Play all audios:
ABSTRACT Using a new red blood cell (RBC) metabolite extraction protocol, we performed a metabolomic analysis on RBCs in rheumatoid arthritis (RA) patients treated or not with methotrexate
(MTX), with the two following objectives: to compare the RBC metabolic profiles of MTX-naïve RA patients and healthy controls (HC), and to investigate whether RBC profiles before and after
MTX treatment in RA differed between responders and non-responders. Plasma analysis was performed in parallel. Metabolites were extracted and identified in RBCs and plasma by liquid
chromatography-mass spectrometry. We compared the metabolomic fingerprints of 31 DMARD-naïve RA patients and 39 HCs. We also compared the RBC and plasma metabolomes of 25 RA patients who
responded or not to MTX therapy before (M0) and after a 3-month treatment period (M3). Significance was determined by Storey’s false discovery rate (FDR) q-values to correct for multiple
testing. RA patients and HCs differed in the metabolomic signature of RBCs. The signature mainly contained amino acids (AA). Eleven metabolites, including 4 metabolites belonging to the
carbohydrate subclass and 2 amino acids (creatine and valine) showed accumulation in RBCs from RA patients. Conversely, citrulline (fold change = 0.83; q = 0.025), histidine (fold change =
0.86; q = 0.014) and ergothioneine (EGT) (fold change = 0.66; q = 0.024), were lower in RBC of RA patients. Five plasma metabolites, including succinic acid and hydroxyproline, were higher
in RA patients, and 7 metabolites, including DHEA sulfate, alanine, threonine and ornithine, were lower. Among RA patients undergoing MTX treatment pre-treatment (M0), EGT values were
significantly lower in non-responders. In conclusion, low RBC levels of EGT, a food-derived AA barely detectable in plasma, characterize DMARD naïve RA patients and lack of response to MTX
treatment. SIMILAR CONTENT BEING VIEWED BY OTHERS A BLOOD-BASED METABOLOMICS TEST TO DISTINGUISH RELAPSING–REMITTING AND SECONDARY PROGRESSIVE MULTIPLE SCLEROSIS: ADDRESSING PRACTICAL
CONSIDERATIONS FOR CLINICAL APPLICATION Article Open access 24 July 2020 COMPARISON OF THE BLOOD, BONE MARROW, AND CEREBROSPINAL FLUID METABOLOMES IN CHILDREN WITH B-CELL ACUTE LYMPHOBLASTIC
LEUKEMIA Article Open access 04 October 2021 URINARY METABOLOMIC PROFILING OF A COHORT OF COLOMBIAN PATIENTS WITH SYSTEMIC LUPUS ERYTHEMATOSUS Article Open access 25 April 2024 INTRODUCTION
Omics sciences are increasingly used to study complex multifactorial diseases. Among these omics, metabolomics study the whole set of metabolites in a biological system that define its
metabolome. Given that metabolites are the final products of cellular regulatory pathways, the metabolome integrates and reflects all the interactions between biochemical entities in cells
or tissues and their environment. Thus, metabolomics enable the qualitative and quantitative description of a biological system’s ultimate response to environmental changes1. With regard to
rheumatoid arthritis (RA), metabolomics can identify specific signatures of the disease (e.g. RA vs. healthy controls (HC)), trace putative metabolicpathways relevant for disease
pathogenesis and treatment, and provide additional information on how patients with RA respond to the environment, including diet and exposure to xenobiotic substances. Several metabolomics
studies have been published in the field of RA and have identified a large number of metabolites as putative markers of RA2, response to treatment3, and disease activity4. Most of them are
involved in glycolysis (lactic acid, glucose), citrate cycle (citric acid), amino acid (leucine, isoleucine, valine, threonine, serine, proline, alanine, histidine) and lipid (cholesterol,
glycerol) metabolisms5. All the RA metabolomic studies were performed on serum, plasma, urine and synovial fluid, but none of these studies examined red blood cells (RBC) as an alternative
matrix. RBCs play a major role in interorgan transport of metabolites during their life span, thereby reflecting exogenous metabolic processes. A case in point is the measure of glycated
hemoglobin A1c, which reflects mean glycemia over the past 3 months. RBCs are basic cells devoid of nucleus, mitochondria and ribosome. They therefore have an elementary metabolism. In this
proof-of-concept study, we developed a metabolite extraction protocol to perform metabolomic analysis on RBCs with three objectives. The first was to characterize any differences in the RBC
metabolic profiles of Disease-Modifying antirheumatic drugs (DMARD)-naïve RA patients and HCs. The second was to determine whether RBC profiles before and after methotrexate (MTX) treatment
in RA patients differed between responders and non-responders. The third was to identify relevant RBC discriminant metabolites. In parallel, we performed metabolomic analysis of the plasma
to demonstrate the diagnostic value of RBC metabolome and to study any association between RBC and plasma metabolomes. RESULTS CLINICAL PARAMETERS RA patients were predominantly female
(71.0%), with a mean age of 50.0 years, and mostly ACPA (92.3%) and RF (95.2%) positive. Disease activity before MTX initiation was moderate to high (mean DAS28-CRP: 4.7 ± 1.4) (Table 1). Of
the 31 initial RA patients, 25 were treated with MTX for at least 3 months. After a 3-month MTX treatment period, 12 patients were classified as responders and 13 as non-responders. The two
groups before treatment did not significantly differ in baseline DAS28-CRP (4.6 ± 1.1 vs. 3.8 ± 1.5, p = 0.17 for responders and non-responders, respectively) or in tender and swollen joint
counts (Supplementary Table S1). RBC METABOLITES ASSOCIATED WITH RA (VS. HEALTHY CONTROLS) Under our conditions, 33 metabolite features from the C18( +) and 74 from the HILIC(-) analysis
matched the accurate masses and retention times of the metabolites included in our chemical database, yielding 105 unique annotated metabolites (See supplementary file). Univariate analysis
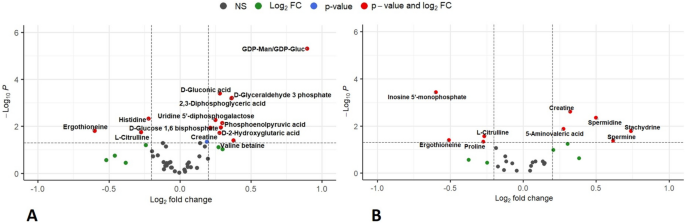
of the differences in mean expression between the two groups showed a significant difference in HILIC(−) analysis (p-value < 0.05) for 14 metabolites (Fig. 1A, Table 2) and all of them
remained significant after correction for multiple testing (FDR q-value < 0.05) (Supplementary Figure S1). Three of them – histidine, ergothioneine (EGT), and citrulline—were found to be
less expressed in RBCs from RA patients (RA/HC ratio: 0.86, 0.66 and 0.83, respectively, with all three FDR qvalue < 0.02). Meanwhile, eleven—including 4 metabolites in the carbohydrate
subclass and 2 amino acids (creatine and valine)—showed accumulation. Although the results obtained under C18( +) conditions were statistically less robust (Fig. 1B, Supplementary Table S2),
the same trend was observed for EGT (RA/HC ratio: 0.70, p = 0.040) and citrulline (RA/HC ratio: 0.83, p = 0.027), showing lower concentrations in the RA group compared to HCs. These
differences did not reach FDR-adjusted significance. In this dataset, 5 metabolites involved in the arginine and proline pathway showed accumulation in RA RBCs compared to HCs (aminovaleric
acid, creatine, spermidine, spermine and stachydrine). Metabolites were also studied in plasma samples from RA patients and HCs (See supplementary Data, tables S3 and S4). We found lower
concentrations of several amino acids in the plasma of RA patients compared to HCs (DHEA, alanine, threonine, ornithine, theophylline) and higher hydroxyproline. HIGH LEVEL OF RBC
ERGOTHIONEINE IS ASSOCIATED WITH FAVORABLE METHOTREXATE (MTX) RESPONSE Since RBC analysis identified EGT as a differentiating metabolite between RA patients and HCs, we investigated whether
the concentration of EGT differed between responders and non-responders before (M0) and/or after (M3) MTX initiation. Of the 31 included patients, 25 completed the follow-up and were
available for analysis. Among the 6 patients that did not complete the study, 4 missed their 3 month follow-up visit and 2 did not tolerate MTX. Those 6 patients, had similar values of
DAS28-CRP (p = 0.47), mean corticosteroid dose (p = 0.21) and EGT levels (p = 0.10) as those that completed their follow-up. A total of 12 patients responded to MTX, while 13 were classified
as non-responders. We found significantly lower EGT values in non-responders at M0 (p = 0.036) when using the HILIC(−) dataset (Fig. 2). A similar trend was observed using C18( +) (p =
0.07, Wilcoxon test). Responders and non-responders did not differ in baseline corticosteroid dose (p = 0.84) or in baseline disease activity expressed by DAS28CRP values (p = 0.17)
(supplementary table 1). Higher that the median EGT was the only variable associated with clinical response to methotrexate (OR 6.00; 95% CI 1.02 to 35.38) in multivariable logistics
regression model taking into account these two confounders. DISCUSSION This study is the first RBC metabolomics characterization in RA. This proof-of-concept study identified EGT—an amino
acid barely detectable in plasma—as a key metabolite that is reduced in untreated RA and particularly in future non-responders to MTX. In alignment with several RA metabolomic studies, our
RBC and plasma analysis shows a predominantly amino acidic RA signature. Since the RBC metabolome is not limited to pathways essential for RBC functions, but also includes metabolites from
external sources such as plasma and other cells, the observed variations in AA concentration may reflect impaired systemic metabolism. We found a significant decrease in EGT, histidine (EGT
precursor), and citrulline in RA patient RBCs vs. HCs. This decrease may be related to the predominance of the ammonia recycling pathway and/or the nitric oxide (NO) pathway involved in RA,
and may reflect increased catabolism and resulting muscle loss6. Interestingly, the plasma metabolome also revealed the potential role of the ammonia-recycling pathway, notably through the
lower ornithine in RA patients. Furthermore, the fatigue frequently experienced by RA patients has been associated with downregulation of the urea cycle that involves citrulline7. Citrulline
concentration was lower in RA patients’ RBC metabolome compared to HCs. Since RBCs do not have any NO synthase activity, the citrulline may be coming from plasma. The presence of
endothelial dysfunction in RA patients, even at an early stage, is well established and it has been suggested that patients may exhibit impaired NO reactivity8. Thus, we can hypothesize that
the citrulline decrease reflects the endothelial dysfunction observed in untreated RA and may be a consequence of decreased NO synthase activity and/or lack of arginine (NO synthase
substrate) intake. The metabolomic analysis performed on plasma in our study is consistent with the relevant literature. In a meta-analysis of 10 publications reporting metabolomic analyses,
Li et al. identified 17 metabolites that could differentiate RA patients and controls5. This study confirmed two of them: threonine and alanine. Of note, these metabolites were
significantly reduced in collagen-induced arthritis mice compared to controls9. We also identified DHEA sulfate as the metabolite with the lowest RA/HC ratio, in alignment with the
literature10. The data obtained from the RBC metabolome analysis is therefore partly consistent with the plasma analysis (involvement of the ammonia recycling pathway), but also identified
other metabolites and pathways that appear to be dysregulated in RA patients, especially EGT. Our analyses showed a lower EGT concentration in RA RBCs compared to HC RBCs, and a higher EGT
concentration in MTX responders compared to non-responders before MTX onset. EGT is a natural AA that derives from histidine. It is strongly associated with a “health conscious food pattern”
that was linked to a reduced risk of cardiometabolic disease in a 3,236-participant study11. EGT cannot be synthesized by humans and is provided through diet. It can be found in
actinobacteria, cyanobacteria, some fungi (such as porcini), oysters and garlic. EGT is a powerful antioxidant and can induce the expression of SIRT112, which appears lower in RA endothelial
and synovial vessels13. EGT uses OCTN1—a dependent organic cation transporter primarily expressed in blood cells including erythrocytes—to enter cells14. EGT is therefore up to 100 times
more concentrated in RBCs than in plasma, where it is barely detectable. Interestingly, a study highlighted a polymorphism of SLC22A4 (coding for OCTN1) strongly associated with RA in East
Asian15 but not Caucasian populations16. The functional consequences of this polymorphism are unknown, as is the actual biological role of EGT. Our result differs from a study showing that
the erythrocyte concentration of ergothioneine in RA patients was higher than in HCs17. This discrepancy may be related to the cohorts used, since the patients included in this previous
study were nearly all treated with MTX and showed low disease activity (i.e. responders). Conversely, our study focused on DMARD-naïve RA patients. If, as our results suggest, patients with
higher levels of EGT have a higher probability of responding to methotrexate treatment, it is conceivable that these patients are also more likely to remain on treatment over the long term.
Therefore, by selecting patients in remission on methotrexate treatment, the other study might have focused on RA patients with the highest levels of EGT. Our work has some limitations,
mainly the absence of matching of RA patients and HCs on potential confounders like smoking status and BMI. Moreover, like most proof-of-concept studies, this work is underpowered to predict
the response to methotrexate. Based on our ratio of responders to non-responders, an ideal number of 59 subjects should have been included to achieve 80% power with an alpha error of 0.05.
Also, our results were not replicated in a validation cohort. However, identifying a metabolic signature that may predict response to methotrexate treatment in early RA is beyond the scope
of this work and would require larger cohorts and adjustments for multiple confounders. Another limitation is the fact that the biological role of EGT and its transporter in the pathogenesis
of RA is still unknown. The observation that RA patients have lower levels of EGT compared to healthy controls, and that those with the lowest levels are also less likely to respond to
methotrexate treatment, is intriguing. This finding warrants mechanistic studies to understand the biological role of this metabolite. Whether EGT may be a marker of refractory RA, a
potential adjuvant to methotrexate treatment, or merely a random bystander cannot be established by this work. However, this completely novel method in rheumatology preliminarily identifies
EGT, a food-derived AA that has antioxidant properties and accumulates in tissues, as a potential metabolite of interest in untreated RA and MTX response. Larger studies are warranted to
bolster these original data. METHODS PATIENTS The study group consisted of 31 prospectively recruited consecutive patients that met the _2010 ACR_-_EULAR_ _Classification Criteria_ for RA
and were DMARD-naïve. All patients were recruited from the Avicenne Rheumatology Department (Bobigny, France). Following an overnight fast, RA patients underwent blood sampling and clinical
assessment before (M0) and 3 months after (M3) MTX initiation. At M3, all patients with a good or moderate DAS28-CRP-based EULAR response or in DAS28-CRP-based remission (i.e. DAS28-CRP <
2.6)) were classified as MTX responders. Only patients who had completed 3 months of MTX treatment and completed their 3-month follow-up visit were included in the longitudinal analysis
(i.e. responders vs. non-responders). Blood samples of age- and sex-matched blood donors (Etablissement Français du Sang) were used as healthy controls (HC). This study was approved by the
local review board of Avicenne Hospital (NI-2016-11-01) and performed in accordance with the Declaration of Helsinki and the institutional guidelines. Informed consent was obtained from all
subjects. PREPARATION OF RBC LYSATES AND RBC AND PLASMA METABOLITE EXTRACTION Preparation of RBC lysates and metabolite extraction were performed as previously described (see supplementary
methods). UNTARGETED METABOLOMICS BY ULTRA-HIGH PERFORMANCE LIQUID CHROMATOGRAPHY (UHPLC) COUPLED WITH HIGH-RESOLUTION MASS SPECTROMETRY (HRMS) AND DATA PROCESSING The ultra-high performance
liquid chromatographic (UHPLC) separation was performed on a Hypersil GOLD C18 1.9 µm, 2.1 mm × 150 mm column (RP) at 30 °C (Thermo Fisher Scientific, les Ulis, France), and HPLC
chromatographic separations were performed on a Sequant ZICpHILIC 5 µm, 2.1 × 150 mm (HILIC) at 15 °C (Merck, Darmstadt, Germany). All chromatographic systems were equipped with an on-line
prefilter (Thermo Fisher Scientific, Courtaboeuf, France). The data processing is described elsewhere18 and reported in the supplementary methods. LC–MS analyses were performed using a U3000
liquid chromatography system coupled to an Exactive mass spectrometer from Thermo Fisher Scientific (Courtaboeuf, France) fitted with an electrospray source. The software interface was
Xcalibur (version 2.1) (Thermo Fisher Scientific, Courtaboeuf, France) (see supplementary methods). Data processing and metabolite annotation procedures are described elsewhere (see
supplementary methods). STATISTICAL ANALYSIS Metabolite concentrations expressed as chromatographic peak areas (HILIC (-) or C18 ( +)) were analyzed using R software (version 4.2.1)19. For a
given metabolite, the normality of the data distributions for each of the two groups—RA and HC—was evaluated by the Shapiro–Wilk test and visual examination of the qqplot graphs. The
equality of variance was tested using the var.test function (R stats library). If the hypothesis of normality of at least one of the two distributions was rejected, the comparison of the
values of the two groups was done by the row_Wilcoxon_two-sample test (R MatrixTest library). Otherwise, this comparison was done by Welch t test (unequal variances) or Student t test.
Significant changes in metabolite levels were tested using the t-test, and False Discovery Rate (FDR) were determined according to Storey and Tibshirani procedure to control false discovery
rate in multiple statistical tests. For the comparison between responders and non-responders, baseline corticosteroid doses and DAS28CRP values were compared with independent sample T test
after checking for normality. A multivariable logistic regression model was used to check the independent association between EGT baseline values and 3-month clinical response to
methotrexate adjusting on baseline corticosteroids and DAS28CRP values. To check for a potential attrition bias, DAS28-CRP, mean corticosteroid dose and EGT level were compared between
patients lost-to-follow-up and those who completed the study by Mann–Whitney test to the sample size. DATA AVAILABILITY The datasets used and/or analysed during the current study available
from the corresponding author on reasonable request. REFERENCES * Semerano, L., Roméo, P.-H. & Boissier, M.-C. Metabolomics for rheumatic diseases: Has the time come?. _Ann. Rheum. Dis._
74, 1325–1326 (2015). Article PubMed Google Scholar * Chu, S. H. _et al._ Circulating plasma metabolites and risk of rheumatoid arthritis in the Nurses’ Health Study. _Rheumatology
(Oxford)_ 59, 3369–3379 (2020). Article CAS PubMed Google Scholar * Priori, R. _et al._ 1H-NMR-based metabolomic study for identifying serum profiles associated with the response to
etanercept in patients with rheumatoid arthritis. _PLoS One_ 10, e0138537 (2015). Article PubMed PubMed Central Google Scholar * Hur, B. _et al._ Plasma metabolomic profiling in patients
with rheumatoid arthritis identifies biochemical features predictive of quantitative disease activity. _Arthritis Res. Ther._ 23, 164 (2021). Article CAS PubMed PubMed Central Google
Scholar * Li, C. _et al._ Metabolomics in the development and progression of rheumatoid arthritis: A systematic review. _Joint Bone Spine_ 87, 425–430 (2020). Article CAS PubMed Google
Scholar * Farrow, M. _et al._ Muscle deterioration due to rheumatoid arthritis: Assessment by quantitative MRI and strength testing. _Rheumatology (Oxford)_ 60, 1216–1225 (2021). Article
PubMed Google Scholar * Surowiec, I. _et al._ Metabolomics study of fatigue in patients with rheumatoid arthritis naïve to biological treatment. _Rheumatol. Int._ 36, 703–711 (2016).
Article CAS PubMed Google Scholar * Bergholm, R. _et al._ Impaired responsiveness to NO in newly diagnosed patients with rheumatoid arthritis. _Arterioscler. Thromb. Vasc. Biol._ 22,
1637–1641 (2002). Article CAS PubMed Google Scholar * He, M. _et al._ Spontaneous ultra-weak photon emission in correlation to inflammatory metabolism and oxidative stress in a mouse
model of collagen-induced arthritis. _J. Photochem. Photobiol. B_ 168, 98–106 (2017). Article CAS PubMed Google Scholar * Lee, Y.-J. _et al._ A discovery of screening markers for
rheumatoid arthritis by liquid chromatography mass spectrometry: A metabolomic approach. _Int. J. Rheum. Dis._ 23, 1353–1362 (2020). Article CAS PubMed Google Scholar * Smith, E. _et
al._ Ergothioneine is associated with reduced mortality and decreased risk of cardiovascular disease. _Heart_ 106, 691–697 (2020). Article CAS PubMed Google Scholar * D’Onofrio, N. _et
al._ Ergothioneine oxidation in the protection against high-glucose induced endothelial senescence: Involvement of SIRT1 and SIRT6. _Free Radic. Biol. Med._ 96, 211–222 (2016). Article
PubMed Google Scholar * Leblond, A. _et al._ Implication of the deacetylase sirtuin-1 on synovial angiogenesis and persistence of experimental arthritis. _Ann. Rheum. Dis._ 79, 891–900
(2020). Article CAS PubMed Google Scholar * Gründemann, D. _et al._ Discovery of the ergothioneine transporter. _Proc. Natl. Acad. Sci. U.S.A._ 102, 5256–5261 (2005). Article ADS
PubMed PubMed Central Google Scholar * Tokuhiro, S. _et al._ An intronic SNP in a RUNX1 binding site of SLC22A4, encoding an organic cation transporter, is associated with rheumatoid
arthritis. _Nat. Genet._ 35, 341–348 (2003). Article CAS PubMed Google Scholar * Lee, Y. H. _et al._ Meta-analysis of SLC22A4 and RUNX1 polymorphisms : Associations with rheumatoid
arthritis susceptibility. _Z. Rheumatol._ 74, 351–358 (2015). Article CAS PubMed Google Scholar * Taubert, D. _et al._ Association of rheumatoid arthritis with ergothioneine levels in
red blood cells: A case control study. _J. Rheumatol._ 33, 2139–2145 (2006). CAS PubMed Google Scholar * Darghouth, D. _et al._ Pathophysiology of sickle cell disease is mirrored by the
red blood cell metabolome. _Blood_ 117, e57-66 (2011). Article CAS PubMed Google Scholar * R Core Team (2021). R: A language and environment for statistical computing. R Foundation for
Statistical Computing, Vienna, Austria. URL https://www.R-project.org/ Download references FUNDING This work was supported by the French Rheumatology Society (Societé Française de
Rhumatologie, SFR) and Entrepreneurs&Go. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Inserm U1125, Université Sorbonne Paris Nord, Bobigny, France Johanna Sigaux, Marie-Christophe
Boissier, Magali Breckler & Luca Semerano * Rheumatology Department, Assistance Publique-Hôpitaux de Paris, GH HUPSSD, Bobigny, France Johanna Sigaux, Marie-Christophe Boissier, Mylène
Petit & Luca Semerano * Service de Pharmacologie et Immuno-Analyse (SPI), Laboratoire d’Etude du Métabolisme des Médicaments, CEA, INRA, Université Paris Saclay, MetaboHUB, 91191,
Gif-Sur-Yvette, France Christophe Junot, Florence Castelli & François Fenaille * Institut de Biologie François Jacob (IBFJ), CEA, 92260, Fontenay-Aux-Roses, France Paul-Henri Roméo
Authors * Johanna Sigaux View author publications You can also search for this author inPubMed Google Scholar * Christophe Junot View author publications You can also search for this author
inPubMed Google Scholar * Marie-Christophe Boissier View author publications You can also search for this author inPubMed Google Scholar * Mylène Petit View author publications You can also
search for this author inPubMed Google Scholar * Magali Breckler View author publications You can also search for this author inPubMed Google Scholar * Florence Castelli View author
publications You can also search for this author inPubMed Google Scholar * François Fenaille View author publications You can also search for this author inPubMed Google Scholar * Paul-Henri
Roméo View author publications You can also search for this author inPubMed Google Scholar * Luca Semerano View author publications You can also search for this author inPubMed Google
Scholar CONTRIBUTIONS PHR, MCB and LS conceptualized the study JS, PHR and LS created the methodology for the study JS, MP, MB, FC, FF carried out the data collection JS and LS did the data
analysis and verified the data JS and CJ supervised the data collection JS and LS wrote the original draft All authors reviewed and edited the manuscript All authors had full access to all
of the data and the final responsibility to submit for publication. CORRESPONDING AUTHOR Correspondence to Marie-Christophe Boissier. ETHICS DECLARATIONS COMPETING INTERESTS The authors
declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional
affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION 1. SUPPLEMENTARY INFORMATION 2. SUPPLEMENTARY INFORMATION 3. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed
under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or
format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material.
You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the
article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use
is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit
http://creativecommons.org/licenses/by-nc-nd/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Sigaux, J., Junot, C., Boissier, MC. _et al._ Red blood cell metabolomics
identify ergothioneine as a key metabolite in DMARD-naïve rheumatoid arthritis and response to methotrexate. _Sci Rep_ 14, 21005 (2024). https://doi.org/10.1038/s41598-024-68477-6 Download
citation * Received: 22 March 2024 * Accepted: 24 July 2024 * Published: 09 September 2024 * DOI: https://doi.org/10.1038/s41598-024-68477-6 SHARE THIS ARTICLE Anyone you share the following
link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature
SharedIt content-sharing initiative KEYWORDS * Rheumatoid arthritis * Metabolomic * Ergothioneine * Diet * Environment