Play all audios:
ABSTRACT Allogeneic platelet-rich plasma (al-PRP) is gaining attention in clinical practice for treating chronic refractory wounds, though research results remain controversial. To assess
the clinical efficacy of al-PRP for chronic refractory wounds. Databases including PubMed, Cochrane Library, Embase, CNKI, SinoMed, VIP, and WFPD were searched for randomized controlled
trials comparing al-PRP with conventional treatments up to October 2023. Two researchers independently screened studies, extracted data, and assessed quality. Statistical analysis was
conducted using RevMan 5.4, and potential publication bias was assessed and corrected using funnel plots and Egger’s test. Twelve studies with 717 cases were included. Meta-analysis showed
al-PRP significantly improved outcomes compared to non-al-PRP treatments: increased healing rate (RR 2.72, 95% CI 1.77–4.19, _p_ < 0.00001), shortened healing time (SMD − 1.03, 95% CI
-1.31 to -0.75, _p_ < 0.00001), improved efficacy rate (RR 1.19, 95% CI 1.10–1.28, _p_ < 0.00001), increased wound shrinkage (MD 35.65%, 95% CI 21.65–49.64, _p_ < 0.00001), and
reduced hospital stays (MD -2.62, 95% CI -4.35 to -0.90, _p_ = 0.003). Al-PRP is a feasible, effective, and safe biological therapy for chronic refractory wounds. _Trial registration_:
PROSPERO Identifier CRD42022374920. SIMILAR CONTENT BEING VIEWED BY OTHERS IMPACT OF HYDROCOLLOID DRESSINGS IN THE PREVENTION OF PRESSURE ULCERS IN HIGH-RISK PATIENTS: A RANDOMIZED
CONTROLLED TRIAL (PENFUP) Article Open access 07 December 2023 CHRONIC WOUNDS TREATED WITH COLD ATMOSPHERIC PLASMAJET VERSUS BEST PRACTICE WOUND DRESSINGS: A MULTICENTER, RANDOMIZED,
NON-INFERIORITY TRIAL Article Open access 07 March 2022 A PROSPECTIVE SINGLE CENTER NON RANDOMIZED CLINICAL TRIAL OF AUTOLOGOUS SKIN CELLS WITH PLATELET RICH PLASMA FOR DIABETIC ULCER AND
TRAUMA INJURIES PATIENTS Article Open access 05 March 2025 INTRODUCTION Chronic refractory wounds, also known as recalcitrant or chronic wounds, lack a universally accepted definition
globally. However, the generally accepted consensus is that a wound can be classified as chronic refractory if it fails to heal after one month of treatment or shows a lack of healing
tendency. Such wounds are characterized by a healing rate of no more than 10-15% per week, or less than 50% contraction within a month1. Chronic refractory wounds encompass a wide range of
types, including but not limited to chronic non-healing wounds due to infection, pressure ulcers, diabetic-related wounds, post-traumatic wounds, arteriovenous ulcers, and skin ulcers caused
by radiation therapy. The incidence of these wounds is positively correlated with age and involves diverse and complex pathological mechanisms with a prolonged disease course2. In China,
the proportion of surgical patients in hospitals with chronic refractory wounds ranges from 1.5 to 3.0%, with the most common types being post-traumatic wounds, pressure ulcers, and
diabetic-related skin ulcers3. Chronic refractory wounds significantly extend hospital stays, increase treatment costs, and impose a substantial economic burden on families and society at
large. Research indicates that chronic refractory wounds from various causes can be effectively treated with subcutaneous injections of autologous platelet-rich plasma (PRP) and topical
application of PRP gel, achieving favorable outcomes. This demonstrates the potential safety and efficacy of autologous PRP in treating these wounds. Patients receiving this treatment have
shown a significant reduction in wound size, with no notable side effects reported. Additionally, PRP’s role in inhibiting the release of inflammatory factors also contributes to reduced
pain and inflammation4,5. However, the use of autologous PRP faces certain limitations. Many elderly patients with chronic wounds often suffer from malnutrition, hypoproteinemia, and
moderate to severe anemia, making it challenging to collect sufficient whole blood to extract PRP. Furthermore, the quality of PRP may be compromised in patients with severe underlying
conditions or poor health, affecting its biological functions. Allogeneic Platelet-Rich Plasma (al-PRP) has emerged as a focal issue in both basic and clinical research as an alternative to
autologous PRP. Several teams have conducted clinical studies using allogeneic PRP for chronic refractory wounds6. However, the results vary and there is a lack of large-scale clinical
trials and comprehensive data analysis, which limits its widespread adoption and promotion. Additionally, although some literature reports that allogeneic PRP can improve tissue recovery in
the short term, clinical outcomes have not shown statistically significant differences7. Therefore, a meta-analysis was conducted on clinical randomized controlled trials (RCTs) comparing
the treatment of chronic refractory wounds with and without the application of al-PRP. This analysis provided evidence-based medical insights drawn from clinical practice, evaluating the
efficacy and safety of al-PRP in treating chronic refractory wounds and exploring new therapeutic approaches for their repair. DATA AND METHODS LITERATURE RETRIEVAL STRATEGY The retrieval
strategy involves decomposing the systematic evaluation questions into keywords or subject terms recognizable by the computer system, following the PICO (participants, interventions,
comparisons, outcomes) principle. Logical operations are then used to form a retrieval query to search Chinese and English literature published from the inception of each database to October
2023. The English databases include the Cochrane Library, PubMed, and EMBASE, while the Chinese databases include CNKI, VIP, Wanfang Patent Database, and the China Biomedical Literature
Service System (SinoMed). For gray literature, searches are conducted through dissertation and thesis databases of various countries, abstracts of academic conferences, clinical trial
registration platforms, as well as manual retrieval and retrospective searches to minimize the risk of missing relevant studies. Retrieval strategy: #1(Plasma) OR (Platelet-Rich) OR
(platelet-rich Plasma) OR (allogeneic platelet-rich plasma) OR (allogeneic platelet-rich plasma) OR (allogeneic platelet) OR (allogeneic platelet-rich plasma gel) OR (allogeneic platelet
gel); #2((Chronic refractory wounds) OR (chronic nonhealing wounds) OR (Chronic refractory wound) OR (chronic nonhealing wound) OR (chronic wound) OR (chronic wounds) OR (Diabetic Foot) OR
(diabetic ulcer) OR (venous ulcer) OR (pressure ulcer); #3(#1AND#2). INCLUSION AND EXCLUSION CRITERIA Inclusion criteria: * i. Subjects: Chronic refractory wounds include infection-induced
wounds, pressure injuries (pressure ulcers), diabetes associated wounds, arterial ulcers, venous ulcers, and radioactive skin ulcers, regardless of age, sex, or race. * ii. Interventions:
Interventions: The experimental group received al-PRP treatment, while the control group received standard routine treatment without al-PRP (including debridement, drainage, decompression,
dressing coverage, etc.); * iii. Research type: RCT; * iv. Outcome indicators: The total efficacy rate, healing rate, wound healing time, wound shrinkage rate after treatment, and length of
hospital stay. Exclusion criteria: * i. Studies on the same research or repeatedly published; * ii. Studies with incomplete data, unable to extract or convert data; * iii. Studies on
combined use of two or more therapeutic measures; * iv. Animal test, review, case report, case analysis and conference abstract and studies unrelated to this study. LITERATURE SCREENING AND
DATA EXTRACTION To ensure data integrity and reliability, improve the efficiency of data extraction, and minimize the impact of subjective factors, two evaluators from different professional
backgrounds independently conduct the inclusion and exclusion process, literature quality evaluation, and design of the data extraction table. A blind extraction method is used. The data
extraction table includes the following information: researcher, year of publication, journal of publication, year of obtaining the research data, total sample size, sample size of the
experimental group and the control group, average age of patients, location of chronic refractory wounds, type of study design, and outcome indicators. BIAS EVALUATION INCLUDED IN THE STUDY
In this study, the Cochrane risk of bias assessment tool was used to evaluate the quality of the included studies. Each study was assessed for bias risk and categorized as “high risk of
bias,” “uncertain risk of bias,” or “low risk of bias.” The assessment focused on six domains and seven items to determine the overall risk of bias for each included study. STATISTICAL
ANALYSIS NoteExpress was used for study screening, and RevMan 5.4 was employed for the meta-analysis. The heterogeneity of the included studies was measured by the I2 statistic. If the
heterogeneity test result was _P_ > 0.10, it indicated that the studies were homogeneous, and a fixed effects model was used. If the heterogeneity test result was _P_ ≤ 0.10, the causes
of heterogeneity were analyzed, and a subgroup analysis was conducted to calculate the combined statistics. If heterogeneity persisted despite these adjustments, a random effects model was
used. When describing the combined results of multiple similar studies, the RR and its 95% CI were used as the combined statistics if the analysis index was a binary variable. For continuous
variables, the MD or the SMD and their 95% CI were used. The combined statistics were assessed using a Z test. If _P_ ≤ 0.05, the combined results were statistically significant. RESULTS
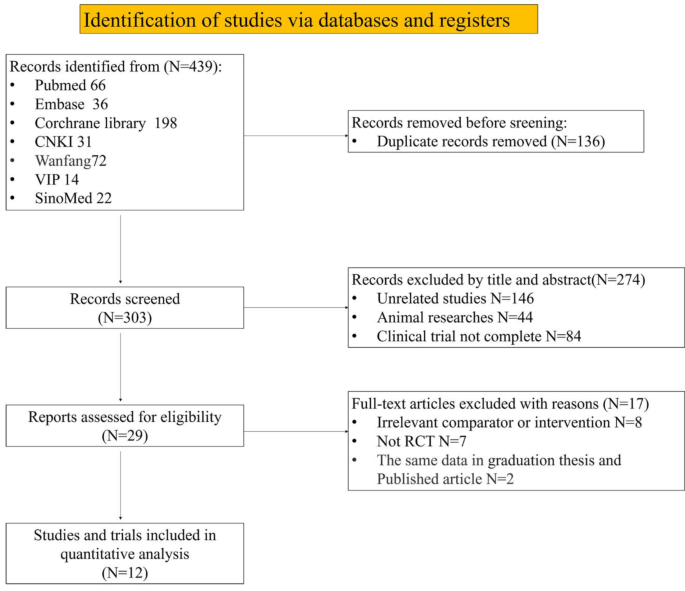
ANALYSIS OF SEARCH RESULTS In accordance with the retrieval strategy, a total of 439 studies were retrieved. The studies were imported into NoteExpress, where duplicate studies were deleted.
Studies that did not meet the inclusion criteria were then removed after reviewing the titles and abstracts. Ultimately, 12 studies were included for quantitative analysis (Fig.
1)8,9,10,11,12,13,14,15,16,17,18,19. BASIC CHARACTERISTICS AND QUALITY EVALUATION OF INCLUDED STUDIES The 12 studies included in this analysis focused on subjects with chronic refractory
wounds, with a total of 717 participants (358 in the experimental group and 359 in the control group). Table 1 details the basic characteristics of these studies, arranged in order of their
publication date. Figures 2 and 3 present the results of the Cochrane risk of bias assessment for the included studies. META-ANALYSIS HEALING RATE OF CHRONIC REFRACTORY WOUNDS A total of
seven studies8,9,10,11,12,17,18 were included in the meta-analysis of the healing rate of chronic refractory wounds, encompassing 360 patients. The heterogeneity test results indicated
heterogeneity among the study groups (_P_ = 0.02, I2=60%); therefore, a random effects model was employed for the analysis. The results (Fig. 4) demonstrated that the healing rate in the
al-PRP experimental group was significantly higher than that in the control group. The RR value was 2.72(95% CI 1.77–4.19), indicating a statistically difference between the two groups (_p_
< 0.00001). TIME REQUIRED FOR WOUND HEALING Nine studies8,11,12,13,15,16,17,18,19, involving a total of 588 patients, were included in the meta-analysis of healing time for chronic
refractory wounds. The heterogeneity test indicated significant variability among the studies (_P_ = 0.01, I2=60%), prompting the use of a random effects model. Due to differing units of
healing time across the studies, the SMD was employed for comparison. The analysis (Fig. 5) revealed that the SMD for the healing time in the al-PRP experimental group compared to the
control group was − 1.03, with a 95% CI of -1.31 to -0.75. This finding indicates that the experimental group experienced significantly shorter healing times than the control group, with a
statistically significant difference (_p_ < 0.00001). EFFICACY RATE OF TREATMENT Seven studies9,10,11,12,15,17,18, comprising a total of 346 patients, were included in the meta-analysis
of the efficacy rate for treating chronic refractory wounds. The heterogeneity test indicated homogeneity among the study groups (_P_ = 0.26, I2=22%), allowing for the use of a fixed effects
model. The analysis results (Fig. 6) showed that the efficacy rate of al-PRP treatment in the experimental group was higher than in the control group. The RR value was 1.19(95% CI
1.10–1.28), indicating a statistically significant difference in the healing rates between the two groups (_p_ < 0.00001). WOUND SHRINKAGE RATE Six studies8,9,10,11,12,14,18, involving a
total of 329 patients, were included in the meta-analysis of wound shrinkage rates for chronic refractory wounds. The heterogeneity test revealed significant variability among the studies
(_P_ < 0.00001, I2=95%), leading to the use of a random effects model for analysis. The results (Fig. 7) indicated that the wound shrinkage rate in the al-PRP experimental group was
35.65% (95% CI 21.65%, 49.64%), which was significantly higher than that in the control group (_p_ < 0.00001). HOSPITAL STAY Two studies15,16, with a combined total of 164 patients, were
included in the meta-analysis of hospital stay duration for patients with chronic refractory wounds. The heterogeneity test indicated homogeneity between the study groups (_P_ = 0.40,
I2=0%), which justified the use of a fixed effects model for comparison. The analysis results (Fig. 8) revealed that the mean difference (MD) in hospital stay duration between the al-PRP
experimental group and the control group was − 2.62 days, with a 95% CI ranging from − 4.35 to -0.90 days. Patients in the experimental group had significantly shorter hospital stays
compared to the control group (_p_ = 0.003). SENSITIVITY ANALYSIS Sensitivity analysis can provide insight into the robustness of the combined effect estimate to a certain extent. In the
analysis of the outcome indicator “wound shrinkage rate,” a one-by-one removal method was employed. It was observed that the heterogeneity originated from the study by Jeong8. Upon its
removal, sensitivity analysis revealed a reduction in statistical heterogeneity between different studies (I2=89%, _P_ < 0.01). The remaining four studies were analyzed using a random
effects model, which still demonstrated a statistically significant difference in wound healing time between the control and experimental groups (_P_ < 0.01). This finding underscores the
robustness and reliability of the meta-analysis results. Similarly, sensitivity analysis was conducted for the other four effect indicators using the one-by-one removal method. The results
consistently indicated statistically significant combined effect estimates, with the direction and significance of the original research results remaining unchanged. This further confirms
the scientific validity and reliability of the meta-analysis results. FUNNEL PLOT ANALYSIS Funnel plot analysis is a method utilized to assess potential publication bias in research
findings. In this study, “wound healing time” was selected as the indicator, and out of the 12 included studies, 9 used this measure for observation. The inclusion results (see Fig. 9) were
randomly distributed throughout the funnel plot, appearing symmetrically across the middle and upper portions. We have conducted the Egger’s test using STATA 15 software, as shown in Fig.
10. The test results indicate a p-value of 0.44, suggesting that there is no significant publication bias among the included studies. DISCUSSION Skin wound healing is a sophisticated and
tightly regulated biological process involving intricate interactions among various cell types, mediators, and signaling pathways20. The spontaneous wound healing process encompasses four
stages, which may overlap or occur sequentially: (1) formation of a dynamic equilibrium of blood platelet embolism; (2) acute inflammatory stage; (3) cell migration and proliferation,
encompassing extracellular matrix (ECM) formation and angiogenesis; (4) remodeling21. These stages are orchestrated by a myriad of cells, cytokines, and growth factors22.Chronic wounds
present challenges in healing due to factors such as bacterial infection, retention of necrotic tissue, compromised blood circulation, inadequate growth factor availability, and disruption
or abnormal apoptosis of the extracellular matrix23. These factors contribute to a significant increase in inflammatory cells and inflammatory mediators during the acute inflammatory phase,
along with excessive secretion of matrix metalloproteinases (MMPs), which degrade the extracellular matrix24. Glycation of the ECM during cell migration, proliferation, and remodeling
results in instability, reducing the content of type I collagen fibers, proteoglycans, and other components of the loose matrix. This inhibits cell proliferation and migration, as well as
the formation of vascular granulation tissue, thereby disrupting the normal wound healing process and leading to prolonged and challenging wound healing25. PRP is a plasma concentrate
obtained from whole blood after centrifugation, comprising a variety of cell components, notably platelets in concentrations exceeding physiological levels26. Easily obtained through a
straightforward centrifugation process, PRP represents a safe, simple, and cost-effective product27. While the molecular mechanisms by which platelet-rich plasma (PRP) promotes wound healing
remain incompletely understood, several potential pathways have been proposed. First, PRP enhances angiogenesis, a critical process in the healing of chronic wounds. The formation of new
capillaries improves blood supply, delivering oxygen, nutrients, and proteins, while facilitating waste removal28. Platelet α-granules, which are rich in growth factors such as
platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), and vascular endothelial growth factor (VEGF), play a crucial role in endothelial cell proliferation, prevention of
apoptosis, increased vascular permeability, and angiogenesis29. Animal studies have shown that allogenic PRP significantly increases the number of regenerating blood vessels, thereby
accelerating wound healing30. Secondly, PRP modulates the inflammatory response in wounds. By reducing pro-inflammatory cytokines such as interleukin-17 A (IL-17 A) and interleukin-1β
(IL-1β), PRP alleviates inflammation, promotes vascular reconstruction, and supports rapid tissue repair31,32. It also regulates the AMP-activated protein kinase (AMPK) signaling pathway,
influencing macrophage behavior by suppressing M1 macrophage differentiation and promoting M2 macrophage polarization33. M2 macrophages clear wound debris, induce CD4 T cell differentiation
into regulatory T cells, and create a microenvironment conducive to tissue regeneration through the release of interleukin-10 (IL-10) and prostaglandin E234,35. Thirdly, PRP stimulates skin
cell proliferation, a key objective in restoring the skin’s barrier function. This involves fibroblast migration, extracellular matrix deposition, and keratinocyte proliferation36. PRP
enhances epidermal stem cell (ESC) stemness and proliferative capacity by upregulating cytokeratin 19 (CK19), thus accelerating epithelialization37. Both in vitro and in vivo studies
indicate that PRP promotes fibroblast proliferation, type I collagen production, and tissue remodeling38,39. Finally, PRP exhibits antibacterial activity, mediated by platelet-derived
components such as antibacterial proteins and innate immune defense peptides40. PRP has shown significant efficacy against drug-resistant bacteria, including methicillin-resistant
Staphylococcus aureus, vancomycin-resistant Enterococcus species, extended-spectrum β-lactamase-producing Klebsiella pneumoniae, and carbapenem-resistant Pseudomonas aeruginosa41,42,43,44.
Meta-analyses on the efficacy of autologous PRP in treating diabetic foot ulcers have demonstrated significant increases in healing rates, reduced healing times, and decreased amputation
rates45. The 2020 China expert consensus on concentrated platelet products in wound repair also highlighted that concentrated platelet products are safe for chronic wounds, effectively
promoting granulation tissue growth and re-epithelialization. These products surpass conventional wound treatments and can be used multiple times37. However, due to the lack of standardized
methods for autologous PRP preparation, variations in preparation techniques and operator expertise result in differing platelet concentrations, leading to variable treatment outcomes.
Additionally, the proportion of individuals over 65 years old is increasing year by year46. Many of these elderly individuals suffer from conditions such as diabetes, cardiovascular and
cerebrovascular diseases, kidney diseases, and mobility impairments. They are often nutritionally deficient, presenting with moderate to severe anemia and hypoproteinemia, which can
compromise platelet function and reduce platelet counts. Platelet counts in individuals over 70 years old are typically about 10% lower than in younger individuals48. Andia49also noted that
the efficacy of autologous PRP injection treatment decreases with age in the context of osteoarthritis. Furthermore, each PRP preparation involves extracting tens to hundreds of milliliters
of whole blood from the patient, potentially increasing their physical burden and exacerbating the underlying condition. Therefore, for patients unable to use autologous PRP due to these
limitations, allogeneic PRP may be a viable alternative. Compared to autologous PRP, allogeneic PRP has broader sourcing options and easier collection methods. It can be derived from the
whole blood of healthy individuals or blood banks, facilitating the extraction of an ample quantity of platelets with robust biological functionality through meticulous screening processes.
However, in vitro studies on allogenic platelet-rich plasma (PRP) have intriguingly suggested that the immune response elicited by allogenic PRP in a therapeutic context is generally minimal
and potentially negligible34. Zhang50 conducted a pioneering in vivo study to assess the immunogenicity of allogenic PRP. In this study, allogenic PRP was intramuscularly injected into
rabbits, resulting in only a slight, statistically insignificant increase in the number and proportion of CD4 + and CD8 + T cells in peripheral blood compared to pre-injection levels.
Furthermore, histological examination of the injection site revealed no significant changes, leading to the conclusion that allogenic PRP exhibits low immunogenicity. Several factors may
contribute to the low immunogenicity observed with allogenic PRP: (1) Limited Exposure to Host Antibodies: As a localized therapeutic agent intended to promote tissue healing, allogeneic PRP
exhibits minimal interaction with host antibodies, thereby reducing the likelihood of triggering an immune response. (2) Altered Antigenic Structure: Platelet activation may modify their
surface antigen structure and expression levels, potentially reducing their immunogenicity51. (3) Complete Degradation and Absorption: Allogeneic PRP is fully degraded and absorbed within a
few weeks, eliminating the potential for a chronic immune response52. (4) Indirect Clinical Validation: The clinical use of allogeneic platelet transfusions further corroborates the low
immunogenicity and high safety profile of allogeneic PRP. These findings suggest that while platelets possess immunogenic antigens, the risk of a significant immune response in the context
of allogeneic PRP therapy is minimal, making it a potentially safe option for clinical applications in tissue healing. Furthermore, to ensure the safe use of allogeneic PRP, performing ABO
and Rh blood typing, along with comprehensive pathogen screening—including HIV, hepatitis B, hepatitis C, and other bloodborne pathogens—is essential. This approach minimizes the risk of
cross-infection and allergic reactions. Furthermore, maintaining strict sterility throughout blood collection, PRP processing, and application is critical to preventing microbial
contamination. This study found that the use of allogeneic PRP in the treatment of chronic refractory wounds is superior to conventional treatment methods without al-PRP. It significantly
increases the healing rate, total efficacy rate, and wound shrinkage rate in patients with chronic refractory wounds, while also shortening the healing time. Therefore, it can effectively
reduce the health risks and economic burden associated with chronic refractory wounds. Regarding the length of hospital stay, only two studies were included in the analysis, which makes the
results less reliable. In all the included studies, neither the experimental group nor the control group reported significant local inflammation, allergies, or other adverse reactions.
Despite the promising results of small-scale studies and our meta-analysis, there remains a significant gap in large-scale clinical trials assessing the efficacy and safety of PRP,
particularly allogeneic PRP. The absence of such studies may be attributed to several factors, including the logistical complexity of organizing multi-center trials, ethical considerations
surrounding the use of allogeneic materials, and the substantial financial investment required to conduct large-scale research. However, these challenges underscore the importance of
pursuing large-scale, multi-center clinical trials in the future. Such studies are essential for providing robust evidence on the safety and effectiveness of allogeneic PRP, thereby
supporting its broader adoption in clinical practice. LIMITATIONS OF THIS STUDY There are still some limitations to this study. Firstly, the RCTs included in the meta-analysis were conducted
in different patient populations and clinical settings. Although the detected heterogeneity is not significant, the risk of potential heterogeneity cannot be entirely ruled out. Secondly,
since al-PRP treatment for chronic refractory wounds is a visible clinical procedure, blinding of doctors, nurses, and patients was not feasible. As a result, the included RCTs did not
implement blinding, which might introduce unavoidable bias. Thirdly, variations in the preparation of PRP allografts, as well as differences in the methods and frequency of platelet
concentration use, were not standardized across studies, contributing to heterogeneity. Additionally, due to the limited number of available studies, we included only 12 publications with a
total of 717 patients, which may have limited our ability to capture the full range of variability and nuances in clinical practice. Lastly, most of the studies included were conducted in
East Asia, which introduces geographical and healthcare system-related limitations. CONCLUSION According to the results of our meta-analysis, the use of allogeneic PRP in the treatment of
chronic refractory wounds is confirmed to be a feasible, effective, and safe biological therapy. However, heterogeneity exists among the analyzed trials. To enhance the reliability of
research findings and provide a robust basis for clinical implementation, it is essential to conduct large-sample, multicenter, and well-designed randomized controlled trials across
different regions and healthcare institutions. DATA AVAILABILITY All data supporting the findings of this study are available within the paper. The data that support the fundings of this
study are also available from the corresponding author upon reasonable request. REFERENCES * Yuan, X. et al. Guideline for the diagnosis and treatment of chronic refractory wounds in
orthopedic trauma patients (version 2023). _Chin. J. Trauma_ 39(06), 481–493 (2023). Google Scholar * Dissemond, J. et al. Efficacy of MMP-inhibiting wound dressings in the treatment of
chronic wounds: A systematic review. _J. Wound Care_ 29(2), 102–118 (2020). Article PubMed Google Scholar * Lu, S. L. Enhance the connotation of establishment of wound healing department.
_Zhonghua Shao Shang Za Zhi_ 28(1), 1–2 (2012). MathSciNet PubMed Google Scholar * Frykberg, R. G. et al. Chronic wounds treated with a physiologically relevant concentration of
platelet-rich plasma gel: A prospective case series. _Ostomy Wound Manag._ 56(6), 36–44 (2010). Google Scholar * Suthar, M., Gupta, S., Bukhari, S. & Ponemone, V. Treatment of chronic
non-healing ulcers using autologous platelet-rich plasma: A case series. _J. Biomed. Sci._ 24(1), 16 (2017). Article PubMed PubMed Central Google Scholar * Oliveira, M. G., Abbade, L. P.
F., Miot, H. A., Ferreira, R. R. & Deffune, E. Pilot study of homologous platelet gel in venous ulcers. _Bras. Dermatol._ 92(4), 499–504 (2017 Jul-Aug). * Scevola, S. et al. Allogenic
platelet gel in the treatment of pressure sores: A pilot study. _Int. Wound J._ 7(3), 184–190 (2010). Article PubMed PubMed Central Google Scholar * Jeong, S. H., Han, S. K. & Kim,
W. K. Treatment of diabetic foot ulcers using a blood bank platelet concentrate[J]. _Plast. Reconstr. Surg._ 125(3), 944–952 (2010). Article CAS PubMed Google Scholar * Shan, G. Q.
_Study on promoting wound healing by homologous platelet gel and its mechanism [D]_ (Southern Medical University, 2012). * Li, Y. H. _Experimental and Clinical Study of Platelet gel
Promoting Wound Healing [D]_ (Guangzhou University of Traditional Chinese Medicine, 2011). * He, S.-y. et al. The treatment of diabetic foot ulcer with allogeneic platelet gel. _Guangdong
Med._, 34(01), 129–131 (2013). Google Scholar * Huang, H. et al. Clinical efficacy of allogeneic platelet-rich gel in refractory skin ulcer. _J. Third Mil. Med. Univ._ 39(19), 1944–1948
(2017). Google Scholar * He, M. et al. Comparison of allogeneic platelet-rich plasma with autologous platelet-rich plasma for the treatment of diabetic lower extremity ulcers. _Cell
Transpl._ 963689720931428 (2020). * Liao, X. et al. Allogeneic platelet-rich plasma therapy as an effective and safe adjuvant method for chronic wounds. _J. Surg. Res._ 246, 284–291 (2020).
Article CAS PubMed Google Scholar * Liu, H. et al. Efficacy of allogeneic platelet-rich on healing and regeneration of diabetic foot wounds. _Chin. J. Blood Transfus._ 34(04), 358–361
(2021). CAS Google Scholar * Liu, H. et al. Efficacy analysis of allogeneic platelet-rich plasma gel in the treatment of chronic refractory wounds. _Chin. J. Aesth Plast. Surg._ 32(12),
741–744 (2021). Google Scholar * Chen, R. et al. Efficacy of allogeneic platelet-rich plasma in treatment of diabetic chronic wound. _Infect. Inflamm. Rep._ 22(01), 10–15 (2021). Google
Scholar * Mimi, G. & Haining, W. Efficacy of allogeneic platelet rich plasma for chronic refractory wounds. J. Clin. Transfus. Lab. Med. 25(3), 368. * Zhang, J., Jia, M., He, J. &
Chenguang, T. The effect of allogeneic platelet-rich plasma on the healing of diabetic foot wounds and its mechanisms. _Chin. J. Clin. Phys._ 51(06), 670–672 (2023). CAS Google Scholar *
Ren, H., Zhao, F., Zhang, Q., Huang, X. & Wang, Z. Autophagy and skin wound healing. _Burns Trauma_ 10, tkac003 (2022). Article PubMed PubMed Central Google Scholar * Gurtner, G. C.,
Werner, S., Barrandon, Y. & Longaker, M. T. Wound repair and regeneration. _Nature_ 453(7193), 314–321 (2008). Article ADS CAS PubMed Google Scholar * Villela, D. L. & Santos,
V. L. Evidence on the use of platelet-rich plasma for diabetic ulcer: A systematic review. _Growth Factors_ 28(2), 111–116 (2010). Article CAS PubMed Google Scholar * Riedel, K., Ryssel,
H., Koellensperger, E., Germann, G. & Kremer, T. Pathophysiologie Der Chronischen Wunde [Pathogenesis of chronic wounds]. _Chirurg_ 79(6), 526–534 (2008). German. Article CAS PubMed
Google Scholar * Zhao, R., Liang, H., Clarke, E., Jackson, C. & Xue, M. Inflammation in chronic wounds. _Int. J. Mol. Sci._ 17(12), 2085 (2016). Article PubMed PubMed Central Google
Scholar * Liao, H., Zakhaleva, J. & Chen, W. Cells and tissue interactions with glycated collagen and their relevance to delayed diabetic wound healing. _Biomaterials_ 30(9), 1689–1696
(2009). Article CAS PubMed PubMed Central Google Scholar * Chamata, E. S., Bartlett, E. L., Weir, D. & Rohrich, R. J. Platelet-rich plasma: Evolving role in plastic surgery. _Plast.
Reconstr. Surg._ 147(1), 219–230 (2021). Article PubMed Google Scholar * Everts, P., Onishi, K., Jayaram, P., Lana, J. F. & Mautner, K. Platelet-rich plasma: New Performance
understandings and therapeutic considerations in 2020. _Int. J. Mol. Sci._ 21(20), 7794 (2020). Article CAS PubMed PubMed Central Google Scholar * Bodnar, R. J. Chemokine regulation of
angiogenesis during Wound Healing. _Adv. Wound Care (New Rochelle)_ 4(11), 641–650 (2015). Article PubMed Google Scholar * Everts, P. A. et al. Profound properties of protein-rich,
platelet-rich plasma matrices as novel, multi-purpose biological platforms in tissue repair, regeneration, and wound healing. _Int. J. Mol. Sci._ 25(14), 7914 (2024). Article CAS PubMed
PubMed Central Google Scholar * Yuan, Z. et al. Comparison of leukocyte-rich and leukocyte-poor platelet-rich plasma on pressure ulcer in a rat model. _J. Burn Care Res._ 44(4), 860–868
(2023). Article PubMed PubMed Central Google Scholar * Pietrzak, W. S. & Eppley, B. L. Platelet-rich plasma: Biology and new technology. _J. Craniofac. Surg._ 16(6), 1043–1054
(2005). Article PubMed Google Scholar * Pengcheng & Xu. _Platelet-Rich Plasma Accelerates skin Wound Healing by Promoting Re-epithelialization_, Vol. 8 (Burns & Trauma, 2020). *
Shi, L. Y. et al. Platelet-rich plasma induces M2 macrophage polarization via regulating AMPK singling pathway. _Zhongguo Shi Yan Xue Ye Xue Za Zhi_ 31(5), 1486–1491 (2023). PubMed Google
Scholar * Papait, A., Cancedda, R., Mastrogiacomo, M. & Poggi, A. Allogeneic platelet-rich plasma affects monocyte differentiation to dendritic cells causing an anti-inflammatory
microenvironment, putatively fostering wound healing. _J. Tissue Eng. Regen Med._ 12(1), 30–43 (2018). Article CAS PubMed Google Scholar * Lei, X. et al. Co-administration of
platelet-rich plasma and small intestinal submucosa is more beneficial than their individual use in promoting acute skin wound healing. _Burns Trauma_ 9, tkab033 (2021). Article PubMed
PubMed Central Google Scholar * Werner, S., Krieg, T. & Smola, H. Keratinocyte-fibroblast interactions in wound healing. _J. Invest. Dermatol._ 127(5), 998–1008 (2007). Article CAS
PubMed Google Scholar * Xu, P. C., He, W. F. & Cheng, B. [Exploration of the mechanism of human platelet-rich plasma in regulating and controlling human epidermal stem cells for
promoting wound re-epithelialization at transcriptome level]. _Zhonghua Shao Shang Za Zhi_ 36(10), 915–922 (2020). Chinese. CAS PubMed Google Scholar * Spanò, R. et al. Platelet-rich
plasma-based bioactive membrane as a new advanced wound care tool. _J. Tissue Eng. Regen Med._ 12(1), e82–e96 (2018). Article PubMed Google Scholar * Liu Chen, Z. Allogeneic platelet-rich
plasma promotes wound collagen synthesis in diabetic rats[J]. _Chin. J. Tissue Eng. Res._ 18(39), 6329–6334 (2014). Google Scholar * Cl, K. et al. Antimicrobial effects of platelet-rich
plasma and platelet-rich fibrin: A scoping review. _Cureus_ 15(12), e51360 (2023). PubMed PubMed Central Google Scholar * Smith, O. J. et al. An evaluation of the bacteriostatic effect of
platelet-rich plasma. _Int. Wound J._ 18(4), 448–456 (2021). Article PubMed PubMed Central Google Scholar * Çetinkaya, R. A. et al. Platelet-rich plasma as an additional therapeutic
option for infected wounds with multi-drug resistant bacteria: In vitro antibacterial activity study. _Eur. J. Trauma Emerg. Surg._ 45(3), 555–565 (2019). Article PubMed Google Scholar *
Farghali, H. A. et al. Antimicrobial action of autologous platelet-rich plasma on MRSA-infected skin wounds in dogs. _Sci. Rep._ 9(1), 12722 (2019). Article ADS PubMed PubMed Central
Google Scholar * Leisi, S. & Farahpour, M. R. Effectiveness of topical administration of platelet-rich plasma on the healing of methicillin-resistant Staphylococcus aureus-infected
full-thickness wound model. _J. Plast. Reconstr. Aesthet. Surg._ 77, 416–429 (2023). Article PubMed Google Scholar * Carter, M. J., Fylling, C. P. & Parnell, L. K. Use of platelet
rich plasma gel on wound healing: A systematic review and meta-analysis. _Eplasty_ 11, e38 (2011). PubMed PubMed Central Google Scholar * Burn and Trauma Branch of Chinese Geriatrics
Society. National expert consensus on application of enriched platelet products in wound repair (2020 version). _Zhonghua Shao Shang Za Zhi_ 36(11), 993–1002 (2020). Google Scholar *
Graves, N., Phillips, C. J. & Harding, K. A narrative review of the epidemiology and economics of chronic wounds. _Br. J. Dermatol._ 187(2), 141–148 (2022). Article CAS PubMed Google
Scholar * Montenont, E., Rondina, M. T. & Campbell, R. A. Altered functions of platelets during aging. _Curr. Opin. Hematol._ 26(5), 336–342 (2019). Article CAS PubMed PubMed Central
Google Scholar * Andia, I. & Maffulli, N. Platelet-rich plasma for managing pain and inflammation in osteoarthritis. _Nat. Rev. Rheumatol._ 9, 721–730 (2013). Article CAS PubMed
Google Scholar * Zhang, Z. Y. et al. The potential use of allogeneic platelet-rich plasma for large bone defect treatment: Immunogenicity and defect healing efficacy. _Cell. Transpl._
22(1), 175–187 (2013). Article Google Scholar * Schaller, M. et al. Filtered platelet concentrates from pooled buffy coats show comparable storage lesions when stored for 9 d at 20–24
degrees C or when supplemented with thromboSol at 2–6 degrees C. _Eur. J. Haematol._ 64(6), 401–410 (2000). Article CAS PubMed Google Scholar * He, M. et al. The role of allogeneic
platelet-rich plasma in patients with diabetic foot ulcer: Current perspectives and future challenges. _Front. Bioeng. Biotechnol._ 10, 993436 (2022). Article PubMed PubMed Central Google
Scholar Download references FUNDING This study was supported from the Program of National Natural Science Foundation of China (82072169, 82272279). AUTHOR INFORMATION Author notes * Yalong
Li, Xingtong Wang and Yucong li contributed equally to this work. AUTHORS AND AFFILIATIONS * Senior Department of Burns and Plastic Surgery, The Fourth Medical Center of PLA General,
Beijing, China Yalong Li, Xingtong Wang, Yucong Li, Dawei Li, Shijie Li & Chuanan Shen Authors * Yalong Li View author publications You can also search for this author inPubMed Google
Scholar * Xingtong Wang View author publications You can also search for this author inPubMed Google Scholar * Yucong Li View author publications You can also search for this author inPubMed
Google Scholar * Dawei Li View author publications You can also search for this author inPubMed Google Scholar * Shijie Li View author publications You can also search for this author
inPubMed Google Scholar * Chuanan Shen View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS L.Y.L. conceived and designed the experiments.
L.Y.L. X.T.W. S.J.L and D.W.L performed the experiments and data analysis. L.Y.L. wrote the manuscript. Y.C.L. and C.A.S. revised the work critically for important intellectual content. All
authors have read and approved the final version of the manuscript. All authors reviewed the manuscript. CORRESPONDING AUTHOR Correspondence to Chuanan Shen. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing,
adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons
licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a
credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted
use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT
THIS ARTICLE CITE THIS ARTICLE Li, Y., Wang, X., Li, Y. _et al._ Efficacy and safety of allogeneic platelet-rich plasma in chronic wound treatment: a meta-analysis of randomized controlled
trials. _Sci Rep_ 14, 25209 (2024). https://doi.org/10.1038/s41598-024-75090-0 Download citation * Received: 19 May 2024 * Accepted: 01 October 2024 * Published: 24 October 2024 * DOI:
https://doi.org/10.1038/s41598-024-75090-0 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * Allogeneic * Chronic refractory wounds * Platelet-rich
plasma * Ulcers * Clinical effect * Meta-analysis