Play all audios:
ABSTRACT mRNA and adenoviral vector vaccine platforms were used for the primary series of COVID-19 vaccines in many countries. However, the distinct immunogenic properties on these platforms
remain less understood. We traced neutralizing antibodies, memory B cells, and T cells longitudinally in cohorts that received either mRNA (BNT162b2 or mRNA-1273) or adenoviral vector
(ChAdOx1) vaccines with homologous or heterologous regimens (total 9 groups, _n_ = 26–28 for each group) at 4 weeks interval. The priming and boosting effects on various immune parameters
were comparably assessed between mRNA and adenoviral vector platforms. We found that initial priming by adenoviral vector vaccine elicited robust T cell responses, but B cell responses,
including antibody titers, were relatively lower than those elicited by mRNA priming. The dissociation between T cell and antibody responses were exaggerated at greater extents after the
homologous booster with the adenoviral vector vaccine, resulting in 5-19-fold lower antibody titers despite comparable spike-specific T cell numbers at day 28 after the boost. Robust IFN-γ
and few IL-2 and IL-5 production characterized T cell functionality primed by adenoviral vector. Boosting with mRNA vaccines restored their IL-2 and IL-5 production at some extents, but the
IL-5 T cell responses elicited by adenoviral vector/mRNA heterologous regimen waned faster than those by mRNA homologous regimen. Thus, our data revealed that the cytokine production of
helper T cells was skewed by adenoviral vector priming, leading to the attenuated IL-2 and IL-5 responses which were prolonged even after mRNA boosting, suggesting an imprinting of T-cell
functionality depending on the vaccine platform used for initial priming. These results highlight the importance of selecting vaccine platforms based on the immunogenic properties. SIMILAR
CONTENT BEING VIEWED BY OTHERS OPTIMISING T CELL (RE)BOOSTING STRATEGIES FOR ADENOVIRAL AND MODIFIED VACCINIA ANKARA VACCINE REGIMENS IN HUMANS Article Open access 12 October 2020
BNT162B2-BOOSTED IMMUNE RESPONSES SIX MONTHS AFTER HETEROLOGOUS OR HOMOLOGOUS CHADOX1NCOV-19/BNT162B2 VACCINATION AGAINST COVID-19 Article Open access 18 August 2022 STRONG IMMUNOGENICITY OF
HETEROLOGOUS PRIME-BOOST IMMUNIZATIONS WITH THE EXPERIMENTAL VACCINE GRAD-COV2 AND BNT162B2 OR CHADOX1-NCOV19 Article Open access 04 November 2021 INTRODUCTION The swift development of
multiple COVID-19 vaccines within one year after viral genome isolation greatly contributed to the pandemic response and the end of Public Health Emergency of International Concern on May
2023. While two types of COVID-19 mRNA vaccines (BNT162b2 and mRNA-1273) have been administered for primary series of vaccination in several countries including Japan, the other platforms of
vaccines, such as adenoviral vector (ChAdOx1 nCoV-19), recombinant protein (NVX-CoV2373), and inactivated (CoronaVac) vaccine were also utilized for the prompt acquisition of herd immunity
in other regions. Numerous studies have been performed to profile immune responses elicited by COVID-19 vaccines and extended our understanding of vaccine-induced immunity as well as their
potential relevance to the vaccine effectiveness. Many immune profiling studies have been performed in mRNA vaccinees1,2,3,4,5,6,7,8,9,10, as the populations in many regions were vaccinated
with mRNA platform at highest percentage. However, it is important to explore the immunogenic properties of other vaccine platforms or prime-boost vaccination regimen by heterologous vaccine
platforms, as the multiple vaccine platforms and regimens are required for the preparedness of the next pandemic caused by unknown pathogen X for which we cannot predict the appropriate
platforms to elicit protective immunity. The main aim of the current COVID-19 vaccination is to durably elicit multiple layers of memory responses that are coordinated by neutralizing
antibodies, memory B, and T cells. Neutralizing antibodies are relatively easy to quantitate by standardized methods in high-throughput manner while the memory B and T cells in the second
layer requires immunological techniques with in vitro culture and flow cytometric analysis hampering standardization and high-throughputness. The neutralizing antibodies serve as the first
line of defense and are shown to correlate with the vaccine effectiveness (VE) within 3 months after the booster when high antibody titers are maintained, providing the basis for using the
neutralizing antibody titers as the immune correlate of protection11,12,13,14. However, the gap between neutralizing antibody titers and VE expands along with an antigenic mismatch between
the vaccine and infected strains occurs as we experienced by the emergence of Omicron variants. Moreover, the waning of neutralizing antibody titers with time enhances the gap, because the
contribution of memory B and T cells on the VE increases in such situation, especially against more severe diseases15. Indeed, the longer persistence of VE against severe diseases relative
to preventing infection is frequently observed16. Therefore, a detailed evaluation of the vaccine-specific immune responses with relevance of VE requires the profiling of not only
neutralizing antibodies but also memory B and T cells. In the early COVID-19 pandemic period, the ChAdOx1 nCoV-19 (AZD1222) vaccine accounted for over one third of all global COVID-19
vaccine doses administered in 2021. Although ChAdOx1 received regulatory approval as a two-dose regimen with 4 to 12 weeks interval longer than homologous mRNA vaccination, an association
with immune thrombocytopenia with the adenoviral vector vaccination hampered the booster vaccination by this platform17,18. As a result, the substantial numbers of individuals primed by a
ChAdOx1 vaccine were boosted with mRNA vaccine. The heterologous prime-boost regimen (ChAdOx1/mRNA vaccine) elicited higher neutralizing antibodies and comparable numbers of IFN-γ-producing
T cells, along with the greater VE19,20. However, the factors underlying the enhanced immunogenic properties by the ChAdOx1 priming remains unknown, even though it was used in more countries
than any other COVID-19 vaccine21. This point can be addressed using the cohorts which were given by the prime-boost regimens with the same time interval with homologous vaccination. Here,
we compared vaccine-elicited neutralizing antibody and memory B and T cell responses in various cohorts which received mRNA (BNT162b2 or mRNA-1273) or adenoviral vector (ChAdOx1) vaccines
with homologous or heterologous regimen with the same 4 weeks interval, elucidating the priming and boosting properties of ChAdOx1 vaccines on T cells which are previously unappreciated.
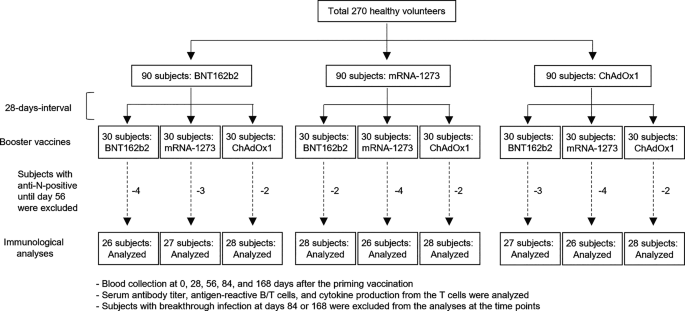
RESULTS STUDY DESIGN We enrolled a total of 270 healthy volunteers as subjects (Fig. 1). The subjects were separated into 9 groups (_n_ = 30) who then received any possible combination of
BNT162b2, mRNA-1273, and ChAdOx1 with a uniform 28-days-interval. We enrolled subjects with > 40 ages for ChAdOx1 doses, because the vaccine was approved for > 40 years adults in Japan
(Fig. S1A and B). Blood samples were collected for analyses on serum antibody titers and peripheral blood mononuclear cells (PBMCs) at 0, 28, 56, 84, and 168 days after the primary
vaccination. We first measured anti-nucleocapsid antibody titers to assess SARS-CoV-2 infection histories throughout the study period (Fig. S1C and D). We excluded 26 subjects (2 to 4
subjects per group) who seroconverted by day 56 from further longitudinal analyses. The subjects seroconverted at day 84 or 168 (1 to 6 subjects per group, 34 subjects in total) were
excluded from the analyses at all time points later than seroconversion. We analyzed anti-spike IgG titers, neutralization titers, memory B cells, and T cells in peripheral blood (Fig. S1A).
For memory B cell analyses, we focused on receptor-binding domain (RBD)-reactive B cells, since RBD is a main target of potently neutralizing antibodies22,23,24,25,26,27. It is important to
mention that the numbers of spike-reactive B cells and RBD-reactive B cells highly correlate each other in the vaccinees7. Memory B cells were detected as CD19+CD20+CD21+CD27+IgM−IgA−IgG+ B
cells that bind SARS-CoV-2 spike and RBD probes (Fig. S2A). Spike-specific T cells were analyzed as CD4+ or CD8+ in CD3+ T cells with activation-induced markers (AIM), CD69 and CD137,
expression after overnight stimulation of PBMCs with spike overlapping peptides (Fig. S2B). The study design with a uniform prime/boost interval and any possible combination provided us with
a rare and relatively straightforward approach to compare the priming/boosting effects between adenoviral vector and mRNA platforms. ROBUST T-CELL RESPONSES BUT ATTENUATED ANTIBODY AND
B-CELL RESPONSES FOLLOWING ADENOVIRAL VECTOR PRIMING First, we compared the immune responses induced by the primary vaccination at 28 days. Among three vaccines, mRNA-1273 was most potent
for eliciting anti-spike titers, neutralization titers, and RBD-reactive memory B cells (Fig. 2A-C). BNT162b2 and ChAdOx1 were comparable for these responses except for anti-spike titers,
where BNT162b2 induced slightly higher titers than ChAdOx1. Regarding T cell responses, mRNA-1273 and ChAdOx1 induced higher frequencies of CD4 and CD8 T cells than BNT162b2 (Fig. 2D),
indicating that the three vaccines have distinct immunogenic properties for priming antibody, T cell, and B cell responses. Overall, adenoviral vector ChAdOx1 induced robust T-cell
responses, although its antibody and B-cell responses were equal or lower than those by two types of mRNA vaccines. To visualize the T-cell biased responses, the ratios of the antibody
responses over the CD4+ T-cell responses were plotted (Fig. 2E). Indeed, the ratios were lower in ChAdOx1 compared to BNT162b2 and mRNA-1273, while the numbers were equivalent among the mRNA
vaccines. The similar analysis from CD8+ T cells revealed more profound reduction in ChAdOx1 compared to BNT162b2 and mRNA-1273 (Fig. 2F), supporting the adenoviral vector-directed biases
for priming T-cell responses over antibody/B-cell responses. ChAdOx1 vaccinees were composed of > 40 years adults only, whereas the mRNA vaccinees included < 40 years adults as well
owing to the distinct age eligibility. To assess the possible biases posed by differential age distribution between the groups, we stratified the mRNA vaccinees into > 40 and < 40 ages
(Fig. 3). Most immune parameters, except RBD B cells from BNT162b2, were comparable between two age groups in the mRNA vaccinees, showing the minor contribution of ages in these parameters.
Indeed, the comparison between adenoviral vector and mRNA vaccinees > 40 ages reproduced the similar trend without the age adjustment (Fig. 2); robust T-cell responses but equal or lower
antibody/B-cell responses were observed after adenoviral vector priming compared to mRNA priming. In sum, the T cell-biased responses over antibody/B-cell responses are adenoviral
vector-dependent event rather than age-dependent events. COMPARABLE T-CELL RESPONSES BUT ATTENUATED ANTIBODY RESPONSES FOLLOWING HOMOLOGOUS ADENOVIRAL VECTOR BOOSTER We next analyzed the
immune parameters induced by the homologous and heterologous booster vaccination with any possible combination. The booster vaccinations recalled the comparable numbers of T-cells by any
combination of adenoviral vector and mRNA vaccines from day 56 (day 28 after the boost) to day 168 (day 140 after the boost) (Fig. 4A). B cell numbers were also within the similar ranges
among the different platform combination (Fig. 4B). In contrast, both spike-binding and neutralizing antibody titers from the homologous ChAdOx1 booster regimen were 5–19 folds lower than
those from any other regimens that include mRNA platform for either priming or boosting at day 56 (Fig. 4C and D). These results indicate that the usage of mRNA vaccines for either priming
or boosting as the heterologous regimens compensated the poor immunogenicity of homologous ChAdOx1 regimen on eliciting antibodies. Antibody titers elicited by heterologous booster regimens
also persisted from day 56 to day 168 as well as those by homologous mRNA booster regimen (Fig. 4E and F). Besides the vaccine platform, the differential antibody titers following homologous
booster regimen were notified between the mRNA vaccines (BNT162b2 vs. mRNA-1273), with higher antibody responses observed at days 56 and 168 from mRNA-1273 group (Fig. 4G and H). The
superior immunogenicity in mRNA-1273 is observed from several studies28,29,30, possibly accounting for the higher antibody responses in this study. CYTOKINE PROFILES OF SPIKE-REACTIVE T
CELLS To investigate mechanisms underlying the differences in antibody responses induced by the ChAdOx1 vaccination, we analyzed the frequencies of circulating follicular helper T (cTfh)
cells, which has been reported to correlate with antibody responses (Fig. S2C). After the primary vaccination, mRNA-1273 and ChAdOx1 induced higher frequencies of cTfh cells in total CD4 T
cells (or in total PBMCs) than BNT162b2 did (Figs. 5A and S3A), which does not explain the difference in antibody titers at the primary vaccination. We confirmed the frequency of
spike-specific cTfh cells were comparable among the three groups before the vaccination (Fig. S4A). Likewise, cTfh cells were almost comparable between the nine groups after the homologous
and heterologous booster vaccination (Figs. 5B and S3B). Hence, we concluded that cTfh cell induction is not determinant for the differential antibody responses in our study. To identify the
T-cell derived correlates for differential antibody responses, we next analyzed cytokines secreted from spike-reactive T cells. We measured major cytokines produced by CD4 T cell subsets,
including Th0 (IL-2), Th1 (IFN-γ), Th2 (IL-4 and IL-5), Th17 (IL-17A), and regulatory T (IL-10) cells. We confirmed the cytokine producing ability of spike-specific T cells were comparable
among the three groups before the vaccination (Fig. S4B), indicating the differences below were induced by the vaccination. After the primary vaccination, the spike-reactive T cells showed
different patterns of cytokine production, indicating that these vaccines induce qualitatively different helper T cell responses (Fig. 5C). Notably, ChAdOx1 priming induced robust
IFN-γ-producing T cells at the levels higher than those by BNT162b2 and comparable to mRNA-1273. mRNA-1273 also elicited T cells that produce other cytokine, such as IL-2 and IL-5, which
were not produced by spike-specific T cells induced by ChAdOx1. Indeed, the ratios of IFN-γ versus IL-2 or IL-5 revealed 2.4–5.5 or 2.3–4.3 folds skewing, respectively, of ChAdOx1 priming
for IFN-γ production (Fig. 5D). Since the provision of IL-2 and IL-5 enhances the antibody responses from in vitro stimulated B cells, the results suggest that induction of IL-2 and/or IL-5
T cells may have contributed to higher antibody responses in the mRNA-1273 vaccinees. After the booster vaccination, the IL-2 and IL-5 production remained low in the homologous ChAdOx-1
group (Fig. 5E), while other groups receiving mRNA vaccines for either priming or boosting showed more elevated IL-2 and IL-5 production. Especially, IL-5 production was significantly lower
in the homologous ChAdOx1 group compared with any other groups. Collectively, these results suggest that IL-5 levels in the stimulated culture may be one of correlates for attenuated
antibody responses observed following homologous ChAdOx1 boosters compared to other booster regimens including mRNA vaccines for either priming or boosting. DURABILITY OF THE IL-5-PRODUCING
T CELLS IS IMPRINTED BY THE PRIMARY VACCINE Finally, we examined kinetics of the IL-5-producing T cells in each group (Fig. 6A). IL-5-producing T cells remained below the detection limit
throughout the period following homologous ChAdOx1 booster; however, IL-5-producing T cells were elicited above the detectable levels in heterologous booster groups at day 28 (Fig. 6A),
indicating the usage of mRNA vaccines for either priming or boosting compensates the immunogenic properties for eliciting IL-5-producing T cells above detectable levels, similar to the
antibody titers. Of note, IL-5-producing T cells detected at day 28 in the heterologous booster (adenoviral vector/mRNA) groups waned to undetectable levels over time (Fig. 6B). In contrast,
the heterologous booster groups with reversed vaccination order sustained higher numbers of IL-5-producing T cells, showing the adenovirus vector priming rather than boosting affected more
profoundly on the waning of IL-5 T cells, the phenomenon similar to immunological imprinting. Such imprinting was specific to the IL-5-producing T cells since cTfh cells and IL-2-producing T
cells were sustained throughout the period (Fig. 6C and D). DISCUSSION We have performed the immune profiling analysis following heterologous booster regimens with a combination of
adenoviral vector and mRNA vaccines, and demonstrated the distinct immunogenic properties by adenoviral vector and mRNA vaccines, particularly in the elicited T-cell functionality and
durability. Adenoviral vector priming induced T-cell responses characterized by robust IFN-γ production with an attenuated IL-2 and IL-5 production and anti-spike antibody titers. This
cytokine skewing appears to have long-lasting effects, as the IL-5 production detected in this study were significantly less durable in individuals primed with the ChAdOx1 vaccine, even
after a subsequent mRNA vaccine boost. IL-2 and IL-5 have been reported to be involved in humoral immune responses. IL-5 is a well-established type 2 helper cytokine for humoral immune
responses, inducing B cell differentiation into antibody-secreting cells31. Although IL-2 is not so established for B cell help functions as IL-5, several studies revealed that IL-2 promotes
B cell proliferation and differentiation into antibody-secreting cells32,33,34. Hence, the attenuated IL-2 and IL-5 T cell responses in adenoviral vector vaccination may contribute to the
modest antibody responses. One of the key implications in this study is to shed new lights on the concept of “immunological imprinting” depending on the initial vaccine platform. The ChAdOx1
adenoviral vector vaccine not only influenced the magnitude of the T-cell response but also appeared to establish a prolonged bias in T-cell functionality that persisted at least after the
boosting with mRNA vaccine platform. This suggests that the initial exposure to a particular vaccine platform could influence the quality of the immune response to future vaccinations,
underscoring the need to understand the immunogenic properties of individual vaccine platforms for applying heterologous vaccine regimens. The balance of T cell responses is regulated by
innate antigen-presenting cells and microenvironmental milieu, such as cytokines35,36. Our results showed that the primary dose of ChAdOx-1 induced higher production of IFN-γ by
spike-stimulated T cells, which could skew Th balance towards Th1. Further, one dose of ChAdOx-1 has been reported to induce trained immunity, where monocytes isolated from ChAdOx-1
vaccinees highly produced IFN-γ in response to stimulation for months after the vaccination37. Hence, one possible scenario is that the IFN-γ-producing T cells and monocytes induced by
primary ChAdOx-1 dose dampen Th2 cell response and impair durability of IL-5-producing T cells after heterologous mRNA booster dose. Our results also stress the importance of immune
profiling studies not only for quantitating neutralizing antibody titers but also for detecting the multiple parameters associated with vaccines-elicited protective immunity. Although
neutralizing antibody titers serve as one of immune correlates of protection in individuals who have completed vaccination regimens (COVID-19, influenza, rabies, mumps, and more)38, the
other immune parameters, such as non-neutralizing antibody titers, B-cell and T-cell responses are also suggested to contribute to VE15. Notably, the VE for severe COVID-19 is maintained for
long periods even after the waning of neutralizing antibody titers16, supporting the contribution of immune parameters other than neutralizing antibody titers for durable protection. The
prolonged skewing of T-cell cytokine production observed in our study suggests that vaccine strategies need to be tailored not just to elicit neutralizing antibody responses in the early
time points, but also to ensure that T-cell and B-cell responses are well-balanced and capable of supporting long-term immune protection which is contributed by multiple layers of immune
responses as represented by antibody, B-cell, and T-cell responses. In conclusion, this study highlights the important roles of the initial vaccine platform in shaping the long-term immune
responses, with significant implications for the design of future vaccination regimens. The concept of vaccine-induced T-cell imprinting should be taken into consideration when planning
booster doses at least one time, as it could influence the durability of the protective immune response and VE as well. Further research into the mechanisms underlying this imprinting and
its impact on other aspects of the immune response, such as antibody and B-cell helper function, will be essential for optimizing vaccine strategies in the emerging and re-emerging
infectious diseases. LIMITATIONS OF THE STUDY We analyzed combinations of three COVID-19 vaccines to compare immunogenic differences of vaccine platforms with well-coordinated cohorts for
comparable study. For more generalizable information about immunogenic properties of vaccine platforms, further analyses on cohorts with other vaccine platforms are needed. We also did not
trace the cohorts after third vaccine dose. Further studies are needed to examine how much extents the imprinting effects of ChAdOx1 priming on IL-5-producing T cells can be alleviated by an
additional mRNA vaccine booster or not. Our study compared the effects of vaccines at clinically used dose (30 µg of BNT162b2, 100 µg of mRNA-1273, and 5 × 1010 viral particles of ChAdOx1)
to evaluate immune responses in real world. The differences in antigen dose may have affected the immune responses, e.g., higher antibody responses in mRNA-1273 than BNT162b2. The responses
may also be affected by differences in conformation of spike antigen, as two-proline mutations are introduced in BNT162b2 and mRNA-1273 to stabilize prefusion conformation of the spike39,
which increase antibody titer40. Further detailed study will be needed for elucidating differences in immune responses induced by each vaccine platform. METHODS HUMAN PARTICIPANTS Healthy
volunteers were recruited and grouped into the nine regimens in order of their first visit. Only the participants with age of > 40 years were included in groups where participants
received ChAdOx1 as a primary or booster vaccine. We obtained written informed consent from all the participants before enrollment. The vaccination was conducted in double-blind. This study
was approved by Certified Review Board of National Center for Global Health and Medicine (NCGM-C-004337-00). All methods were performed in accordance with the principles of the Declarations
of Helsinki. SAMPLE PREPARATION Blood samples were longitudinally collected from the participants at PS Clinic (Fukuoka, Japan). For serum samples, blood was collected in Venoject II
(Terumo) and left at room temperature for 30 min. After centrifuge at 4 °C and 3000 rpm for 30 min., serum samples were collected and stored at -80 °C. PBMCs were prepared as previously
described41. Briefly, blood was collected in Vacutainer CPT tubes (BD biosciences), followed by centrifugation at 1800 g for 20 min. PBMCs suspended in plasma were transferred into conical
tubes followed by further centrifugation at 300 g for 15 min. PBMC pellets were washed with PBS three times followed by cryopreservation in CELLBANKER 1 plus (Zenogen pharma). ECLIA Serum
anti-spike and nucleocapsid protein antibody titers were measured as cut-off index (COI) values using Cobas e411 (Roche) with Elecsys Anti-SARS-CoV-2 S (Roche) and Elecsys Anti-SARS-CoV-2
(Roche), respectively, according to the manufacturer’s instruction. VIRAL NEUTRALIZATION ASSAY Serum neutralization titers against authentic SARS-CoV-2 viruses were measured as described
previously9,42. Briefly, serum samples were serially diluted (2-fold dilutions starting from 1:5) in Dulbecco’s modified Eagle’s medium (D-MEM) supplemented with 2% fetal bovine serum (FBS)
and 100 U/mL penicillin/streptomycin, and were mixed with 100 TCID50 SARS-CoV-2 WK-521 (hCoV-19/Japan/TY-WK-521/2020, ancestral strain), followed by incubation at 37 °C for 1 h. The
virus-plasma mixtures were placed on VeroE6/TMPRSS2 cells (JCRB1819) seeded in 96-well plates and cultured at 37 °C with 5% CO2 for 5 days. After the culture, the cells were fixed with 20%
formalin (Fujifilm Wako Pure Chemicals) and were stained with crystal violet solution (Sigma-Aldrich). The mean cut-off dilution index with > 50% cytopathic effect from 2 to 4
multiplicate series was presented as the neutralizing titers. FLOW CYTOMETRY RBD-reactive B cells were analyzed by flow cytometry as described previously43. Briefly, cryopreserved PBMCs were
thawed at 37 °C and immediately washed with D-MEM supplemented with 2% FBS. The PBMCs were suspended in D-MEM supplemented with 2% FBS containing the spike- and RBD-probes and 10 µM biotin,
followed by incubation for 30 min at room temperature. The cells were then incubated with D-MEM supplemented with 2% FBS containing fluorochrome-conjugated antibodies against surface
antigens, 10 µM biotin, and Brilliant stain buffer plus (BD Biosciences), and incubated for 30 min at room temperature followed by washing. The cells were suspended in D-MEM supplemented
with 2% FBS. Flow cytometry was performed with FACSymphony A3 (BD Biosciences). Data were analyzed with FlowJo software (BD Biosciences). AIM ASSAY Spike-specific T cells were analyzed using
AIM assay as previously described44. Briefly, cryopreserved PBMCs were suspended in R10 medium [RPMI1640 supplemented with 10% heat-inactivated human AB serum (Sigma-Aldrich), 10 mM Hepes
(Thermo Fisher Scientific), 1% minimum essential medium non-essential amino acid (Thermo Fisher Scientific), 1 mM L-glutamine (Thermo Fisher Scientific), and 100 U/mL penicillin/streptomycin
(Thermo Fisher Scientific) ] and incubated at 0.5–1.5 × 106 cells/well in 96-well U-bottom plates with or without overlapping (15 oligomers with 11 amino acids overlap) peptide pools
spanning the Wuhan spike glycoprotein (spike-OLPs) at 37 °C for 16 h in a 5% CO2 incubator. The spike-OLPs were purchased from JPT (PM-WCPV-S-1) and Myltenyi Biotec (PepTivator SARS-CoV-2
Prot_S Complete), reconstituted with dimethyl sulfoxide (DMSO) and distilled water, respectively, and used at a final concentration of 1 µg/ml. Supernatants were harvested for cytokine
quantification. Cells were washed with staining buffer (PBS supplemented with 2% fetal bovine serum and 0.01% NaN3), incubated with Fc Receptor Blocking Solution (Human TruStain FcX,
Biolegend) on ice for 20 min, and then stained for 1 h on ice with the following antibodies: CD3-BUV805 (SK7, BD Biosciences), CD4-BV480 (SK3, BD Biosciences), CD8-BUV496 (RPA-T8, BD
Biosciences), CD69-FITC (FN50, BioLegend), CD137-APC (4B4-1, BioLegend), and CD185-PE-Cy7 (J252D4, BioLegend). After staining, cells were washed twice with staining buffer and then subjected
to flow cytometry using a FACSymphony A3 (BD Biosciences). Data were saved as FCS files and analyzed using FlowJo software (v. 10.8.0, BD Biosciences), and the frequencies of spike-specific
CD4 T cells and cTfh cells in total CD4 T cells, and spike-specific CD8 T cells in total CD8 T cells were calculated. We also calculated the frequency of cTfh cells in total PBMCs. The
concentrations of cytokines secreted into the culture supernatant were quantified using the cytometric bead array kit (BD Biosciences) according to the manufacturer’s instructions. Culture
supernatant was diluted two-fold for analysis. Data were acquired using a FACSCanto II cytometer (BD Biosciences) and analyzed using FCAP Array Software Version 3.0 (BD Biosciences). The
cytokine quantities in the supernatant were normalized by the number of PBMCs. The data represent the amount of cytokines produced by one million PBMCs. STATISTICAL ANALYSIS Statistical
analyses were performed using Prism software (GraphPad). Detailed methods used for the statistical analyses are indicated in figure legends. MATERIALS AVAILABILITY All unique and stable
materials generated in this study are available from the lead contact under a Material Transfer Agreement. DATA AVAILABILITY The datasets used and/or analyzed during the current study are
available from the corresponding author on reasonable request. REFERENCES * Goel, R. R. et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. _Science_
374, abm0829 (2021). Article PubMed PubMed Central Google Scholar * Painter, M. M. et al. Rapid induction of antigen-specific CD4+ T cells is associated with coordinated humoral and
cellular immunity to SARS-CoV-2 mRNA vaccination. _Immunity_ https://doi.org/10.1016/j.immuni.2021.08.001 (2021). Article PubMed PubMed Central Google Scholar * Cho, A. et al.
Anti-SARS-CoV-2 receptor-binding domain antibody evolution after mRNA vaccination. _Nature_ 600, 517–522 (2021). Article ADS CAS PubMed PubMed Central Google Scholar * Sokal, A. et al.
mRNA vaccination of Naive and COVID-19-recovered individuals elicits potent memory B cells that recognize SARS-CoV-2 variants. _Immunity_ 54, 2893–2907 (2021). Article CAS PubMed PubMed
Central Google Scholar * Mazzoni, A. et al. First-dose mRNA vaccination is sufficient to reactivate immunological memory to SARS-CoV-2 in subjects who have recovered from COVID-19. _J.
Clin. Invest._ 131, e149150 (2021). * Tong, P. et al. Memory B cell repertoire for recognition of evolving SARS-CoV-2 Spike. _Cell_ 184, 4969–4980 (2021). Article CAS PubMed PubMed
Central Google Scholar * Goel, R. R. et al. Distinct antibody and memory B cell responses in SARS-CoV-2 Naïve and recovered individuals following mRNA vaccination. _Sci. Immunol._ 6,
eabi6950 (2021). Article PubMed PubMed Central Google Scholar * Mateus, J. et al. Low-dose mRNA-1273 COVID-19 vaccine generates durable memory enhanced by cross-reactive T cells.
_Science_ eabj9853 (2021). * Kotaki, R. et al. SARS-CoV-2 Omicron-neutralizing memory B-cells are elicited by two doses of BNT162b2 mRNA vaccine. _Sci. Immunol._ eabn8590 (2022). * Takano,
T. et al. Distinct immune cell dynamics correlate with the immunogenicity and reactogenicity of SARS-CoV-2 mRNA vaccine. _Cell. Rep. Med._ https://doi.org/10.1016/j.xcrm.2022.100631 (2022).
Article PubMed PubMed Central Google Scholar * Polack, F. P. et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. _N. Engl. J. Med._ 383, 2603–2615 (2020). Article CAS
PubMed Google Scholar * Baden, L. R. et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. _N. Engl. J. Med._ 384, 403–416 (2021). Article CAS PubMed Google Scholar *
Gilbert, P. B. et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. _Science_ 375, 43–50 (2022). Article ADS CAS PubMed Google Scholar * Earle,
K. A. et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. _Vaccine_ 39, 4423–4428 (2021). Article CAS PubMed PubMed Central Google Scholar * Khoury, D. S. et
al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. _Nat. Med._ 27, 1205–1211 (2021). Article CAS PubMed Google Scholar *
Arashiro, T. et al. COVID-19 vaccine effectiveness against severe COVID-19 requiring oxygen therapy, invasive mechanical ventilation, and death in Japan: A multicenter case-control study
(MOTIVATE study). _Vaccine_ 42, 677–688 (2024). Article CAS PubMed Google Scholar * Schultz, N. H. et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. _N. Engl. J.
Med._ 384, 2124–2130 (2021). Article CAS PubMed Google Scholar * Greinacher, A. et al. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. _N. Engl. J. Med._ 384, 2092–2101
(2021). Article CAS PubMed Google Scholar * Nordström, P., Ballin, M. & Nordström, A. Effectiveness of heterologous ChAdOx1 nCoV-19 and mRNA prime-boost vaccination against
symptomatic Covid-19 infection in Sweden: A nationwide cohort study. _Lancet Reg. Health Eur._ 11, 100249 (2021). Article PubMed PubMed Central Google Scholar * Pozzetto, B. et al.
Immunogenicity and efficacy of heterologous ChAdOx1-BNT162b2 vaccination. _Nature_ 600, 701–706 (2021). Article ADS CAS PubMed Google Scholar * Shoemaker, K. et al. Long-term safety and
immunogenicity of AZD1222 (ChAdOx1 nCoV-19): 2-year follow-up from a phase 3 study. _Vaccines_ 12, 883 (2024). Article PubMed PubMed Central Google Scholar * Barnes, C. O. et al.
SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies. _Nature_ 588, 682–687 (2020). Article ADS CAS PubMed PubMed Central Google Scholar * Zost, S. J. et al.
Potently neutralizing and protective human antibodies against SARS-CoV-2. _Nature_ 584, 443–449 (2020). Article ADS CAS PubMed PubMed Central Google Scholar * Piccoli, L. et al.
Mapping neutralizing and immunodominant sites on the SARS-CoV-2 spike receptor-binding domain by structure-guided high-resolution serology. _Cell_ 183, 1024–1042e21 (2020). Article CAS
PubMed PubMed Central Google Scholar * Rogers, T. F. et al. Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. _Science_ 369,
956–963 (2020). Article ADS CAS PubMed PubMed Central Google Scholar * Andreano, E. et al. Extremely potent human monoclonal antibodies from COVID-19 convalescent patients. _Cell_ 184,
1821–1835 (2021). Article CAS PubMed PubMed Central Google Scholar * Cao, Y. et al. Potent neutralizing antibodies against SARS-CoV-2 identified by high-throughput single-cell
sequencing of convalescent patients’ B cells. _Cell_ 182, 73–84 (2020). Article CAS PubMed PubMed Central Google Scholar * Dickerman, B. A. et al. Comparative effectiveness of BNT162b2
and mRNA-1273 vaccines in US Veterans. _N. Engl. J. Med._ 386, 105–115 (2022). Article CAS PubMed Google Scholar * Puranik, A. et al. Comparative effectiveness of mRNA-1273 and BNT162b2
against symptomatic SARS-CoV-2 infection. _Med_ 3, 28–41e8 (2022). Article CAS PubMed Google Scholar * Abu-Raddad, L. J., Chemaitelly, H., Bertollini, R. & National Study Group for
COVID-19 Vaccination. Effectiveness of mRNA-1273 and BNT162b2 vaccines in Qatar. _N. Engl. J. Med._ 386, 799–800 (2022). Article CAS PubMed Google Scholar * Takatsu, K. Interleukin 5 and
B cell differentiation. _Cytokine Growth Factor Rev._ 9, 25–35 (1998). Article CAS PubMed Google Scholar * Mingari, M. C. et al. Human interleukin-2 promotes proliferation of activated
B cells via surface receptors similar to those of activated T cells. _Nature_ 312, 641–643 (1984). Article ADS CAS PubMed Google Scholar * Le Gallou, S. et al. IL-2 requirement for
human plasma cell generation: Coupling differentiation and proliferation by enhancing MAPK-ERK signaling. _J. Immunol._ 189, 161–173 (2012). Article PubMed Google Scholar * Hipp, N. et
al. IL-2 imprints human Naive B cell fate towards plasma cell through ERK/ELK1-mediated BACH2 repression. _Nat. Commun._ 8, 1443 (2017). Article ADS PubMed PubMed Central Google Scholar
* Zhou, L., Chong, M. M. W. & Littman, D. R. Plasticity of CD4+ T cell lineage differentiation. _Immunity_ 30, 646–655 (2009). Article CAS PubMed Google Scholar * Geginat, J. et
al. Plasticity of human CD4 T cell subsets. _Front. Immunol._ 5, 630 (2014). Article PubMed PubMed Central Google Scholar * Murphy, D. M. et al. Trained immunity is induced in humans
after immunization with an adenoviral vector COVID-19 vaccine. _J. Clin. Invest._. https://doi.org/10.1172/JCI162581 (2023). * Plotkin, S. A. Correlates of protection induced by vaccination.
_Clin. Vaccine Immunol._ 17, 1055–1065 (2010). Article CAS PubMed PubMed Central Google Scholar * Wrapp, D. et al. Cryo-EM structure of the 2019-nCoV Spike in the prefusion
conformation. _Science_ 367, 1260–1263 (2020). Article ADS CAS PubMed PubMed Central Google Scholar * Mercado, N. B. et al. Single-shot Ad26 vaccine protects against SARS-CoV-2 in
rhesus macaques. _Nature_ 586, 583–588 (2020). Article ADS CAS PubMed PubMed Central Google Scholar * Kotaki, R. et al. Repeated Omicron exposures redirect SARS-CoV-2-specific memory B
cell evolution toward the latest variants. _Sci. Transl. Med._ 16, eadp9927 (2024). Article CAS PubMed Google Scholar * Moriyama, S. et al. Temporal maturation of neutralizing
antibodies in COVID-19 convalescent individuals improves potency and breadth to circulating SARS-CoV-2 variants. _Immunity_ 54, 1841–1852 (2021). Article CAS PubMed PubMed Central Google
Scholar * Onodera, T. et al. CD62L expression marks SARS-CoV-2 memory B cell subset with preference for neutralizing epitopes. _Sci. Adv._ 9, eadf0661 (2023). Article CAS PubMed PubMed
Central Google Scholar * Terahara, K. et al. SARS-CoV-2-specific CD4+ T cell longevity correlates with Th17-like phenotype. _iScience_ 25, 104959 (2022). Article ADS CAS PubMed PubMed
Central Google Scholar Download references ACKNOWLEDGEMENTS We thank Akira Dosaka, Eriko Izumiyama, Rieko Iwaki, Megumi Koda, Saori Tachibana, and Ryoko Itami at Research Center for Drug
and Vaccine Development, NIID for their technical support. We thank Akiko Sataka and Rena Sakamoto at Department of Pathology, NIID for their support for the antibody measurement. We also
thank Hiroko Kumashiro at Clinical Research Division, SOUSEIKAI medical group for coordinating clinical research. This study was supported by the Japan Agency for Medical Research and
Development (JP21nf0101637 to TS, YT, and TT, JP24gm1810004 to YT, JP243fa627005 to YT, JP243fa627009 to YT, and JP243fa727002 to YT). AUTHOR INFORMATION Author notes * Masanori Isogawa,
Taishi Onodera, Akira Ainai and Ryutaro Kotaki contributed equally to this work. AUTHORS AND AFFILIATIONS * Research Center for Vaccine Development, National Institute of Infectious
Diseases, Japan Institute for Health Security, Tokyo, Japan Masanori Isogawa, Taishi Onodera, Ryutaro Kotaki & Yoshimasa Takahashi * Department of Virology II, National Institute of
Infectious Diseases, Japan Institute for Health Security, Tokyo, Japan Masanori Isogawa * Department of Infectious Disease Pathology, National Institute of Infectious Diseases, Japan
Institute for Health Security, Tokyo, Japan Akira Ainai, Takayuki Kanno, Shinji Saito, Minoru Tobiume, Kenzo Tokunaga & Tadaki Suzuki * Department of Preventive Medicine, Faculty of
Medicine, Saga University, Saga, Japan Megumi Hara * Clinical Epidemiology Research Center, SOUSEIKAI Medical Group (Medical Co. LTA), Fukuoka, Japan Yoshio Hirota * PS Clinic, SOUSEIKAI
Medical Group (Medical Co. LTA), Fukuoka, Japan Tomomi Tsuru * Department of Infectious Disease Pathobiology, Graduate School of Medicine, Chiba University, Chiba, Japan Tadaki Suzuki *
Institute for Vaccine Research and Development, Hokkaido University, Hokkaido, Japan Yoshimasa Takahashi Authors * Masanori Isogawa View author publications You can also search for this
author inPubMed Google Scholar * Taishi Onodera View author publications You can also search for this author inPubMed Google Scholar * Akira Ainai View author publications You can also
search for this author inPubMed Google Scholar * Ryutaro Kotaki View author publications You can also search for this author inPubMed Google Scholar * Takayuki Kanno View author publications
You can also search for this author inPubMed Google Scholar * Shinji Saito View author publications You can also search for this author inPubMed Google Scholar * Minoru Tobiume View author
publications You can also search for this author inPubMed Google Scholar * Kenzo Tokunaga View author publications You can also search for this author inPubMed Google Scholar * Megumi Hara
View author publications You can also search for this author inPubMed Google Scholar * Yoshio Hirota View author publications You can also search for this author inPubMed Google Scholar *
Tadaki Suzuki View author publications You can also search for this author inPubMed Google Scholar * Yoshimasa Takahashi View author publications You can also search for this author inPubMed
Google Scholar * Tomomi Tsuru View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Conceptualization: Y.H., T.S., Y.T., and T.T. Funding
acquisition: Y.H., T.S., Y.T., and T.T. Investigation: M.I., T.O., A.A., R.K., T.K., S.S., M.T., K.T., M.H. Methodology: M.I., T.O., and A.A. Project administration: T.S., Y.T., and T.T.
Resources: M.H., Y.H., T.S., Y.T., and T.T. Supervision: T.S., Y.T., and T.T. Visualization: M.I., T.O., A.A., and R.K. Writing – original draft: R.K. and Y.T. Writing – review &
editing: M.I., T.O., A.A., T.K., S.S., M.T., K.T., M.H., Y.H., T.S., and T.T. CORRESPONDING AUTHORS Correspondence to Tadaki Suzuki, Yoshimasa Takahashi or Tomomi Tsuru. ETHICS DECLARATIONS
COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps
and institutional affiliations. ELECTRONIC SUPPLEMENTARY MATERIAL Below is the link to the electronic supplementary material. SUPPLEMENTARY MATERIAL 1 RIGHTS AND PERMISSIONS OPEN ACCESS
This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and
reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you
modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in
this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative
Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a
copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Isogawa, M., Onodera, T., Ainai, A. _et al._
Prolonged effects of adenoviral vector priming on T-cell cytokine production in heterologous adenoviral vector/mRNA COVID-19 vaccination regimens. _Sci Rep_ 15, 18684 (2025).
https://doi.org/10.1038/s41598-025-00054-x Download citation * Received: 05 December 2024 * Accepted: 24 April 2025 * Published: 28 May 2025 * DOI: https://doi.org/10.1038/s41598-025-00054-x
SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy
to clipboard Provided by the Springer Nature SharedIt content-sharing initiative