _et al._
Ti-in-quartz thermobarometer and TiO2 solubility in rhyolitic melts: New experiments and parametrization. _Earth Plan. Sci. Lett._ 538, 1–13 (2020). Article Google Scholar * Wark, D. A.
& Watson, E. B. TitaniQ: A titanium-in-quartz geothermometer. _Contrib. Mineral. Petrol._ 152, 743–754 (2006). Article ADS CAS Google Scholar * Osborne, Z. R. _et al._ TitaniQ
revisited: Expanded and improved Ti-in-quartz solubility model for thermobarometry. _Contrib. Mineral. Petrol._ 177, 31 (2022). Article ADS CAS Google Scholar * Lanzillo, N. A., Watson,
E. B., Thomas, J. B., Nayak, S. K. & Curioni, A. Near-surface controls on the composition of growing crystals: Car-parrinello molecular dynamics (CPMD) simulations of Ti energetics and
diffusion in alpha quartz. _Geochim. Cosmochim. Acta_ 131, 33–46 (2014). Article ADS CAS Google Scholar * Antignano, A. & Manning, C. E. Rutile solubility in H2O, H2O-SiO2, and
H2ONaAlSi3O8 fluids at 0.7–2.0 GPa and 700–1000 °C: Implications for mobility of nominally insoluble elements. _Chem. Geol._ 255, 283–293 (2008). Article ADS CAS Google Scholar * Hayden,
L. A. & Watson, E. B. Rutile saturation in hydrous siliceous melts and its bearing on Ti-thermometry of quartz and zircon. _Earth Plan. Sci. Lett._ 258, 561–568 (2007). Article ADS
CAS Google Scholar * Steele-MacInnis, M., Lecumberri-Sanchez, P. & Bodnar, R. J. HokieFlincs_H2O-NaCl: A Microsoft Excel spreadsheet for interpreting microthermometric data from fluid
inclusions based on the PVTX properties of H2O–NaCl. _Comput. Geosci._ 49, 334–337 (2012). Article ADS Google Scholar * Johannes, W. & Holtz, F. _Petrogenesis and Experimental
Petrology of Granite Rocks_ (Springer, 1996). Book Google Scholar * Shinohara, H. Exsolution of immiscible vapor and liquid phases from a crystallizing silicate melt: Implications for
chlorine and metal transport. _Geochim. Cosmochim. Acta_ 40, 5215–5221 (1994). Article ADS Google Scholar * Webster, J. D. The exsolution of magmatic hydrosaline chloride liquids. _Chem.
Geol._ 40, 33–48 (2004). Article ADS Google Scholar * Schmidt, C., Romer, R. L., Wohlgemuth-Ueberwasser, C. & Appelt, O. Partitioning of Sn and W between granitic melt and aqueous
fluid. _Ore Geol. Rev._ 117, 103263 (2020). Article Google Scholar * Xu, R., Romer, R. L. & Glodny, J. External fluids cause alteration and metal redistribution in the granite-hosted
Tangziwa Sn-Cu deposit, Gejiu district, China. _Lithos_ 382, 105937 (2021). Article Google Scholar * Schmidt, C. Formation of hydrothermal tin deposits: Raman spectroscopic evidence for an
important role of aqueous Sn (IV) species. _Geochim. Cosmochim. Acta_ 220, 499–511 (2017). Article ADS Google Scholar * Hezarkhani, A., Williams-Jones, A. E. & Gammons, C. H. Factors
controlling copper solubility and chalcopyrite deposition in the Sungun porphyry copper deposit, Iran. _Miner. Deposita_ 34, 770–783 (1999). Article ADS CAS Google Scholar * Mao, J. W.
_et al._ Metallogenic regularity and minerogenetic series of ore deposits in Inner Mongolia and adjacent areas. _Miner. Deposits_ 32, 715–729 (2013) ((IN CHINESE WITH ENGLISH ABSTRACT)). CAS
Google Scholar * Shu, Q. H. _et al._ Regional metallogeny of Mo-bearing deposits in northeastern China, with new Re-Os dates of porphyry Mo deposits in the northern Xilamulun district.
_Econ. Geol._ 111, 1783–1798 (2016). Article Google Scholar * Shu, Q. H., Chang, Z. S. & Mavrogenes, J. Fluid compositions reveal fluid nature, metal deposition mechanisms, and
mineralization potential: An example at the Haobugao Zn-Pb skarn. China. _Geology_ 49, 473–477 (2021). Article ADS CAS Google Scholar * Zhai, D. G., Liu, J. J., Zhang, A. L. & Sun,
Y. Q. U-Pb, Re-Os and 40Ar/39Ar geochronology of porphyry Sn±Cu±Mo and polymetallic (Ag-Pb-Zn-Cu) vein mineralization at Bianjiadayuan, Inner Mongolia, NE China: Implications for discrete
mineralization events. _Econ. Geol._ 112, 2041–2059 (2017). Article Google Scholar * Zhou, Z. H., Gao, X., Ouyang, H. G., Liu, J. & Zhao, J. Q. Formation mechanism and intrinsic
genetic relationship between tin-tungstenlithium mineralization and peripheral lead-zinc-silver-copper mineralization: Exemplified by Weilasituo tin-tungsten-lithium polymetallic deposit,
Inner Mongolia. _Miner. Deposits_ 4, 1297–1306 (2019) ((IN CHINESE WITH ENGLISH ABSTRACT)). Google Scholar * Zhang, J. _et al._ Shale gas resource prospect of late Permian Linxi formation
in the middle-southern part of the Da Hinggan Mountains. _Geol. Bull. China_ 32, 1297–1306 (2013) ((IN CHINESE WITH ENGLISH ABSTRACT)). Google Scholar * Wang, Q., Zhao, G. C., Han, Y. G.
& Yao, J. L. Detrital zircon constraints on late Paleozoic tectonism of the Bogda region (NW China) in the southern Central Asian Orogenic Belt. _Geosci. Front._ 11, 1533–1548 (2020).
Article CAS Google Scholar * Whitney, D. L. & Evans, B. W. Abbreviations for names of rock-forming minerals. _Am. Mineral._ 95, 185–187 (2010). Article ADS CAS Google Scholar *
Sheppard, S.M.F. Characterization and isotopic variations in natural waters. In Stable _Isotopes in High Temperature Geological Processes. Rev Mineral, vol. 16 _(ed. Valley, J. W. _et al_.)
165–184 (Springer, 1986). * Craig, H. Isotopic variations in meteoric waters. _Science_ 133, 1702–1703 (1961). Article ADS CAS PubMed Google Scholar * Savin, S. M. & Epstein, S. The
oxygen and hydrogen isotope geochemistry of clay minerals. _Geochim. Cosmochim. Acta_ 34, 25–42 (1970). Article ADS CAS Google Scholar * Shelton, K. L., So, C. S. & Chang, J. S.
Gold-rich mesothermal vein deposits of the Republic of Korea; geochemical studies of the Jungwon gold area. _Econ. Geol._ 40, 1221–1237 (1988). Article Google Scholar * Shelton, K. L., So,
C. S., Haeussler, G. T., Chi, S. J. & Lee, K. Y. Geochemical studies of the Tongy-oung gold-silver deposits, Republic of Korea: Evidence of meteoric water dominance in a Te-bearing
epithermal system. _Econ. Geol._ 40, 1114–1132 (1990). Article Google Scholar * Taylor, B. E. Degassing of H2O from rhyolite magma during eruption and shallow intrusion, and the isotopic
composition of magmatic water in hydrothermal systems. In _Magmatic Contributions to Hydrothermal Systems. Geol Surv Japan, Reports, Tsukuba, vol. 279_ (ed. Hedenquist, J. W.) 190–194
(1992). * Chang, Y. & Lai, Y. Study on characteristics of ore-forming fluid and chronology in the Yindu Zn-Pb-Ag polymetallic ore deposit, Inner Mongolia. _J. Beijing Normal Univ. (Nat.
Sci.)_ 46, 581–593 (2010) ((IN CHINESE WITH ENGLISH ABSTRACT)). Google Scholar Download references ACKNOWLEDGEMENTS This work was financially supported by the National Natural Science
Foundation of China (Grant No. 41772084), the Science & Technology Fundamental Resources Investigation Program (Grant Nos. 2022YF101900 and 2022YF101901), and a grant from the China
Scholarship Council (Grant No. 201908110162). Boyang Li, Xu Fu, Kexiang Wang, Peng Sheng, Dawei Jiang and Hucan Jiang are thanked for their field support. Cooperation of KB was supported by
the Czech Science Foundation, project No. P210/19/05198S, and by RVO 67985831 of the Institute of Geology of the Czech Academy of Sciences. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * MNR
Key Laboratory of Metallogeny and Mineral Assessment, Institute of Mineral Resources, Chinese Academy of Geological Sciences, Beijing, 100037, China Xu Gao, Zhenhua Zhou & Jingwen Mao *
Institut für Mineralogie, Leibniz Universität Hannover, Callinstr. 3, 30167, Hannover, Germany Xu Gao & François Holtz * Research School of Earth Sciences, Australian National
University, 142 Mills Rd, Canberra, ACT, 2601, Australia Zhenhua Zhou * Institute of Geology of the Czech Academy of Sciences, Rozvojová 269, 16500, Praha 6, Czech Republic Karel Breiter *
GFZ German Research Centre for Geosciences, Telegrafenberg, 14473, Potsdam, Germany Rolf L. Romer * School of Chemical Engineering, The University of Adelaide, Adelaide, SA, 5005, Australia
Nigel J. Cook Authors * Xu Gao View author publications You can also search for this author inPubMed Google Scholar * Zhenhua Zhou View author publications You can also search for this
author inPubMed Google Scholar * Karel Breiter View author publications You can also search for this author inPubMed Google Scholar * Jingwen Mao View author publications You can also search
for this author inPubMed Google Scholar * Rolf L. Romer View author publications You can also search for this author inPubMed Google Scholar * Nigel J. Cook View author publications You can
also search for this author inPubMed Google Scholar * François Holtz View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Experiments
conceived, performed and manuscript written by X.G. and Z.H.Z. K.B., R.L.R. and F.H. contributed to the interpretation of quartz geochemistry results. N.J.C. commented on the sphalerite
geochemistry interpretation. K.B., R.L.R., J.W.M., N.J.C. and F.H. contributed to the framework, modification and revision of the text. CORRESPONDING AUTHOR Correspondence to Zhenhua Zhou.
ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional
claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative
Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the
original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in
the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your
intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence,
visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Gao, X., Zhou, Z., Breiter, K. _et al._ Magmatic-hydrothermal fluid evolution
of the tin-polymetallic metallogenic systems from the Weilasituo ore district, Northeast China. _Sci Rep_ 14, 3006 (2024). https://doi.org/10.1038/s41598-024-53579-y Download citation *
Received: 28 August 2023 * Accepted: 02 February 2024 * Published: 06 February 2024 * DOI: https://doi.org/10.1038/s41598-024-53579-y SHARE THIS ARTICLE Anyone you share the following link
with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative KEYWORDS * In situ LA-ICP-MS trace element analysis * H-O isotopes * Fluid mixing * Physico-chemical conditions * Sn-polymetallic ore systems