Play all audios:
ABSTRACT Understanding the mechanism of catalytic hydrogenation at the local environment requires chemical and topographic information involving catalytic sites, active hydrogen species, and
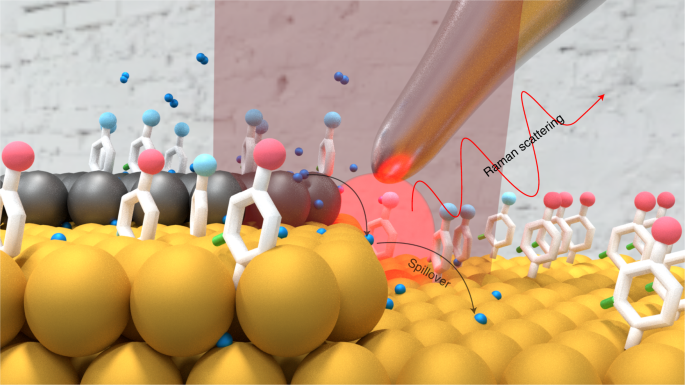
their spatial distribution. Here we used tip-enhanced Raman spectroscopy (TERS) to study the catalytic hydrogenation of chloronitrobenzenethiol on a well-defined Pd(submonolayer)/Au(111)
bimetallic catalyst (\(p_{\rm{H}_{2}}\) = 1.5 bar, 298 K), where the surface topography and chemical fingerprint information were simultaneously mapped with nanoscale resolution (~10 nm).
TERS imaging of the surface after catalytic hydrogenation confirms that the reaction occurs beyond the location of Pd sites. The results demonstrate that hydrogen spillover accelerates
hydrogenation at Au sites as far as 20 nm from the bimetallic Pd/Au boundary. Density functional theory was used to elucidate the thermodynamics of interfacial hydrogen transfers. We
demonstrate TERS to be a powerful analytical tool that provides a unique approach to spatially investigate the local structure–reactivity relationship in catalysis. Access through your
institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio
journals Get Nature+, our best-value online-access subscription $29.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 digital issues and online access to articles
$119.00 per year only $9.92 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are
calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS
REAL-SPACE IMAGING OF A PHENYL GROUP MIGRATION REACTION ON METAL SURFACES Article Open access 21 February 2023 SMALL MOLECULE BINDING TO SURFACE-SUPPORTED SINGLE-SITE TRANSITION-METAL
REACTION CENTRES Article Open access 01 December 2022 SINGLE HYDROGEN ATOM MANIPULATION FOR REVERSIBLE DEPROTONATION OF WATER ON A RUTILE TIO2 (110) SURFACE Article Open access 19 January
2021 DATA AVAILABILITY The original data used in this publication are made available in a curated data archive at ETH Zurich (https://www.researchcollection.ethz.ch) under
https://doi.org/10.3929/ethz-b-000423837, or are available from the corresponding authors upon reasonable request. Source data are provided with this paper. CODE AVAILABILITY The MATLAB
codes used for processing the data are made available in a curated data archive at ETH Zurich (https://www.researchcollection.ethz.ch) under https://doi.org/10.3929/ethz-b-000423837, or are
available from the corresponding authors upon reasonable request. REFERENCES * Buurmans, I. L. C. & Weckhuysen, B. M. Heterogeneities of individual catalyst particles in space and time
as monitored by spectroscopy. _Nat. Chem._ 4, 873–886 (2012). CAS PubMed Google Scholar * Sambur, J. B. et al. Sub-particle reaction and photocurrent mapping to optimize catalyst-modified
photoanodes. _Nature_ 530, 77–80 (2016). CAS PubMed Google Scholar * Agarwal, N. et al. Aqueous Au–Pd colloids catalyze selective CH4 oxidation to CH3OH with O2 under mild conditions.
_Science_ 358, 223–227 (2017). CAS PubMed Google Scholar * Cárdenas-Lizana, F. et al. Pd-promoted selective gas phase hydrogenation of _p_-chloronitrobenzene over alumina supported Au.
_J. Catal._ 262, 235–243 (2009). Google Scholar * Lucci, F. R. et al. Controlling hydrogen activation, spillover, and desorption with Pd−Au single-atom alloys. _J. Phys. Chem. Lett._ 7,
480–485 (2016). CAS PubMed Google Scholar * Marcinkowski, M. et al. Controlling a spillover pathway with the molecular cork effect. _Nat. Mater._ 12, 523–528 (2013). CAS PubMed Google
Scholar * Huizinga, T. & Prins, R. Behavior of titanium (3+) centers in the low- and high-temperature reduction of platinum/titanium dioxide, studied by ESR. _J. Phys. Chem._ 85,
2156–2158 (1981). CAS Google Scholar * Briggs, N. M. et al. Identification of active sites on supported metal catalysts with carbon nanotube hydrogen highways. _Nat. Commun._ 9, 3827
(2018). PubMed PubMed Central Google Scholar * Kyriakou, G. et al. Isolated metal atom geometries as a strategy for selective heterogeneous hydrogenations. _Science_ 335, 1209–1212
(2012). CAS PubMed Google Scholar * Miller, J. T. et al. Hydrogen temperature-programmed desorption (H2 TPD) of supported platinum catalysts. _J. Catal._ 143, 395–408 (1993). CAS Google
Scholar * Karim, W. et al. Catalyst support effects on hydrogen spillover. _Nature_ 541, 68–71 (2017). CAS PubMed Google Scholar * van Lent, R. et al. Site-specific reactivity of
molecules with surface defects—the case of H2 dissociation on Pt. _Science_ 363, 155–157 (2019). PubMed Google Scholar * Dong, J. et al. In situ Raman spectroscopic evidence for oxygen
reduction reaction intermediates at platinum single-crystal surfaces. _Nat. Energy_ 4, 60–67 (2019). CAS Google Scholar * Zhong, J. et al. Probing the electronic and catalytic properties
of a bimetallic surface with 3 nm resolution. _Nat. Nanotechnol._ 12, 132–136 (2017). CAS PubMed Google Scholar * Zhang, R. et al. Chemical mapping of a single molecule by
plasmon-enhanced Raman scattering. _Nature_ 498, 82–86 (2013). CAS PubMed Google Scholar * Lee, J., Crampton, K. T., Tallarida, N. & Apkarian, V. A. Visualizing vibrational normal
modes of a single molecule with atomically confined light. _Nature_ 568, 78–82 (2019). CAS PubMed Google Scholar * van Schrojenstein Lantman, E. M., Deckert-Gaudig, T., Mank, A. J. G.,
Deckert, V. & Weckhuysen, B. M. Catalytic processes monitored at the nanoscale with tip-enhanced Raman spectroscopy. _Nat. Nanotechnol._ 7, 583–586 (2012). PubMed Google Scholar *
Herrero, E., Buller, L. J. & Abruña, H. D. Underpotential deposition at single crystal surfaces of Au, Pt, Ag and other materials. _Chem. Rev._ 101, 1897–1930 (2001). CAS PubMed Google
Scholar * Lin, L. et al. A highly CO-tolerant atomically dispersed Pt catalyst for chemoselective hydrogenation. _Nat. Nanotechnol._ 14, 354–361 (2019). CAS PubMed Google Scholar * Pan,
M. et al. Model studies of heterogeneous catalytic hydrogenation reactions with gold. _Chem. Soc. Rev._ 42, 5002–5013 (2013). CAS PubMed Google Scholar * Kibler, L. A., Kleinert, M.,
Randler, R. & Kolb, D. M. Initial stages of Pd deposition on Au (_hkl_). Part I: Pd on Au (111). _Surf. Sci._ 443, 19–30 (1999). CAS Google Scholar * Chen, C., Hayazawa, N. &
Kawata, S. A 1.7 nm resolution chemical analysis of carbon nanotubes by tip-enhanced Raman imaging in the ambient. _Nat. Commun._ 5, 3312 (2014). PubMed Google Scholar * Su, H. et al.
Probing the local generation and diffusion of active oxygen species on a Pd/Au bimetallic surface by tip-enhanced Raman spectroscopy. _J. Am. Chem. Soc._ 142, 1341–1347 (2020). CAS PubMed
Google Scholar * Lopez, N., Łodziana, Z., Illas, F. & Salmeron, M. When Langmuir is too simple: H2 dissociation on Pd(111) at high coverage. _Phys. Rev. Lett._ 93, 146103 (2004). PubMed
Google Scholar * Groß, A. & Dianat, A. Hydrogen dissociation dynamics on precovered Pd surfaces: Langmuir is still right. _Phys. Rev. Lett._ 98, 206107 (2007). PubMed Google Scholar
* Lauhon, L. J. & Ho, W. Direct observation of the quantum tunneling of single hydrogen atoms with a scanning tunneling microscope. _Phys. Rev. Lett._ 89, 079901 (2002). Google Scholar
* Marshall, S. et al. Controlled selectivity for palladium catalysts using self-assembled monolayers. _Nat. Mater._ 9, 853–858 (2010). CAS PubMed Google Scholar * Abazari, R.,
Heshmatpour, F. & Balalaie, S. Pt/Pd/Fe trimetallic nanoparticle produced via reverse micelle technique: synthesis, characterization, and its use as an efficient catalyst for reductive
hydrodehalogenation of aryl and aliphatic halides under mild conditions. _ACS Catal._ 3, 139–149 (2013). CAS Google Scholar * de Pedro, Z. M., Casas, J. A., Gomez-Sainero, L. M. &
Rodriguez, J. J. Hydrodechlorination of dichloromethane with a Pd/AC catalyst: reaction pathway and kinetics. _Appl. Catal. B_ 98, 79–85 (2010). Google Scholar * Qian, X., Emory, S. R.
& Nie, S. Anchoring molecular chromophores to colloidal gold nanocrystals: surface-enhanced Raman evidence for strong electronic coupling and irreversible structural locking. _J. Am.
Chem. Soc._ 134, 2000–2003 (2012). CAS PubMed PubMed Central Google Scholar * Coq, B., Ferrat, G. & Figueras, F. Conversion of chlorobenzene over palladium and rhodium catalysts of
widely varying dispersion. _J. Catal._ 101, 434–445 (1986). CAS Google Scholar * Stadler, J., Schmid, T. & Zenobi, R. Nanoscale chemical imaging using top-illumination tip-enhanced
Raman spectroscopy. _Nano Lett._ 10, 4514–4520 (2010). CAS PubMed Google Scholar * Clavilier, J., Faure, R., Guinet, G. & Durand, R. Preparation of monocrystalline Pt microelectrodes
and electrochemical study of the plane surfaces cut in the direction of the {111} and {110} planes. _J. Electroanal. Chem._ 107, 205–209 (1979). Google Scholar * Weiss, E. A. et al.
Si/SiO2-templated formation of ultrafast metal surfaces on glass, polymer, and solder supports: their use as substrates for self-assembled monolayers. _Langmuir_ 23, 9686–9694 (2007). CAS
PubMed Google Scholar * Zhang, Y.-J. et al. Probing the electronic structure of heterogeneous metal interfaces by transition metal shelled gold nanoparticle-enhanced Raman spectroscopy.
_J. Phys. Chem. C._ 120, 20684–20691 (2016). CAS Google Scholar * Gyr, L., Klute, F. D., Franzke, J. & Zenobi, R. Characterization of a nitrogen-based dielectric barrier discharge
ionization source for mass spectrometry reveals factors important for soft ionization. _Anal. Chem._ 91, 6865–6871 (2019). CAS PubMed Google Scholar * Szczerbiński, J., Gyr, L., Kaeslin,
J. & Zenobi, R. Plasmon-driven photocatalysis leads to products known from E-beam and X-ray-induced surface chemistry. _Nano Lett._ 18, 6740–6749 (2018). PubMed Google Scholar *
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. _Phys. Rev. B_ 54, 11169 (1996). CAS Google Scholar *
Klimeš, J., Bowler, D. R. & Michaelides, A. Chemical accuracy for the van der Waals density functional. _J. Phys. Condens. Matter_ 22, 022201 (2010). PubMed Google Scholar * Carrasco,
J., Klimeš, J. & Michaelides, A. The role of van der Waals forces in water adsorption on metals. _J. Chem. Phys._ 138, 024708 (2013). PubMed Google Scholar * Berland, K. et al. van der
Waals forces in density functional theory: a review of the vdW-DF method. _Rep. Prog. Phys._ 78, 066501 (2015). PubMed Google Scholar * Klimeš, J., Bowler, D. R. & Michaelides, A. van
der Waals density functionals applied to solids. _Phys. Rev. B_ 83, 195131 (2011). Google Scholar * Henkelman, G., Uberuaga, B. P. & Jónsson, H. A climbing image nudged elastic band
method for finding saddle points and minimum energy paths. _J. Chem. Phys._ 113, 9901 (2000). CAS Google Scholar Download references ACKNOWLEDGEMENTS This work was supported financially by
the European Research Council program (grant number 741431—2DNanoSpec), the Natural Science Foundation of China (grant numbers 21925404, 21775127 and 21703181), the Fundamental Research
Funds for the Central Universities (20720190044) and MOST (2019YFA0705402). L.-Q.Z. was financially supported by the Chinese Scholarship Council for a PhD student fellowship. H.Y. was
financially supported by the Sino‐Swiss Science and Technology Cooperation program (grant number EG22‐122016). W.F. and J.O.R. are supported by the Swiss National Science Foundation (project
number 175696.) We thank A. Rossi (ETH Zurich) and G. Cossu (ETH Zurich) for help with the XPS measurements. DFT computations were supported by the High-Performance Computing Team at ETH
Zurich. H.Y. and L.-Q.Z. also thank A. Begley, J.B. Metternich, J. Szczerbińsky and J.A. van Bokhoven (all from ETH Zurich) for insightful discussions. H.Y. thanks W.-Q. Li (Xiamen
University) for the coverage measurements. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Chemistry and Applied Biosciences, ETH Zurich, Zurich, Switzerland Hao Yin, Li-Qing
Zheng, Wei Fang, Yin-Hung Lai, Nikolaus Porenta, Guillaume Goubert, Jeremy O. Richardson & Renato Zenobi * State Key Laboratory of Physical Chemistry of Solid Surfaces, Collaborative
Innovation Center of Chemistry for Energy Materials (iChEM), College of Chemistry and Chemical Engineering, College of Energy, College of Materials, Xiamen University, Xiamen, China Hao Yin,
Hua Zhang, Hai-Sheng Su, Bin Ren & Jian-Feng Li Authors * Hao Yin View author publications You can also search for this author inPubMed Google Scholar * Li-Qing Zheng View author
publications You can also search for this author inPubMed Google Scholar * Wei Fang View author publications You can also search for this author inPubMed Google Scholar * Yin-Hung Lai View
author publications You can also search for this author inPubMed Google Scholar * Nikolaus Porenta View author publications You can also search for this author inPubMed Google Scholar *
Guillaume Goubert View author publications You can also search for this author inPubMed Google Scholar * Hua Zhang View author publications You can also search for this author inPubMed
Google Scholar * Hai-Sheng Su View author publications You can also search for this author inPubMed Google Scholar * Bin Ren View author publications You can also search for this author
inPubMed Google Scholar * Jeremy O. Richardson View author publications You can also search for this author inPubMed Google Scholar * Jian-Feng Li View author publications You can also
search for this author inPubMed Google Scholar * Renato Zenobi View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS R.Z. and J.-F.L. supervised
the project. L.-Q.Z. conceived of the ideas. L.-Q.Z. and H.Y. designed the experiments. H.Y., L.-Q.Z. and N.P. performed the experiments. W.F. and J.O.R. performed the DFT calculations.
Y.-H.L. and L.-Q.Z. performed the TPD-MS experiments. G.G., H.-S.S. and B.R. contributed to the electrochemistry. H.Y., L.-Q.Z. and W.F. wrote the manuscript with the help of G.G. and H.Z.
All authors discussed the results and commented on the manuscript. CORRESPONDING AUTHORS Correspondence to Li-Qing Zheng, Jeremy O. Richardson, Jian-Feng Li or Renato Zenobi. ETHICS
DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in
published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Figs. 1–25, discussion and Tables 1 & 2. SUPPLEMENTARY DATA 1 Cartesian
coordinates (Å) for the optimized geometries in DFT calculations. SOURCE DATA SOURCE DATA FIG. 1 Cyclic voltammetry data and Raman signals for Fig. 1. SOURCE DATA FIG. 2 Raw spectrum data
without background subtraction for Fig. 2. SOURCE DATA FIG. 3 Statistical source data for Fig. 3. SOURCE DATA FIG. 4 Statistical source data for Fig. 4. SOURCE DATA FIG. 5 Statistical source
data for Fig. 5. SOURCE DATA FIG. 6 Statistical source data for Fig. 6. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Yin, H., Zheng, LQ., Fang, W.
_et al._ Nanometre-scale spectroscopic visualization of catalytic sites during a hydrogenation reaction on a Pd/Au bimetallic catalyst. _Nat Catal_ 3, 834–842 (2020).
https://doi.org/10.1038/s41929-020-00511-y Download citation * Received: 21 January 2020 * Accepted: 06 August 2020 * Published: 21 September 2020 * Issue Date: October 2020 * DOI:
https://doi.org/10.1038/s41929-020-00511-y SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative