Play all audios:
Chronic histiocytic intervillositis (CHI) is a recurrent placental lesion where maternal macrophages infiltrate the intervillous space. Its cause is unknown, though due to similarities to
rejected allografts one hypothesis is that CHI represents maternal–fetal rejection. Here, virtual crossmatching was applied to healthy pregnancies and those with a history of CHI. Anti-HLA
antibodies, measured by Luminex, were present in slightly more controls than CHI (8/17 (47.1%) vs 5/14 (35.7%)), but there was no significant difference in levels of sensitisation or fetal
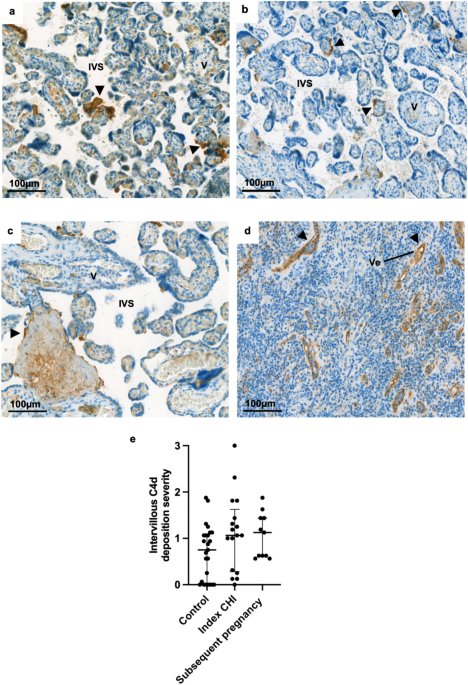
specific antibodies. Quantification of immunohistochemical staining for HLA-Class II was increased in syncytiotrophoblast of placentas with CHI (Grade 0.44 [IQR 0.1–0.7]) compared to healthy
controls (0.06 [IQR 0–0.2]) and subsequent pregnancies (0.13 [IQR 0–0.3]) (P = 0.0004). HLA-Class II expression was positively related both to the severity of CHI (r = 0.67) and C4d
deposition (r = 0.48). There was no difference in overall C4d and HLA-Class I immunostaining. Though increased anti-HLA antibodies were not evident in CHI, increased expression of HLA-Class
II at the maternal–fetal interface suggests that they may be relevant in its pathogenesis. Further investigation of antibodies immediately after diagnosis is warranted in a larger cohort of
CHI cases to better understand the role of HLA in its pathophysiology.
Throughout healthy human pregnancy the fetus is tolerated by the maternal immune system despite possessing genetically foreign paternal human leukocyte antigen (HLA). This ability of the
semi-allogeneic fetus to evade mounting an inflammatory response has led the conceptus to be coined as the ‘most successful graft’1. In pregnancy, there are several mechanisms which promote
maternal–fetal tolerance, including specialised placental HLA expression and maternal immune cell adaptations2,3,4,5. The failure of these tolerogenic processes has been linked to the
pathogenesis of multiple inflammatory placental disorders resulting in adverse pregnancy outcomes such as preterm birth, miscarriage and stillbirth6,7,8.
One example of a placental lesion with an immune etiology is chronic histiocytic intervillositis (CHI), in which maternal macrophages infiltrate the intervillous space9. CHI is associated
with fetal growth restriction (FGR), and in severe cases can result in miscarriage or stillbirth10. Though the cause of CHI is unknown, previous case reports and series have proposed an
antibody-mediated component, given its increased incidence in women with autoimmune disease11,12, high recurrence rate13,14, evidence of increased anti-HLA antibodies15,16, and in select
cases deposition of complement split product C4d15,17. In addition, some cases of CHI also exhibit accompanying fibrin deposition10 and anti-paternal T cells have been detected in several
women with the disorder16. As many of these pathological features are also common to rejected allografts, CHI is hypothesised to represent maternal–fetal rejection and is consequently
treated with immunosuppressive medication12,18,19,20. Despite suggestions of antibody involvement from case reports, CHI’s rarity (~ 0.17% of pregnancies10) has made conducting larger scale
studies into its pathophysiology difficult. Research into the condition is further complicated by the fact its diagnosis can only be made after delivery of the placenta, in most cases when
the health or survival of the fetus has already been compromised.
For decades, predicting risk of rejection in recipients of solid organ transplants has been possible via laboratory crossmatching techniques which informs clinical management and facilitates
maintenance of a foreign graft for a sustained period. In this study, we aimed to apply virtual crossmatching techniques to cases of CHI to investigate the possibility of placental
rejection and characterise the HLA expression profile in inflamed placentas. In doing so, we hypothesised that affected women would exhibit increased evidence of humoral involvement
including high titre anti-HLA antibodies and deposition of C4d, similar to that observed in the rejection of a transplanted organ.
Participants were retrospectively identified by searching medical records at Saint Mary’s Hospital, Manchester, UK, for women with a diagnosis of CHI in a previous pregnancy. Cases of CHI
with archived placental tissue available for analysis from the Paediatric and Perinatal Histopathology Department were included. Placental tissue from cases of CHI and healthy controls were
received as formalin-fixed paraffin embedded (FFPE) blocks, and accompanying hospital records collected where available, detailing maternal demographic data and obstetric history. For
certain participants, accompanying medical history could not be retrieved for the study as they had delivered at another centre or records were unavailable at the time of retrieval. These
cases were excluded from statistical analysis of demographic characteristics and pregnancy outcome, and analysis was restricted to blood and/or placental tissue. An initial subset of FFPE
placental tissue from healthy pregnancies was provided for the study by the Paediatric and Perinatal Histopathology Department, for which matched maternal blood was not collected. Pregnancy
outcomes consisted of livebirth, miscarriage (fetal death 80% are highly sensitised and prove more challenging to find a suitable donor, as > 80% of donors are considered unsuitable.
In participants with FSAs, results of T and B cell flow cytometry crossmatch (FXCM) and complement-dependent cytotoxicity (CDC) crossmatch were predicted using a formula developed by the
MRITL24. HLA specificities and respective FSA MFI values were inputted to give an estimation of positive or negative result. Where results were in between the in-house cut-off values for a
positive or negative result, these were described as equivocal, and could not be reliably predicted.
All statistical analysis and creation of graphs was undertaken using GraphPad Prism v9 (GraphPad Software, USA). Normality was determined by the Shapiro–Wilk test. Continuous demographic
data were analysed using ordinary one-way ANOVA with Dunn’s multiple comparisons test or Kruskal–Wallis test for normally distributed and non-normally distributed data, respectively.
Interobserver agreement of C4d and HLA staining was determined by calculation of the weighted Kappa between reviewer scores and grading was analysed via Kruskal–Wallis test with Dunn’s
multiple comparisons where this was significant. Categorical demographic data and proportions of antibody-positive participants were analysed using Fisher’s exact test. For %cRF values the
Mann–Whitney test was run to determine statistical differences. Statistical significance was set at P