Play all audios:
ABSTRACT Early prediction of patient responses to neoadjuvant chemotherapy (NACT) is essential for the precision treatment of early breast cancer (EBC). Therefore, this study aims to
noninvasively and early predict pathological complete response (pCR). We used dynamic ultrasound (US) imaging changes acquired during NACT, along with clinicopathological features, to create
a nomogram and construct a machine learning model. This retrospective study included 304 EBC patients recruited from multiple centers. All enrollees had completed NACT regimens, and
underwent US examinations at baseline and at each NACT cycle. We subsequently determined that percentage reduction of tumor maximum diameter from baseline to third cycle of NACT serves to
independent predictor for pCR, enabling creation of a nomogram (\(\text{AUC}=0.75\)). Our predictive accuracy further improved (\(\text{AUC}=0.868\)) by combining dynamic US data and
clinicopathological features in a machine learning model. Such models may offer a means of accurately predicting NACT responses in this setting, helping to individualize patient therapy. Our
study may provide additional insights into the US-based response prediction by focusing on the dynamic changes of the tumor in the early and full NACT cycle. SIMILAR CONTENT BEING VIEWED BY
OTHERS MULTIPARAMETRIC ULTRASOUND EXAMINATION FOR RESPONSE ASSESSMENT IN BREAST CANCER PATIENTS UNDERGOING NEOADJUVANT THERAPY Article Open access 28 January 2021 PREDICTING BREAST CANCER
RESPONSE TO NEOADJUVANT TREATMENT USING MULTI-FEATURE MRI: RESULTS FROM THE I-SPY 2 TRIAL Article Open access 27 November 2020 PREDICTING RESPONSE TO NEOADJUVANT CHEMOTHERAPY WITH LIQUID
BIOPSIES AND MULTIPARAMETRIC MRI IN PATIENTS WITH BREAST CANCER Article Open access 20 January 2024 INTRODUCTION Neoadjuvant chemotherapy (NACT) is systemic treatment administered prior to
surgery. In patients with early breast cancer (EBC), NACT may mitigate surgical damage and broaden surgical possibilities, improving eligibility for such options as breast-conserving surgery
and exempted axillary node dissection1. Individual NACT regimens may also be adjusted in accord with observed patients’ drug sensitivities2. In recent years, indications for NACT have
increased substantially3,4,5,6, especially in terms of triple-negative breast cancer (TNBC) and HER2-enriched (HER2+) breast cancer7. Patient-level analysis has shown that achieving
pathological complete response (pCR) after NACT is associated with increased event-free survival (EFS) and overall survival (OS) compared with patients with non-pCR2,8,9,10. However, only
5–38% of patients with EBC actually achieve pCR, with the majority having residual disease, meaning failure of NACT2,8,9. It is particularly important that therapeutic responses are
accurately evaluated, because ineffective NACT will potentially increase resistant heterogeneous clones, inducing drug resistance and compounding toxic side effects. Treatment strategies
should thus be revised timely to reduce unnecessary drug burdens and avoid suboptimal surgical timing. Hence, early and accurate identification of tumor response to NACT is critical for
precision treatment decision-making and individualization of surgical procedures. Mammography, ultrasound (US), and magnetic resonance imaging (MRI) are often regularly undertaken in
patients receiving NACT for purpose of therapeutic response monitoring. MRI is currently deemed the most accurate approach to detect tumor response to NACT11,12,13,14, but procedural
drawbacks (i.e., high cost and discomfort during testing) limit its use in this setting. Mammography is less accurate than US in predicting residual tumor size after NACT15,16. Liquid
biopsy, such as circulating tumor cells (CTCs) and circulating tumor DNA (ctDNA) may readily reflect the efficacy of NACT in early-stage17,18. Unfortunately, liquid biopsy is clinically
problematic, owing to its invasiveness, low content, the insufficient sensitivity of next-generation sequencing technology (NGS), and a lack of adequate medical evidence. Various benefits of
US afforded in non-invasiveness, economy, operability, accessibility, and freedom from radiation makes it the best method for frequent, dynamic monitoring of NACT efficacy. Studies have
shown that US is a useful tool for early assessment of pCR after NACT, even though it is less reliable in predicting poor pathological outcomes19. Although some studies have utilized US and
MRI parameters acquired before NACT to predict pCR20, tumor characteristics are apt to change during NACT21. It is thus doubtful that a single pre- or post-NACT static state is an accurate
representation of patients’ response to NACT. While not entirely foolproof, dynamic changes in tumors during NACT may offer clues for predicting responses to NACT. In recent years,
artificial intelligence has been invoked for tumor epidemiologic analysis, auxiliary tumor imaging diagnostics, and prognostic predictive models. Existing research aimed at machine learning
methods has relied on mammography, MRI, and US imaging to predict the efficacy of NACT in patients with breast cancer22,23,24. Herein, we have constructed a nomogram and a support vector
machine (SVM) for early prediction of pCR, based on both clinicopathological characteristics of EBC patients and longitudinal dynamic changes in US images during NACT. Our intent was to help
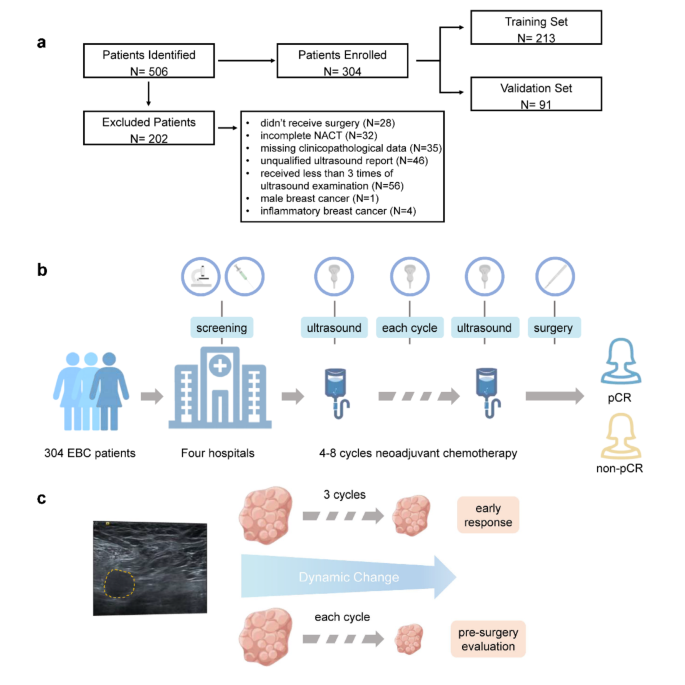
clinicians make critical treatment decisions and formulate appropriate surgical plans. METHODS PATIENT POPULATION This multicenter retrospective study enrolled 506 patients with EBC from
four medical centers between October 2013 and December 2021. All had been diagnosed with primary breast cancer and treated with NACT. We excluded 202 of these candidates on the following
grounds: no surgery performed (\(\text{n}=28\)), incomplete NACT (\(\text{n}=32\)), missing clinicopathological data (\(\text{n}=35\)), unqualified US report (\(\text{n}=46\)), less than
three US examinations (\(\text{n}=56\)), male breast cancer (\(\text{n}=1\)), and inflammatory breast cancer (\(\text{n}=4\)). Ultimately, 304 patients remained for randomization to training
and validation sets at a 7:3 ratio. The study design is shown in Fig. 1. Figure 1 was created by Figdraw (https://www.figdraw.com/static/index.html). The study was approved by the ethics
committee of Second Affiliated Hospital of Dalian Medical University. All methods were performed in accordance with the relevant guidelines and regulations. Informed consent has been
obtained from patients. CLINICAL AND PATHOLOGICAL EVALUATIONS We collected patient clinical characteristics at baseline (before NACT), including age and clinical tumor/nodal stage. Data on
hormone receptors (estrogen receptor, ER; progesterone receptor, PR) and other markers (HER2 and Ki67) were obtained from pre-treatment core needle biopsies. Hormone receptor positivity
(either ER+ or PR+) was defined as ≥ 1% of tumor cells staining positively in immunohistochemistry (IHC)25. Tumors with immunohistochemical HER2 3+ or HER2 2+ with HER2 gene amplification
confirmed by fluorescent in situ hybridization (FISH) were considered HER2+26. The Ki67 index was denoted by percentage of stained cells27. In accord with the above criteria, we classified
tumors as HR+/HER2+, HR−/HER2+, HR+/HER2−, or HR−/HER2−. NEOADJUVANT CHEMOTHERAPY AND PATHOLOGICAL RESPONSE Each patient was treated with 4–8 cycles of NACT, based on anthracycline (A)
and/or taxanes (T). Some patients received carboplatin (Cb) as well. The patients with HER2+ tumors were treated with trastuzumab or trastuzumab plus pertuzumab in conjunction with
chemotherapy. Biopsy specimens acquired before NACT and surgical specimens assessed after NACT were used to evaluate pathological response, applying the Miller-Payne system28. Pathological
complete response (pCR) was indicated by the absence of invasive tumor cells in both breast and axillary lymph nodes, and ductal carcinoma in situ could be present (ypT0/is ypN0)29,30,31.
ULTRASOUND EXAMINATIONS All US scans of breast were performed by three experienced radiologists independently. All radiologists were required to undergo a unified training in operating
technology according to the guidelines of the American College of Radiology (BI-RADS lexicon) and the expert consensus of the National Ultrasound Quality Control Centre. The largest radial
section of the lesion (longitudinal section) and the largest section vertical to it (transverse section) were required to be obtained. Two maximum diameters vertical to each other were
measured in the longitudinal section of the lesion, and then the transverse diameter was measured in the transverse section. The maximum tumor diameter was selected from the two sections.
Volumes were calculated from recorded three maximum diameters. Patients routinely underwent US examinations at baseline and at every cycle of NACT to determine maximum tumor diameters and
volumes over time. To measure early dynamic changes of tumors, we defined \(\Delta {T}_{x}\) as \(({T}_{0}-{T}_{x})/ {T}_{0}\), where \({T}_{0}\) and \({T}_{x}\) were maximum tumor diameters
at baseline and at NACT cycle _x_, respectively. NOMOGRAM DEVELOPMENT Logistic regression was done to identify associations of clinical, pathological, and US parameters with pCR. We
reserved variables of significance (_p_ < 0.05) in univariate analysis for multivariate analysis, determining independent predictive factors of pCR. The identified factors provided a
basis for nomogram construction. Predictive accuracy of \(\Delta {T}_{x}\) was assessed by the receiver operating characteristic (ROC) curve, deriving sensitivity and specificity from the
area under the curve (AUC). Values of AUC (range, 0–1) indicate perfect concordance at 1, no better than chance at 0.5, and discordance at 032. Internal validation of the nomogram was
performed by a calibration method and by the AUC in the training set. External validation was conferred by performing AUC in the validation set. The calibration curve, assessed by
Hosmer–Lemeshow goodness-of-fit test (_p_ > 0.05 indicating good fit), and the decision curve analysis (DCA) served to evaluate predictive model utility. The final nomogram was conducted
using R software (v4.2.1; The R Foundation for Statistical Computing, Vienna, Austria). MACHINE LEARNING MODEL The samples were randomly divided into a training set and a validation set in a
7:3 ratio. Various parameters, including ER, PR, HER2, and Ki67 status; NACT regimen; and maximum tumor diameters/volumes by cycle enabled the construction of our machine learning model.
Data were preprocessed in three steps, the first being observed dynamic tumor changes. For this, we excluded any samplings examined less than three times. At the second step, data were
standardized to eliminate differences between features. Considering the imbalance of data types, the pCR sampling was extended as part of the training set for step three, using the Synthetic
Minority Over-sampling Technique (SMOTE) method. We then constructed the pCR prediction model using the support vector machine (SVM). SVM is a binary classification model, transforming
classification issues into convex quadratic programming for problem solving. The radial basis function (RBF) was used as the kernel in SVM. To avoid overfitting, we conducted fivefold
cross-validation when selecting parameters for RBFs. This model was evaluated by precision, recall, and F1-score, all obtained from the confusion matrix. To assess the predictive performance
of the model, ROC curves and AUC were calculated. The process was conducted using Python (v3.8.12; Python Software Foundation). STATISTICAL ANALYSIS We compared patient characteristics of
the training and validation sets. Mann–Whitney _U_ test was applied to compare \(\Delta {T}_{x}\) between pCR and non-pCR patients at different NACT cycles. The Hosmer–Lemeshow test was used
to assess the fitness of the nomogram. All statistical tests were two-sided, driven by standard softwares (SPSS v25.0 [IBM Corp, Armonk, NY, USA] and R v4.2.1). RESULTS PATIENT
CHARACTERISTICS We randomly assigned 304 EBC patients to a training (\(\text{n}=213\)) or a validation (\(\text{n}=91\)) set. Among all enrollees, 85 (28.0%) achieved pCR. Clinical
characteristics of the patient population are summarized in Table 1. More than half (53.0%) of those achieving pCR received anthracycline and taxane agents as chemotherapy, and HER2+ tumors
were more often associated with pCR (HR+/HER2+, 28.2%; HR−/HER2+, 33.0%). The pCR rate in HR+/HER2− subtype was 28.2%. Patients with HR−/HER2− subtype were least likely (10.6%) to achieve
pCR. PREDICTORS OF PATHOLOGICAL COMPLETE RESPONSE (PCR) Logistic regression was performed to explore predictors of pCR, analyzing potential relations with patient age, \({T}_{0}\), \(\Delta
{T}_{x}\), ER, PR, HER2, Ki67, and NACT regimen (Table 2). In univariate analysis, smaller \({T}_{0}\) values were associated with higher probability of pCR (odds ratio
\([\text{OR}]=0.828\); 95% confidence interval [CI], 0.711–0.965; \(p=0.016\)). Likewise, HR (ER/PR) status, HER2 status, and NACT regimen (all _p_ < 0.001), as well as Ki67 expression
level (\(p=0.003\)), emerged as significant predictors of pCR. To determine the feasibility of predicting therapeutic response through early tumor dynamic changes, we analyzed percentages of
change in \({T}_{x}\) (as shown by US) relative to baseline values across initial four NACT cycles. In Fig. 2a, \(\Delta {T}_{x}\) values at respective NACT cycles are shown to compare
between pCR and non-pCR. Significant differences were apparent in cycles 2–4. Furthermore, \(\Delta {T}_{x}\) was confirmed as a significant predictor of pCR. Despite the importance of early
response prediction, the earliest values were not the best. Figure 2b demonstrates that \(\Delta {T}_{3}\) and \(\Delta {T}_{4}\) surpassed \(\Delta {T}_{1}\) and \(\Delta {T}_{2}\) in
terms of capacity to predict pCR, which was shown by AUC. Additionally, the ROC shows that \(\Delta {T}_{3}\) and \(\Delta {T}_{4}\) had similar AUC. Due to the aim of early response
evaluation, we subsequently selected change in maximum tumor diameter at third cycle as the preferred metric, denoted as \(\Delta {T}_{3}\) (\(\text{OR}=9.518, 95\%\; \text{CI}:
3.327{-}27.232; p<0.001\)). ROC analysis yielded a cut-off value of 29.9% for \(\Delta {T}_{3}\). Multivariate logistic regression analysis was next carried out in search of independent
predictors for pCR. The forest plot is shown as Fig. 3. Relative to ER+ patients, those with ER- status were more likely to achieve pCR (\(\text{OR}=0.417\), \(95\%\; \text{CI}:
0.192{-}0.907\); \(p=0.027\)). In addition, \({T}_{0}\) (\(\text{OR}=0.706\), \(95\%\; \text{CI}: 0.582{-}0.857\); \(p<0.001\)) and \(\Delta {T}_{3}\) (\(\text{OR}=10.795, 95\%\;
\text{CI}: 3.077{-}37.880; p<0.001\)) displayed significant associations with pCR, as did Ki67 expression (\(\text{OR}=4.843\), \(95\%\; \text{CI}: 1.202{-}19.519\); \(p=0.027\)). Once
adjusted for ER status, Ki67 expression, \({T}_{0}\), and \(\Delta {T}_{3}\), a significantly higher rate of pCR was also evident after carboplatin-based (vs anthracycline-based) treatment
(\(\text{OR}=7.928\), \(95\%\; \text{CI}: 1.567{-}40.112\); \(p=0.012\)). Although significance was not reached, patients receiving taxane-based (\(\text{OR}=3.083\)) or anthracycline plus
taxane-based (\(\text{OR}=1.376\)) regimen seemed to have a higher odds ratio than those administered anthracycline-based treatment (\(\text{OR}=1.0\)). NOMOGRAM CONSTRUCTION AND VALIDATION
Our nomogram incorporated significant predictors of pCR (\({T}_{0}\), ER status, Ki67 expression, NACT regimen) established by multivariate logistic regression analysis, with the addition of
\(\Delta {T}_{3}\) (Fig. 4). Each variable was assigned a point value (top scale), the sum of which reflected pCR probability (bottom scale). Nomogram validation by ROC analysis generated
AUC values of 0.75 in both training and validation sets (training set, 95% CI: 0.67–0.83; validation set, 95% CI: 0.64–0.86), suggesting good predictive efficiency (Fig. 5a,b). The
calibration curve for pCR probability also showed good agreement between predicted and observed results (Fig. 5c). The Hosmer–Lemeshow test indicated no significant deviation of obtained
results from an ideal fitting (\(p=0.502\)). We additionally conducted a DCA to assess predictive performances of four separate models (Fig. 6). Model 1 was developed using ER status, Ki67
expression, and NACT regimen; Model 2 and Model 3 were created using predictors in Model 1 plus \({T}_{0}\) or \(\Delta {T}_{3}\); and Model 4 encompassed all stated predictors. Ultimately,
the greatest net benefit in predicting NACT response was conveyed by Model 4. Relative to Model 2 and Model 3, there were significant gains in predictive efficiency, supporting \(\Delta
{T}_{3}\) as a driving factor for improved predictive performance. In Fig. 7, we have presented two patients presented with similar clinical characteristics but with differing tumor dynamic
trends during NACT. After three treatment cycles, Patient 2 registered a higher \(\Delta {T}_{3}\) than that of Patient 1 (84% vs 27%). The total points calculated by nomogram of Patient 1
and Patient 2 are respectively 150 and 167, and Patient 2 had a higher probability of pCR compared to Patient 1. Pathological results upon NACT completion were confirmatory of pCR in Patient
2 but not in Patient 1, underscoring the utility \(\Delta {T}_{3}\) as a relatively sensitive index of NACT response. MACHINE LEARNING MODEL During NACT, dynamic changes of US images should
not be underestimated. We performed several machine learning algorithms to construct the prediction models, including support vector machines (SVM), decision tree, random forest (RF),
gradient boosting machine (GBM), and fully connected neural network (Table 3). The results showed that the SVM exhibited the best predictive performance (\(\text{AUC}=0.868\)). Consequently,
our final endeavor was constructing a SVM model driven by clinicopathological data and US parameters at each treatment cycle, including maximum tumor diameters and volumes. The model’s
precision, recall and F1 scores were 77.0%, 71.0%, and 74.0%, respectively. The confusion matrix is provided as Fig. 8. DISCUSSION Cure is the ultimate goal of treatment in patients with
EBC. Breast cancer is a systemic disease requiring systemic intervention, including chemotherapy and targeted therapy33. However, the therapeutic effects will vary according to individual,
some responding to systemic therapy and others left unaffected. Multicenter prospective clinical studies, such as CREATE-X and KATHERINE, have confirmed that intensified follow-up adjuvant
therapy in proven non-responders may improve long-term survival34,35. Early response evaluations during treatment or even at baseline are therefore essential for optimal outcomes. NACT is an
individual drug sensitivity assessment platform. Patients insensitive to standard NACT regimens must be detected as soon as possible to avoid unnecessary toxicity exposures and extra costs.
There is an unmet clinical need for a biomarker to distinguish patient subsets by pCR and non-pCR. Non-pCR patients calls for treatment revision or expeditious surgical management. Imaging
methods and liquid biopsy have been previously utilized during a number of studies to evaluate patient response early. In the PHERGain trial, 18F-fluorodeoxyglucose (FDG)-positron emission
tomography (PET) was performed to metabolically detect HER2+ breast cancer patients, enabling dual targeted therapies and avoiding chemotherapy after just two cycles of treatment36. Dynamic
contrast-enhanced (DCE)-MRI performed after two cycles of NACT may also help predict pathological responses in the setting of breast cancer37. Liquid biopsies were undertaken in the
WSG-ADAPT-TP trial to determine patient responses in early-stage, defined as 30% decline in Ki67 expression or low cellularity38. Also, ctDNA and circulating free DNA (cfDNA) have known
merit as predictive and prognostic factors39,40,41. The current contention in China is that MRI and PET-computed tomography (CT), as well as secondary biopsies, may be costly and
inordinately traumatic for patients. US imaging is a noninvasive and cost-effective method for examining the breast. In the GeparTrio trial, US served to identify non-responders who
initially received taxanes/anthracycline/cyclophosphamide (TAC) regimen. Switching to TAC plus vinorelbine/capecitabine (TAC-NX) improved their prognosis, without cross-drug resistance or
NACT prolongation42. Similarly, US imaging has facilitated early NACT response prediction in the Neo-ALTTO trial43; and some small-scale studies have focused on its role in early response
evaluation44,45, without a uniform consensus on timing or criteria of such efforts. In the present study, we primarily addressed early tumor shrinkage in the nomogram, validating tumor
reduction after three cycles of NACT as a basis for early response determinations. This finding indicated that the concept of early prediction does not imply that the earlier the prediction,
the more accurate it will be. The changes of the tumor after three cycles of NACT may prove to be a more accurate predictor of the response than the earlier assessments. Interestingly, the
cut-off value of 29.9% is just comparable to the definition of partial response (PR) by RECIST standards (version 1.1)46. This outcome readily demonstrates that early US examinations during
NACT are beneficial, ensuring timely modifications of therapeutic regimens. Machine learning has been extensively applied to therapeutic response evaluations, revealing tumor characteristics
that escape the naked eye47,48,49. One such model has already been reported for handling dynamic longitudinal US imaging data to predict pCR early during NACT in patients with HER2+ breast
cancer50. Several other studies have concentrated on pre-, mid-, and post-NACT features of US-delineated NACT response, while failing to explore dynamic tumor changes51,52. Herein, we
assessed US parameters at each cycle of NACT to fully capture tumor dynamics in our prediction model. To the best of our knowledge, it is the first time to investigate the predictive
efficacy of dynamic changes in ultrasound-measured tumor maximum diameter throughout the entire NACT cycle. We also input static elements, including hormone receptor (ER/PR) status, HER2
status, Ki67 expression, and NACT regimen. By integrating both dynamic and static features, predictive performance was heightened (\(\text{AUC}=0.868\)) to levels reached elsewhere, capable
of reliably guiding therapeutic decisions. Furthermore, other researchers have reported that MRI has the potential to predict pCR to NACT, with AUC values ranging from 0.7 to 0.953,54. This
suggests that our US-based prediction models are comparable to other MRI-based prediction models. Although MRI is considered to have the highest sensitivity for breast cancer detection and
the high accuracy to predict the response to NACT13,55, it is a costly procedure, rendering it impractical for frequent scanning during NACT. In comparison, US is a more cost-effective and
widely available modality, and therefore more suitable to be the routine NACT monitoring tool. Clinicopathological parameters also figured prominently in response evaluation. We identified
other independent predictors of pCR, including ER status, Ki67 expression, \({T}_{0}\), and NACT regimen. The nomogram reached an AUC of 0.75. Patients with ER− (vs ER+) tumors displayed a
comparatively higher rate of pCR, aligning with past studies and perhaps translating to less NACT sensitivity of ER+ patients32. Ki67 is a biomarker of cellular proliferation with predictive
ramifications56. In our study, the higher the expression level of Ki67, the more likely pCR would be achieved, albeit with no consensus on a related cut-off value. \({T}_{0}\) emerged as an
independent predictor as well, and larger tumors had worse responses. Carboplatin is a DNA alkylating agent often administered for TNBC, which bears higher rates of BRCA1/2 mutation57,58.
Patients treated with carboplatin and paclitaxel, with or without anthracycline, were more inclined to pCR, as opposed to recipients of anthracycline-based regimens. However, 80% of those
treated with carboplatin were HER2+ and additionally received targeted therapy. We did add \(\Delta {T}_{3}\) to the nomogram, and \(\Delta {T}_{3}\) distinctly contributed to improved
predictive performance in the subsequent DCA. There are certain study limitations to acknowledge. The prediction models were constructed based on retrospective data, which may have biases.
To eliminate potential biases, patients were enrolled from multiple centers and objective variables were selected for multivariate analysis. This was done in order to minimize the effect of
these biases on the results. The incorporation of prospective data from more clinical trials would improve the clinical evidence supporting the validity of our model. Another drawback is the
small number of patients enrolled, knowing that various molecular subtypes commonly influence treatment and therapeutic effects. Moreover, because trastuzumab (Herceptin) has been widely
used in China since 2017, some patients with HER2+ tumors did not receive targeted therapy. Furthermore, intra- and inter-observer variability is also typically high for US imaging, which
may potentially impact the accuracy and reliability of US results. In light of the aforementioned considerations, all radiologists were required to follow standard guidelines and protocols
in order to minimize the potential US variability. In the future, more work is planned to refine this prediction model, especially an expanded patient sampling with broader clinical
implications. Prospective clinical trials are ultimately needed to optimize our prediction model. In conclusion, we have proposed a method for timely prediction of NACT responses. This
simple graphic representation is intuitive and may assist clinicians in rendering expeditious, individualized therapeutic decisions. We have also constructed an analogous machine learning
model that combines dynamic US changes and static clinicopathological characteristics. It may well serve as a reference for preoperative clinical decision-making. Given their overall
clinical potential, validation is warranted for both models through future clinical trials. DATA AVAILABILITY The medical data used in this study are not publicly available due to patient
privacy considerations. Interested users may request access to the data for research purposes, through contacting the corresponding author. Institutional approvals of data sharing will be
required along with signed data use agreements and/or material transfer agreements. REFERENCES * Korde, L. A. et al. Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for
breast cancer: ASCO guideline. _J. Clin. Oncol._ 39, 1485–1505. https://doi.org/10.1200/jco.20.03399 (2021). Article CAS PubMed PubMed Central MATH Google Scholar * Cortazar, P. et al.
Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. _Lancet_ 384, 164–172. https://doi.org/10.1016/s0140-6736(13)62422-8 (2014).
Article PubMed MATH Google Scholar * Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised
trials. _Lancet Oncol._ 19, 27–39. https://doi.org/10.1016/s1470-2045(17)30777-5 (2018). * Clough, K. B. et al. Rates of neoadjuvant chemotherapy and oncoplastic surgery for breast cancer
surgery: A French national survey. _Ann. Surg. Oncol._ 22, 3504–3511. https://doi.org/10.1245/s10434-015-4378-6 (2015). Article PubMed MATH Google Scholar * Mougalian, S. S. et al. Use
of neoadjuvant chemotherapy for patients with stage I to III breast cancer in the United States. _Cancer_ 121, 2544–2552. https://doi.org/10.1002/cncr.29348 (2015). Article PubMed MATH
Google Scholar * Khokher, S., Mahmood, S., Qureshi, M. U., Khan, S. A. & Chaudhry, N. A. “Initial clinical response” to neoadjuvant chemotherapy: an in-vivo chemosensitivity test for
efficacy in patients with advanced breast cancer. _Asian Pac. J. Cancer Prev._ 12, 939–946 (2011). PubMed Google Scholar * Miglietta, F., Dieci, M. V., Griguolo, G. & Guarneri, V.
Neoadjuvant approach as a platform for treatment personalization: focus on HER2-positive and triple-negative breast cancer. _Cancer Treat. Rev._ 98, 102222.
https://doi.org/10.1016/j.ctrv.2021.102222 (2021). Article CAS PubMed Google Scholar * Fisher, B. et al. Effect of preoperative chemotherapy on the outcome of women with operable breast
cancer. _J. Clin. Oncol._ 41, 1795–1808. https://doi.org/10.1200/jco.22.02571 (2023). Article CAS PubMed MATH Google Scholar * Esserman, L. J. et al. Pathologic complete response
predicts recurrence-free survival more effectively by cancer subset: results from the I-SPY 1 TRIAL–CALGB 150007/150012, ACRIN 6657. _J. Clin. Oncol._ 30, 3242–3249.
https://doi.org/10.1200/jco.2011.39.2779 (2012). Article PubMed PubMed Central MATH Google Scholar * Pierga, J. Y. _et al._ Prognostic factors for survival after neoadjuvant
chemotherapy in operable breast cancer. the role of clinical response. _Eur. J. Cancer_ 39, 1089–1096. https://doi.org/10.1016/s0959-8049(03)00069-8 (2003). * Balu-Maestro, C. et al. Imaging
in evaluation of response to neoadjuvant breast cancer treatment benefits of MRI. _Breast Cancer Res. Treat._ 72, 145–152. https://doi.org/10.1023/a:1014856713942 (2002). Article CAS
PubMed Google Scholar * Park, S. H. et al. Diffusion-weighted MR imaging: pretreatment prediction of response to neoadjuvant chemotherapy in patients with breast cancer. _Radiology_ 257,
56–63. https://doi.org/10.1148/radiol.10092021 (2010). Article PubMed MATH Google Scholar * Gu, Y. L., Pan, S. M., Ren, J., Yang, Z. X. & Jiang, G. Q. Role of magnetic resonance
imaging in detection of pathologic complete remission in breast cancer patients treated with neoadjuvant chemotherapy: a meta-analysis. _Clin. Breast Cancer_ 17, 245–255.
https://doi.org/10.1016/j.clbc.2016.12.010 (2017). Article CAS PubMed Google Scholar * Janssen, L. M. et al. MRI to assess response after neoadjuvant chemotherapy in breast cancer
subtypes: a systematic review and meta-analysis. _NPJ Breast Cancer_ 8, 107. https://doi.org/10.1038/s41523-022-00475-1 (2022). Article CAS PubMed PubMed Central MATH Google Scholar *
Dialani, V., Chadashvili, T. & Slanetz, P. J. Role of imaging in neoadjuvant therapy for breast cancer. _Ann. Surg. Oncol._ 22, 1416–1424. https://doi.org/10.1245/s10434-015-4403-9
(2015). Article PubMed Google Scholar * Keune, J. D. et al. Accuracy of ultrasonography and mammography in predicting pathologic response after neoadjuvant chemotherapy for breast cancer.
_Am. J. Surg._ 199, 477–484. https://doi.org/10.1016/j.amjsurg.2009.03.012 (2010). Article PubMed PubMed Central MATH Google Scholar * Magbanua, M. J. M. et al. Circulating tumor DNA
in neoadjuvant-treated breast cancer reflects response and survival. _Ann. Oncol._ 32, 229–239. https://doi.org/10.1016/j.annonc.2020.11.007 (2021). Article CAS PubMed Google Scholar *
Magbanua, M. J. M. et al. Circulating tumor DNA and magnetic resonance imaging to predict neoadjuvant chemotherapy response and recurrence risk. _NPJ Breast Cancer_ 7, 32.
https://doi.org/10.1038/s41523-021-00239-3 (2021). Article CAS PubMed PubMed Central Google Scholar * Wang, X. _et al._ Early prediction of pathological outcomes to neoadjuvant
chemotherapy in breast cancer patients using automated breast ultrasound. _Chin. J. Cancer Res._ 28, 478–485. https://doi.org/10.21147/j.issn.1000-9604.2016.05.02 (2016). * Chen, P. et al.
Multivariable models based on baseline imaging features and clinicopathological characteristics to predict breast pathologic response after neoadjuvant chemotherapy in patients with breast
cancer. _Breast Care (Basel, Switzerland)_ 17, 306–315. https://doi.org/10.1159/000521638 (2022). Article PubMed MATH Google Scholar * Park, Y. H. et al. Chemotherapy induces dynamic
immune responses in breast cancers that impact treatment outcome. _Nat. Commun._ 11, 6175. https://doi.org/10.1038/s41467-020-19933-0 (2020). Article ADS CAS PubMed PubMed Central MATH
Google Scholar * Skarping, I., Larsson, M. & Förnvik, D. Analysis of mammograms using artificial intelligence to predict response to neoadjuvant chemotherapy in breast cancer
patients: proof of concept. _Eur. Radiol._ 32, 3131–3141. https://doi.org/10.1007/s00330-021-08306-w (2022). Article CAS PubMed Google Scholar * Cain, E. H. et al. Multivariate machine
learning models for prediction of pathologic response to neoadjuvant therapy in breast cancer using MRI features: a study using an independent validation set. _Breast Cancer Res. Treat._
173, 455–463. https://doi.org/10.1007/s10549-018-4990-9 (2019). Article CAS PubMed MATH Google Scholar * Jiang, M. et al. Ultrasound-based deep learning radiomics in the assessment of
pathological complete response to neoadjuvant chemotherapy in locally advanced breast cancer. _Eur. J. Cancer_ 147, 95–105. https://doi.org/10.1016/j.ejca.2021.01.028 (2021). Article CAS
PubMed MATH Google Scholar * Hammond, M. E. et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of
estrogen and progesterone receptors in breast cancer. _J. Clin. Oncol._ 28, 2784–2795. https://doi.org/10.1200/jco.2009.25.6529 (2010). Article PubMed PubMed Central MATH Google Scholar
* Wolff, A. C. et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline
focused update. _J. Clin. Oncol._ 36, 2105–2122. https://doi.org/10.1200/jco.2018.77.8738 (2018). Article CAS PubMed MATH Google Scholar * Gerdes, J. _et al._ Cell cycle analysis of a
cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. _J. Immunol. (Baltimore, MD.: 1950)_ 133, 1710–1715 (1984). * Ogston, K. N. et al. A new
histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. _Breast_ 12, 320–327.
https://doi.org/10.1016/s0960-9776(03)00106-1 (2003). Article PubMed MATH Google Scholar * Fumagalli, D. et al. A common language in neoadjuvant breast cancer clinical trials: proposals
for standard definitions and endpoints. _Lancet Oncol._ 13, e240-248. https://doi.org/10.1016/s1470-2045(11)70378-3 (2012). Article PubMed MATH Google Scholar * Provenzano, E. _et al._
Standardization of pathologic evaluation and reporting of postneoadjuvant specimens in clinical trials of breast cancer: recommendations from an international working group. _Mod. Pathol._
28, 1185–1201. https://doi.org/10.1038/modpathol.2015.74 (2015). * Huang, M. et al. Association of pathologic complete response with long-term survival outcomes in triple-negative breast
cancer: a meta-analysis. _Cancer Res._ 80, 5427–5434. https://doi.org/10.1158/0008-5472.Can-20-1792 (2020). Article CAS PubMed MATH Google Scholar * Jin, X. et al. A nomogram for
predicting pathological complete response in patients with human epidermal growth factor receptor 2 negative breast cancer. _BMC Cancer_ 16, 606. https://doi.org/10.1186/s12885-016-2652-z
(2016). Article CAS PubMed PubMed Central MATH Google Scholar * Harbeck, N. Breast cancer is a systemic disease optimally treated by a multidisciplinary team. _Nat. Rev. Dis. Primers_
6, 30. https://doi.org/10.1038/s41572-020-0167-z (2020). Article PubMed Google Scholar * Masuda, N. et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. _N.
Engl. J. Med._ 376, 2147–2159. https://doi.org/10.1056/NEJMoa1612645 (2017). Article CAS PubMed MATH Google Scholar * von Minckwitz, G. et al. Trastuzumab emtansine for residual
invasive HER2-positive breast cancer. _N. Engl. J. Med._ 380, 617–628. https://doi.org/10.1056/NEJMoa1814017 (2019). Article Google Scholar * Pérez-García, J. M. et al. Chemotherapy
de-escalation using an (18)F-FDG-PET-based pathological response-adapted strategy in patients with HER2-positive early breast cancer (PHERGain): a multicentre, randomised, open-label,
non-comparative, phase 2 trial. _Lancet Oncol._ 22, 858–871. https://doi.org/10.1016/s1470-2045(21)00122-4 (2021). Article CAS PubMed Google Scholar * Tateishi, U. et al. Neoadjuvant
chemotherapy in breast cancer: prediction of pathologic response with PET/CT and dynamic contrast-enhanced MR imaging–prospective assessment. _Radiology_ 263, 53–63.
https://doi.org/10.1148/radiol.12111177 (2012). Article PubMed MATH Google Scholar * Harbeck, N. et al. De-escalation strategies in human epidermal growth factor receptor 2
(HER2)-positive early breast cancer (BC): final analysis of the West German Study Group adjuvant dynamic marker-adjusted personalized therapy trial optimizing risk assessment and therapy
response prediction in early BC HER2- and hormone receptor-positive phase II randomized trial-efficacy, safety, and predictive markers for 12 weeks of neoadjuvant trastuzumab emtansine with
or without endocrine therapy (ET) versus trastuzumab plus ET. _J. Clin. Oncol._ 35, 3046–3054. https://doi.org/10.1200/jco.2016.71.9815 (2017). Article CAS PubMed Google Scholar * Zhou,
Q. et al. Persistence of ctDNA in patients with breast cancer during neoadjuvant treatment is a significant predictor of poor tumor response. _Clin. Cancer Res._ 28, 697–707.
https://doi.org/10.1158/1078-0432.Ccr-21-3231 (2022). Article CAS PubMed MATH Google Scholar * Ma, G. et al. Identification of the plasma total cfDNA level before and after chemotherapy
as an indicator of the neoadjuvant chemotherapy response in locally advanced breast cancer. _Cancer Med._ 9, 2271–2282. https://doi.org/10.1002/cam4.2906 (2020). Article CAS PubMed
PubMed Central MATH Google Scholar * Magbanua, M. J. M. et al. Clinical significance and biology of circulating tumor DNA in high-risk early-stage HER2-negative breast cancer receiving
neoadjuvant chemotherapy. _Cancer Cell_ 41, 1091-1102.e1094. https://doi.org/10.1016/j.ccell.2023.04.008 (2023). Article CAS PubMed PubMed Central Google Scholar * von Minckwitz, G. et
al. Response-guided neoadjuvant chemotherapy for breast cancer. _J. Clin. Oncol._ 31, 3623–3630. https://doi.org/10.1200/jco.2012.45.0940 (2013). Article MATH Google Scholar * Di Cosimo,
S. et al. The use of breast imaging for predicting response to neoadjuvant lapatinib, trastuzumab and their combination in HER2-positive breast cancer: Results from Neo-ALTTO. _Eur. J.
Cancer_ 89, 42–48. https://doi.org/10.1016/j.ejca.2017.10.036 (2018). Article CAS PubMed MATH Google Scholar * Adrada, B. E. et al. Early ultrasound evaluation identifies excellent
responders to neoadjuvant systemic therapy among patients with triple-negative breast cancer. _Cancer_ 127, 2880–2887. https://doi.org/10.1002/cncr.33604 (2021). Article PubMed MATH
Google Scholar * Hong, J. et al. Early response and pathological complete remission in Breast Cancer with different molecular subtypes: a retrospective single center analysis. _J. Cancer_
11, 6916–6924. https://doi.org/10.7150/jca.46805 (2020). Article CAS PubMed PubMed Central MATH Google Scholar * Eisenhauer, E. A. _et al._ New response evaluation criteria in solid
tumours: revised RECIST guideline (version 1.1). _Eur. J. Cancer_ 45, 228–247. https://doi.org/10.1016/j.ejca.2008.10.026 (2009). * Bitencourt, A. G. V. et al. MRI-based machine learning
radiomics can predict HER2 expression level and pathologic response after neoadjuvant therapy in HER2 overexpressing breast cancer. _EBioMedicine_ 61, 103042.
https://doi.org/10.1016/j.ebiom.2020.103042 (2020). Article PubMed PubMed Central MATH Google Scholar * Tahmassebi, A. et al. Impact of machine learning with multiparametric magnetic
resonance imaging of the breast for early prediction of response to neoadjuvant chemotherapy and survival outcomes in breast cancer patients. _Investig. Radiol._ 54, 110–117.
https://doi.org/10.1097/rli.0000000000000518 (2019). Article Google Scholar * Gillies, R. J., Kinahan, P. E. & Hricak, H. Radiomics: Images are more than pictures, they are data.
_Radiology_ 278, 563–577. https://doi.org/10.1148/radiol.2015151169 (2016). Article PubMed Google Scholar * Liu, Y. et al. Early prediction of treatment response to neoadjuvant
chemotherapy based on longitudinal ultrasound images of HER2-positive breast cancer patients by Siamese multi-task network: A multicentre, retrospective cohort study. _EClinicalMedicine_ 52,
101562. https://doi.org/10.1016/j.eclinm.2022.101562 (2022). Article PubMed PubMed Central MATH Google Scholar * Eun, N. L., Son, E. J., Gweon, H. M., Kim, J. A. & Youk, J. H.
Prediction of axillary response by monitoring with ultrasound and MRI during and after neoadjuvant chemotherapy in breast cancer patients. _Eur. Radiol._ 30, 1460–1469.
https://doi.org/10.1007/s00330-019-06539-4 (2020). Article CAS PubMed Google Scholar * Zhu, Q. et al. Early assessment window for predicting breast cancer neoadjuvant therapy using
biomarkers, ultrasound, and diffuse optical tomography. _Breast Cancer Res. Treat._ 188, 615–630. https://doi.org/10.1007/s10549-021-06239-y (2021). Article CAS PubMed PubMed Central
MATH Google Scholar * Liu, Z. et al. Radiomics of multiparametric MRI for pretreatment prediction of pathologic complete response to neoadjuvant chemotherapy in breast cancer: a
multicenter study. _Clin. Cancer Res._ 25, 3538–3547. https://doi.org/10.1158/1078-0432.Ccr-18-3190 (2019). Article CAS PubMed MATH Google Scholar * Huang, Y. et al. Longitudinal
MRI-based fusion novel model predicts pathological complete response in breast cancer treated with neoadjuvant chemotherapy: a multicenter, retrospective study. _EClinicalMedicine_ 58,
101899. https://doi.org/10.1016/j.eclinm.2023.101899 (2023). Article PubMed PubMed Central MATH Google Scholar * Mann, R. M., Cho, N. & Moy, L. Breast MRI: state of the art.
_Radiology_ 292, 520–536. https://doi.org/10.1148/radiol.2019182947 (2019). Article PubMed MATH Google Scholar * Zhang, J. et al. A Novel model incorporating tumor stiffness, blood flow
characteristics, and Ki-67 expression to predict responses after neoadjuvant chemotherapy in breast cancer. _Front. Oncol._ 10, 603574. https://doi.org/10.3389/fonc.2020.603574 (2020).
Article PubMed PubMed Central Google Scholar * Hurley, J. et al. The use of neoadjuvant platinum-based chemotherapy in locally advanced breast cancer that is triple negative:
retrospective analysis of 144 patients. _Breast Cancer Res. Treat._ 138, 783–794. https://doi.org/10.1007/s10549-013-2497-y (2013). Article CAS PubMed Google Scholar * Farmer, H. et al.
Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. _Nature_ 434, 917–921. https://doi.org/10.1038/nature03445 (2005). Article ADS CAS PubMed MATH Google
Scholar Download references FUNDING This study was supported by the “1 + X” program cross-disciplinary innovation project (2022JCXKYB07), and Wu Jieping Medical Foundation
(320.6750.2022-19-81). AUTHOR INFORMATION Author notes * These authors contributed equally: Xinyi Wang and Yuting Zhang. AUTHORS AND AFFILIATIONS * Department of Breast Surgery, Second
Affiliated Hospital of Dalian Medical University, No. 467 Zhongshan Road, Shahekou District, Dalian, China Xinyi Wang, Yuting Zhang, Nan Wu, Shan Wang, Hong Chen, Tianyang Zhou, Ying Zhang
& Jia Wang * Faculty of Electronic Information and Electrical Engineering, Dalian University of Technology, Dalian, China Mengting Yang & Pan Qin * Department of Breast and Thyroid
Surgery, Affiliated Zhongshan Hospital of Dalian University, Dalian, China Xiaolan Wang & Dianlong Zhang * Department of Breast Surgery, The First Hospital of China Medical University,
Shenyang, China Zining Jin, Ang Zheng, Fan Yao & Feng Jin Authors * Xinyi Wang View author publications You can also search for this author inPubMed Google Scholar * Yuting Zhang View
author publications You can also search for this author inPubMed Google Scholar * Mengting Yang View author publications You can also search for this author inPubMed Google Scholar * Nan Wu
View author publications You can also search for this author inPubMed Google Scholar * Shan Wang View author publications You can also search for this author inPubMed Google Scholar * Hong
Chen View author publications You can also search for this author inPubMed Google Scholar * Tianyang Zhou View author publications You can also search for this author inPubMed Google Scholar
* Ying Zhang View author publications You can also search for this author inPubMed Google Scholar * Xiaolan Wang View author publications You can also search for this author inPubMed Google
Scholar * Zining Jin View author publications You can also search for this author inPubMed Google Scholar * Ang Zheng View author publications You can also search for this author inPubMed
Google Scholar * Fan Yao View author publications You can also search for this author inPubMed Google Scholar * Dianlong Zhang View author publications You can also search for this author
inPubMed Google Scholar * Feng Jin View author publications You can also search for this author inPubMed Google Scholar * Pan Qin View author publications You can also search for this author
inPubMed Google Scholar * Jia Wang View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS XW and YZ conceived this study, performed the data
analyses and wrote the manuscript. MY and PQ performed the machine learning part. NW, SW, HC, TZ, and YZ assisted revising the manuscript. ZJ, AZ, FY, XW, and FJ provided a portion of the
data. JW designed the study and was the director for the fund. All authors read and approved the final manuscript. CORRESPONDING AUTHOR Correspondence to Jia Wang. ETHICS DECLARATIONS
COMPETING INTERESTS The authors declare that they have no competing interests. ETHICS DECLARATIONS This is a retrospective study approved by the ethics committee of Second Affiliated
Hospital of Dalian Medical University. All methods were performed in accordance with the relevant guidelines and regulations. Informed consent has been obtained from patients. ADDITIONAL
INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This
article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction
in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the
licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article
are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and
your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this
licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Wang, X., Zhang, Y., Yang, M. _et al._ Dynamic
ultrasound-based modeling predictive of response to neoadjuvant chemotherapy in patients with early breast cancer. _Sci Rep_ 14, 31644 (2024). https://doi.org/10.1038/s41598-024-80409-y
Download citation * Received: 22 August 2024 * Accepted: 18 November 2024 * Published: 30 December 2024 * DOI: https://doi.org/10.1038/s41598-024-80409-y SHARE THIS ARTICLE Anyone you share
the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative KEYWORDS * Breast cancer * Neoadjuvant chemotherapy * Early response prediction * Ultrasound * Nomogram * Support vector machine