Play all audios:
ABSTRACT Abnormal brain aging is suggested in epilepsy. Given the brain network dysfunction in epilepsy, the white matter tracts, which primarily interconnect brain regions, could be of
special importance. We focused on white matter brain aging in diverse forms of epilepsy and comorbid psychosis. We obtained brain diffusion tensor imaging (DTI) data at 3 T-MRI in 257
patients with epilepsy and 429 healthy subjects. The tract-based fractional anisotropy values of the healthy subjects were used to build a brain-age prediction model, and we calculated the
brain-predicted age difference (brain-PAD: predicted age—chronological age) of all subjects. As a result, almost all epilepsy categories showed significantly increased brain-PAD (p <
0.001), including temporal lobe epilepsy (TLE) with no MRI-lesion (+ 4.2 yr), TLE with hippocampal sclerosis (+ 9.1 yr), extratemporal focal epilepsy (+ 5.1 yr), epileptic encephalopathy or
progressive myoclonus epilepsy (+ 18.4 yr), except for idiopathic generalized epilepsy (IGE). Patients with psychogenic non-epileptic seizures also presented increased brain-PAD. In TLE,
interictal psychosis significantly raised brain-PAD by 8.7 years. In conclusion, we observed increased brain aging in most types of epilepsy, which was generally consistent with brain
morphological aging results in previous studies. Psychosis may accelerate brain aging in TLE. These findings may suggest abnormal aging mechanisms in epilepsy and comorbid psychotic
symptoms. SIMILAR CONTENT BEING VIEWED BY OTHERS CORTICAL THINNING OVER TWO YEARS AFTER FIRST-EPISODE PSYCHOSIS DEPENDS ON AGE OF ONSET Article Open access 11 March 2022 INVESTIGATING BRAIN
AGING TRAJECTORY DEVIATIONS IN DIFFERENT BRAIN REGIONS OF INDIVIDUALS WITH SCHIZOPHRENIA USING MULTIMODAL MAGNETIC RESONANCE IMAGING AND BRAIN-AGE PREDICTION: A MULTICENTER STUDY Article
Open access 07 March 2023 PREDICTING AGING TRAJECTORIES OF DECLINE IN BRAIN VOLUME, CORTICAL THICKNESS AND FRACTIONAL ANISOTROPY IN SCHIZOPHRENIA Article Open access 03 January 2023
INTRODUCTION Epilepsy is a common but diverse disorder of the brain, characterized by various types of recurrent seizures due to abnormal and excessive neuronal activities1, which affects
over 45 million people globally2. Various forms of psychiatric comorbidities can occur in epilepsy, such as depression, anxiety, or psychosis3. Despite recent progress in treatments, the
burden of epilepsy is still high, which is shown by the high disability-adjusted life-years (DALYs) of 182.6 per 100,000 population due to premature mortality and unhealthy life with
disability2. To develop further advanced treatment and care for people with epilepsy, it is essential to reveal the pathophysiology of the brain in epilepsy. Additionally, as the quality of
life in people with epilepsy can be affected not only by seizures but also by psychiatric comorbidities4, the underlying mechanisms of comorbidities should also be elucidated. Neuroimaging
is a powerful tool to investigate the human brain less invasively and widely used for neurological and psychiatric disorders. While its main clinical role is the detection of seizure focus
in epilepsy5, it is also highly expected to establish imaging biomarkers6. In particular, machine learning is an emerging trend in this field to provide optimal algorithms or uncover hidden
patterns7. The advantage of machine learning over conventional methods is accurate, automated, and fast pattern learning. Neuroimaging-based brain-age estimation is an advanced technique to
calculate an individual’s brain age using neuroimaging and machine learning models8. In fact, many brain disorders, such as dementia or neurodegeneration, are associated with aging9, and
thus, brain-age estimation is expected to be a novel biomarker for neuropsychiatric disorders8. Additionally, previous conventional neuroimaging studies on psychosis in epilepsy failed to
provide consistent results10, and thus, advanced machine learning may also help overcome such problems. In our previous study on brain aging in epilepsy11, we observed higher brain-age than
chronological age by over four years in most types of epilepsy, based on the morphological features of the brain using 3D T1-weighted MRI. In addition, we found a significant effect of
interictal psychosis on the increase in brain-age in temporal lobe epilepsy (TLE)11. Psychosis is a serious comorbidity presenting hallucination or delusion, and in fact, epilepsy has a
7.8-fold higher prevalence than the general population12. The neurobiological mechanism of psychosis in epilepsy is still less understood, although several studies have reported structural,
metabolic, or network abnormalities of the brain13. White matter (WM) tracts, which comprise axon fibers, primarily connect neurons and transmit information between different brain regions,
organizing the network of the brain. Given the increasing evidence showing that epilepsy is a brain network disorder14, WM microstructure may provide further significant evidence on epilepsy
and its comorbidities. Brain-age can be estimated by various modalities of neuroimaging, including morphological, microstructural, and functional images. A previous study investigated WM
brain-age of TLE with hippocampal sclerosis (HS) and reported an increase in brain-age by 2–10 years using diffusion tensor imaging (DTI)15, although the sample size was relatively small (N
= 35) and other types of epilepsy were not examined. In the current study, we applied WM-based brain-age estimation to patients with various forms of epilepsy and interictal psychosis,
hypothesizing that WM brain-age in most types of epilepsy may present an increase compared to healthy subjects, particularly in TLE with HS or with psychosis, reflecting an abnormal aging
process, as in our previous study based on morphological brain features11. METHODS SUBJECTS We analyzed the DTI data of the cohort of our previous study11, which consisted of 437 adults with
epilepsy or psychogenic non-epileptic seizures (PNES) and 1196 healthy controls (HCs). Of those, DTI data from the same protocol were available in 257 patients and 429 healthy subjects. The
inclusion and exclusion criteria are the same as our previous brain-age study on epilepsy11. All patients underwent careful clinical diagnosis by board-certified epileptologists based on
seizure semiology, scalp EEG, and high-resolution MRI inspection. The inclusion criteria and number of subjects in each epilepsy category are described in Table 1. The initial categorization
of epilepsy at this stage was as follows: (1) TLE with no visible lesion (i.e., visually normal) on MRI (TLE-NL), (2) TLE with hippocampal sclerosis (TLE-HS), (3) extra-temporal lobe focal
epilepsy (Ext-FE), (4) idiopathic generalized epilepsy (IGE), (5) progressive myoclonus epilepsy or epileptic encephalopathy (symptomatic generalized epilepsy) (PME/EE), and (6) PNES without
any epileptic seizures (PNES). The following exclusion criteria were applied to all patients: (1) a significant medical history of acute encephalitis, meningitis, severe head trauma,
ischemic encephalopathy, or brain surgery; and (2) suspicious epileptogenic lesions (e.g., tumor, vascular malformation, destructive lesion) on MRI other than unilateral HS or focal cortical
dysplasia (FCD). We assessed the existence of interictal psychosis only in patients with TLE. Because TLE has the highest prevalence of psychosis, this investigation of psychosis was
originally planned for TLE patients. The presence or history of interictal psychosis was diagnosed based on the Diagnostic and Statistical Manual of Mental Disorders, 4th edition criteria16
by a board-certified psychiatrist (DS). Of the 173 patients with TLE, 19 were diagnosed with interictal psychosis; the others had no psychotic episodes. Patients with postictal psychosis
were excluded, as the current study focused on interictal psychosis. To build and estimate the brain age model, we used MRI scans at our center from 429 healthy controls (HCs) with no
history of neurological or psychiatric diseases and no use of medication affecting the central nervous system. No possible structural anomalies or abnormalities affecting the analysis were
visually found in the controls on MRI. The 429 HCs were aged between 20 and 85 years (median 53, IQR: 23) and comprised 172 men and 257 women. As in the previous study11, the age and sex
distributions were different between each group of patients and HCs, but we included all available samples to establish a reliable brain age model. All participants gave written informed
consent. This study was performed in accordance with the Declaration of Helsinki and approved by the Institutional Review Board at the National Center of Neurology and Psychiatry Hospital.
MRI ACQUISITION All MRI scans were performed on a 3.0-T MR system with a 32-channel coil (Philips Medical Systems, Best, The Netherlands). The parameters of the DTI sequence were as follows:
repetition time (TR), 6700 ms; echo time (TE), 58 ms; flip angle, 90°; effective slice thickness, 3.0 mm with no gap; slices, 60; matrix, 80 × 78; and field of view (FOV), 24 × 24 cm. The
DTI was acquired along 15 non-collinear directions with a diffusion-weighted factor b of 1000 s/mm2, and one image was acquired without diffusion gradient. To increase the signal-to-noise
ratio, we adopted the number of excitations (NEX) of 2 for DTI acquisition. Three-dimensional (3D) sagittal T1-weighted images were obtained by the following protocol: TR, 7.12 ms; TE, 3.4
ms; flip angle, 10°; NEX, 1; effective slice thickness, 0.6 mm with no gap; slices, 300; matrix, 260 × 320; and FOV, 26 × 24 cm. Additionally, transverse turbo spin echo T2-weighted imaging
and coronal fluid-attenuated inversion recovery (FLAIR) imaging were also obtained for visual MRI assessment. MRI PROCESSING Initially, the DTI data were processed with tract-based spatial
statistics (TBSS), using the PANDA toolbox v.1.3.1 (https://www.nitrc.org/projects/panda/)17 running within the MATLAB (The MathWorks, Natick, MA, USA) and FMRIB Software Library (FSL)
version 5.0.11. After eddy current correction and brain extraction, the TBSS pipeline provided atlas-based region-of-interest (ROI) analysis using all tracts of the Johns Hopkins University
(JHU) atlas. Anatomical accuracy of the automated ROI locations was visually confirmed. The FA threshold for TBSS was set at 0.20. The PANDA toolbox calculated mean FA values within each
tract of the atlas in each patient18. The quality of raw and processed DTI data was visually checked, and we confirmed no problematic error nor artifact. BRAIN-AGE MODEL The mean FA values
within all the 20 ROIs of the JHU atlas tracts were used to establish the WM brain-age model. To predict brain age, we employed a Support Vector Regression (SVR) algorithm with a linear
kernel, implemented using MATLAB_R2020b software. The SVR algorithm was chosen because of its established effectiveness in predicting brain age based on brain characteristics19. The
prediction model made use of FA values from ROI-based measurements and the variable of sex as independent variables, while the actual age was the dependent variable. To assess the accuracy
of the prediction, we conducted a tenfold cross-validation strategy on a training set of 429 HCs, measuring the mean absolute error (MAE) as an indicator. A validated bias adjustment
technique was used to calculate unbiased brain age values20. The final prediction model was developed using the entire training set (N = 429) and subsequently used to estimate brain age
values for different patient categories. GROUP COMPARISONS AND CLINICAL CORRELATIONS OF THE BRAIN PREDICTED AGE DIFFERENCE Based on the age predicted by the DTI-based SVR model, we
calculated each participant’s brain predicted age difference (brain-PAD: predicted age—chronological age). A brain-PAD value close to zero suggests that the subject is following a healthy
brain aging trajectory. Conversely, a negative brain-PAD value implies a younger-looking brain, while a positive brain-PAD value implies an older-looking brain. First, we compared the mean
brain-PAD among the six categories of patients and the HCs. Additionally, correlations of the brain-PAD with disease duration or onset age were investigated within each category except for
the PME/EE and PNES groups, which had a small sample size for within-group correlation analysis. Furthermore, we assessed the effect of interictal psychosis on WM brain aging by comparing
TLE patients with and without psychosis. STATISTICS Statistical analyses were performed using SPSS software, version 25.0 (SPSS Japan, Tokyo). Parametric or non-parametric distributions of
variables were examined by the Shapiro–Wilk test. The mean brain-PAD was compared via analysis of covariance (ANCOVA) with age and sex as covariates, followed by Bonferroni correction.
Because onset age or disease duration in each group did not show a normal distribution, the correlations of the brain-PAD with these parameters were analyzed by a non-parametric method
(i.e., Spearman’s rank correlation coefficient) with Bonferroni correction for multiple comparisons. While the main scope of this study was to estimate WM-based brain-age in patients with
various types of epilepsy and interictal psychosis, we also performed an additional analysis on seizure burdens and effects of antiseizure medications (ASMs) on brain-age. Since
fundamentally different seizures occur across the various epilepsy types, this analysis included only TLE patients. The associations of the brain-PAD with the number of ASMs, seizure
freedom, or the presence or history of focal to bilateral tonic–clonic seizures (FBTCS) were evaluated. This study included various types of analysis, and thus not all analyses had the
sample sizes validated. However, the total sample sizes were demonstrated to be sufficient (95%) to detect differences of 0.25 effect sizes among six categories of patients and HCs based on
G*Power 3.1.9.421. Clinical parameters other than brain-PAD were compared by a Mann–Whitney U-test for continuous variables and a Pearson’s χ2 test for binary parameters. A p-value < 0.05
was deemed significant. RESULTS CLINICAL DEMOGRAPHICS The clinical demographics are presented in Table 2. Each group showed differing distributions of age, sex, and disease onset/duration.
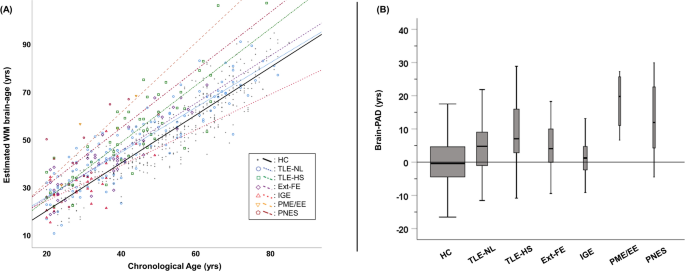
Most patients had refractory seizures, except those in the IGE group. BRAIN AGE PREDICTION MODEL IN HCS Figure 1A contains each individual’s predicted age and chronological age. The SVR
brain age prediction model showed a MAE of 5.57 years in HCs, and the predicted age in HCs was highly correlated with their chronological age (rs = 0.92, p < 0.001). The mean (± SD)
brain-PAD in HCs was 0.03 (± 7.1) years. BRAIN-PAD IN THE SIX CATEGORIES OF PATIENTS As shown in the Fig. 1B and Table 2, while IGE did not show any significant change in brain-PAD, all the
other groups presented significantly increased brain -PAD compared with HCs (p < 0.001). There was particularly increased brain aging (> + 9 years) in the TLE-HS, PME/EE, and PNES
groups, while TLE-NL and Ext-FE showed a moderate increase (+ 4–5 years). We also found a significant negative correlation between onset age and brain-PAD in the TLE-NL group (rs = − 0.24,
FWE p = 0.036), while there was no other significant correlation of brain-PAD with onset age or duration of disease. PSYCHOSIS VERSUS NON-PSYCHOSIS IN TLE The clinical demographics and
brain-PAD results of TLE with and without psychosis are shown in Table 3 and Fig. 2. On average, the TLE with interictal psychosis group showed significantly higher brain-PAD (+ 13.4 years)
than those without psychosis (+ 4.7 years). This difference was statistically significant even after correcting for the presence of hippocampal sclerosis (p < 0.001) or for the number of
ASMs (p < 0.001). For differentiating psychotic from non-psychotic patients in TLE, the area under the receiver operating characteristic (ROC) curve of the brain-PAD was 0.753 (Fig. 2B).
SEIZURE AND MEDICATION BURDEN IN TLE The brain-PAD was not significantly different between seizure-free (N = 14) and not seizure-free (N = 159) patients with TLE (p = 0.438, ANCOVA with age
and sex as covariates), nor between patients with (N = 38) and without (N = 135) history of FBTCS (p = 0.287). On the other hand, there was a significant correlation between the brain-PAD
and the number of ASMs (rs = 0.28, p < 0.001, Spearman’s rank correlation, Fig. 3). DISCUSSION In the current study, we performed DTI-based brain age analysis to reveal WM aging in
patients with various types of epilepsy. As a result, we observed that most types of epilepsy presented increased WM microstructure brain-age except for IGE (Table 1). Our findings on
increased WM brain-age in TLE (i.e., + 4.2–9.1 years) were consistent with a previous study with a smaller sample size reporting + 2.2–10.9 years increase15. Additionally, in our previous
study based on structural MRI11, we found increased brain-age by + 4.7 years in TLE-NL, + 8.8 years in TLE-HS, + 5.6 years in Ext-FE, + 8.9 years in IGE, + 21.2 years in PME/EE, and + 10.6
years in PNES. Overall, these estimated brain-ages were in line with the current findings based on WM microstructures, while there seems to be a distinct difference between morphological and
microstructural brain-age scores in the IGE group. It is well known that IGE tends to preferably respond to pharmacotherapy, and 80% of the IGE group achieved seizure freedom in our study.
Possibly, WM brain-age might be more affected by refractory seizures, compared with morphological brain-age in IGE. On the other hand, in the TLE group, we observed no significant effect of
seizure burden, i.e., seizure freedom or FBTCS, on the brain-PAD. The relationships between seizure burden and WM brain aging might be complicated across different epilepsy types and require
further elucidation. Association between neurological and psychiatric conditions and abnormal brain aging processes has been increasingly reported, suggesting strong relationships not only
in dementia but also in epilepsy and other brain disorders8. In fact, various biological parameters, including telomere length or the epigenetic clock, have been applied to estimate an
individual’s aging process that may not be uniform even within an individual22. Given the complexity of the aging process, precise and accurate evaluation of brain aging is necessary for
reliable neuropsychiatric biomarkers. In this regard, it is considered important to estimate the brain-age and confirm findings using different modalities, including the DTI-based method.
Particularly, recent evidence has strongly suggested that epilepsy is a brain network disorder14, and thus the white matter tracts can provide significant information on the brain with
epilepsy, as WM primarily interconnects various brain regions. In addition, we observed that interictal psychosis further increased brain-age by 8.7 years in patients with TLE (Table 2 and
Fig. 2), which was independent of HS or ASMs. This difference might be slightly greater than that of morphological brain-age (+ 10.9 years in TLE with psychosis, + 5.3 years in TLE without
psychosis). Also, according to a recent meta-analysis by the ENIGMA consortium23, the average brain-PAD based on morphological features was + 3.55 years in patients with schizophrenia.
Compared with these findings, the effect of psychosis on WM brain-age in TLE appears greater. Possibly, WM brain-age may be a more sensitive marker for psychosis, and in fact, DTI-based
studies reported + 5–8 years increase of brain-age in schizophrenia24,25,26. Alternatively, compared with schizophrenia, psychosis of epilepsy typically occurs more than 10 years after the
onset of epilepsy27,28, and thus, more damage may have accumulated or there may be different mechanisms. The pathophysiology of comorbid psychosis in epilepsy is still far from clear, and
regional brain morphological abnormalities have been reported less consistently10. More recently, network abnormalities29, atrophy of the hippocampal tail30, and increased glucose metabolism
in the upper cerebellum31 have been reported, but further evidence is needed. Our findings on WM brain-age may contribute to this issue. There was also a significant negative correlation
between brain-PAD and onset age of epilepsy in the TLE-NL group (rs = − 0.24, FWE p = 0.036), which means that later onset TLE may present less abnormality of brain aging. This finding
agrees with our previous study on morphological brain-age (rs = − 0.44)11. Elderly-onset epilepsy has been attracting attention recently, showing different characteristics from younger-onset
cases in terms of several clinical features, e.g., subtle seizure symptoms, less EEG abnormality, or favorable drug response32. WM brain-age may reflect the different neural mechanisms of
TLE-NL with various onset ages. We also observed a significant effect of ASMs on WM brain aging, while the relationship between interictal psychosis and the brain-PAD was significant even
after correction for the number of ASMs. The impact of ASMs on brain structure has been reported, which may be associated with synapse, myelination and cortical thinning33,34. Although the
causal relationship is still unclear in this study, our finding would suggest the need of further thorough investigations on the effects of ASMs on brain structure and function. The study
has several limitations. The age and sex distribution, sample size, and diagnostic criteria for each group differed, although some of these differences were corrected by statistics. Since
the preferred age of onset differs for each epilepsy syndrome, it was inevitable that there would be differences in the age of each category, and it was difficult to set up a control group
that matched all groups. Therefore, all healthy data were set as the control group. While the largest group, i.e., TLE, comprised 173 patients in total, some categories included only 5–10
patients. Thus, our results with small sample sizes must be carefully interpreted with caution. The lack of psychiatric and neuropsychological information other than psychosis, e.g., mood
disorders or intellectual disability, is another limitation. Particularly, brain functional changes could occur prior to structural ones, and this limitation may make it difficult to
interpret the results from MRI. Furthermore, a cross-sectional design may not answer questions about causality and early predictability. Further longitudinal investigation with more detailed
clinical evaluation is desirable to address these limitations. CONCLUSION We observed increased WM brain aging in most types of epilepsy except for IGE, which was generally consistent with
findings of brain morphological aging in previous studies. In TLE, interictal psychosis significantly raised the WM brain-age by 8.7 years. These findings may suggest abnormal aging
mechanisms in epilepsy and comorbid psychotic symptoms. DATA AVAILABILITY Data not included in the article will be made available from the corresponding author to qualified researchers on
reasonable request subject to ethics approval. REFERENCES * Scheffer, I. E. _et al._ ILAE classification of the epilepsies: Position paper of the ILAE commission for classification and
terminology. _Epilepsia_ 58, 512–521. https://doi.org/10.1111/epi.13709 (2017). Article PubMed PubMed Central Google Scholar * Collaborators, G. B. D. E. Global, regional, and national
burden of epilepsy, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. _Lancet Neurol._ 18, 357–375. https://doi.org/10.1016/S1474-4422(18)30454-X (2019). Article
Google Scholar * Berg, A. T., Altalib, H. H. & Devinsky, O. Psychiatric and behavioral comorbidities in epilepsy: A critical reappraisal. _Epilepsia_ 58, 1123–1130.
https://doi.org/10.1111/epi.13766 (2017). Article PubMed PubMed Central Google Scholar * Gilliam, F., Hecimovic, H. & Sheline, Y. Psychiatric comorbidity, health, and function in
epilepsy. _Epilepsy Behav._ 4(Suppl 4), S26-30 (2003). Article PubMed Google Scholar * Sone, D. Making the invisible visible: Advanced neuroimaging techniques in focal epilepsy. _Front.
Neurosci._ 15, 699176. https://doi.org/10.3389/fnins.2021.699176 (2021). Article PubMed PubMed Central Google Scholar * van Vliet, E. A. _et al._ WONOEP appraisal: Imaging biomarkers in
epilepsy. _Epilepsia_ 58, 315–330. https://doi.org/10.1111/epi.13621 (2017). Article PubMed Google Scholar * Sone, D. & Beheshti, I. Clinical application of machine learning models
for brain imaging in epilepsy: A review. _Front. Neurosci._ 15, 684825. https://doi.org/10.3389/fnins.2021.684825 (2021). Article PubMed PubMed Central Google Scholar * Sone, D. &
Beheshti, I. Neuroimaging-based brain age estimation: A promising personalized biomarker in neuropsychiatry. _J. Pers. Med._ 12, 1850. https://doi.org/10.3390/jpm12111850 (2022). Article
PubMed PubMed Central Google Scholar * Hou, Y. _et al._ Ageing as a risk factor for neurodegenerative disease. _Nat. Rev. Neurol_ 15, 565–581. https://doi.org/10.1038/s41582-019-0244-7
(2019). Article PubMed Google Scholar * Allebone, J., Kanaan, R. & Wilson, S. J. Systematic review of structural and functional brain alterations in psychosis of epilepsy. _J. Neurol.
Neurosurg. Psychiatry_ 89, 611–617. https://doi.org/10.1136/jnnp-2017-317102 (2018). Article PubMed Google Scholar * Sone, D. _et al._ Neuroimaging-based brain-age prediction in diverse
forms of epilepsy: A signature of psychosis and beyond. _Mol. Psychiatry_ 26, 825–834. https://doi.org/10.1038/s41380-019-0446-9 (2021). Article PubMed Google Scholar * Clancy, M. J.,
Clarke, M. C., Connor, D. J., Cannon, M. & Cotter, D. R. The prevalence of psychosis in epilepsy; A systematic review and meta-analysis. _BMC Psychiatry_ 14, 75.
https://doi.org/10.1186/1471-244X-14-75 (2014). Article PubMed PubMed Central Google Scholar * Sone, D. Neurobiological mechanisms of psychosis in epilepsy: Findings from neuroimaging
studies. _Front. Psychiatry_ 13, 1079295. https://doi.org/10.3389/fpsyt.2022.1079295 (2022). Article PubMed PubMed Central Google Scholar * Royer, J. _et al._ Epilepsy and brain network
hubs. _Epilepsia_ https://doi.org/10.1111/epi.17171 (2022). Article PubMed Google Scholar * Chen, C. L. _et al._ Premature white matter aging in patients with right mesial temporal lobe
epilepsy: A machine learning approach based on diffusion MRI data. _NeuroImage. Clin._ 24, 102033. https://doi.org/10.1016/j.nicl.2019.102033 (2019). Article PubMed PubMed Central Google
Scholar * Association, A. P. _DSM-IV Diagnostic and Statistical Manual of Mental Disorders_ 4th edn. (American Psychiatric Press, 1994). Google Scholar * Cui, Z., Zhong, S., Xu, P., He, Y.
& Gong, G. PANDA: A pipeline toolbox for analyzing brain diffusion images. _Front. Hum. Neurosci._ 7, 42. https://doi.org/10.3389/fnhum.2013.00042 (2013). Article ADS PubMed PubMed
Central Google Scholar * Wakana, S. _et al._ Reproducibility of quantitative tractography methods applied to cerebral white matter. _NeuroImage_ 36, 630–644.
https://doi.org/10.1016/j.neuroimage.2007.02.049 (2007). Article PubMed Google Scholar * Mishra, S., Beheshti, I. & Khanna, P. A review of neuroimaging-driven brain age estimation for
identification of brain disorders and health conditions. _IEEE Rev. Biomed. Eng._ 16, 371–385. https://doi.org/10.1109/RBME.2021.3107372 (2023). Article PubMed Google Scholar * Beheshti,
I., Nugent, S., Potvin, O. & Duchesne, S. Bias-adjustment in neuroimaging-based brain age frameworks: A robust scheme. _NeuroImage Clin._ 24, 102063.
https://doi.org/10.1016/j.nicl.2019.102063 (2019). Article PubMed PubMed Central Google Scholar * Faul, F., Erdfelder, E., Lang, A. G. & Buchner, A. G*Power 3: A flexible statistical
power analysis program for the social, behavioral, and biomedical sciences. _Behav. Res. Methods_ 39, 175–191 (2007). Article PubMed Google Scholar * Cole, J. H., Marioni, R. E., Harris,
S. E. & Deary, I. J. Brain age and other bodily “ages”: Implications for neuropsychiatry. _Mol. Psychiatry_ 24, 266–281. https://doi.org/10.1038/s41380-018-0098-1 (2019). Article
PubMed Google Scholar * Constantinides, C. _et al._ Brain ageing in schizophrenia: Evidence from 26 international cohorts via the ENIGMA Schizophrenia consortium. _Mol. Psychiatry_ 28,
1201–1209. https://doi.org/10.1038/s41380-022-01897-w (2023). Article PubMed Google Scholar * Shahab, S. _et al._ Brain structure, cognition, and brain age in schizophrenia, bipolar
disorder, and healthy controls. _Neuropsychopharmacology_ 44, 898–906. https://doi.org/10.1038/s41386-018-0298-z (2019). Article PubMed Google Scholar * Wang, J. _et al._ White matter
brain aging in relationship to schizophrenia and its cognitive deficit. _Schizophr. Res._ 230, 9–16. https://doi.org/10.1016/j.schres.2021.02.003 (2021). Article PubMed PubMed Central
Google Scholar * Xi, Y. B. _et al._ Neuroimaging-based brain-age prediction of first-episode schizophrenia and the alteration of brain age after early medication. _Br. J. Psychiatry_
https://doi.org/10.1192/bjp.2021.169 (2021). Article PubMed Google Scholar * Nadkarni, S., Arnedo, V. & Devinsky, O. Psychosis in epilepsy patients. _Epilepsia_ 48(Suppl 9), 17–19.
https://doi.org/10.1111/j.1528-1167.2007.01394.x (2007). Article PubMed Google Scholar * Kanner, A. M. & Rivas-Grajales, A. M. Psychosis of epilepsy: A multifaceted neuropsychiatric
disorder. _CNS Spectr._ 21, 247–257. https://doi.org/10.1017/S1092852916000250 (2016). Article PubMed Google Scholar * Sone, D. _et al._ Disrupted white matter integrity and structural
brain networks in temporal lobe epilepsy with and without interictal psychosis. _Front. Neurol._ 11, 556569. https://doi.org/10.3389/fneur.2020.556569 (2020). Article PubMed PubMed Central
Google Scholar * Allebone, J. _et al._ Bilateral volume reduction in posterior hippocampus in psychosis of epilepsy. _J. Neurol. Neurosurg. Psychiatry_ 90, 688–694.
https://doi.org/10.1136/jnnp-2018-319396 (2019). Article PubMed Google Scholar * Sone, D., Sato, N., Shigemoto, Y., Kimura, Y. & Matsuda, H. Upper cerebellar glucose hypermetabolism
in patients with temporal lobe epilepsy and interictal psychosis. _Epilepsia Open_ https://doi.org/10.1002/epi4.12645 (2022). Article PubMed PubMed Central Google Scholar * Sen, A.,
Jette, N., Husain, M. & Sander, J. W. Epilepsy in older people. _Lancet_ 395, 735–748. https://doi.org/10.1016/S0140-6736(19)33064-8 (2020). Article PubMed Google Scholar *
Ikonomidou, C. & Turski, L. Antiepileptic drugs and brain development. _Epilepsy Res._ 88, 11–22. https://doi.org/10.1016/j.eplepsyres.2009.09.019 (2010). Article CAS PubMed Google
Scholar * Crespo Pimentel, B. _et al._ Sodium valproate is associated with cortical thinning of disease-specific areas in juvenile myoclonic epilepsy. _J. Neurol. Neurosurg. Psychiatry_
https://doi.org/10.1136/jnnp-2024-333703 (2021). Article Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by grants from the Japan Society for the Promotion of
Science (KAKENHI; No. JP21K15720), the Japan Epilepsy Research Foundation (JERF TENKAN 22007), and the Uehara Memorial Foundation (all to DS). AUTHOR INFORMATION Author notes * These authors
contributed equally: Daichi Sone and Iman Beheshti. AUTHORS AND AFFILIATIONS * Department of Radiology, National Center of Neurology and Psychiatry, 4-1-1 Ogawa-Higashi, Kodaira, Tokyo,
187-8551, Japan Daichi Sone, Yoko Shigemoto, Yukio Kimura, Noriko Sato & Hiroshi Matsuda * Department of Psychiatry, Jikei University School of Medicine, Tokyo, Japan Daichi Sone *
Department of Human Anatomy and Cell Science, Rady Faculty of Health Sciences, Max Rady College of Medicine, University of Manitoba, Winnipeg, MB, Canada Iman Beheshti * Drug Discovery and
Cyclotron Research Center, Southern Tohoku Research Institute for Neuroscience, Fukushima, Japan Hiroshi Matsuda Authors * Daichi Sone View author publications You can also search for this
author inPubMed Google Scholar * Iman Beheshti View author publications You can also search for this author inPubMed Google Scholar * Yoko Shigemoto View author publications You can also
search for this author inPubMed Google Scholar * Yukio Kimura View author publications You can also search for this author inPubMed Google Scholar * Noriko Sato View author publications You
can also search for this author inPubMed Google Scholar * Hiroshi Matsuda View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS DS organized the
whole study. DS, NS, and HM were involved in the study concept and design. DS, NS, YS and YK performed recruitment and data acquisition. DS and IB analyzed data and wrote the initial
manuscript. NS, YS, YK and HM contributed to critical supervision. All authors read and approved the submitted version. CORRESPONDING AUTHOR Correspondence to Daichi Sone. ETHICS
DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional claims
in published maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0
International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s)
and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material
derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a
credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted
use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/. Reprints and permissions
ABOUT THIS ARTICLE CITE THIS ARTICLE Sone, D., Beheshti, I., Shigemoto, Y. _et al._ White matter brain-age in diverse forms of epilepsy and interictal psychosis. _Sci Rep_ 14, 19156 (2024).
https://doi.org/10.1038/s41598-024-70313-w Download citation * Received: 02 October 2023 * Accepted: 14 August 2024 * Published: 19 August 2024 * DOI:
https://doi.org/10.1038/s41598-024-70313-w SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative