Play all audios:
ABSTRACT Bicyclo[1.1.1]pentanes (BCPs) are highly strained carbocycles that have fascinated the chemical community for decades because of their unique structure. Despite the immense interest
in this scaffold and extensive synthetic efforts, the construction of BCP derivatives still relies substantially on the manipulation of dimethyl bicyclo[1.1.1]pentane-1,3-dicarboxylate.
Furthermore, BCPs that contain a proximal stereocentre are underrepresented in the literature and their generation requires stoichiometric chiral auxiliaries. Here we explore
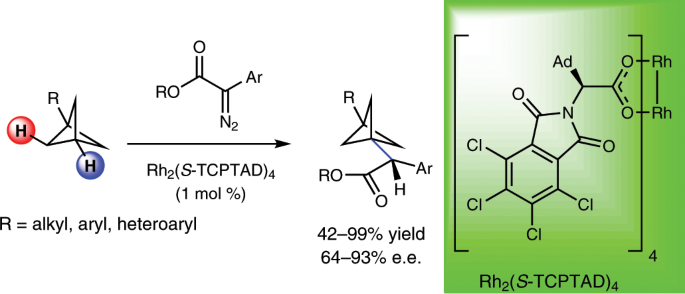
enantioselective C–H functionalization of BCPs as a conceptually innovative strategy that provides access to chiral substituted BCPs. For this purpose, enantioselective intermolecular _sp_3
C–H insertion reactions of donor/acceptor diazo compounds catalysed by the chiral dirhodium complex, Rh2(TCPTAD)4, were employed to forge new C–C bonds at the tertiary position of a variety
of BCPs. This work also establishes that highly strained molecules can undergo direct C–H insertion without losing the integrity of their carbocyclic framework. Access through your
institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio
journals Get Nature+, our best-value online-access subscription $32.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 digital issues and online access to articles
$119.00 per year only $9.92 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are
calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS
DIFUNCTIONALIZATION OF BICYCLO[1.1.0]BUTANES ENABLED BY MERGING C−C CLEAVAGE AND RUTHENIUM-CATALYSED REMOTE C−H ACTIVATION Article Open access 17 February 2025 ASYMMETRIC SYNTHESIS OF
ATROPISOMERS FEATURING CYCLOBUTANE BORONIC ESTERS FACILITATED BY RING-STRAINED B-ATE COMPLEXES Article Open access 30 December 2024 ENANTIOSELECTIVE SYNTHESIS OF 2-SUBSTITUTED
BICYCLO[1.1.1]PENTANES VIA SEQUENTIAL ASYMMETRIC IMINE ADDITION OF BICYCLO[1.1.0]BUTANES AND SKELETAL EDITING Article 28 January 2025 DATA AVAILABILITY Crystallographic data for the
structures reported in this Letter have been deposited at the Cambridge Crystallographic Data Centre, under deposition nos. 1906391 and 1906395. Copies of the data can be obtained free of
charge via www.ccdc.cam.ac.uk/data_request/cif. Complete experimental procedures and compound characterization data are available in the Supplementary Information; any other data is
available from the authors on request. REFERENCES * Levin, M. D., Kaszynski, P. & Michl, J. Bicyclo[1.1.1]pentanes, [_n_]staffanes, [1.1.1]propellanes, and tricyclo[2.1.0.02,5]pentanes.
_Chem. Rev._ 100, 169–234 (2000). CAS PubMed Google Scholar * Wiberg, K. B. The concept of strain in organic chemistry. _Angew. Chem. Int. Ed._ 25, 312–322 (1986). Google Scholar *
Locke, G. M., Bernhard, S. S. R. & Senge, M. O. Nonconjugated hydrocarbons as rigid-linear motifs: isosteres for material sciences and bioorganic and medicinal _Chemistry. Chem. Eur. J._
25, 4590-4647 (2018). * Kanazawa, J. & Uchiyama, M. Recent advances in the synthetic chemistry of bicyclo[1.1.1]pentane. _Synlett_ 30, 1–11 (2019). CAS Google Scholar * Stepan, A. F.
et al. Application of the bicyclo[1.1.1]pentane motif as a nonclassical phenyl ring bioisostere in the design of a potent and orally active γ-secretase inhibitor. _J. Med. Chem._ 55,
3414–3424 (2012). CAS PubMed Google Scholar * Westphal, M. V., Wolfstädter, B. T., Plancher, J.-M., Gatfield, J. & Carreira, E. M. Evaluation of _tert_-butyl isosteres: case studies
of physicochemical and pharmacokinetic properties, efficacies, and activities. _ChemMedChem_ 10, 461–469 (2015). CAS PubMed Google Scholar * Makarov, I. S., Brocklehurst, C. E.,
Karaghiosoff, K., Koch, G. & Knochel, P. Synthesis of bicyclo[1.1.1]pentane bioisosteres of internal alkynes and _para_‐disubstituted benzenes from [1.1.1]propellane. _Angew. Chem. Int.
Ed._ 56, 12774–12777 (2017). CAS Google Scholar * Barbachyn, M. R. et al. U-87947E, a protein quinolone antibacterial agent incorporating a bicyclo[1.1.1]pent-1-yl (BCP) subunit. _Bioorg.
Medicinal Chem. Lett._ 3, 671–676 (1993). CAS Google Scholar * Goh, Y. L., Cui, Y. T., Pendharkar, V. & Adsool, V. A. Toward resolving the resveratrol conundrum: synthesis and in vivo
pharmacokinetic evaluation of BCP–resveratrol. _ACS Med. Chem. Lett_. 8, 516−520 (2017). * Measom, N. D. et al. Investigation of a bicyclo[1.1.1]pentane as a phenyl replacement within an
LpPLA2 Inhibitor. _ACS Med. Chem. Lett._ 8, 43–48 (2017). CAS PubMed Google Scholar * Kaszynski, P., Friedli, A. & Michl, J. Toward a molecular-size tinkertoy construction set.
Preparation of terminally functionalized [n]staffanes from [1.1.1]propellane. _J. Am. Chem. Soc._ 114, 601–620 (1992). CAS Google Scholar * Wiberg, K. B. & Connor, D. S.
Bicyclo[1.1.1]pentane. _J. Am. Chem. Soc._ 88, 4437–4441 (1966). CAS Google Scholar * Wiberg, K. B. & Waddell, S. T. Reactions of [1.1.1]propellane. _J. Am. Chem. Soc._ 112, 2194–2216
(1990). CAS Google Scholar * Bunker, K. D., Sach, N. W., Huang, Q. & Richardson, P. F. Scalable synthesis of 1-bicyclo[1.1.1]pentylamine via a hydrohydrazination reaction. _Org. Lett._
13, 4746–4748 (2011). CAS PubMed Google Scholar * Goh, Y. L. et al. A new route to bicyclo[1.1.1]pentan-1-amine from 1-azido-3- iodobicyclo[1.1.1]pentane. _Org. Lett._ 16, 1884–1887
(2014). CAS PubMed Google Scholar * Kanazawa, J., Maeda, K. & Uchiyama, M. Radical multicomponent carboamination of [1.1.1]propellane. _J. Am. Chem. Soc._ 139, 17791–17794 (2017). CAS
PubMed Google Scholar * Gianatassio, R. et al. Strain-release amination. _Science_ 351, 241–246 (2016). CAS PubMed PubMed Central Google Scholar * Lopchuk, J. M. et al.
Strain-release heteroatom functionalization: development, scope, and stereospecificity. _J. Am. Chem. Soc._ 139, 3209–3226 (2017). CAS PubMed PubMed Central Google Scholar * Della, E. W.
& Taylor, D. K. Synthesis of some bridgehead–bridgehead-disubstituted bicyclo[1.1.1]pentanes. _J. Org. Chem._ 59, 2986–2996 (1994). CAS Google Scholar * Rehm, J. D. D., Ziemer, B.
& Szeimies, G. A facile route to bridgehead disubstituted bicyclo[1.1.1]pentanes involving palladium-catalyzed cross-coupling reactions. _Eur. J. Org. Chem_. 1999, 2079–2085 (1999). *
Messner, M., Kozhushkov, S. I. & de Meijere, A. Nickel- and palladium-catalyzed cross-coupling reactions at the bridgehead of bicyclo[1.1.1]pentane derivatives—a convenient access to
liquid crystalline compounds containing bicyclo[1.1.1]pentane moieties. _Eur. J. Org. Chem_. 2000, 1137–1155 (2000). * Matos, J. L. M., Vásquez-Céspedes, S., Gu, J., Oguma, T. & Shenvi,
R. A. Branch-selective addition of unactivated olefins into imines and aldehydes. _J. Am. Chem. Soc._ 140, 16976–16981 (2018). CAS PubMed PubMed Central Google Scholar * Caputo, D. F. J.
et al. Synthesis and applications of highly functionalized 1-halo-3-substituted bicyclo[1.1.1]pentanes. _Chem. Sci._ 9, 5295–5300 (2018). CAS PubMed PubMed Central Google Scholar *
Schelp, R. A. & Walsh, P. J. Synthesis of BCP benzylamines from 2-azaallyl anions and [1.1.1]propellane. _Angew. Chem. Int. Ed_. 55, 15857–15861 (2018). * Ni, S. et al. A general amino
acid synthesis enabled by innate radical cross-coupling. _Angew. Chem. Int. Ed._ 57, 14560–14565 (2018). CAS Google Scholar * Pellicciari, R. et al.
(S)-(+)-2-(3'-Carboxybicyclo[1.1.1]pentyl)-glycine, a structurally new group I metabotropic glutamate receptor antagonist. _J. Med. Chem._ 39, 2874–2876 (1996). CAS PubMed Google
Scholar * Pritz, S., Pätzel, M., Szeimies, G., Dathe, M. & Bienert, M. Synthesis of a chiral amino acid with bicyclo[1.1.1]pentane moiety and its incorporation into linear and cyclic
antimicrobial peptides. _Org. Biomol. Chem._ 5, 1789–1794 (2007). CAS PubMed Google Scholar * Filosa, R. et al. Design, synthesis and biological evaluation of novel
bicyclo[1.1.1]pentane-based omega-acidic amino acids as glutamate receptors ligands. _Bioorg. Medicinal Chem._ 17, 242–250 (2009). CAS Google Scholar * Kokhan, S. O. et al. Design,
synthesis, and application of an optimized monofluorinated aliphatic label for peptide studies by solid-state 19F NMR spectroscopy. _Angew. Chem. Int. Ed._ 55, 14788–14792 (2016). CAS
Google Scholar * Mikhailiuk, P. K. et al. Conformationally rigid trifluoromethyl-substituted α-amino acid designed for peptide structure analysis by solid-state 19F NMR spectroscopy.
_Angew. Chem. Int. Ed._ 45, 5659–5661 (2006). CAS Google Scholar * Wong, M. L. J., Mousseau, J. J., Mansfield, S. J. & Anderson, E. A. Synthesis of enantioenriched α-chiral
bicyclo[1.1.1]pentanes. _Org. Lett_. 7, 2408–2411 (2019). * Wiberg K. B. & Williams, V. Z. Jr. Bicyclo[1.1.1]pentane derivatives. _J. Org. Chem._ 35, 366–369 (1970). Google Scholar *
Della, E. W., Grob, C. A. & Taylor, D. K. Bridgehead carbocations: a solvolytic study of 1-bromobicyclo[1.1.1]pentane and its bridgehead-substituted derivatives. _J. Am. Chem. Soc._ 116,
6159–6166 (1994). CAS Google Scholar * Gutekunst, W. R. & Baran, P. S. C–H Functionalization logic in total synthesis. _Chem. Soc. Rev._ 40, 1976–1991 (2011). CAS PubMed Google
Scholar * Davies, H. M. L. & Morton, D. Guiding principles for site selective and stereoselective intermolecular C–H functionalization by donor/acceptor rhodium carbenes. _Chem. Soc.
Rev._ 40, 1857–1869 (2011). CAS PubMed Google Scholar * Guptill, D. M. & Davies, H. M. L. 2,2,2-Trichloroethyl aryldiazoacetates as robust reagents for the enantioselective C–H
functionalization of methyl ethers. _J. Am. Chem. Soc._ 136, 17718–17721 (2014). CAS PubMed Google Scholar * Liao, K., Negretti, S., Musaev, D. G., Bacsa, J. & Davies, H. M. L.
Site-selective and stereoselective functionalization of unactivated C–H bonds. _Nature_ 533, 230–234 (2016). CAS PubMed Google Scholar * Liao, K. et al. Site-selective and stereoselective
functionalization of non-activated tertiary C–H bonds. _Nature_ 551, 609–613 (2017). CAS PubMed Google Scholar * Liao, K. et al. Design of catalysts for site-selective and
enantioselective functionalization of non-activated primary C–H bonds. _Nat. Chem._ 10, 1048–1055 (2018). CAS PubMed PubMed Central Google Scholar * Liu, W. et al. Catalyst-controlled
selective functionalization of unactivated C–H bonds in the presence of electronically activated C–H bonds. _J. Am. Chem. Soc._ 140, 12247–12255 (2018). CAS PubMed Google Scholar * Fu,
J., Ren, Z., Bacsa, J., Musaev, D. G. & Davies, H. M. L. Desymmetrization of cyclohexanes by site- and stereoselective C–H functionalization. _Nature_ 564, 395–399 (2018). CAS PubMed
Google Scholar * Qin, C. & Davies, H. M. L. Role of sterically demanding chiral dirhodium catalysts in site-selective C–H functionalization of activated primary C–H bonds. _J. Am. Chem.
Soc._ 136, 9792–9796 (2014). CAS PubMed Google Scholar * Auberson, Y. P. et al. Improving nonspecific binding and solubility: bicycloalkyl groups and cubanes as para-phenyl bioisosteres.
_ChemMedChem_ 12, 590–598 (2017). CAS PubMed Google Scholar * _Biphenyl-4-yl(phenyl)acetic Acid_ Section 7 (PubChem, accessed 7 November 2018);
https://pubchem.ncbi.nlm.nih.gov/compound/226171#section=Biological-Test-Results * _2-Phenyl-2-(4-phenylphenyl)ethanamine_ Section 6 (PubChem, acessed 7 November 2018);
https://pubchem.ncbi.nlm.nih.gov/compound/44719858#section=BioAssay-Results * Davies, H. M. L., Hansen, T. & Churchill, M. R. Catalytic asymmetric C–H activation of alkanes and
tetrahydrofuran. _J. Am. Chem. Soc._ 122, 3063–3070 (2000). CAS Google Scholar * Della, E. W. & Schiesser, C. H. Hyperconjugation in strained bridgehead cyclobutyl cations: an ab
initio study of bicyclo[1.1.1]pent-1-yl cubyl and norcubyl cations. _J. Chem. Soc. Chem. Commun._ 1994, 417–419 (1994). Google Scholar * Wiberg, K. B. & McMurdie, N. Formation and
reactions of bicyclo[1.1.1]pentyl-1 cations. _J. Am. Chem. Soc._ 116, 11990–11998 (1994). CAS Google Scholar * Wiberg, K. B. & McMurdie, N. Mechanism of the solvolysis of
bicyclo[1.1.1]pentyl-1 derivatives. _J. Org. Chem._ 58, 5603–5604 (1993). CAS Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by the NSF under the Center for
C–H Functionalization (grant no. CHE-1700982) and the NIGMS of the NIH under award nos. F32GM130020 (Z.J.G.) and F32GM122218 (J.N.S.). The content is solely the responsibility of the authors
and does not necessarily represent the views of the NSF or NIH. Additional financial support was provided by Novartis. Instrumentation used in this work was supported by the National
Science Foundation (grant nos. CHE 1531620 and CHE 1626172). Computational resources were provided by the UCLA Institute for Digital Research and Education (IDRE). We wish to thank the
members of the NSF Center for C–H Functionalization (grant no. CHE-1700982), especially J.Du. Bois . and N. Chiappini, for helpful discussions regarding this work. We thank J. Bacsa and T.
Pickel at the Emory X-ray Crystallography Facility for the X-ray structural analysis. We thank S. Skolnik and J. Poirier at the Novartis Institutes for BioMedical Research for carrying out
solubility and logD measurements. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Chemistry, Emory University, Atlanta, GA, USA Zachary J. Garlets & Huw M. L. Davies *
Department of Chemistry and Biochemistry, University of California, Los Angeles, CA, USA Jacob N. Sanders & K. N. Houk * Novartis Institutes for Biomedical Research Inc., Cambridge, MA,
USA Hasnain Malik & Christian Gampe Authors * Zachary J. Garlets View author publications You can also search for this author inPubMed Google Scholar * Jacob N. Sanders View author
publications You can also search for this author inPubMed Google Scholar * Hasnain Malik View author publications You can also search for this author inPubMed Google Scholar * Christian
Gampe View author publications You can also search for this author inPubMed Google Scholar * K. N. Houk View author publications You can also search for this author inPubMed Google Scholar *
Huw M. L. Davies View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Z.J.G. performed the synthetic experiments. J.N.S. and K.N.H. conducted
the computational studies. H.M. and C.G. evaluated the biologically relevant compounds and conducted the comparison studies. Z.J.G. and H.M.L.D. designed and analysed the synthetic
experiments and prepared the manuscript. All authors contributed to the final draft of the manuscript. CORRESPONDING AUTHOR Correspondence to Huw M. L. Davies. ETHICS DECLARATIONS COMPETING
INTERESTS H.M.L.D. is a named inventor on a patent entitled ‘Dirhodium catalyst compositions and synthetic processes related thereto’ (US 8,974,428, issued 10 March 2015). The other authors
declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Methods, Figs. 1–3, Tables 1–16 and references. COMPOUND 8D Crystallographic Data for Compound 8d. COMPOUND 29
Crystallographic Data for Compound 29. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Garlets, Z.J., Sanders, J.N., Malik, H. _et al._ Enantioselective
C–H functionalization of bicyclo[1.1.1]pentanes. _Nat Catal_ 3, 351–357 (2020). https://doi.org/10.1038/s41929-019-0417-1 Download citation * Received: 08 April 2019 * Accepted: 09 December
2019 * Published: 27 January 2020 * Issue Date: April 2020 * DOI: https://doi.org/10.1038/s41929-019-0417-1 SHARE THIS ARTICLE Anyone you share the following link with will be able to read
this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative