Play all audios:
ABSTRACT The nucleocapsid (N) protein of SARS-CoV-2 is known to participate in various host cellular processes, including interferon inhibition, RNA interference, apoptosis, and regulation
of virus life cycles. Additionally, it has potential as a diagnostic antigen and/or immunogen. Our research focuses on examining structural changes caused by mutations in the N protein. We
have modeled the complete tertiary structure of native and mutated forms of the N protein using Alphafold2. Notably, the N protein contains 3 disordered regions. The focus was on
investigating the impact of mutations on the stability of the protein's dimeric structure based on binding free energy calculations (MM-PB/GB-SA) and RMSD fluctuations after MD
simulations. The results demonstrated that 28 mutations out of 37 selected mutations analyzed, compared with wild-type N protein, resulted in a stable dimeric structure, while 9 mutations
led to destabilization. Our results are important to understand the tertiary structure of the N protein dimer of SARS-CoV-2 and the effect of mutations on it, their behavior in the host
cell, as well as for the research of other viruses belonging to the same genus additionally, to anticipate potential strategies for addressing this viral illness․ SIMILAR CONTENT BEING
VIEWED BY OTHERS A CORE NETWORK IN THE SARS-COV-2 NUCLEOCAPSID NTD MEDIATES STRUCTURAL INTEGRITY AND SELECTIVE RNA-BINDING Article Open access 09 December 2024 STRUCTURAL, ENERGETIC AND
LIPOPHILIC ANALYSIS OF SARS-COV-2 NON-STRUCTURAL PROTEIN 9 (NSP9) Article Open access 26 November 2021 EXPOSING STRUCTURAL VARIATIONS IN SARS-COV-2 EVOLUTION Article Open access 11 November
2021 INTRODUCTION The SARS-CoV-2 virus contains a single-stranded RNA genome packaged in a 100-nm-diameter membrane-bound virion. The viral genome is made up of a positive sense,
single-stranded RNA that encodes four structural proteins—spike (S), envelope (E), membrane (M), and nucleocapsid (N)—along with 9 auxiliary proteins and 14 open reading frames (ORFs) that
encode 16 nonstructural proteins, making up a replicase complex. One of the most abundant structural proteins in cells infected with viruses is protein N, which is very conservative in the
CoV genus1,2,3. The primary function of the N protein is to encapsulate the viral RNA into long-stranded ribonucleocapsid (RNP) complexes, as well as to participate in the assembly of the
virus by interacting with the M protein of the virus genome. Additionally, it has been demonstrated that the CoV N protein participates in host cellular processes that control the viral life
cycles, also the N protein is an immunodominant antigen of the host cell immune system1,3,4,5. The N protein is encoded by the 9th ORF of the virus, consists of 419 amino acids, and has a
modular structure that can be divided into intrinsically disordered regions (IDRs) and conservative structural regions2,4. IDRs include 3 modules: N-arm (1–48 aa), central Ser/Arg rich
linker region (LKR) (176–247aa), and C-tail (366–419 aa), while conservative structural regions include two modules: N-terminal domain (NTD) (49–175 aa) and C-terminal domain (CTD) (248–365
aa). In the primary structure, NTD and CTD are connected by LKR and are surrounded by N-arm and C-tail1,3. The presence of disordered regions in viral proteins is commonly linked to viral
infectivity and pathogenicity. Disordered regions participate in liquid–liquid phase separation, which is also typical of N protein, which exhibits concentration-dependent liquid–liquid
phase separation in the presence of RNA6,7. The N protein is known to exhibit functional activity when present in a dimeric structure1,8. In the host cell, the N protein typically undergoes
phosphorylation, mainly occurring in its linker region. Additionally, it interacts with several viral proteins such as Membrane (M ) and non-structural protein (Nsp33), and host proteins
including GTPase-activating protein SH3 domain-binding protein 1/2 (G3BP-1/2), heterogeneous nuclear ribonucleoproteins (hnRNPs), NLR family PYRIN domain containing-3 (NLRP3), 14-3-3, and
others.3,6,9. The data revealed that the regions spanning amino acid positions 164 to 205, exhibited the highest number of mutations compared to the total number of amino acids among the N
amino acid sequences, and the second-highest frequency of mutations was observed in the regions spanning amino acid positions 205 to 24610. Some mutations can have a positive impact on
pathogenesis, such as R203K/G204R mutations11,12,13, but at the same time, these mutations decrease the structural stability and flexibility of the N protein. Also, there are data about the
absence of a significant correlation between N protein mutations and infection rates14. Some variants containing the R203K/G204R mutations in the N protein often exhibit concurrent
mutations, including the spike protein’s N501Y and E484K, among others13. Based on the above-mentioned, it is indeed challenging to establish a clear correlation between specific mutations
and pathogenesis or disease severity. Taking into account that viral proteins do not mutate singularly, and the impact on pathogenesis is likely influenced by the combination of multiple
mutations in different proteins, it becomes a complicated task. Therefore, determining the relationship between mutations and disease outcomes requires a comprehensive analysis of various
mutation combinations rather than focusing on individual mutations in isolation12,15. Due to the presence of multiple disordered regions within the N protein, including the N-arm, LKR, and
C-tail1,2,4, acquiring its comprehensive tertiary structure through experimental techniques like X-ray crystallography has presented formidable challenges16,17,18. As of now, there is no
established protocol for elucidating the structural conformation of disordered proteins. However, it's pertinent to highlight that the complete monomeric structure of the N protein
became accessible in the Protein Data Bank (PDB) on January 2023, utilizing electron microscopy with a resolution of 4.57 Å (PDBID: 8FD5). Herein, we used computational approaches to
understand how mutations impact the stabilization of SARS-CoV-2 N protein dimeric structure. We performed molecular modeling for the complete tertiary structure of wild-type and mutated N
proteins, molecular dynamic simulations, binding free energy and RMSD (root mean square deviation) calculations, and structural rearrangements among wild-type and mutated proteins. The data
obtained from this study are essential to understanding the tertiary structure of the N protein of SARS-CoV-2 and the impact of mutations on protein dimeric structure. Moreover, the findings
shed light on the behavioral dynamics of these mutations within host cellular environments. Such insights are crucial and could expand the scope of research on viruses of a similar genus.
RESULTS We used Alphafold2 (AF) for modeling and as we mentioned, there is no X-ray crystallographic model of the N protein complete tertiary structure, only 2 domains are available, thus we
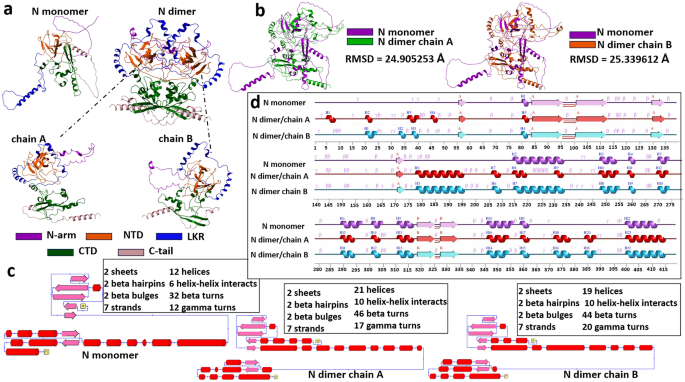
based our further validation process on 2 known X-ray crystallographic domains: NTD (_PDBID:7N0R_), and CTD (_PDBID:6WZQ_). Figure 1a represents the N protein monomer, dimer, dimer’s chain
A and chain B complete modeled structures. It can be seen from the tertiary structure that the monomer disordered regions (N-arm, LKR, C-tail), besides not having the characteristic elements
of the secondary structure are scattered and not compactly assembled, and in the case of the dimer, the structure is different. We separated the dimer into 2 monomers (chain A and chain B)
that make up the dimer and compared them with the N monomer (Fig. 1b). Superimposition of modeled proteins on these domains and RMSD score comparison was performed using ICM Pro software.
RMSD comparison showed that RMSD [AF_N_monomer vs AF_N_ dimer_chainA] = 24.905253 Å and RMSD [AF_N_monomer vs AF_N _dimer_chainB] = 25.339612 Å. Given the limitation of observing structural
rearrangements within tertiary structures, we opted to visualize these alterations in secondary structural elements as a more effective means of analysis and visualization. We used the
PDBSum server to understand what kind of elements of secondary structures are presented in the modeled protein. The differences in the structure are also shown by the analysis of the
topology and the secondary structure elements (Fig. 1c,d). It is important to note that in both monomer and dimer models, there is an alpha helix between 400 and 412 amino acids (Fig. 1d),
which is the C-tail and one of the disordered regions of N protein. In the case of dimer, there are several short alpha helixes formed, the most noticeable of which are between 178 and 196
amino acids in chain A and B and between 215 and 234 amino acids in chain B and monomer (Fig. 1d). Figure 2a and b represent the pLDDT confidence measure in the B factor field obtained by
ChimeraX. The pLDDT score represents a pivotal instrument within the domain of protein structural prediction. Its primary function is to provide an estimate of the confidence levels
associated with individual amino acid residues within predicted protein configurations․ Employing a continuum ranging from 0 to 100, with scores exceeding 90 signifying a robust degree of
assurance and those falling below 50 indicating a diminished level of confidence, the pLDDT score plays a critical role in visualizing AlphaFold2 models. Specifically, it imparts a color
scheme where regions of heightened confidence are typically rendered in gradients of blue, contrasting with lower confidence regions, which are often portrayed in the hues of yellow, orange,
or red. The pLDDT score evaluates whether the predicted positioning of a given residue aligns with the distances between its C-alpha atom and neighboring C-alpha atoms (within a 15 Å range)
as observed in the true protein structure19. In the computational models presented (Fig. 2a and b), regions characterized by hues of yellow and orange signify pLDDT scores ranging from 50
to 70 which indicate IDRs within the protein structure. The manifestation of such IDRs is a documented attribute and is deemed characteristic within this molecular framework. The N monomer
exhibited an average pLDDT score of 65.54, while the dimer was characterized by an average iptm + ptm score of 0.466. The PAE plot functions similarly to a map, highlighting the differences
between the predicted and actual positions of various segments of a protein. PAE represents the absolute error in the relative positioning of residues, quantified in Ångströms (0–30)․ Colors
are used to represent these disparities, with dark green indicating minimal differences and white representing larger discrepancies. When examining this map, we expect most points along the
diagonal line to be close to dark green because this is where the predicted and actual positions should closely align. If we observe distinct clusters of similar colors elsewhere on the
map, it signifies that the model is highly confident in those regions. An outstanding model would display an entirely dark green map, signifying highly accurate predictions of the
protein's structure19. The plot c displayed in Fig. 2c represents a PAE plot specifically designed to depict the characteristics of the N-dimer model. On January 2023 Casasanta M et al.
released the N protein monomer model using cryo-electron microscopy (Cryo-EM) (_PDBID:8FD5_) with 4.57 Å resolution20. Our investigation entailed a comprehensive examination of the N
monomers' domains within our AF models in superimposition with the analogous domains derived from Cryo-EM and X-ray crystallography methodologies. The results are presented in Fig. 3.
To check the accuracy of the AF models we compared them with X-ray and with CryoEM structures (Fig. 3a–c). Through calculations and comparisons, we have established the significant utility
of AF modeling. Specifically, when we superimposed AF-NTD and AF-CTD models onto X-ray domains, we observed remarkably low RMSD scores (Fig. 3a and c), measuring at 1.94 Å and 1.89 Å,
respectively. However, in the case of IDR, where no X-ray model was available for reference, we conducted a comparison solely with the Cryo-EM model (Fig. 3b), resulting in a considerably
higher RMSD score exceeding 23.4 Å. This elevated RMSD score can be attributed to the absence of structured regions in the AF and Cryo-EM models, with Cryo-EM models demonstrating greater
assembly of disordered regions, a behavior consistent with expectations. The N protein acquires functional activity when it adopts a dimeric structure. Consequently, in subsequent
experiments, we used the AF dimeric configuration. To assess the reliability of the AF dimeric structure, we considered not only the pLDDT and PAE scores but also examined whether its
dimerization domain contributed to the formation of the dimer. Furthermore, we conducted a comparison of structures with the X-ray model (PDBID:6WZQ, PDBID:7N0R), which was originally
associated with the oligomeric structure (Fig. 3d). When comparing the AF-NTD dimeric structure with X-ray dimeric structures, we observed a higher RMSD score for the NTD dimer as
represented in Fig. 3d. This elevated RMSD score can be attributed to the fact that the NTD does not participate in dimerization. However, in a separate comparison where we compared the
NTD-dimer chains with X-ray monomeric structures, we found a significantly lower RMSD score of 1.16 Å. This lower RMSD score suggests a closer structural resemblance between the NTD-dimer
chains and the X-ray monomeric structures. In contrast, for the CTD dimer, the RMSD score was 0.8 Å, which aligns with our expectations. This low RMSD score indicates a close match between
the AF-CTD dimeric structure and the X-ray dimeric structures, suggesting that the CTD is actively involved in dimerization. We evaluated several metrics such as pLDDT, PAE, RMSD scores,
along with tertiary and secondary structure elements, and topologies. These metrics uniformly confirmed the precision and dependability of the AF models. Apart from the precision concerns of
AF modeling, our main objective was to understand how mutations in the N protein impact the stability of its dimeric formation. For our study, we chose 34 single mutations (D3L, Q9L, P13L,
D63G, I157T, Q160R, P168Q, A173S, R185C, S186F, S197A, S197L, S202N, R203E, R203M, T205A, T205I, A208S, G215C, S235F, K256N, T265I, A267V, T296I, F307V, A308S, M322I, P326L, K374N, D377Y,
Q384H, D401Y, S413I, Q418H) and 3 combined mutations, each distinctive to specific strains of concern. These include the Alpha strain (lineage B.1.1.7) with mutations D3L, S235F, R203K,
G204R; the Gamma strain (lineage P.1) and Omicron strain (lineage B1.1.529) both featuring R203K, G204R mutations, and the Delta strain (lineage B1.617.2) identified by mutations D63G,
R203M, D377Y. It is also important to note that the R203K/G204R mutants occur together10,16, and we also observed this in the single-protein model. T205I is associated with the Beta strain
(lineage B.1.351) so we observed T205I single mutation as a Beta strain-related mutation. Thus, we have had 38 models, 1 for native N protein (N wild type) and 37 for mutated proteins. Data
for N protein mutations were obtained from the previous study21 and the CoV-Lineages Report22. Figure 4 represents all selected mutations for further study- according to their location: it
can be seen that the vast majority of mutations are located in the disordered regions N-arm -3 mutations, LKR-14 mutations, C-tail-6 mutations, and in the functional domains—NTD-5 and CTD-8
mutations. For further experiments, the native N protein was chosen as the control model. The supplementary Table S1 represents the RMSD scores for all models compared with the native N
protein structure. The strain-specific protein tertiary structures were looked at, as shown in Fig. 5. The data analysis reveals notable distinctions between the mutant models and the native
N protein structure, as observed through structural superimposition. Figure 5a represents the existence of variations in the secondary structure elements within each strain-specific model
which illustrates the impact of the mutation on the structure. All mentioned rearrangements affect on tertiary structure of strain-specific models (Fig. 5b). An in-depth examination of the
mutated variants revealed that all the studied mutations had an impact on the secondary structure elements of the protein, resulting in structural rearrangements such as loop-to-α-helix,
loop-to-β-sheet, β-sheet-to-loop, and α-helix-to-loop transitions, as shown in Supplementary Fig. S1. We employed the PDBSum website for this structural analysis23. All studies were
performed at the level of tertiary structure, but for visualization and analysis, the tertiary structure was represented as a secondary structure. Since the server performs calculations at
the monomer level, we separated the model dimers by monomer chains and then considered them. The models utilized in our research were selectively phosphorylated at several distinct sites:
S23, S180, S186, S188, S194, S197, S201, S202, S206, T24, T198, T205, T265, and T391. After the phosphorylation process, these modified structures were then subjected to MD simulations. Each
simulation was conducted for a substantial duration of 100 ns with a simulation time step of 2 fs. To determine the protein structure stability, ΔG values, as well as RMSD and ΔG
fluctuation graphs, were considered and presented in Figs. 6, 7 and Supplementary Table S1. The average number of atoms in each of the systems, together with water and ions (Na+ and Cl−), is
about 123,000 atoms, and the protein part of the systems is about 12,800 atoms. From the graphs of RMSD fluctuations (Fig. 6) for native N and all mutated models, it can be seen that the
structure of the native N protein remained stable, while the mutant forms also showed instability of the structure, or stability at the beginning of the dynamics, then instability, and vice
versa. Instability was observed especially in D3L, S186F, S197L, R203E, K256N, F307V models. Using the trajectories obtained from molecular dynamics, we also calculated the binding free
energy of the monomers that make up the dimer in the systems by the MM/PBSA and MM/GBSA methods. ΔG values results are presented in Supplementary Table S1. In Fig. 7, we present a series of
graphs that depict the ΔG values computed using the MM/PBSA methodology across all systems under investigation. For these calculations, every 10th frame was selected, resulting in a total of
500 frames being considered for both the native and all associated mutated models. From the data obtained by the MM/GBSA method (Supplementary Table S1), the strongest interaction of
monomers in the dimer is observed in the case of the P326L mutation (ΔG = − 848.7848 kcal/mol), in the case when the following value is N wild-type (ΔG = − 765.8 ), the worst value is S202N
(ΔG = 1331.8977), and strongest interaction by MM/PBSA data is Q418H (ΔG = − 643.6706), the weakest match with MM/GBSA data and it’s S202N (ΔG = 1477.9502). The more negative the value of
ΔG, the more stable the system is, and vice versa. By data in Supplementary Table S1, according to MM/PBSA N wild-type ΔG = − 587.1434, and for Q418H ΔG = − 643.6706, T205A:ΔG = − 625.9514,
P168Q:ΔG = − 615.9578, Q384H:ΔG = − 600.1673, P326L:ΔG = − 598.1866, D3L:ΔG = − 587.1434, which means that these mutations led to a significant stabilization of the dimer structure. And the
mutations R203K/G204R (ΔG = 74.8293), S197L (ΔG = 490.5904), D63G (ΔG = 665.7818), K256N (ΔG = 751.8934), D377Y (ΔG = 994.0435), S202N (ΔG = 1477.9502) caused destabilization. As
demonstrated by MM/PBSA analyses, it was found that the dimer structure was stable in case of 28 mutations (Q418H, T205A, P168Q, Q384H, P326L, D3L, G215C, S197A, A267V, S235F, S413I, T265I,
Q160R, M322I, F307V, D401Y, A308S, alpha, P13L, delta, R185C, A173S, A208S, I157T, T205I, Q9L, R203E, S186F), and unstable in case of 9 mutations (K374N, T296I, R203M, R203K_G204R, S197L,
D63G, K256N, D377Y, S202N), Fig. 8. DISCUSSION The N protein, widely distributed within the viral genome, assumes a key role in a spectrum of fundamental biological functions, including RNA
packaging, viral assembly, replication, and transcription1,2,4,6. The multifaceted involvement of the N protein in these essential processes underscores its significance at the core of the
virus life cycle. However, studying the structure of N protein is complicated by the presence of disordered regions characterized by inherent conformational flexibility and a lack of
well-defined tertiary structures. The study of such disordered regions is of paramount importance, given their propensity to contribute to the functional versatility of the protein,
particularly in mediating various protein–protein interactions and adaptations during viral processes. The primary objective of our research was to elucidate the impact of mutations in the N
protein on the stability of its dimeric conformation. The N protein attains its functional activity in a dimeric arrangement. Given the pivotal role of the N protein in interactions with
both viral and host cell proteins, comprehending the potential influence of mutations on its dimeric structure assumes critical importance. Before scrutinizing the effects of the mutations,
a prerequisite was the modeling of the complete structure of the N protein, a task rendered challenging by the presence of disordered regions that are inherently flexible. Employing the
Alphafold2 technique, we executed the structural modeling and validated it by comparative analysis with the extant two X-ray structures of 2 domains: NTD and CTD, as well as
cross-verification with models derived from cryo-EM. The modeling encompassed both the monomeric and dimeric forms of the N protein, aiming to discern potential disparities between the two.
Visualization of the tertiary structure and identification of secondary structural elements were pivotal in this comparative analysis. As illustrated in Fig. 1, we observed a pattern in the
monomers that constitute the dimeric structure. Specifically, secondary structure elements were found to manifest in disorder regions within the monomers forming the dimer compared to the
monomer model. An intriguing observation emerged when examining the dimeric structure itself—the disorder regions within the dimer exhibited a greater degree of assembly. We hold the view
that the disorder regions in the protein tend to be more organized and assembled when part of a dimeric structure7. Subsequent experimental investigations focused exclusively on the dimeric
structure. The impact of a total 34 individual mutations, along with an additional 3 combined mutations, was systematically studied utilizing computational methodologies. This analytical
approach afforded a comprehensive understanding of the nuanced effects of these mutations on the stability and conformational dynamics of the N protein's dimeric structure. The
R203M/R203K, D377Y, D63G, G215C, G204R, D3L, S235F, and Q9L mutations included in our study are among the top 10 mutations of the N protein with the highest frequency worldwide10. MD
simulations and free binding energy calculations within the dimers showed that 9 of the 37 mutant models selected led to the destabilization of the dimer structure and 28 to stabilization.
The R203K/G204R mutation caused destabilization, which was also shown by other authors13,14,24. These mutations have been particularly studied because it has been found in a larger number of
lineages. The escalated infectiousness of the R203K/G204R mutant virus was experimentally verified by Wu et al., as evidenced by heightened viral replication observed in the 203K/204R
virus13. This enhanced replication was observed across various cell lines and primary upper airway tissues both humans and hamsters. The heightened efficiency in viral replication suggests a
potential for increased virulence and fitness. Similar associations with heightened fitness and disease severity have been observed in other mutations linked to high infectivity, including
D614G and N510Y in Spike (S) protein. Despite the distinct locations of the S protein and N protein, both being structural components of the virion, notable similarities between D614G and
R203K/G204R exist concerning the consequences of the virus's properties. In article from Mourier et al. has shown that R203K/G204R has higher viral loads in COVID-19 patients in Saudi
Arabia24. R203K/G204R mutations increased along with the mutation of the Spike Y501N protein. R203K/G204R mutation increased oligomerization potential and RNA-binding affinity compared with
wild-type N. Also, they showed that R203K/G204R mutations lead to significant changes in protein structure (destabilize) and potentially enhance the protein's ability to bind RNA and
alter its response to serine phosphorylation events. In any case, the authors did not find an association between the R203K/G204R mutations' high load and mortality, because even though
the virus had a high load, it did not affect mortality, since it is possible that other factors played a role, such as a change in the course of treatment, or a person's susceptibility
to infection. the complications that occurred24. Anyway, making definitive conclusions about the correlation between disease severity and mutation based on a single mutation within one
protein is challenging. The reason is that strains typically possess multiple complex mutations in various proteins, both structural and non-structural. During our study, 63.8% of selected
mutations were located in disorder regions, of which 60.86% were in the LKR part, which is involved in protein–protein interactions. About this, Abavisani et al. also note that the majority
of N protein mutations occur in 164 to 205 AA and the second highest mutations frequency in N 205 to 246 AA, which also include in the basis of the disorder sections10. The study by Oulas et
al. demonstrates that the P13L mutation can influence both transmissibility and death rates. Specifically, this mutation is associated with a decrease in the number of deaths and cases per
million15,25. For the P13L mutation, our experiments showed that it leads to structural stabilization. Making definitive conclusions about the correlation between disease severity and
mutation based on a single mutation within one protein is challenging. The reason is that strains typically possess multiple complex mutations in various proteins, both structural and
non-structural, and more research is needed, because the same R203K/G204R mutation, which has been reported in several articles to affect virulence, pathogenesis, and fitness10,11,13,24, is
found in the base together with the spike protein N510Y mutation, which also enhances viral attachment and infectivity10,24․ In conclusion, our findings hold significance in elucidating the
tertiary structure of the N protein dimer and comprehending the consequences of mutations on its conformation. Our study provides insights into the behavioral dynamics of these mutations
within the host cell. It is known from the literature that intrinsically disordered proteins (IDPs) undergo a disorder-to-order transition state during protein–protein interactions7,26,27.
We advance a hypothesis suggesting that in the dimeric state characterized by protein–protein interaction, secondary structure elements appear in the disordered parts of the protein, and the
protein is assembled in its tertiary structure. As illustrated in Fig. 1d and Supplementary Fig. S1, our findings indicate that, compared with N monomer secondary structure elements, in
dimers, the secondary structure elements appear in disorder regions. And compared to the wild-type dimer N structure, models featuring the D63G, Q160R, S197L, S186F, S202N, S203E, R203M,
K256N, T296I, S413I, Q418H, alpha and delta mutations (35,1% of all models) exhibit a reduced presence of secondary structure elements within the disordered regions (Supplementary Fig. S1).
From which D63G, S197L, S202N, R203M, K256N, and T296I mutations (46,15%) led to the destabilization of the structure. This allows us to assume mutations that caused destabilization, are
less likely to generate secondary structure elements in disordered regions of the protein. Our results contribute not only to an understanding of the SARS-CoV-2 N protein but also offer
valuable hints for the investigation of conservative proteins in other viruses within the same genus. Our research establishes a basis for proactive strategies designed to address the
pathogenesis of this viral illness as the presence of disordered regions in viral proteins is commonly associated with viral infectivity and pathogenicity, as these regions typically play an
essential role in the binding process with targets7. Certainly, in the context of single protein mutations, drawing unequivocal conclusions regarding their impact on virus flexibility,
virulence, and disease progression among patients is challenging. Comprehensive investigations are essential, extending beyond the examination of individual mutations within a single
protein. Instead, a more intricate approach involves conducting studies at the level of multiple mutations within proteins that play crucial roles in the virus's life cycle. This
encompasses an exploration of the collective effects of mutations across various proteins involved in the virus life cycle. METHODS MODELING To carry out the research, we used Alphafold2
version 2.1.2 for protein structure prediction19,28. The amino acid sequence of the SARS-CoV-2 N protein (UniProt ID: P0DTC9) was used for molecular modeling. PHOSPHORYLATION AND MUTATIONS
All mutations were obtained using the ICM-Pro software29, and then the models were phosphorylated at the following sites—S23, S180, S186, S188, S194, S197, S201, S202, S206, T24, T198, T205,
T265, T39130. MD SIMULATIONS, ANALYSIS (MM/PB(GB)SA, RMSD) The resulting systems were subjected to 100 ns molecular dynamics (MD) simulations using AMBER2031,32. All molecular dynamics (MD)
simulations were conducted in water environments using the ff19SB force field for the solute, as it has been demonstrated to synergize optimally with the OPC water model, which offers
enhanced accuracy33,34,35. The solvation box, housing the water molecules, was defined as a cubic periodic boundary to ensure homogeneous spatial distribution and to mitigate potential edge
effects throughout the simulation trajectories. The integration step for the leap-frog algorithm was fixed at 2 fs. Simultaneously, the system was maintained at 309.75 K under standard
atmospheric pressure. The binding free energy was calculated by the MM/PB(GB)SA method, which is based on the determination of the energy of the protein–ligand complex, the individual
energies of the protein and the ligand, and then the determination of the energy difference36,37. Molecular dynamics simulations were executed across all the prepared systems, each spanning
a duration of 100 ns. For every individual system, a consistent sampling rate was maintained, resulting in the acquisition of 5,000 frames. The binding free energy (ΔG) is calculated
according to Eq. (1)36,37. $$\Delta G={\Delta G}_{complex}-{\Delta G}_{receptor}-{\Delta G}_{ligand}$$ (1) Since each system consists of an N protein dimer, the role of the receptor and the
ligand were each of the monomers: receptor (monomer1)—the monomer with amino acid residues 1–419, and ligand (monomer2)—the monomer with amino acid residues 420–838, Eq. (2). $$\Delta
G={\Delta G}_{complex}-{\Delta G}_{monomer1}-{\Delta G}_{monomer2}$$ (2) Typically, the quality of a model is assessed based on its energy or similarity to a reference structure—RMSD
(usually atoms in the protein backbone). RSMD analysis of all obtained trajectories was performed using the CPPTRAJ program. RMSD can be used to identify significant changes in protein
structure relative to the starting point. The equilibrium nature of the RMSD curve indicates that the protein was equilibrated38. For studying structural rearrangements (loop-α-helix,
loop-β-sheet, β-sheet-loop, and α-helix-loop) the PDBSum website was used23. For visualization, we have used ICM-Pro software, and for graph building Gnuplot and ChimeraX have been
used39,40. DATA AVAILABILITY The datasets used and/or analysed during the current study available from the corresponding author on reasonable request. REFERENCES * Bai, Z., Cao, Y., Liu, W.
& Li, J. The SARS-COV-2 nucleocapsid protein and its role in viral structure, biological functions, and a potential target for drug or vaccine mitigation. _Viruses_ 13, 1115 (2021).
Article CAS PubMed PubMed Central Google Scholar * Kopecky-Bromberg, S. A., Martínez-Sobrido, L., Frieman, M. B., Baric, R. & Palese, P. Severe acute respiratory syndrome
coronavirus open reading frame (ORF) 3B, ORF 6, and nucleocapsid proteins function as interferon antagonists. _J. Virol._ 81, 548–557 (2007). Article CAS PubMed Google Scholar * Khan, A.
_et al._ Structural insights into the mechanism of RNA recognition by the N-terminal RNA-binding domain of the SARS-CoV-2 nucleocapsid phosphoprotein. _Comput. Struct. Biotechnol. J._ 18,
2174–2184 (2020). Article CAS PubMed PubMed Central Google Scholar * Giri, R. _et al._ Understanding COVID-19 via comparative analysis of dark proteomes of SARS-CoV-2, human SARS and
bat SARS-like coronaviruses. _Cell. Mol. Life Sci._ 78, 1655–1688 (2020). Article PubMed PubMed Central Google Scholar * Mu, J. _et al._ SARS-CoV-2-encoded nucleocapsid protein acts as a
viral suppressor of RNA interference in cells. _Sci. China Life Sci._ 63, 1413–1416 (2020). Article PubMed PubMed Central Google Scholar * Tugaeva, K. V. _et al._ The mechanism of
SARS-COV-2 nucleocapsid protein recognition by the human 14-3-3 proteins. _J. Mol. Biol._ 433, 166875 (2021). Article CAS PubMed PubMed Central Google Scholar * Tenchov, R. & Zhou,
Q. Intrinsically disordered proteins: Perspective on COVID-19 infection and drug discovery. _ACS Infect. Dis._ 8, 422–432 (2022). Article CAS PubMed Google Scholar * Zhou, R., Zeng, R.,
Von Brunn, A. & Lei, J. Structural characterization of the C-terminal domain of SARS-CoV-2 nucleocapsid protein. _Mol. Biomed._ https://doi.org/10.1186/s43556-020-00001-4 (2020). Article
PubMed PubMed Central Google Scholar * Ni, X., Han, Y., Zhou, R., Zhou, Y. & Lei, J. Structural insights into ribonucleoprotein dissociation by nucleocapsid protein interacting with
non-structural protein 3 in SARS-CoV-2. _Commun. Biol._ https://doi.org/10.1038/s42003-023-04570-2 (2023). Article PubMed PubMed Central Google Scholar * Abavisani, M. _et al._
Mutations in SARS-CoV-2 structural proteins: A global analysis. _Virol. J._ https://doi.org/10.1186/s12985-022-01951-7 (2022). Article PubMed PubMed Central Google Scholar * Johnson, B.
A. _et al._ Nucleocapsid mutations in SARS-CoV-2 augment replication and pathogenesis. _PLoS Pathog._ 18, e1010627 (2022). Article CAS PubMed PubMed Central Google Scholar * Khan, M. Z.
_et al._ An overview of viral mutagenesis and the impact on pathogenesis of SARS-CoV-2 variants. _Front. Immunol._ https://doi.org/10.3389/fimmu.2022.1034444 (2022). Article PubMed PubMed
Central Google Scholar * Wu, H. _et al._ Nucleocapsid mutations R203K/G204R increase the infectivity, fitness, and virulence of SARS-CoV-2. _Cell Host Microbe_ 29, 1788-1801.e6 (2021).
Article CAS PubMed PubMed Central Google Scholar * Rahman, M. S. _et al._ Evolutionary dynamics of SARS-CoV-2 nucleocapsid protein and its consequences. _J. Med. Virol._ 93, 2177–2195
(2020). Article PubMed Google Scholar * Dang, S., Ren, L. & Wang, J. Functional mutations of SARS-CoV-2: Implications to viral transmission, pathogenicity and immune escape. _Chin.
Med. J._ 135, 1213–1222 (2022). Article CAS PubMed PubMed Central Google Scholar * Oldfield, C. J. & Dunker, A. K. Intrinsically disordered proteins and intrinsically disordered
protein regions. _Annu. Rev. Biochem._ 83, 553–584 (2014). Article CAS PubMed Google Scholar * Uversky, V. N., Gillespie, J. R. & Fink, A. L. Why are “natively unfolded” proteins
unstructured under physiologic conditions?. _Proteins_ 41, 415–427 (2000). Article CAS PubMed Google Scholar * Wilson, C. J., Choy, W. & Karttunen, M. AlphaFold2: A role for
disordered protein/region prediction?. _Int. J. Mol. Sci._ 23, 4591 (2022). Article CAS PubMed PubMed Central Google Scholar * Jumper, J. _et al._ Highly accurate protein structure
prediction with AlphaFold. _Nature_ 596, 583–589 (2021). Article ADS CAS PubMed PubMed Central Google Scholar * Casasanta, M. A. _et al._ Structural insights of the SARS-COV-2
nucleocapsid protein: Implications for the inner-workings of rapid antigen tests. _Microsc. Microanal._ 29, 649–657 (2022). Article ADS Google Scholar * Avetyan, D. _et al._ Molecular
analysis of SARS-COV-2 lineages in Armenia. _Viruses_ 14, 1074 (2022). Article CAS PubMed PubMed Central Google Scholar * Cov-Lineages. https://cov-lineages.org/. * Team, E. W.PDBSum
generate. https://www.ebi.ac.uk/thornton-srv/databases/pdbsum. * Mourier, T. _et al._ SARS-CoV-2 genomes from Saudi Arabia implicate nucleocapsid mutations in host response and increased
viral load. _Nat. Commun._ https://doi.org/10.1038/s41467-022-28287-8 (2022). Article PubMed PubMed Central Google Scholar * Oulas, A. _et al._ Generalized linear models provide a
measure of virulence for specific mutations in SARS-CoV-2 strains. _PLoS ONE_ 16, e0238665 (2021). Article CAS PubMed PubMed Central Google Scholar * Lindström, I. & Dogan, J.
Dynamics, conformational entropy, and frustration in protein–protein interactions involving an intrinsically disordered protein domain. _ACS Chem. Biol._ 13, 1218–1227 (2018). Article
PubMed Google Scholar * Saurabh, S., Nadendla, K., Purohit, S. S., Sivakumar, P. M. & Çetinel, S. Fuzzy drug targets: Disordered proteins in the drug-discovery realm. _ACS Omega_ 8,
9729–9747 (2023). Article CAS PubMed PubMed Central Google Scholar * Evans, R. _et al._ Protein complex prediction with AlphaFold-Multimer. _BioRxiv Cold Spring Harb. Lab._
https://doi.org/10.1101/2021.10.04.463034 (2021). Article Google Scholar * Abagyan, R., Totrov, M. & Kuznetsov, D. ICM - A new method for protein modeling and design: Applications to
docking and structure prediction from the distorted native conformation. _J. Comput. Chem._ 15, 488–506 (1994). Article CAS Google Scholar * Tung, H. Y. L. & Limtung, P. Mutations in
the phosphorylation sites of SARS-CoV-2 encoded nucleocapsid protein and structure model of sequestration by protein 14-3-3. _Biochem. Biophys. Res. Commun._ 532, 134–138 (2020). Article
CAS PubMed PubMed Central Google Scholar * Case, D. I. _et al._ AMBER (2020). * Karplus, M. Molecular Dynamics simulations of biomolecules. _Acc. Chem. Res._ 35, 321–323 (2002). Article
CAS PubMed Google Scholar * Izadi, S., Anandakrishnan, R. & Onufriev, A. V. Building water models: A different approach. _J. Phys. Chem. Lett._ 5, 3863–3871 (2014). Article CAS
PubMed PubMed Central Google Scholar * Raguette L., Cuomo, A., Belfon, K., Tian, C., Wu, Q. & Simmerling, C. Updated Amber force field parameters for phosphorylated amino acids for
ff14SB and ff19SB. (2020) (IN PREP). * Tian, C. _et al._ ff19SB: Amino-acid-specific protein backbone parameters trained against quantum mechanics energy surfaces in solution. _J. Chem.
Theory Comput._ 16, 528–552 (2019). Article PubMed Google Scholar * Kumari, R., Kumar, R. & Lynn, A. M. g_mmpbsa—A GROMACS tool for high-throughput MM-PBSA calculations. _J. Chem.
Inf. Model._ 54, 1951–1962 (2014). Article CAS PubMed Google Scholar * Massova, I. & Kollman, P. A. Combined molecular mechanical and continuum solvent approach (MM-PBSA/GBSA) to
predict ligand binding. _Perspect. Drug Discov. Des._ 18, 113–135 (2000). Article CAS Google Scholar * Kufareva, I. & Abagyan, R. Methods of protein structure comparison. In _Methods
in Molecular Biology_ 231–257 (2011) https://doi.org/10.1007/978-1-61779-588-6_10. * Williams, T., et algnuplot 5.2. An interactive plotting program. (2017). http://www.gnuplot.info/. *
Pettersen, E. F. _et al._ UCSF ChimeraX: Structure visualization for researchers, educators, and developers. _Protein Sci._ 30, 70–82 (2020). Article PubMed PubMed Central Google Scholar
Download references ACKNOWLEDGEMENTS We are grateful to Dr. Arsen Arakelyan and the Institute of Molecular Biology’s Human Genomics Laboratory and Bioinformatics group for providing
mutation data related to the N protein. The work was supported by the Higher Education and Science Committee RA, in the frames of the research projects №21AG-1F057, 22AA-1F026, and
Foundation Armenia (Switzerland). This work used the Cirrus UK National Tier2 HPC Service at EPCC (http://www.cirrus.ac.uk) funded by the University of Edinburgh and EPSRC (EP/P020267/1). We
also thank the Joint Institute for Nuclear Research (Dubna, Russia) for providing HPC resources. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Laboratory of Computational Modeling of
Biological Processes, Institute of Molecular Biology of the National Academy of Sciences of the Republic of Armenia (NAS RA), 0014, Yerevan, Armenia Nelli Muradyan, Vahram Arakelov, Arsen
Sargsyan, Adrine Paronyan, Grigor Arakelov & Karen Nazaryan * Russian-Armenian University, 0051, Yerevan, Armenia Arsen Sargsyan, Adrine Paronyan, Grigor Arakelov & Karen Nazaryan
Authors * Nelli Muradyan View author publications You can also search for this author inPubMed Google Scholar * Vahram Arakelov View author publications You can also search for this author
inPubMed Google Scholar * Arsen Sargsyan View author publications You can also search for this author inPubMed Google Scholar * Adrine Paronyan View author publications You can also search
for this author inPubMed Google Scholar * Grigor Arakelov View author publications You can also search for this author inPubMed Google Scholar * Karen Nazaryan View author publications You
can also search for this author inPubMed Google Scholar CONTRIBUTIONS G.A. conceived the experiments, N.M. conducted the experiments, N.M. and G.A. analysed the experiments, N.M. and V.A.
prepared figures. N.M. wrote the draft. All authors: K.N., V.A., G.A., A.P., A.S., and N.M. reviewed the manuscript. K.N. supervision. CORRESPONDING AUTHOR Correspondence to Grigor Arakelov.
ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under
a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate
credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article
are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons
licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of
this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Muradyan, N., Arakelov, V., Sargsyan, A. _et al._ Impact of
mutations on the stability of SARS-CoV-2 nucleocapsid protein structure. _Sci Rep_ 14, 5870 (2024). https://doi.org/10.1038/s41598-024-55157-8 Download citation * Received: 30 October 2023 *
Accepted: 21 February 2024 * Published: 11 March 2024 * DOI: https://doi.org/10.1038/s41598-024-55157-8 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative