Play all audios:
ABSTRACT _Perilla frutescens_ (L.) Britton, a member of the Lamiaceae family, stands out as a versatile plant highly valued for its unique aroma and medicinal properties. Additionally, _P.
frutescens_ seeds are rich in Îś-linolenic acid, holding substantial economic importance. While the nuclear and chloroplast genomes of _P. frutescens_ have already been documented, the
complete mitochondrial genome sequence remains unreported. To this end, the sequencing, annotation, and assembly of the entire Mitochondrial genome of _P. frutescens_ were hereby conducted
using a combination of Illumina and PacBio data. The assembled _P. frutescens_ mitochondrial genome spanned 299,551 bp and exhibited a typical circular structure, involving a GC content of
45.23%. Within the genome, a total of 59 unique genes were identified, encompassing 37 protein-coding genes, 20 tRNA genes, and 2 rRNA genes. Additionally, 18 introns were observed in 8
protein-coding genes. Notably, the codons of the _P. frutescens_ mitochondrial genome displayed a notable A/T bias. The analysis also revealed 293 dispersed repeat sequences, 77 simple
sequence repeats (SSRs), and 6 tandem repeat sequences. Moreover, RNA editing sites preferentially produced leucine at amino acid editing sites. Furthermore, 70 sequence fragments (12,680
bp) having been transferred from the chloroplast to the mitochondrial genome were identified, accounting for 4.23% of the entire mitochondrial genome. Phylogenetic analysis indicated that
among Lamiaceae plants, _P. frutescens_ is most closely related to _Salvia_ _miltiorrhiza_ and _Platostoma chinense_. Meanwhile, inter-species Ka/Ks results suggested that Ka/Ks \(<1\)
for 28 PCGs, indicating that these genes were evolving under purifying selection. Overall, this study enriches the mitochondrial genome data for _P. frutescens_ and forges a theoretical
foundation for future molecular breeding research. SIMILAR CONTENT BEING VIEWED BY OTHERS ASSEMBLY AND ANALYSIS OF THE COMPLETE MITOCHONDRIAL GENOME OF _LEONURUS JAPONICUS_ (LAMIACEAE)
Article Open access 18 April 2025 COMPARATIVE CHLOROPLAST GENOME ANALYSIS OF FOUR _POLYGONATUM_ SPECIES INSIGHTS INTO DNA BARCODING, EVOLUTION, AND PHYLOGENY Article Open access 01 October
2023 THE CHARACTERISATION OF THE COMPLETE MITOCHONDRIAL GENOME OF _POLYGONATUM KINGIANUM_ REVEALS RECOMBINATION MEDIATED BY REPEATS ASSOCIATED WITH DNA REPLICATION Article Open access 06
June 2025 INTRODUCTION The mitochondrial genome is relatively small, highly conserved, and densely packed with genes, while also containing highly variable non-coding regions? In plant
research, in addition to its role in energy metabolism, the mitochondrial genome generally affects plant fertility, also playing an important role in plant development and stress
resistance1. Comparative analyses of mitochondrial genome sequences, structures, and functions reveal the complexity of plant mitochondrial genomes, providing a basis for deciphering their
classification and evolution, and facilitating the exploration of cytoplasmic male sterility mechanisms2,3. However, research on complete plant mitochondrial genomes lags far behind that of
complete plastid genomes. The NCBI database hosts nearly asting with only 673 complete plant mitochondrial genomes4, which is attributed 13,000 complete plastid genomes, contrasting with
only 673 complete plant mitochondrial genomes4, which is attributed to the complex structure of plant Mitochondria. They are challenging to purify and often face interference from
chloroplasts and other organelles, complicating genome assembly. In addition, significant differences are observed in the structure and content of plant mitochondrial genomes, as well as in
nucleotide substitution rates and repeat sequences3,5. Therefore, a complete description of the plant mitochondrial genome remains a bottleneck in evolutionary biology, yet most plant
systematic studies focus on nuclear and plastid genomes6. For example, while the nuclear and plastid genomes of_ Perilla frutescens _ have been sequenced and assembled, its mitochondrial
genome remains unknown7,8,9. With the development of high-throughput sequencing technology and the rise of next-generation systems genomics, many software programs applicable to
mitochondrial genome sequencing and assembly, such as GetOrganelle10, Mitofiner11, GSAT12, and PMAT13 (https://github.com/bichangwei/PMAT), have been developed, making mtochondrial genome
sequencing and assembly more accurate and efficient14. Besides, in land plants, mitochondrial genomes typically contain exogenous genes or fragments due to horizontal gene transfer or
intracellular gene transfer15,16. As the mitochondrial genome undergoes continuous recombination, this sequence transfer between genomes also occurs17. This can result in cytoplasmic male
sterility (CMS), where plants cannot produce viable pollen grains due to a mismatch between the nuclear and cytoplasmic genomes. However, female fertility is maintained, as observed in
Brassica, rice, and other plants17,18,19. Additionally, CMS is often inherited maternally and is commonly associated with abnormal open reading frames (ORFs) and RNA editing in the
mitochondrial genome20. These ORFs are often found near functional mitochondrial genes and are co-transcribed with them, affecting the mitochondrial function21. CMS-based hybridization
techniques have been utilized to breed offspring with significantly improved yield, stress resistance, and adaptability, making this approach a promising way to maintain crop productivity22.
Therefore, mitochondrial genomes are a valuable source of genetic information for plant systematics and necessary cellular process research. They have significant implications for species
evolution research, species identification, and genetic transformation23. _Perilla frutescens_ (L.) Britt. belongs to the Lamiaceae family, and is an annual upright herb. It is renowned as a
traditional dual-purpose medicinal and edible plant, prized for its distinct aroma24. Notably, its leaves, stems, and fruits all possess medicinal properties. _P. frutescens_ is widely
cultivated in China and Southeast Asia, ranging from Indonesia in the south to Japan in the east25. Current research on _P. frutescens _mainly focuses on its pharmacological effects and
chemical composition of its compound perillaldehyde26. Currently, 400 compounds have been isolated from _P. frutescens_ leaves, including terpenoids, flavonoids, alkaloids, steroids,
quinines, and phenolic compounds, all of, which hold significant potential for various applications27,28,29. People have discovered the pharmacological functions of _P. frutescens_
attributed to secondary metabolites present in different parts of the plant, such as anti-allergic, anti-depressive, lipid-lowering, hepatoprotective, neuroprotective, anti-inflammatory,
anti-cancer, antioxidant and antibacterial activities30,31,32,33. In addition, common oil crops such as soybean, rapeseed, peanut, and olive generally have an \(\alpha\)-linolenic acid
content of less than 5\(\%\)34,35. Conversely, _P. frutescens_ seeds boast a high oil content of 45–55\(\%\), with a rich content of unsaturated fatty acids, accounting for more than
90\(\%\) of the total oil content. Among which, \(\alpha\)-linolenic acid has the highest content, reaching 55 65\(\%\)36. Consequently, _P. frutescens_ is considered a natural resource
possessing significant economic and medicinal value. Despite being a plant of significant medicinal and economic value, the mitochondrial genome of _P. frutescens_ frutescens has not yet
been assembled and analyzed. This crucial step is essential for comprehending its genetic composition and realizing its full potential for diverse applications. In this study, sequencing and
annotation of the _P. frutescens_ mitochondrial genome were conducted, and mitochondrial genome features, RNA editing, and codon bias were comprehensively analyzed. Besides, systematic
evolutionary analysis was also carried out to provide essential background information for further understanding the genetics of this plant. Overall, this research lays the groundwork for
future studies on molecular breeding strategies for _P. frutescens_. RESULTS MITOCHONDRIAL GENE ORGANIZATION AND FEATURES OF _P. FRUTESCENS_ In this study, the mitochondrial genome was
sequenced using Illumina and Pacbio sequencing platforms. A total of 248,479,765 clean reads (Q30 = 89.78\(\%\)) were generated from the second-generation sequencing platform, while the
third-generation sequencing platform produced 7,853,744 clean reads. A total of 63,122,358,854 bases were generated. The subreads possessed an N50 value of 9597 bp and an N90 value of 4224
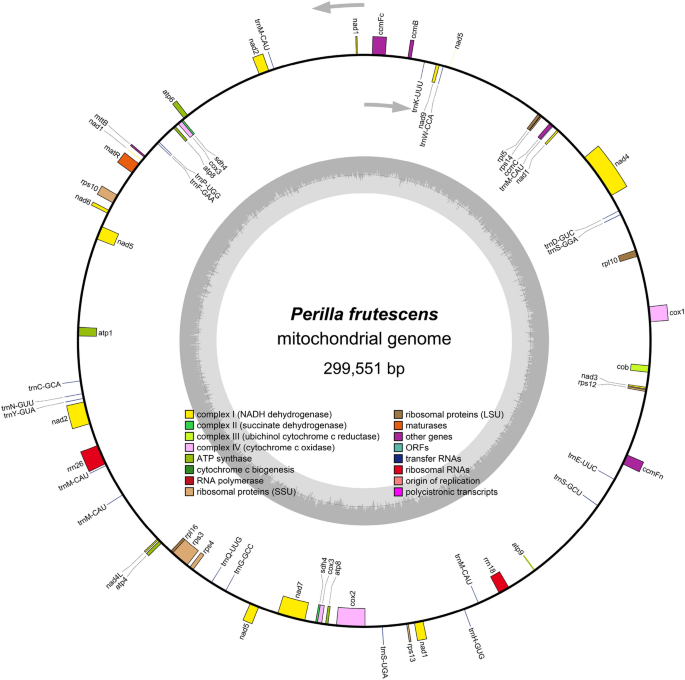
bp. The longest subread possessed a length of 65,388 bp. The complete circular mitochondrial DNA molecule of _P. frutescens_ with a length of 299,551 bp was obtained through de novo assembly
(Fig. 1). The nucleotide composition of _P. frutescens_ mitochondrial DNA was 27.3\(\%\) A, 27.5\(\%\) T, 22.5\(\%\) G, and 22.7\(\%\) C, with a GC content of 45.23\(\%\). Ultimately, the
_P. frutescens_ mitochondrial genome sequence was archived in CNGB with the accession number AA059311.1. The mitochondrial genome of _ P. frutescens_ encompasses a total of 59 genes,
consisting of 37 protein-coding genes (PCGs), 20 transfer RNAs (_trn_As), and 2 ribosomal RNAs (rRNAs) (refer to Table 1). The essential genes include 6 _ATP_ synthase genes (_atp_1, _atp_4,
_atp_6, _atp_8, and _atp_9), 9 _NAD_H dehydrogenase genes (_nad_1, _nad_2, _nad_3, _nad_4, _nad_4L, _nad_5, _nad_6, _nad_7, and _nad_9), 4 cytochrome c biogenesis genes (_ccm_B, _ccm_C,
_ccm_FC, and _ccm_FN), 4 cytochrome c oxidase genes (_cox_1, _cox_2, and _cox_3), 1 Maturases gene (_mat_R), 1 membrane transport protein gene (_mtt_B), and 1 ubiquinol cytochrome c
reductase gene (_cob_). The variable genes include 3 large subunits of ribosomal protein (_rpl_16, _rpl_10, _rpl_5), 6 small subunits of ribosomal protein (_rps_10, _rps_12, _rps_13,
_rps_14, _rps_3, _rps_4), and 2 succinate dehydrogenases (_sdh_4). Herein, notably, the genes _atp_8, _cox_3, and sdh4 were observed in duplicate copies. Introns were found in the _nad_1,
_nad_2, _nad_5 (with four introns each), _nad_4, and _nad_7 genes (with three introns each), as well as in _ccm_FC, _cox_1, _cox_2, _rps_10, and _rps_3 (each containing one intron).
Furthermore, the _sdh_4 sequences were identified as pseudogenes. Among the transfer RNA genes, _trn_M-CAU was duplicated 5 times, while the remaining _trn_A genes existed as identical
copies within the mitochondrial genome. REPEAT SEQUENCE ANALYSIS In the plant mitochondrial genome, there are abundant repetitive sequences, including simple sequence repeats (SSRs), tandem
repeats, and dispersed repeats37. In the _P. frutescens_ mitochondrial genome, two different types of dispersed repeats, i.e., Forward and Palindromic, were identified, totaling 293
dispersed repeats with a length greater than or equal to 20 bp. No reverse or complementary repeat sequences were detected. The dispersed repeat sequences were visualized using the Circos
software package. The results uncovered the wide distribution of these repeats in the intergenic regions of these repeats in the intergenic regions of the entire genome (Fig. 2A and
Supplementary Table S1). Besides, a total of 293 dispersed repeat sequences were identified, with a combined length of 22,2651 bp, accounting for 3.76\(\%\) of the total mitochondrial genome
length. Among these repeats, there were 151 forward repeats and 142 palindromic repeats (Fig. 2B). The longest forward repeat sequence was 5545 bp, while the longest palindromic repeat
sequence was 222 bp. Further analysis demonstrated 20–29 bp repeats as the most common type, accounting for 51.53\(\%\) of the total repeat occurrences. The presence of these dispersed
repeat sequences might be related to the structure and function of the mitochondrial genome. Microsatellites (simple repeat sequences, SSRs) are typically tandem sequences of up to 6 base
pairs in eukaryotic genomes38. In this investigation, a total of 77 SSRs were identified in the mitogenome of _P. frutescens_ (Fig. 2C), dominated by tetrameric repeats, accounting for
27.35\(\%\) (27) of the total count, followed by dimeric repeats (20), monomeric repeats (11), trimeric repeats (9), pentameric repeats (6), and hexameric repeats (5). Adenine (A) repeats
took up the highest proportion (90.91\(\%\)) within the monomeric SSRs, while AG repeats occupied the highest proportion among dimeric repeats (65.00\(\%\)) (Fig. 2D). None of the repeat
types exhibited sequences with a repeat length exceeding 20 base pairs. These widely distributed SSRs provided rich potential molecular markers for the identification and genetic research of
_ P. frutescens_ plants. Additionally, tandem repeats, also known as satellite DNA, are characterized by repeat lengths of 1–200 bases with varying numbers of repeats39. Herein, 6 tandem
repeat sequences with lengths between 34 and 60 base pairs were identified in the _P. frutescens_ mitochondrial genome, all of which were present in the intergenic regions (Supplementary
Table S2). HOMOLOGOUS SEQUENCE ANALYSIS OF ORGANELLAR GENOMES The mitochondrial genome of _P. frutescens_ (299,551 bp) is approximately 1.96 times larger than the chloroplast genome (152,593
bp). However, compared to the chloroplast genes, the distribution of mitochondrial genes in _P. frutescens_ is relatively sparse (Fig. 3 and Supplementary Table S3). In this study, based on
sequence similarity between chloroplast and mitochondrial genomes, 70 chloroplast-like segments potentially involved in gene transfer were identified in the mitochondrial genome (Fig. 3).
These inserted segments were distributed throughout the mitochondrial genome, with a total length of 12,680 bp, accounting for 4.23\(\%\) of the entire mitochondrial genome. The longest
sequence (875 bp) was transferred from the cp genome’s _psb_A gene to the intergenic regions of the mitochondrial genes _nad_2 and _atp_6, while the second longest sequence (820 bp) was
transferred from the cp genome’s _pet_A gene to the intergenic regions of the mitochondrial genes _nad_2 and _atp_6. These transferred sequences were predominantly located in the
mitochondrial genome’s intergenic spacers (27), ribosomal RNA genes (20), and transfer RNAs (19). In the _P. frutescens_ chloroplast genome, 22 _rrn_16S and _rrn_23S sequences were inserted
into the mitochondrial genome, with most of them being transferred to _rrn_18 or _rrn_26, except for 4 segments having been transferred to the IGS region. In the remaining chloroplast-like
sequences, 15 _trn_A genes were completely transferred (_trn_P-UGG (2), _trn_F-GAA, _trn_S-GGA, _trn_T-GGU, _trn_D-GUC, _trn_S-GCU (2), _trn_Q-UUG, _trn_H-GUG, _trn_M-CAU (2), _trn_N-GUU,
_trn_N-GUU, and _trn_W-CCA). Apart from _rps_11, which has been fully transferred to the mitochondrial genome _cox_1 and _rps_10’s IGS, the rest were partial sequences from the chloroplast
genome (_psb_N, _psb_B, _psb_E, _pet_A, _acc_D, _rps_4, _ycf_3, _Psa_, _Psa_B, _psb_C, _rpo_B, _atp_A, _psb_A, _rpl_2, _rpl_23, _ycf_2, _nad_F, and _nad_H) transferred to partial genes or
IGS of the mitochondrial genome. CONDON USAGE AND RSCU ANALYSIS The total length of 34 PCGs in _P. frutescens_ is 34,059 bp, containing a total of 9729 codons. The number of encoded codons
varies from 8 to 389, with a total of 64 codons encoding 20 amino acids, including the stop codons (UAG (*), UAA (*), and UGA (*)). After excluding the three stop codons and the unbiased
methionine (Met) and threonine (Thr), 31 codons among the 64 codons displayed a preference with RSCU values exceeding 1, indicating a higher priority for these codons. The GCU codon for
Alanine (Ala) had the highest frequency of occurrence, with an average RSCU value of 1.61. The AUG codon coded for Methionine (Met) exhibited the highest frequency of occurrence, as
indicated by an average RSCU value of 1.89. Among the analyzed codons, the remaining 31 codons displayed a relatively low bias, as indicated by their RSCU values less than 1 (Fig. 4).
Meanwhile, results showed that codons ending with A or U had RSCU values mostly greater than 1.0, while those ending with C or G had RSCU values mostly less than 1. Additionally, codon usage
was typically strongly biased towards A or T(U) at the third codon position, which was also observed in other plant mitochondrial genomes. SELECTIVE PRESSURE ANALYSES The Ka/Ks values of
common protein-coding genes in the _P. frutescens_ mitochondrial genome were calculated and compared with 9 Lamiaceae species (_Ajuga reptans_, _Lavandula angustifolia_, _Platostoma
chinense_, _Pogostemon heyneanus_, _Prunella vulgaris_, _Rotheca serrata_, _Scutellaria barbata_, _Scutellaria franchetiana_, and _ Vitex trifolia_) (Fig. 5). The average Ka/Ks value for 32
identical protein-coding genes was 0.33. Deeper investigation into the selective pressures on specific genes uncovered that except for _ccm_Fn, _mtt_B, _rps_10, and _mat_R showed Ka/Ks
ratios > 1 compared to the other 9 plants, indicating that they might be undergoing positive selection. Most genes exhibited a negative selection effect (Ka/Ks < 1) compared to the
other 9 plants, suggesting that most protein-coding genes in the _P. frutescens_ mitochondrial genome were highly conserved during molecular evolution. RNA EDITING SITES PREDICTION RNA
editing refers to the process of adding, removing, or substituting bases in the coding region of transcribed RNA, which occurs in all eukaryotes15. In the present study, a total of 559 RNA
editing sites were predicted within the mitochondrial genome’s 34 protein-coding genes (PCGs) of _P. frutescen_. The distribution of these RNA editing sites varied among different genes,
with the nad4 gene having the highest number at 48, followed by _ccm_B with 42 sites, making them the top two genes in terms of RNA editing sites (Fig. 6, Supplementary Table S4).
Additionally, _mtt_B, _ccm_C, _nad_7, _nad_2, _ccm_FN, and _nad_5 each exhibited over 30 editing sites. Conversely, genes like _rps_14,_ rpl_2, and _rps_7 presented the fewest editing sites,
with only two C-to-U edits observed. Meanwhile, a total of 56 types of codon transitions were identified, yielding 25 different amino acid changes. Among these codon transition types, the
UCA\(\rightarrow\)UUA transition occurred the most frequently, occurring at 64 sites. The translated amino acids were significantly affected by RNA editing events, with 558 RNA editing sites
leading to non-synonymous substitutions and alterations in encoded amino acids, accounting for 99.83% of the total changes. Furthermore, the most common substitutions involved proline (Pro)
and serine (Ser) being replaced by leucine (Leu) 100 and 99 times, respectively. Additionally, serine (Ser) was frequently substituted by phenylalanine (Phe) in 76 cases. Collectively,
these three substitutions accounted for 49.23\(\%\) of all observed changes. In comparison to the one synonymous substitution RNA editing event, these widespread RNA editing events held
considerable significance in protein translation. PHYLOGENETIC ANALYSIS Reconstructing the phylogenetic tree based on conservative mitochondrial protein-coding genes is instrumental in
elucidating the molecular evolutionary relationships among green plants. To determine the classification status of the _P. frutescens _mitochondrial genome, _P. frutescens_ was hereby
analyzed phylogenetically with 59 species, including 44 dicotyledonous plants (including 20 species of Lamiaceae), 13 monocotyledonous plants, 1 gymnosperm, and 1 bryophyte (outgroup). A
total of 24 protein-coding genes corresponding to each species were also analyzed. Based on the concatenated dataset, both ML and BI phylogenies generated identical tree topologies, with
nearly all nodes exhibiting high support values (ultrafast bootstrap (UFboot) = 100; SH-aLRT values (SH-aLRT) = 100; posterior probability (PP) = 1.00). The results indicated that the
phylogenetic tree strongly supported the separation of dicots and monocots, as well as that of angiosperms and gymnosperms (Fig. 7). Furthermore, the clade distributions of Asparagales,
Lamiales, Fabales, Brassicales, Alismatales, Poales, Cucurbitales, Gentianales, Malvales, Caryophyllales, Vitales, Rosales, Solanales, Apiales, Butomaceae, Zosteraceae, Marchantiophyta,
Malpighiales, Ginkgoales and Arecales were well resolved in the phylogenetic tree. Relationships in the phylogenetic tree were found to be consistent with the traditional classification
relationships of these species, indicating the congruence of traditional and molecular classifications at the family level. _P. frutescens_ belonging to the Nepetoideae subfamily of the
Lamiaceae family shared a highly similar topology to that of the phylogenetic reconstruction inferred from plastid genome and nuclear genome sequences. The species tree topology implemented
in ASTRAL was basically congruent with the result of the concatenation analyses, with high local posterior probability (LPP) values at most nodes. Overall, the present analysis of the _P.
frutescens_ mitochondrial genome provides a valuable foundation for future studies on the phylogenetic relationships of species. DISCUSSION Mitochondria are essential organelles in
eukaryotic cells. They are crucial for cellular respiration and energy metabolism, playing key roles in regulating important cellular activities such as differentiation, apoptosis, growth,
and division40,41. The complex organization, diverse structures, dynamic non-coding sequences, and high levels of repetitive sequences in plant mitochondrial genomes all pose challenges in
the study of plant mitochondria4,15,42,43. Herein, considering the much higher than copy number of plant organelle genomes compared to the corresponding nuclear genome, Illumina and PacBio
sequencing technologies were combined to first describe the basic characteristics of the _P. frutescens _genome. The mitochondrial genome of _P. frutescens_ was demonstrated as a circular
structure with a total length of 299,551 bp. The genome sizes of Lamiaceae plants ranged from 271,618 to 729,504 bp, and the mitochondrial genome of _P. frutescens_ was relatively small
compared to other species in the Lamiaceae family. Its genome size was similar to the published mitochondrial genome of Prunella vulgaris (274,779 bp) in the Lamiaceae family44. The
mitochondrial genome of _P. frutescens_, similar to most Lamiaceae plants, predominantly exhibited a circular structure, which might be attributed to the relative rarity of homologous
recombination events, leading to structural conservation. However, some previously assembled mitochondrial genomes of Lamiaceae plants exhibited multi-chromosomal or multi-branch structures.
For instance, _Ajuga decumbens_ (374,491 bp) and _Teucrium ornatum_ (608,646 bp) each had up to 8 and 9 isolated scaffolds, respectively. The occurrence of such diverse chromosomal
structures in mitochondrial genomes within the same family highlighted the evolutionary adaptability and complexity of plant mitochondrial genomes45. Nevertheless, The mitochondrial genome
of angiosperms averages 475 kb, and the mitochondrial genome of Lamiaceae has a smaller and more conserved structure compared to other angiosperms? Besides, the depth and length of
sequencing data during the assembly process also significantly impacted the assembly results4, warranting further investigation upon the mitochondrial genome structures of _Ajuga decumbens_
and _Teucrium ornatum_. The GC content of the mitotic genome of _P. frutescens_ was 45.23\(\%\), which was highest in the rRNA genes. The result was consistent with other Lamiaceae species
such as _Scutellaria tsinyunensis_ (45.09\(\%\))46, _Rotheca serrata_ (45.53%)47, and _P. vulgaris _(43.92%)44. This suggested that the GC content of the mitotic genome was relatively
conserved during the evolutionary process in plants. In angiosperms, the mitotic genome encoded 24 core protein-coding genes, including _atp_1,_ atp_4, _atp_6, _atp_8, _atp_9, _ccm_B,
_ccm_C, _ccm_Fc, _ccm_Fn, _cob_, _cox_1, _cox_2, _cox_3, _mat_R, _mtt_B, _nad_1, _nad_2, _nad_3, _nad_4, _nad_4L, _nad_5, _nad_6, _nad_7, and _nad_9, most being respiratory protein genes48.
In the mitochondrial genome of _P. frutescens_, a total of 59 genes were identified, including 37 protein-coding genes (26 were core genes), 20 tRNA genes, and 2 rRNA genes. The types and
quantities of these genes were consistent with the core mitochondrial genes of most Lamiaceae plants (Supplementary Table S5)44,46,49. The coding region of the _P. frutescens_ mitochondrial
genome accounted for only 19.23\(\%\) of its total length, with over 80\(\%\) being non-coding regions. This might result from the gradual increase of repeat sequences in the mitochondrial
genome during evolution47. The calculation of non-synonymous substitutions (Ka) and synonymous substitutions (Ks) plays a critical role in phylogenetic reconstruction and understanding the
evolutionary dynamics of protein-coding sequences among closely related species44. In genetics, the Ka/Ks ratio serves as a key indicator to determine whether specific protein-coding genes
are subject to selection pressure during evolution. A Ks value equaling Ka, or the Ka/Ks ratio equaling 1 suggests neutral selection. A Ka/Ks ratio greater than 1 indicates positive
selection, where the non-synonymous substitution rate (Ka) exceeds the synonymous substitution rate (Ks). Conversely, negative selection appears if Ks surpasses Ka or the Ka/Ks ratio is less
than 150. Herein, few protein-coding genes were affected by positive selection, aligning with existing research reports3,43,46,51,52. The Ka/Ks analysis of the mitochondrial genome
indicated that widely present PCGs (protein-coding genes) in mitochondrial were retained. However, genes with Ka/Ks > 1, such as _ccm_Fn, _mtt_B, _rps_10, and _mat_R were also detected,
indicating that positive selection played a role in the evolutionary history of these coding genes. Besides, high Ka/Ks genes were considered important in the study of gene selection and
evolution in Lamiaceae plants. The transfer of genes between chloroplast and mitochondrial genomes is an important feature of plant mitochondrial genome evolution53. When exogenous genes are
inserted into the mitotic genome, they are preferentially inserted into intergenic regions54,55. This phenomenon is a major reason for the observed differences in the number of coding genes
in mitochondrial genomes among different plant species. Therefore, tracking gene transfer matters considerably in exploring the evolution of plant mitochondrial genomes. The length of
chloroplast genome-integrated DNA into the mitotic genome varies among plant species, but generally falls within the range of 1–12\(\%\) of the angiosperm plastome sequence56, such as in
_Garcinia mangostana_ L. (1.7\(\%\))57 and _cucurbita pepo_ (11.6\(\%\))58. In this study, 70 migrating segments with a total length of 12,680 bp were identified, accounting for 4.23\(\%\)
of the entire mitochondrial genome. In the evolutionary history of mitochondrial genomes, widespread loss events of tRNA were observed, and the lost tRNAs in mitochondrial genomes could be
replaced by tRNAs from other organelles59. In this study, it was discovered that the chloroplast rps11 and 15 tRNA genes completely transferred to the Mitochondrial genome, a common
phenomenon in plants featuring frequent transfer of tRNA from chloroplasts to mitochondria60, and that chloroplast-derived tRNAs might have potential functional complementation. Furthermore,
a significant amount of chloroplast gene fragments migrating to the mitochondrial genome were also identified. These chloroplast-derived fragments contained genes playing important roles in
chloroplast function. However, their functionality in the mitochondrial genome remains unclear in this study. Two possible explanations were proposed for the fate of exogenous genes in the
mitochondrial genome: (1) Transferred genes typically lost their functionality, while naturally functional copies coexisted in the mitochondrial genome61; (2) Natural genes were lost from
the mitochondrial genome, and exogenous copies might have a function in maintaining normal cellular operations62. Hence, understanding the patterns of sequence transfer plays an important
role in tracing ancient recombination events and structural variations in plant mitochondrial genomes, requiring more attention. In plants, RNA editing enzymes catalyze conversion of
specific nucleotide positions in mitochondrial RNA sequences from C to U or U to C. RNA editing holds much significance in regulating mitochondrial gene expression and function by inducing
alterations in RNA sequences, thereby influencing the translated protein products63,64,65. RNA editing is an important source of variation in angiosperm mitochondrial genomes66.
Additionally, the mechanism of crop male sterility has been associated with RNA editing processes. Herein, in the mitochondrial genome of _P. frutescens_, 559 RNA editing sites were
identified, primarily occurring at the first and second positions of codons, with the editing method being cytosine <=> uracil (C <=> U). The preference for G/C codons might be
attributed to their high binding energy, which played a role in maintaining translation accuracy67. By identifying RNA editing sites, researchers could gain valuable insights into the
functional implications of predicting newly encoded codons. Notably, RNA editing events produced stop codons in the _rps_10 gene, and start codons in three genes (_cox_2, _nad_4L, and
_rps_10), which could often be related to the production of highly conserved and homologous proteins found in other species, thereby promoting efficient gene expression in mitochondria.
Furthermore, RNA editing events were found to be least common in ribosomal protein genes like _rps_14, while genes like _nad_5, _mtt_B, and _ccm_B exhibited higher frequencies of RNA editing
events. These findings suggested the crucial role of RNA editing in plant adaptation to environmental changes and signal transduction68,69. Codon usage bias is an important factor
reflecting the evolution of mitochondrial genomes70. Generally, factors such as mutation, natural selection, and phylogenetic relationships may lead to differences in codon usage
preferences71. In the mitochondrial genome of _P. frutescens_, 30 codons with bias (RSCU > 1) were hereby identified, and these codons used more A/T bases. Therefore, upcoming research
should account for codon usage biases influenced by mitochondrial RNA editing sites in the development of male sterile materials, thereby facilitating advancements in genetic breeding
programs. Repeat sequences are widely present in mitochondrial genomes and are usually crucial for molecular recombination, structural variation, and extreme differences in mitotic genome
size72. These sequences also serve as important sources of information for population development and evolutionary analysis markers73. In this study, a total of 293 repeats, including 141
pairs of reverse complementary repeats and 152 pairs of forward repeats, were observed, possibly revealing frequent molecular recombination in mitochondrial genomes. This frequent
recombination could play a crucial role in dynamically changing the structure and conformation of the mitochondrial genome during the evolutionary process. Simple sequence repeats (SSRs)
represent a distinctive class of repetitive DNA sequences characterized by high repetitiveness, extensive polymorphism, and frequent co-dominance, and are abundantly dispersed throughout
plant genomes38. SSR markers provide useful information for analyzing genetic diversity, genetic relationships, and population structures in crop species germplasm. In the present study, 77
SSRs were distributed in different genomic regions of _P. frutescens_ mitotic genomes. The most abundant SSR type was tetrameric repeats (27.35\(\%\)), followed by dimeric repeats
(20\(\%\)), consistent with previous studies on mitochondrial genomes. Moreover, a nearly universal A/T bias was also observed in the mitochondrial genome of _P. frutescens_. These SSRs
consisted of motifs rich in A and T, also consistent with previous observations. The findings further confirmed the correlation between the AT content of the complete mitotic genome and
SSRs. Extensive collection of genetic resources in _P. frutescens_ could be found74. While numerous plant genetic resources have been collected in gene banks worldwide, the large sample size
and lack of sufficient information on population structure and genetic diversity still terribly hinder the successful utilization of the genetic potential of plant genetic
resources25,74,75. Currently, efforts have shifted from the collection of plant genetic resources to the identification of genetic diversity and core germplasm in _P. frutescens_74,76,77,78.
The identified SSR sequences will provide useful information for the effective protection and utilization of genetic resources and the selection of useful genetic resources for_ P.
frutescens _breeding programs. METHODS PLANT SAMPLING, DNA EXTRACTING AND SEQUENCING In December 2023, fresh leaves of _P. frutescens_ were collected at the Baiyun Experimental Base of
Guangdong Academy of Agricultural Sciences (N23\(^\circ\)07\(\prime\)03\(\prime \prime\), E113\(^\circ\)08\(\prime\)36\(\prime \prime\)). The plant had been grown for 6 months. The collected
leaves were immediately cooled with liquid nitrogen and stored in an ultra-low temperature freezer at \(-80\; ^{\circ }\)C. Total DNA was extracted using a plant genomic DNA kit (TSINGKE
Biotech, Beijing, China), and the quality and quantity of the extracted DNA were assessed using a NanoDrop One spectrophotometer (Thermo Fisher Scientific, Massachusetts, USA) and the Qubit
dsDNA HS assay kit on a Qubit 3.0 fluorometer (TSINGKE Biotech, Beijing, China), as well as agarose gel electrophoresis. Subsequently, the extracted DNA samples were shipped in dry ice to
Wuhan BMK Tech Solutions Company Limited (Qingdao, China) for SMRT and Illumina sequencing. GENOME ASSEMBLY AND ANNOTATION The raw read data were filtered and corrected using the Pacbio RS
II sequencing technology, while sequencing adapters and low-quality sequences were filtered out using the SMRT Analysis (v2.3.0) with default settings to obtain clean reads. Then, the mt
genome sequence of _P. frutescens_ was extracted from the filtered reads containing chloroplast and mitochondrial genomes. Besides, the NCBI chloroplast genome data were used for BLAST79
filtering of reads containing chloroplast genomes, and reads with a match percentage greater than 90% were removed. Following that, the third-generation assembly software Canu80 was employed
to correct the obtained third-generation data, and Bowtie2 (v2.3.5.1) was adopted to align the second-generation data with the corrected sequences. Using default parameters, Unicycler
(v0.4.8)81 concatenated the aforementioned second-generation data and the corrected third-generation data, yielding a circular mitochondrial genome of _P. frutescens_. The average depth of
the assembled mitochondrial genome was 152.25\(\times\) (Supplementary Table S6). Furthermore, for mitochondrial genome annotation, the following steps were performed: BLAST was used to
compare encoded proteins and rRNA with published plant mitochondrial sequences, followed by manual adjustments based on closely related species. tRNA annotation was conducted using
tRNAscan-SE with default settings (http://lowelab.ucsc.edu/tRNAscan-SE/)82. ORFs were annotated using Open Reading Frame Finder (http://www.ncbi.nlm.nih.gov/gorf/gorf.html). Besides, the
circular mitochondrial genome map was visualized using the online software Draw Cellelle Genome Map83. Following these steps, the assembly and annotation of the _P.frutescens_ mitochondrial
genome could be accomplished, providing essential foundational data for further research. REPEAT SEQUENCE ANALYSIS Three types of repeat sequences (simple, tandem, and dispersed) were
detected in the mitochondrial genome. Simple repeat sequence analysis was performed using the online software MISA (https://webblast.ipk-gatersleben.de/misa/)44. In this analysis, 10, 5, 4,
3, 3, 3, and 3 repeat sequences were identified with 1, 2, 3, 4, 5, and 6 base pairs, respectively. Tandem repeat sequences with a length exceeding 6 bp and a repeat unit match of over 95 %
were detected using the online tool Tandem Repeats Finder v4.09 (http://tandem.bu.edu/trf/trf.submit.options.html)84. The following parameters were involved: 2 7 7 80 10 50 2000 -f -d -m.
Dispersed repeat sequences were identified using BLASTN (v2.10.1) with parameters of word size 7, e-value 1e-5. Redundancy, and tandem repeats were removed. Circos v0.69-5
(http://circos.ca/software/download/)85 was used for visualizing these repeat sequences. Thorough repeat sequence analyses could provide valuable insights into the repeat elements in the _P.
frutescens_ mitochondrial genome, illuminating its genomic organization and evolution. EXPLORATION OF CHLOROPLAST TO MITOCHONDRION DNA TRANSFORMATION, RNA EDITING, CODON USAGE PATTERNS, AND
SELECTION PRESSURE In this investigation, an online cloud platform was utilized to analyze the codon composition of the _P. frutescens_ mitochondrial genome, screen for unique CDS, and
determine the relative synonymous codon usage (RSCU) of each gene. The chloroplast genome sequence of _P. frutescens_ was obtained from the NCBI Organelle Genome Resources database. By
employing the BLAST software on NCBI, homologous fragments between the mitochondrial and chloroplast genomes were identified, and the screening criteria were set at match rate \(\ge\)
70\(\%\), E-value \(\le\) 1e−5, and length \(\ge\)30 bp. The results were visualized using Circos (v0.69-5). The Deepred-mt was used for the prediction of RNA editing of the mitochondrial
genomes. Furthermore, genbank files of 9 Lamiaceae species were imported into the Ka/Ks cloud tool to calculate Ka/Ks values for 32 shared proteins. Subsequently, one-way analysis of
variance on the Ka/Ks ratios of the 32 protein-coding genes was conducted using the R programming language. PHYLOGENETIC INFERENCE A phylogenetic tree was plotted using the mitochondrial
genomes of 50 species downloaded from NCBI, with _Marchantia paleacea_ taken as the outgroup. To ensure comparability, these mitochondrial genomes were re-annotated using the previously
described tools. PhyloSuite (v.1.2.1)86 was utilized to identify and extract 30 orthologous mitochondrial genes from the analyzed species. Besides, the corresponding nucleotide sequences
were aligned using MAFFT (v.7.450)87. Subsequently, these aligned sequences were concatenated and utilized to construct the phylogenetic tree. ModelFind was used to build the best model with
default parameters, and the maximum likelihood (ML) analysis with 1000 bootstrap replicates was performed in RAxML (v.8.2.4)88. Furthermore, Bayesian inference (BI) analysis was conducted
using MrBayes (v.3.2.6)89. The Markov chain Monte Carlo method was adopted for 200,000 generations, with tree sampling conducted every 1000 generatiotabns for precision. The first 20\(\%\)
of trees were discarded as burn-in, while the remaining were used to generate the consensus tree. For the ASTRAL90analysis, 24 mitochondrial gene trees were inferred separately using RAxML ,
utilizing the GTRGAMMA model alongside 100 bootstrap replicates. To enhance the accuracy of species-tree inference, branches (<10\(\%\) bootstrap support) within these gene trees were
pruned subsequently, the collapsed gene trees were imported into ASTRAL-III with the default settings. This process yielded an estimated topology of the species tree with branch lengths and
local posterior probabilities (LPP) as branch support values. STATEMENT OF PLANT COLLECTION We hereby declare that _Perilla frutescens_ is not a plant species covered by the IUCN Policy
Statement on Research Involving Species at Risk of Extinction and the Convention on the Trade in Endangered Species of Wild Fauna and Flora. The botanical collection work involved in this
research has obtained the necessary permits and approvals from relevant local institutions, and strict compliance with applicable laws and guidelines has been ensured. Moreover, we have
minimized the impact on the environment and ecosystems during the collection process, and made every effort to maintain the survival and reproductive capacity of the _P. frutescens_ plants.
CONCLUSIONS In this research, the sequencing and assembly of the mitochondrial genome of _P. frutescens_ were successfully completed, shedding light on a comprehensive comparison of the
organelle genome. This breakthrough is anticipated to provide a broader perspective for the investigation of gene transfer between mitochondrial and plastids. Additionally, through
phylogenetic analysis of the mitochondrial genomes of this species and 50 other taxa, the evolutionary status of _P. frutescens_ was definitively determined. Overall, the findings offer
valuable insights to lay the groundwork for future research on genetic variation, systematic evolution, and breeding of _P. frutescens_, thereby facilitating the cultivation, development,
and utilization of this valuable plant. DATA AVAILIBILITY The complete mitochondrial genome of _P. frutescens_ generated in this study was submitted to the NGDC database
(https://ngdc.cncb.ac.cn/genbase/search/gb/C_AA059311.1) with GeneBase accession number C_AA059311.1. The associated BioProject are PRJNA1108956. REFERENCES * Aliyari Rad, S., Dehghanian,
Z., Asgari Lajayer, B., Nobaharan, K. & Astatkie, T. Mitochondrial respiration and energy production under some abiotic stresses. _J. Plant Growth Regul._ 41, 3285–3299.
https://doi.org/10.1007/s00344-021-10512-1 (2022). Article CAS Google Scholar * Marèchal, A. & Brisson, N. Recombination and the maintenance of plant organelle genome stability. _N.
Phytol._ 186, 299–317. https://doi.org/10.1111/j.1469-8137.2010.03195.x (2010) (PMID: 20180912). Article CAS MATH Google Scholar * Gualberto, J. M. _et al._ The plant mitochondrial
genome: Dynamics and maintenance. _Biochimie_ 100, 107–120. https://doi.org/10.1016/j.biochi.2013.09.016 (2014) (PMID: 24075874). Article CAS PubMed MATH Google Scholar * Wang, J. _et
al._ Plant organellar genomes: Much done, much more to do. _Trends Plant Sci._https://doi.org/10.1016/j.tplants.2023.12.014 (2024). Article PubMed Google Scholar * Day, D. A.
Mitochondrial structure and function in plants. In _Plant Mitochondria: From Genome to Function. Collection-title: Advances in Photosynthesis and Respiration_, vol. 17 (eds. Day, D. A., _et
al._) 1–11 (Springer Netherlands, 2004). https://doi.org/10.1007/978-1-4020-2400-9_1 . * Møller, I. M., Rasmusson, A. G. & Van Aken, O. Plant mitochondria—Past, present and future.
_Plant J._ 108, 912–959. https://doi.org/10.1111/tpj.15495 (2021). Article CAS PubMed Google Scholar * Lang, Y., Zhang, Z., Jiang, J., Cao, T. & Tian, J. Characterization of the
chloroplast genome of _Perilla frutescens_ (lamiaceae), an herb plant from china. _Mitochondrial DNA Part B_ 5, 1550–1551. https://doi.org/10.1080/23802359.2020.1742597 (2020). Article MATH
Google Scholar * Zhang, Y. _et al._ Incipient diploidization of the medicinal plant perilla within 10,000 years. _Nat. Commun._ 12, 5508. https://doi.org/10.1038/s41467-021-25681-6
(2021). Article ADS CAS PubMed PubMed Central MATH Google Scholar * Tamura, K. _et al._ A highly contiguous genome assembly of red perilla (_Perilla frutescens_) domesticated in
Japan. _DNA Res. Int. J. Rapid Publ. Rep. Genes Genomes._ 30, dsac044, https://doi.org/10.1093/dnares/dsac044 (2023). * Jin, J.-J. _et al._ Getorganelle: A fast and versatile toolkit for
accurate de novo assembly of organelle genomes. _Genome Biol._ 21, 241. https://doi.org/10.1186/s13059-020-02154-5 (2020). Article PubMed PubMed Central MATH Google Scholar * Allio, R.
_et al._ Mitofinder: Efficient automated large-scale extraction of mitogenomic data in target enrichment phylogenomics. _Mol. Ecol. Resour._ 20, 892–905.
https://doi.org/10.1111/1755-0998.13160 (2020). Article CAS PubMed PubMed Central MATH Google Scholar * He, W., Xiang, K., Chen, C., Wang, J. & Wu, Z. Master graph: An essential
integrated assembly model for the plant mitogenome based on a graph-based framework. _Brief. Bioinform._ 24, bbac522. https://doi.org/10.1093/bib/bbac522 (2023) (PMID: 36644898). Article
PubMed MATH Google Scholar * Bi, C. _et al._ Pmat: An efficient plant mitogenome assembly toolkit using low coverage hifi sequencing data. _Hortic. Res._https://doi.org/10.1093/hr/uhae023
(2024). Article PubMed PubMed Central Google Scholar * Kuang, W. M. & Yu, L. Mitogenome assembly strategies and software applications in the genome era. _Yi Chuan = Hereditas_ 41,
979–993. https://doi.org/10.16288/j.yczz.19-227 (2019). * Ma, Q. _et al._ Assembly and comparative analysis of the first complete mitochondrial genome of acer truncatum bunge: A woody
oil-tree species producing nervonic acid. _BMC Plant Biol._ 22, 29. https://doi.org/10.1186/s12870-021-03416-5 (2022). Article CAS PubMed PubMed Central Google Scholar * Roulet, M. E.
_et al._ Multichromosomal structure and foreign tracts in the ombrophytum subterraneum (balanophoraceae) mitochondrial genome. _Plant Mol. Biol._ 103, 623–638.
https://doi.org/10.1007/s11103-020-01014-x (2020) (PMID: 32440763). Article CAS PubMed MATH Google Scholar * Wang, M. _et al._ Mitochondrial genome comparison and phylogenetic analysis
of dendrobium (orchidaceae) based on whole mitogenomes. _BMC Plant Biol._ 23, 586. https://doi.org/10.1186/s12870-023-04618-9 (2023). Article CAS PubMed PubMed Central MATH Google
Scholar * Singh, S. _et al._ Elucidating mitochondrial DNA markers of Ogura-based CMS lines in Indian cauliflowers (brassica oleracea var botrytis l) and their floral abnormalities due to
diversity in cytonuclear interactions. _Front. Plant Sci._ 12, 631489. https://doi.org/10.3389/fpls.2021.631489 (2021). Article PubMed PubMed Central Google Scholar * Wu, Z. _et al._
Mitochondrial genome and transcriptome analysis of five alloplasmic male-sterile lines in brassica juncea. _BMC Genom._ 20, 348. https://doi.org/10.1186/s12864-019-5721-2 (2019). Article
MATH Google Scholar * Okazaki, M., Kazama, T., Murata, H., Motomura, K. & Toriyama, K. Whole mitochondrial genome sequencing and transcriptional analysis to uncover an rt102-type
cytoplasmic male sterility-associated candidate gene derived from oryza rufipogon. _Plant Cell Physiol._ 54, 1560–1568. https://doi.org/10.1093/pcp/pct102 (2013) (PMID: 23852329). Article
CAS PubMed Google Scholar * Cheng, Q. _et al._ Complete mitochondrial genome sequence and identification of a candidate gene responsible for cytoplasmic male sterility in celery (apium
graveolens l). _Int. J. Mol. Sci._ 22, 8584. https://doi.org/10.3390/ijms22168584 (2021). Article CAS PubMed PubMed Central MATH Google Scholar * Melonek, J. _et al._ The genetic basis
of cytoplasmic male sterility and fertility restoration in wheat. _Nat. Commun._ 12, 1036. https://doi.org/10.1038/s41467-021-21225-0 (2021). Article ADS CAS PubMed PubMed Central MATH
Google Scholar * Wang, Y. _et al._ Characterization and phylogenetic analysis of the complete mitochondrial genome sequence of photinia serratifolia. _Sci. Rep._ 13, 770.
https://doi.org/10.1038/s41598-022-24327-x (2023). Article ADS CAS PubMed PubMed Central MATH Google Scholar * Yu, H. _et al._ Phytochemical and phytopharmacological review of
_Perilla frutescens_ L. (labiatae), a traditional edible-medicinal herb in China. _Food Chem. Toxicol._ 108, 375–391. https://doi.org/10.1016/j.fct.2016.11.023 (2017). Article ADS CAS
PubMed MATH Google Scholar * Park, D. H., Sa, K. J., Lim, S. E., Ma, S. J. & Lee, J. K. Genetic diversity and population structure of _Perilla frutescens_ collected from Korea and
China based on simple sequence repeats (SSRS). _Genes Genom._ 41, 1329–1340. https://doi.org/10.1007/s13258-019-00860-4 (2019) (PMID: 31468347). Article CAS Google Scholar * Liu, S. _et
al._ A comprehensive review of the botany, ethnopharmacology, phytochemistry, pharmacology, toxicity and quality control of _Perillae fructus_. _J. Ethnopharmacol._ 304, 116022.
https://doi.org/10.1016/j.jep.2022.116022 (2023). Article CAS PubMed MATH Google Scholar * Wu, X. _et al._ _Perilla frutescens_: A traditional medicine and food homologous plant. _Chin.
Herb. Med._ 15, 369–375. https://doi.org/10.1016/j.chmed.2023.03.002 (2023). Article PubMed PubMed Central MATH Google Scholar * Hou, T. _et al._ _Perilla frutescens_: A rich source of
pharmacological active compounds. _Molecules_ 27, 3578. https://doi.org/10.3390/molecules27113578 (2022). Article CAS PubMed PubMed Central MATH Google Scholar * Aochen, C. _et al._
_Perilla frutescens_ L.: A dynamic food crop worthy of future challenges. _Front. Nutr._https://doi.org/10.3389/fnut.2023.1130927 (2023). Article PubMed PubMed Central MATH Google
Scholar * Ahmed, H. M. Ethnomedicinal, phytochemical and pharmacological investigations of _Perilla frutescens_ (L.) britt. _Molecules_ 24, 102. https://doi.org/10.3390/molecules24010102
(2018). Article CAS PubMed PubMed Central MATH Google Scholar * Zhou, X.-J. _et al._ Structural characterisation and antioxidant activity evaluation of phenolic compounds from
cold-pressed _Perilla frutescens_ var. arguta seed flour. _Food Chem._ 164, 150–157, https://doi.org/10.1016/j.foodchem.2014.05.062 (2014). * Ha, T. J. _et al._ Isolation and identification
of phenolic compounds from the seeds of _Perilla frutescens_ (l.) and their inhibitory activities against \(\alpha\)-glucosidase and aldose reductase. _Food Chem._ 135, 1397–1403.
https://doi.org/10.1016/j.foodchem.2012.05.104 (2012). Article CAS PubMed MATH Google Scholar * Meng, L., Lozano, Y. F., Gaydou, E. M. & Li, B. Antioxidant activities of polyphenols
extracted from _Perilla frutescens_ varieties. _Molecules (Basel, Switzerland)_ 14, 133–140. https://doi.org/10.3390/molecules14010133 (2008). Article CAS PubMed PubMed Central Google
Scholar * Lee, D.-S., Noh, B.-S., Bae, S.-Y. & Kim, K. Characterization of fatty acids composition in vegetable oils by gas chromatography and chemometrics. _Anal. Chim. Acta_ 358,
163–175. https://doi.org/10.1016/S0003-2670(97)00574-6 (1998). Article CAS MATH Google Scholar * Li, S.-S. _et al._ Fatty acid composition of developing tree peony (paeonia section
moutan dc) seeds and transcriptome analysis during seed development. _BMC Genom._ 16, 208. https://doi.org/10.1186/s12864-015-1429-0 (2015). Article CAS Google Scholar * Huang, J. _et
al._ Effect of microwave pretreatment of perilla seeds on minor bioactive components content and oxidative stability of oil. _Food Chem._ 388, 133010.
https://doi.org/10.1016/j.foodchem.2022.133010 (2022). Article CAS PubMed MATH Google Scholar * Li, J. _et al._ Complete mitochondrial genome of agrostis stolonifera: Insights into
structure, codon usage, repeats, and rna editing. _BMC Genom._ 24, 466. https://doi.org/10.1186/s12864-023-09573-1 (2023). Article CAS MATH Google Scholar * Srivastava, S., Avvaru, A.
K., Sowpati, D. T. & Mishra, R. K. Patterns of microsatellite distribution across eukaryotic genomes. _BMC Genom._ 20, 153. https://doi.org/10.1186/s12864-019-5516-5 (2019). Article
Google Scholar * Fang, L. _et al._ Deeprepeat: Direct quantification of short tandem repeats on signal data from nanopore sequencing. _Genome Biol._ 23, 108.
https://doi.org/10.1186/s13059-022-02670-6 (2022). Article CAS PubMed PubMed Central MATH Google Scholar * Friedman, J. R. & Nunnari, J. Mitochondrial form and function. _Nature_
505, 335–343. https://doi.org/10.1038/nature12985 (2014). Article ADS CAS PubMed PubMed Central MATH Google Scholar * Alberts, B. _et al._ The mitochondrion. In _Molecular Biology of
the Cell_, 4th edn. (Garland Science, 2002). * Skippington, E., Barkman, T. J., Rice, D. W. & Palmer, J. D. Miniaturized mitogenome of the parasitic plant _Viscum scurruloideum_ is
extremely divergent and dynamic and has lost all nad genes. _Proc. Natl. Acad. Sci._https://doi.org/10.1073/pnas.1504491112 (2015). Article PubMed PubMed Central Google Scholar *
Putintseva, Y. A. _et al._ Siberian larch (_Larix sibirica ledeb_) mitochondrial genome assembled using both short and long nucleotide sequence reads is currently the largest known
mitogenome. _BMC Genom._ 21, 1–12. https://doi.org/10.1186/s12864-020-07061-4 (2020). Article Google Scholar * Sun, Z. _et al._ Assembly and analysis of the mitochondrial genome of
_Prunella vulgaris_. _Front. Plant Sci._ 14, 1237822. https://doi.org/10.3389/fpls.2023.1237822 (2023). Article PubMed PubMed Central Google Scholar * Nosek, J. & Tomáška, Ä.
Mitochondrial genome diversity: Evolution of the molecular architecture and replication strategy. _Curr. Genet._ 44, 73–84. https://doi.org/10.1007/s00294-003-0426-z. * Liu, F. _et al._
Episodic and guanine–cytosine-biased bursts of intragenomic and interspecific synonymous divergence in ajugoideae (lamiaceae) mitogenomes. _New Phytol._ 228, 1107–1114.
https://doi.org/10.1111/nph.16753 (2020) (PMID: 32558926). Article CAS PubMed MATH Google Scholar * Fang, X. & Zhang, S. The complete chloroplast genome sequence of _Rotheca
myricoides_ (hochst.) steane mabb., a traditional medicinal plant. _Mitochondrial DNA Part B_ 6, 2699–2700. https://doi.org/10.1080/23802359.2021.1966340 (2021). Article PubMed PubMed
Central MATH Google Scholar * Li, J. _et al._ Assembly of the complete mitochondrial genome of an endemic plant, scutellaria tsinyunensis, revealed the existence of two conformations
generated by a repeat-mediated recombination. _Planta_ 254, 36. https://doi.org/10.1007/s00425-021-03684-3 (2021) (PMID: 34302538). Article CAS PubMed Google Scholar * Yu, Y., Li, H.-T.,
Wu, Y.-H. & Li, D.-Z. Correlation analysis reveals an important role of gc content in accumulation of deletion mutations in the coding region of angiosperm plastomes. _J. Mol. Evol._
89, 73–80. https://doi.org/10.1007/s00239-020-09987-5 (2021) (PMID: 33433638). Article ADS CAS PubMed MATH Google Scholar * Nardi, F., Carapelli, A. & Frati, F. Repeated regions in
mitochondrial genomes: Distribution, origin and evolutionary significance. _Mitochondrion_ 12, 483–491. https://doi.org/10.1016/j.mito.2012.07.105 (2012). Article CAS PubMed MATH Google
Scholar * Fay, J. C. & Wu, C.-I. Sequence divergence, functional constraint, and selection in protein evolution. _Annu. Rev. Genom. Hum. Genet._ 4, 213–235.
https://doi.org/10.1146/annurev.genom.4.020303.162528 (2003) (PMID: 14527302). Article CAS MATH Google Scholar * Pazos, F. & Valencia, A. Protein co-evolution, co-adaptation and
interactions. _EMBO J._ 27, 2648–2655. https://doi.org/10.1038/emboj.2008.189 (2008). Article CAS PubMed PubMed Central MATH Google Scholar * Qiao, Y., Zhang, X., Li, Z., Song, Y.
& Sun, Z. Assembly and comparative analysis of the complete mitochondrial genome of bupleurum chinense dc. _BMC Genom._ 23, 664. https://doi.org/10.1186/s12864-022-08892-z (2022).
Article CAS MATH Google Scholar * Zhang, T. _et al._ Characterization and comparative analyses of mitochondrial genomes in single-celled eukaryotes to shed light on the diversity and
evolution of linear molecular architecture. _Int. J. Mol. Sci._ 22, 2546. https://doi.org/10.3390/ijms22052546 (2021). Article CAS PubMed PubMed Central MATH Google Scholar * Wang, D.
_et al._ Transfer of chloroplast genomic DNA to mitochondrial genome occurred at least 300 mya. _Mol. Biol. Evol._ 24, 2040–2048. https://doi.org/10.1093/molbev/msm133 (2007) (PMID:
17609537). Article CAS PubMed MATH Google Scholar * Sloan, D. B. & Wu, Z. History of plastid DNA insertions reveals weak deletion and at mutation biases in angiosperm mitochondrial
genomes. _Genome Biol. Evol._ 6, 3210–3221. https://doi.org/10.1093/gbe/evu253 (2014). Article CAS PubMed PubMed Central MATH Google Scholar * Turmel, M., Otis, C. & Lemieux, C.
Mitochondrion-to-chloroplast DNA transfers and intragenomic proliferation of chloroplast group ii introns in gloeotilopsis green algae (ulotrichales, ulvophyceae). _Genome Biol. Evol._ 8,
2789–2805. https://doi.org/10.1093/gbe/evw190 (2016). Article CAS PubMed PubMed Central MATH Google Scholar * Mower, J. P., Sloan, D. B. & Alverson, A. J. Plant mitochondrial
genome diversity: The genomics revolution. In _Plant Genome Diversity Volume 1: Plant Genomes, their Residents, and their Evolutionary Dynamics_ (eds. Wendel, J. F., _et al._) 123–144
(Springer, 2012). https://doi.org/10.1007/978-3-7091-1130-79. * Wee, C.-C. _et al._ Mitochondrial genome of _Garcinia mangostana_ l. variety mesta. _Sci. Rep._ 12, 9480.
https://doi.org/10.1038/s41598-022-13706-z (2022). Article ADS CAS PubMed PubMed Central MATH Google Scholar * Alverson, A. J. _et al._ Insights into the evolution of mitochondrial
genome size from complete sequences of Citrullus lanatus and Cucurbita pepo (cucurbitaceae). _Mol. Biol. Evol._ 27, 1436–1448. https://doi.org/10.1093/molbev/msq029 (2010). Article CAS
PubMed PubMed Central MATH Google Scholar * Kubo, T. & Newton, K. J. Angiosperm mitochondrial genomes and mutations. _Mitochondrion_ 8, 5–14.
https://doi.org/10.1016/j.mito.2007.10.006 (2008) (PMID: 18065297). Article CAS PubMed MATH Google Scholar * Wang, Y. _et al._ Empty pericarp21 encodes a novel PPR-DYW protein that is
required for mitochondrial RNA editing at multiple sites, complexes I and V biogenesis, and seed development in maize. _PLoS Genet._ 15, e1008305.
https://doi.org/10.1371/journal.pgen.1008305 (2019). Article CAS PubMed PubMed Central Google Scholar * Wang, Y. _et al._ Maize PPR-E proteins mediate RNA C-to-U editing in mitochondria
by recruiting the trans deaminase pcw1. _Plant Cell_ 35, 529–551. https://doi.org/10.1093/plcell/koac298 (2023). Article PubMed MATH Google Scholar * Liu, R. _et al._ The DYW-subgroup
pentatricopeptide repeat protein ppr27 interacts with zmmorf1 to facilitate mitochondrial RNA editing and seed development in maize. _J. Exp. Bot._ 71, 5495–5505.
https://doi.org/10.1093/jxb/eraa273 (2020) (PMID: 32531050). Article CAS PubMed MATH Google Scholar * Chen, Z. _et al._ Plant mitochondrial genome evolution and cytoplasmic male
sterility. _Crit. Rev. Plant Sci._ 36, 55–69. https://doi.org/10.1080/07352689.2017.1327762 (2017). Article CAS MATH Google Scholar * Yan, J., Zhang, Q. & Yin, P. RNA editing
machinery in plant organelles. _Sci. China Life Sci._ 61, 162–169. https://doi.org/10.1007/s11427-017-9170-3 (2018). Article CAS PubMed MATH Google Scholar * Hao, W. _et al._ RNA
editing and its roles in plant organelles. _Front. Genet._ 12, 757109. https://doi.org/10.3389/fgene.2021.757109 (2021). Article CAS PubMed PubMed Central Google Scholar * Takenaka, M.,
Zehrmann, A., Verbitskiy, D., Härtel, B. & Brennicke, A. RNA editing in plants and its evolution. _Annu. Rev. Genet._ 47, 335–352. https://doi.org/10.1146/annurev-genet-111212-133519
(2013) (PMID: 24274753). Article CAS PubMed Google Scholar * Parvathy, S. T., Udayasuriyan, V. & Bhadana, V. Codon usage bias. _Mol. Biol. Rep._ 49, 539–565.
https://doi.org/10.1007/s11033-021-06749-4 (2022). Article CAS PubMed Google Scholar * Zhao, S. _et al._ Comparative analysis of chloroplast genome of meconopsis (papaveraceae) provides
insights into their genomic evolution and adaptation to high elevation. _Int. J. Mol. Sci._ 25, 2193. https://doi.org/10.3390/ijms25042193 (2024). Article CAS PubMed PubMed Central MATH
Google Scholar * Chevigny, N., Schatz-Daas, D., Lotfi, F. & Gualberto, J. M. DNA repair and the stability of the plant mitochondrial genome. _Int. J. Mol. Sci._ 21, 328.
https://doi.org/10.3390/ijms21010328 (2020) . Article CAS PubMed PubMed Central Google Scholar * Novák, P. _et al._ Repeat-sequence turnover shifts fundamentally in species with large
genomes. _Nat. Plants_ 6, 1325–1329. https://doi.org/10.1038/s41477-020-00785-x (2020). Article CAS PubMed MATH Google Scholar * Sa, K. J. _et al._ Construction of a core collection of
native Perilla germplasm collected from South Korea based on ssr markers and morphological characteristics. _Sci. Rep._ 11, 23891. https://doi.org/10.1038/s41598-021-03362-0 (2021). Article
ADS CAS PubMed PubMed Central Google Scholar * Zhang, H. _et al._ Genetic diversity, phylogenetic structure and development of core collections in melilotus accessions from a chinese
gene bank. _Sci. Rep._ 9, 13017. https://doi.org/10.1038/s41598-019-49355-y (2019). Article ADS CAS PubMed PubMed Central Google Scholar * Salgotra, R. K. & Chauhan, B. S. Genetic
diversity, conservation, and utilization of plant genetic resources. _Genes_ 14, 174. https://doi.org/10.3390/genes14010174 (2023). Article CAS PubMed PubMed Central MATH Google Scholar
* Ma, S. J., Sa, K. J., Hong, T. K. & Lee, J. K. Genetic diversity and population structure analysis in _Perilla frutescens_ from northern areas of China based on simple sequence
repeats. _Genet. Mol. Res. GMR_https://doi.org/10.4238/gmr16039746 (2017). Article PubMed Google Scholar * Ha, Y. J., Sa, K. J. & Lee, J. K. Identifying SSR markers associated with
seed characteristics in perilla (_Perilla frutescens_ L.). _Physiol. Mol. Biol. Plants_ 27, 93–105. https://doi.org/10.1007/s12298-021-00933-3 (2021). Article CAS PubMed PubMed Central
Google Scholar * Park, H., Sa, K. J., Hyun, D. Y., Lee, S. & Lee, J. K. Identifying SSR markers related to seed fatty acid content in Perilla crop (_Perilla frutescens_ L.). _Plants_
10, 1404. https://doi.org/10.3390/plants10071404 (2021). Article CAS PubMed PubMed Central MATH Google Scholar * Zaru, R., Orchard, S. & Consortium, U. Uniprot tools: Blast, align,
peptide search, and id mapping. _Curr. Protoc._ 3, e697. https://doi.org/10.1002/cpz1.697 (2023). Article Google Scholar * Koren, S. _et al._ Canu: Scalable and accurate long-read
assembly via adaptive k-mer weighting and repeat separation. _Genome Res._ 27, 722–736. https://doi.org/10.1101/gr.215087.116 (2017). Article CAS PubMed PubMed Central MATH Google
Scholar * Wick, R. R., Judd, L. M., Gorrie, C. L. & Holt, K. E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. _PLoS Comput. Biol._ 13, e1005595.
https://doi.org/10.1371/journal.pcbi.1005595 (2017). Article ADS CAS PubMed PubMed Central Google Scholar * Zou, Q. _et al._ Improving trnascan-se annotation results via ensemble
classifiers. _Mol. Inform._ 34, 761–770. https://doi.org/10.1002/minf.201500031 (2015) (PMID: 27491037). Article CAS PubMed Google Scholar * Greiner, S., Lehwark, P. & Bock, R.
Organellargenomedraw (ogdraw) version 1.3.1: Expanded toolkit for the graphical visualization of organellar genomes. _Nucleic Acids Res._ 47, W59–W64. https://doi.org/10.1093/nar/gkz238
(2019). Article CAS PubMed PubMed Central Google Scholar * Benson, G. Tandem repeats finder: A program to analyze DNA sequences. _Nucleic Acids Res._ 27, 573–580.
https://doi.org/10.1093/nar/27.2.573 (1999). Article CAS PubMed PubMed Central MATH Google Scholar * Krzywinski, M. _et al._ Circos: An information aesthetic for comparative genomics.
_Genome Res._ 19, 1639–1645. https://doi.org/10.1101/gr.092759.109 (2009). Article CAS PubMed PubMed Central MATH Google Scholar * Zhang, D. _et al._ Phylosuite: An integrated and
scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. _Mol. Ecol. Resour._ 20, 348–355. https://doi.org/10.1111/1755-0998.13096
(2020) (PMID: 31599058). Article PubMed MATH Google Scholar * Katoh, K., Rozewicki, J. & Yamada, K. D. Mafft online service: Multiple sequence alignment, interactive sequence choice
and visualization. _Brief. Bioinform._ 20, 1160–1166. https://doi.org/10.1093/bib/bbx108 (2019). Article CAS PubMed Google Scholar * Ronquist, F. & Huelsenbeck, J. P. Mrbayes 3:
Bayesian phylogenetic inference under mixed models. _Bioinformatics (Oxford, England)_ 19, 1572–1574. https://doi.org/10.1093/bioinformatics/btg180 (2003). Article CAS PubMed MATH Google
Scholar * Ronquist, F. & Huelsenbeck, J. P. Mrbayes 3: Bayesian phylogenetic inference under mixed models. _Bioinformatics (Oxford, England)_ 19, 1572–1574.
https://doi.org/10.1093/bioinformatics/btg180 (2003) (PMID: 12912839). Article CAS PubMed MATH Google Scholar * Zhang, C., Rabiee, M., Sayyari, E. & Mirarab, S. ASTRAL-III:
polynomial time species tree reconstruction from partially resolved gene trees. _BMC Bioinform._ 19, 153. https://doi.org/10.1093/bioinformatics/btu462 (2018). Article CAS Google Scholar
Download references ACKNOWLEDGEMENTS This work was supported by the Guangdong Provincial Science and Technology Plan Project (2019B030316008), Guangdong Provincial Science and Technology
Plan Project (2023B1212060038), and Guangdong Provincial Agricultural Research and Technology Promotion Demonstration Project (Yue Cai Nong [2022] No. 16). AUTHOR INFORMATION Author notes *
These authors contributed equally: Ru Wang and Yongjian Luo. AUTHORS AND AFFILIATIONS * Key Laboratory of Crops Genetics and Improvement of Guangdong Province, Crops Research Institute,
Guangdong Academy of Agricultural Sciences, Guangzhou, 510640, China Daoshou Qiu * Hubei Minzu University, School of Forestry and Horticulture, Enshi, 445000, China Ru Wang & Yongjian
Luo * Heilongjiang Bayi Agricultural University, Daqing, 163319, China Zheng Lan Authors * Ru Wang View author publications You can also search for this author inPubMed Google Scholar *
Yongjian Luo View author publications You can also search for this author inPubMed Google Scholar * Zheng Lan View author publications You can also search for this author inPubMed Google
Scholar * Daoshou Qiu View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS R.W., (Ru Wang) and Y.L., (Yongjian Luo) designed the study, defined
sampling, obtained samples, and obtained funding. R.W and Y.L., (Yongjian Luo) annotated plastomes and performed comparative and phylogenetic analyses. R.W assembled Illumina sequences.
Z.L.,(Zheng Lan) and R.W., participated in the technical guidance and language revision of the paper. R.W., Z.L., and Q.D (Daoshou Qiu) interpreted the results and co-wrote the manuscript.
CORRESPONDING AUTHOR Correspondence to Daoshou Qiu. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer
Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY TABLES. RIGHTS AND PERMISSIONS OPEN
ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format,
as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third
party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the
article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the
copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Wang, R., Luo, Y., Lan, Z.
_et al._ Insights into structure, codon usage, repeats, and RNA editing of the complete mitochondrial genome of _Perilla frutescens_ (Lamiaceae). _Sci Rep_ 14, 13940 (2024).
https://doi.org/10.1038/s41598-024-64509-3 Download citation * Received: 01 March 2024 * Accepted: 10 June 2024 * Published: 17 June 2024 * DOI: https://doi.org/10.1038/s41598-024-64509-3
SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to
clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * Lamioideae * Sequence characteristics * Phylogenetic analysis * Mitochondrion * RSCU * RNA editing