Play all audios:
ABSTRACT The oxidation of methane, the main component of natural gas, to selectively form oxygenated chemical feedstocks using molecular oxygen has been a long-standing grand challenge in
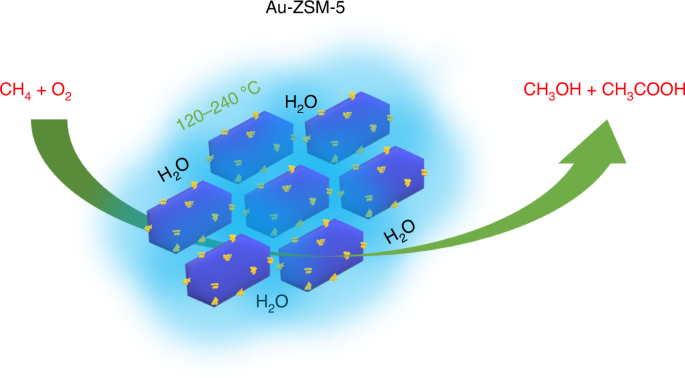
catalysis. Here, using gold nanoparticles supported on the zeolite ZSM-5, we introduce a method to oxidize methane to methanol and acetic acid in water at temperatures between 120 and 240 °C
using molecular oxygen in the absence of any added coreductant. Electron microscopy reveals that the catalyst does not contain gold atoms or clusters, but rather gold nanoparticles are the
active component, while a mechanism involving surface adsorbed species is proposed in which methanol and acetic acid are formed via parallel pathways. Access through your institution Buy or
subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get
Nature+, our best-value online-access subscription $32.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 digital issues and online access to articles $119.00 per
year only $9.92 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during
checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS METHYL RADICAL
CHEMISTRY IN NON-OXIDATIVE METHANE ACTIVATION OVER METAL SINGLE SITES Article Open access 15 September 2023 IDENTIFYING THE NATURE OF THE ACTIVE SITES IN METHANOL SYNTHESIS OVER CU/ZNO/AL2O3
CATALYSTS Article Open access 04 August 2020 WATER ENABLES MILD OXIDATION OF METHANE TO METHANOL ON GOLD SINGLE-ATOM CATALYSTS Article Open access 22 February 2021 DATA AVAILABILITY All
data used in this publication are available free of charge from Cardiff University via https://doi.org/10.17035/d.2021.0142278187 or available from the authors upon reasonable request.
Source data are provided with this paper. REFERENCES * Hammer, G. et al. _Natural Gas in Ullmann’s Encyclopaedia of Industrial Chemistry_ (Wiley-VCH, 2012). Google Scholar * Gesser, H. D.,
Hunter, N. R. & Prakash, C. B. The direct conversion of methane to methanol by controlled oxidation. _Chem. Rev._ 85, 235–244 (1985). Article CAS Google Scholar * Conley, B. L. et al.
Design and study of homogenous catalysts for the selective, low temperature oxidation of hydrocarbons. _J. Mol. Cat. A Chem._ 251, 8–23 (2006). Article CAS Google Scholar * Hargreaves,
J. S. J., Hutchings, G. J. & Joyner, R. W. Control of product selectivity in the partial oxidation of methane. _Nature_ 348, 428–429 (1990). Article CAS Google Scholar * Periana, R.
A. et al. A mercury-catalyzed, high-yield system for the oxidation of methane to methanol. _Science_ 259, 340–343 (1993). Article CAS PubMed Google Scholar * Periana, R. A. et al.
Platinum catalysts for the high-yield oxidation of methane to a methanol derivative. _Science_ 280, 560–564 (1998). Article CAS PubMed Google Scholar * Sobolev, V. I., Dubkov, K. A.,
Panna, O. V. & Panov, G. I. Selective oxidation of methane to methanol on a FeZSM-5 surface. _Catal. Today_ 24, 251–252 (1995). Article CAS Google Scholar * Starokon, E. V. et al.
Oxidation of methane to methanol on the surface of FeZSM-5 zeolite. _J. Catal._ 300, 47–54 (2013). Article CAS Google Scholar * Starokon, E. V., Parfenov, M. V., Pirutko, L. V., Abornev,
S. I. & Panov, G. I. Room-temperature oxidation of methane by α-oxygen and extraction of products from the FeZSM-5 surface. _J. Phys. Chem. C_ 115, 2155–2161 (2011). Article CAS Google
Scholar * Parfenov, M. V., Starokon, E. V., Pirutko, L. V. & Panov, G. I. Quasicatalytic and catalytic oxidation of methane to methanol by nitrous oxide over FeZSM-5 zeolite. _J.
Catal._ 318, 14–21 (2014). Article CAS Google Scholar * Hammond, C. et al. Direct catalytic conversion of methane to methanol in an aqueous medium by using copper-promoted Fe-ZSM-5.
_Angew. Chem. Int. Ed._ 51, 5129–5133 (2012). Article CAS Google Scholar * Yu, T. et al. Highly selective oxidation of methane into methanol over Cu-promoted monomeric Fe/ZSM-5. _ACS
Catal._ 11, 6684–6691 (2021). Article CAS Google Scholar * Narsimhan, K. et al. Methane to acetic acid over Cu-exchanged zeolites: mechanistic insights from a site-specific carbonylation
reaction. _J. Am. Chem. Soc._ 137, 1825–1832 (2015). Article CAS PubMed PubMed Central Google Scholar * Shan, J., Li, M., Allard, L. F., Lee, S. & Flytzani-Stephanopoulos, M. Mild
oxidation of methane to methanol or acetic acid on supported isolated rhodium catalysts. _Nature_ 551, 605–608 (2017). Article CAS PubMed Google Scholar * Tang, Y. et al. Single rhodium
atoms anchored in micropores for efficient transformation of methane under mild conditions. _Nat. Commun._ 9, 1231 (2018). Article PubMed PubMed Central Google Scholar * Jin, Z. et al.
Hydrophobic zeolite modification for in situ peroxide formation in methane oxidation to methanol. _Science_ 367, 193–197 (2020). Article CAS PubMed Google Scholar * Agarwal, N. et al.
Aqueous Au-Pd colloids catalyze selective CH4 oxidation to CH3OH with O2 under mild conditions. _Science_ 358, 223–226 (2017). Article CAS PubMed Google Scholar * Groothaert, M. H.,
Smeets, P. J., Sels, B. F., Jacobs, P. A. & Schoonheydt, R. A. Selective oxidation of methane by the bis(μ-oxo)dicopper core stabilized on ZSM-5 and mordenite zeolites. _J. Am. Chem.
Soc._ 127, 1394–1395 (2005). Article CAS PubMed Google Scholar * Patrick, T. et al. Isothermal cyclic conversion of methane into methanol over copper-exchanged zeolite at low
temperature. _Angew. Chem. Int. Ed._ 55, 5467–5471 (2016). Article Google Scholar * Grundner, S. et al. Single-site trinuclear copper oxygen clusters in mordenite for selective conversion
of methane to methanol. _Nat. Commun._ 6, 7546 (2015). Article PubMed Google Scholar * Sushkevich, V. L., Palagin, D., Ranocchiari, M. & van Bokhoven, J. A. Selective anaerobic
oxidation of methane enables direct synthesis of methanol. _Science_ 356, 523–527 (2017). Article CAS PubMed Google Scholar * Narsimhan, K., Iyoki, K., Dinh, K. & Román-Leshkov, Y.
Catalytic oxidation of methane into methanol over copper-exchanged zeolites with oxygen at low temperature. _ACS Cent. Sci._ 2, 424–429 (2016). Article CAS PubMed PubMed Central Google
Scholar * Dinh, K. T. et al. Continuous partial oxidation of methane to methanol catalyzed by diffusion-paired copper dimers in copper-exchanged zeolites. _J. Am. Chem. Soc._ 141,
11641–11650 (2019). Article CAS PubMed Google Scholar * Koishybay, A. & Shantz, D. F. Water is the oxygen source for methanol produced in partial oxidation of methane in a flow
reactor over Cu-SSZ-13. _J. Am. Chem. Soc._ 142, 11962–11966 (2020). Article CAS PubMed Google Scholar * Sarv, P. et al. Mobility of the acidic proton in Brønsted sites of H-Y,
H-mordenite, and H-ZSM-5 zeolites, studied by high-temperature 1H MAS NMR. _J. Phys. Chem._ 99, 13763–13768 (1995). Article CAS Google Scholar * Herzing, A. A., Kiely, C. J., Carley, A.
F., Landon, P. & Hutchings, G. J. Identification of active gold nanoclusters on iron oxide supports for CO oxidation. _Science_ 321, 1331–1335 (2008). Article CAS PubMed Google
Scholar * He, Q. et al. Population and hierarchy of active species in gold iron oxide catalysts for carbon monoxide oxidation. _Nat. Commun._ 7, 12905 (2016). Article CAS PubMed PubMed
Central Google Scholar * Jin, R. et al. Low temperature oxidation of ethane to oxygenates by oxygen over iridium-cluster catalysts. _J. Am. Chem. Soc._ 141, 18921–18925 (2019). Article
CAS PubMed Google Scholar * Gouget, A. et al. Increased dispersion of supported gold during methanol carbonylation. _J. Am. Chem. Soc._ 131, 6973–6975 (2009). Article Google Scholar *
Denisov, E. T. & Shestakov, A. F. Reactions of alkoxy and peroxy radicals with carbon monoxide. _Kinet. Catal._ 49, 1–10 (2008). Article CAS Google Scholar * Boronat, M., Concepción,
P. & Corma, A. Unravelling the nature of gold surface sites by combining IR spectroscopy and DFT calculations. Implications in catalysis. _J. Phys. Chem. C_ 113, 16772–16784 (2009).
Article CAS Google Scholar * Liu, Z.-P., Hu, P. & Alavi, A. Catalytic role of gold in gold-based catalysts: a density functional theory study on the CO oxidation on gold. _J. Am.
Chem. Soc._ 124, 14770–14779 (2002). Article CAS PubMed Google Scholar * Cooper, C. M. & Wiezevich, P. J. Effects of temperature and pressure on the upper explosive limit of
methane-oxygen mixtures. _Ind. Eng. Chem._ 21, 1210–1214 (1929). Article CAS Google Scholar * Liu, M. et al. Improved WATERGATE pulse sequences for solvent suppression in NMR
spectroscopy. _J. Magn. Reson._ 132, 125–129 (1998). Article CAS Google Scholar * Sarradin, P.-M. & Caprais, J.-C. Analysis of dissolved gases by headspace sampling gas chromatography
with column and detector switching. Preliminary results. _Anal. Commun._ 33, 371–373 (1996). Article CAS Google Scholar * Kresse, G. & Hafner, J. _Ab initio_ molecular dynamics for
liquid metals. _Phys. Rev. B_ 47, 558–561 (1993). Article CAS Google Scholar * Kresse, G. & Furthmüller, J. Efficient iterative schemes for _ab initio_ total-energy calculations using
a plane-wave basis set. _Phys. Rev. B_ 54, 11169–11186 (1996). Article CAS Google Scholar * Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple.
_Phys. Rev. Lett._ 77, 3865–3868 (1996). Article CAS Google Scholar * Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate _ab initio_ parametrization of density
functional dispersion correction (DFT-D) for the 94 elements H-Pu. _J. Chem. Phys._ 132, 154104 (2010). Article PubMed Google Scholar * Kresse, G. & Joubert, D. From ultrasoft
pseudopotentials to the projector augmented-wave method. _Phys. Rev. B_ 59, 1758–1775 (1999). Article CAS Google Scholar * Blöchl, P. E. Projector augmented-wave method. _Phys. Rev. B_
50, 17953–17979 (1994). Article Google Scholar * Mills, G., Jónsson, H. & Schenter, G. K. Reversible work transition state theory: application to dissociative adsorption of hydrogen.
_Surf. Sci._ 324, 305–337 (1995). Article CAS Google Scholar * Hjorth Larsen, A. et al. The atomic simulation environment—a Python library for working with atoms. _J. Phys. Condens.
Matter_ 29, 273002 (2017). Article PubMed Google Scholar * Henkelman, G. & Jónsson, H. A dimer method for finding saddle points on high dimensional potential surfaces using only first
derivatives. _J. Chem. Phys._ 111, 7010–7022 (1999). Article CAS Google Scholar * Kästner, J. & Sherwood, P. Superlinearly converging dimer method for transition state search. _J.
Chem. Phys._ 128, 014106 (2008). Article PubMed Google Scholar * Thetford, A., Hutchings, G. J., Taylor, S. H. & Willock, D. J. The decomposition of H2O2 over the components of
Au/TiO2 catalysts. _Proc. R. Soc. Math. Phys. Eng. Sci._ 467, 1885–1899 (2011). CAS Google Scholar Download references ACKNOWLEDGEMENTS This work was financially supported by the National
Natural Science Foundation of China (grants U1932218, 21872170, 21733013 and 22061130202), as part of the Key projects of international partnership plan for foreign cooperation programme
(112942KYSB20180009). J.X. thanks the Royal Society and the Newton Fund for Royal Society—Newton Advanced Fellowship. G.J.H. acknowledges the support from the Chinese Academy of Sciences
President’s International Fellowship Initiative (grant no. 2019DM0015). Q.H. thanks the National Research Foundation Singapore for support under its NRF Fellowship (NRF-NRFF11-2019-0002). We
thank Cardiff University and the Max Planck Centre for Fundamental Heterogeneous Catalysis (FUNCAT) for financial support. G.J.H., D.J.W. and C.R.A.C. thank the Engineering and Physical
Sciences Research Council for funding this work (grant reference codes EP/P033695/1 and EP/L027240/1). Via our membership of the United Kingdom’s HEC Materials Chemistry Consortium, which is
funded by the Engineering and Physical Sciences Research Council (EP/L000202 and EP/R029431), this work used the ARCHER UK National Supercomputing Service (http://www.archer.ac.uk) and the
UK Materials and Molecular Modelling Hub, which is partially funded by the Engineering and Physical Sciences Research Council (EP/P020194), for computational resources. AUTHOR INFORMATION
AUTHORS AND AFFILIATIONS * National Centre for Magnetic Resonance in Wuhan, State Key Laboratory of Magnetic Resonance and Atomic and Molecular Physics, Innovation Academy for Precision
Measurement Science and Technology, Chinese Academy of Sciences, Wuhan, China Guodong Qi, Xingling Zhao, Feng Deng & Jun Xu * University of Chinese Academy of Sciences, Beijing, China
Guodong Qi, Xingling Zhao & Jun Xu * Max Planck-Cardiff Centre on the Fundamentals of Heterogeneous Catalysis FUNCAT, Cardiff Catalysis Institute, School of Chemistry, Cardiff
University, Cardiff, UK Thomas E. Davies, Ali Nasrallah, Mala A. Sainna, Richard J. Lewis, Matthew Quesne, C. Richard A. Catlow, David J. Willock, Donald Bethell, Mark J. Howard, Barry A.
Murrer, Brian Harrison & Graham J. Hutchings * National University of Singapore, Singapore, Singapore Alexander G. R. Howe & Qian He * Department of Materials Science and
Engineering, Lehigh University, Bethlehem, PA, USA Christopher J. Kiely Authors * Guodong Qi View author publications You can also search for this author inPubMed Google Scholar * Thomas E.
Davies View author publications You can also search for this author inPubMed Google Scholar * Ali Nasrallah View author publications You can also search for this author inPubMed Google
Scholar * Mala A. Sainna View author publications You can also search for this author inPubMed Google Scholar * Alexander G. R. Howe View author publications You can also search for this
author inPubMed Google Scholar * Richard J. Lewis View author publications You can also search for this author inPubMed Google Scholar * Matthew Quesne View author publications You can also
search for this author inPubMed Google Scholar * C. Richard A. Catlow View author publications You can also search for this author inPubMed Google Scholar * David J. Willock View author
publications You can also search for this author inPubMed Google Scholar * Qian He View author publications You can also search for this author inPubMed Google Scholar * Donald Bethell View
author publications You can also search for this author inPubMed Google Scholar * Mark J. Howard View author publications You can also search for this author inPubMed Google Scholar * Barry
A. Murrer View author publications You can also search for this author inPubMed Google Scholar * Brian Harrison View author publications You can also search for this author inPubMed Google
Scholar * Christopher J. Kiely View author publications You can also search for this author inPubMed Google Scholar * Xingling Zhao View author publications You can also search for this
author inPubMed Google Scholar * Feng Deng View author publications You can also search for this author inPubMed Google Scholar * Jun Xu View author publications You can also search for this
author inPubMed Google Scholar * Graham J. Hutchings View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS J.X. and G.J.H. conceived the
research idea and organized the research programmes. G.Q. and R.J.L. prepared catalyst samples; G.Q., X.Z. and F.D. performed the catalytic experiments and NMR analysis; T.E.D., Q.H. and
A.G.R.H. obtained electron microscopy data under the direction of C.J.K.; D.J.W., A.N., M.A.S. and M.Q. carried out most of the computational chemistry calculations. D.B. and M.J.H. provided
mechanistic interpretation of results along with C.R.A.C. and D.J.W., who integrated experimental and computational insights. B.A.M. and B.H. provided advice on the industrial context of
the work. J.X., G.J.H. and D.J.W. wrote the paper, and all authors discussed the results and the various revisions of the manuscript. J.X. and G.J.H. contributed equally to this work.
CORRESPONDING AUTHORS Correspondence to Jun Xu or Graham J. Hutchings. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. PEER REVIEW INFORMATION _Nature
Catalysis_ thanks the anonymous reviewers for their contribution to the peer review of this work. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Figs. 1–25, Tables 1–15, Notes 1 and 2 and
references. SOURCE DATA SOURCE DATA FIG. 1 Statistical source data. SOURCE DATA FIG. 2 Statistical source data. SOURCE DATA FIG. 5 Statistical source data. RIGHTS AND PERMISSIONS Reprints
and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Qi, G., Davies, T.E., Nasrallah, A. _et al._ Au-ZSM-5 catalyses the selective oxidation of CH4 to CH3OH and CH3COOH using O2. _Nat Catal_
5, 45–54 (2022). https://doi.org/10.1038/s41929-021-00725-8 Download citation * Received: 10 February 2021 * Accepted: 12 November 2021 * Published: 06 January 2022 * Issue Date: January
2022 * DOI: https://doi.org/10.1038/s41929-021-00725-8 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable
link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative