Play all audios:
Chinese herbal formulas including the lung-cleaning and toxicity-excluding (LCTE) soup have played an important role in treating the ongoing COVID-19 pandemic (caused by SARS-CoV-2) in
China. Applying LCTE outside of China may prove challenging due to the unfamiliar rationale behind its application in terms of Traditional Chinese Medicine. To overcome this barrier, a
biochemical understanding of the clinical effects of LCTE is needed. Here, we explore the chemical compounds present in the reported LCTE ingredients and the proteins targeted by these
compounds via a network pharmacology analysis. Our results indicate that LCTE contains compounds with the potential to directly inhibit SARS-CoV-2 and inflammation, and that the compound
targets proteins highly related to COVID-19’s main symptoms. We predict the general effect of LCTE is to affect the pathways involved in viral and other microbial infections,
inflammation/cytokine response, and lung diseases. Our work provides a biochemical basis for using LCTE to treat COVID-19 and its main symptoms.
Towards the end of December 2019, a new type of pneumonia (COVID-19), first reported in Wuhan, China, was identified. It is caused by the novel coronavirus SARS-CoV-2 and transmitted from
human-to-human1,2,3. This virus has impacted countries worldwide within a very short time, prompting the World Health Organization (WHO) to pronounce it a worldwide pandemic on March 14,
2020. According to the WHO Daily Report, there have been a total of 142,649 confirmed COVID-19 cases with 5393 deaths in nearly 135 countries, areas or territories as of this writing on
March 14, 2020. This pandemic is ongoing, so quickly identifying new preventive and therapeutic agents is a top priority.
Specific vaccines and antiviral agents are the most effective methods for preventing and treating viral infections, yet no such anti-SARS-CoV-2 agents are currently available. Development of
these treatments may require months or years, meaning that a more immediate treatment should be found if at all possible. Herbs used in Traditional Chinese Medicine (TCM) present a
potentially valuable resource to this end. The effectiveness of herbal treatment in controlling contagious disease was demonstrated during the 2003 severe acute respiratory syndrome (SARS)
outbreak4. As such, the Chinese government has encouraged the use of herbal medication in fighting this new viral pneumonia, which has brought about good clinical results5. The State
Administration of TCM reported to official media (chinadaily.com.cn, March 6, 2020) that up to February 17 a total of 60,107 patients with SARS-CoV-2 infections, accounting for 85.2% of the
infections, had been treated with TCM nationwide. The Chinese government, encouraged by the evident clinical benefits5, has recommended several herbal formulas in its continuously modified
plans to prevent and treat SARS-CoV-2 infection. Among these the lung-cleaning and toxicity-excluding soup formula (LCTE, called Qing Fei Pai Du Tang in China) has the highest
recommendation, based on its clinical effectiveness6 and simple preparation (the preparation protocol is provided as Supplementary Data 1). Containing 20 herbal plants and one mineral
component (detailed in the following section), LCTE has been officially recommended by Chinese government since February 6, 2020 and remains on the list of recommended treatments in the 7th,
most recent version of the National Plan for Preventing and Treating SARS-CoV-2 Infection.
At present, the occurrence rate for COVID-19 infection is declining in China while soaring in some other parts of the world. The successful application of LCTE in China suggests this formula
as an attractive means for fighting COVID-19. Yet, the rationale for LCTE application may be difficult to understand when explained in terms of TCM, making it less approachable to modern
medical society, especially outside of China. This barrier severely limits the potential benefits gained by introducing LCTE treatment to medical professionals treating COVID-19 worldwide.
Fortunately, thanks to advancements in identifying the chemical compounds contained in Chinese herbs7,8,9 and the emergence of network pharmacology technology10, the mechanisms behind LCTE’s
effects may be explained through chemical biology. It is well realized that the therapeutic effects of herbal treatments are due to the pharmacological compounds contained in them11. For
example, it has been proven that the anti-malarial effect of Artemisia apiacea is due to its component artemisinin12. Likewise, the curative effects of LCTE likely reside in the chemical
compounds contained in the formula’s plant ingredients. Network pharmacology highlights a multiple drug component/multiple target model over the single component/single target model in drug
development and assessment10. The use of this modeling has evolved and expanded over the last several decades13. Traditional Chinese herbal formulas, which potentially contain many active
compounds, are a typical example of the network pharmacology paradigm14,15.
In this work, we investigate the potential working mechanisms behind LCTE’s effectiveness in modern biochemical language. We achieved this by screening the compounds of related plants and
undertaking a network pharmacology analysis. Our work shows that two of the main compounds contained in LCTE have the potential to directly inhibit SARS-CoV-2, and that most of the active
constituent compounds are anti-inflammatory. Moreover, these compounds evidently target the proteins related to the main symptoms of COVID-19. The general in vivo therapeutic effect of LCTE
is predicted to be regulation of pathways related to viral and other microbial infection, inflammation/cytokine response, and lung diseases.
It is well held that Chinese herbal plants contain bioactive compounds and that the therapeutic effects of herbal treatments are achieved via compound/target interaction. We started our work
by researching the chemical compounds contained in LCTE and their protein targets. The LCTE formula consists of 20 herbal ingredients and one mineral material (raw gypsum, Rudis Gypsi
Miscueris; Table 1). The main component of raw gypsum is inorganic CaSO4·2H2O, and the inclusion of gypsum in the formula is understandable considering findings that it reduces body
temperature16 and attenuates heat-induced hypothalamic inflammation via down-regulation of IL-1β17. As such, we focused our study on the 20 herbal plants used to formulate LCTE. We found a
complete chemical compound list for each plant as recorded in three Chinese herbal databases7,8,9 (detailed in the Methods section). The listed compounds were then filtered using ADME
(absorption, distribution, metabolism, and excretion) indices to find which might be absorbable via oral administration18. ADME filtration for oral bioavailability is necessary in that most
TCM formulations are prepared by boiled herbs with water and the resultant soup is then orally administered. The passing rates ranged from 0.026 to 0.37 with an average of 0.11 (Table 1),
indicating that the majority of the compounds present in the related plants would not be absorbed. After finding which compounds were most likely to be absorbed, we again checked the three
Chinese herbal databases to find which proteins are affected by each of these compounds. Each herbal ingredient, their total number of compounds, number of orally absorbable compounds, and
their number of protein targets are listed in Table 1. More detailed information on the plant contained compounds passing filtration and the proteins targeted by compounds are available in
Supplementary Data 2 and Supplementary Data 3.
In all, the 20 herbal ingredients in LCTE contain 207 different chemical components absorbable through oral administration. In total, 27 of these components are present in at least 2 of the
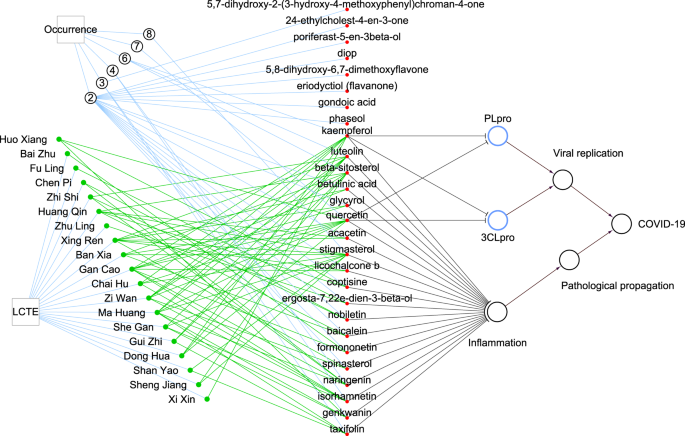
plant sources. Five components were of high concurrence, existing in 6 or more plant sources (Supplementary Data 4). Stigmasterol was present in eight plants, quercetin was present in seven
plants, and luteolin, beta-sitosterol, and kaempferol were present in six plants (Fig. 1). Also worth noting is that we recently found two of the five most prevalent compounds, kaempferol
and quercetin, have the potential to directly inhibit papain-like protease (PLpro) and 3C-like protease (3CLpro), two enzymes critical to the replication of the COVID-19-causing pathogen
SARS-CoV-219.
The circled number indicates the number of occurrences of compounds contained in multiple plants, for example, quercetin is contained in seven plants. Black lines with a blunt end indicate
that the source vertex has inhibiting effects on the target vertex, while those ending in an arrow have activation effects. Green networks connect herbal ingredients to the main chemical
compounds contained in the plants. PLpro and 3 CLpro stand for papain-like protease and 3C-like protease of SARS-CoV-2, respectively.
COVID-19’s basic pathology is viral-caused inflammation. Based on publications in PubMed, we found that 19 of the 27 compounds present in 2 or more of the LCTE herbal plants possess
anti-inflammatory properties (Supplementary Data 4). The indication is that the main compounds of LCTE have the potential to directly inhibit SARS-CoV-2 and down-regulate inflammation (Fig.
1).
Fifteen main symptoms have been reported in COVID-19 patients20,21. These include fever, cough, myalgia, fatigue, dyspnea, among others, and may be catalogued as general, respiratory and
digestive symptoms. The LCTE formula’s clinical effectiveness in relieving these symptoms has been reported in China6. To understand the molecular basis for LCTE’s relief of COVID-19
symptoms, we used DisGeNET22 to download the proteins related to each symptom and then studied the correspondence between these and the protein targets of LCTE’s orally-absorbable compounds.
The exact proteins related to each symptom and the results for the DisGeNet search query ‘viral respiratory infection’ are available in Supplementary Data 5.
Hypergeometric distribution probability computation showed that the component-targeted proteins were significantly enriched in 11 of the symptoms-related proteins (adjusted P 30%, >–0.4,
>0.18, and >3 h, respectively as recommended18. The values of these four indices can be obtained from the TCMSP database. Each compound passing ADME screening was cross-checked in the three
aforementioned Chinese herbal databases to find the compound’s protein targets.
The proteins related to the main symptoms of COVID-19 were found through searching the DisGeNET (https://www.disgenet.org/)22 database using the name of each symptom as input. Since COVID-19
is a viral respiratory infection, we also searched the DisGeNET database using the input ‘viral respiratory infection’ to find the related proteins.
Literature about the anti-inflammation properties of the main compounds was retrieved by searching PubMed with each chemical compound’s name and ‘inflammation’ as query terms.
Enrichment of the COVID-19 symptom-related proteins targeted by LCTE ingredients were assessed by the probability (P) value of hypergeometric distribution:
k represents the number of overlapping proteins between the protein set targeted by LCTE-contained compounds and the set of protein related to each symptom or ‘viral respiratory infection’.
M represents the number of proteins in the set related to a single symptom or ‘viral respiratory infection’, n stands for the number of proteins in the set targeted by LCTE ingredients. N
(the total number of proteins) was set as 17,549, which is the number of genes included in the latest version of DisGeNET RDF (v6.0). The adjusted P value was obtained with the p.adjust
function of R package stats.
The constructions, degree calculations, and frequencies of vertices in the shortest pathways of the plants-compound-protein-symptom network were performed using R package igraph (v1.2.4.2)
and the results are presented with Cytoscape (v3.7.2). The network constructions are based on using these data as edges: herbal plants and their corresponding chemical compounds, chemical
compounds and their affected proteins, and proteins related to each symptom. For specific symptoms or ‘viral respiratory infection’, the network was constructed independently. When
constructing a network for a specific symptom or ‘viral respiratory infection’, the proteins used were different in that only the proteins related to the symptom or ‘viral respiratory
infection’ were included in the construction. The shortest pathways are the ones with the least vertices between the vertex of “LCTE” and the vertex of the symptom or ‘viral respiratory
infection’. This information was extracted by the function ‘get.all.shortest.paths’ of R package igraph. After extracting the shortest pathways, the frequency of each vertex in the shortest
pathways was calculated.
To evaluate the superiority of LCTE in treatment, in this study, for each symptom and for ‘viral respiratory infection’, we compared the total number of shortest pathways in the actual LCTE
network to 1,000,000 random networks similar to the original. LCTE has one unique combination of compounds in that each distinct compound appears a certain number of times in the formula;
i.e.: 1 of these compounds appears eight times, 1 appears seven times, 3 appear six times, 2 appear four times, 3 appear three times, 17 appear twice, and a majority, 180 compounds, are
present only once. Our control networks were constructed based on random combinations of these compounds. Each random combination of compounds was made by selecting only from the 207
compounds found in the real LCTE formula. The frequency ratios remained the same as well, but we randomly assigned which compound occurred at which frequency. This process was accomplished
via the sampling without replacement method in which we randomly drew 1, 1, 3, 2, 3, and 17 compounds and assigned them with frequencies of 8, 7, 6, 4, 3, and 2 times, respectively. The
remaining 180 compounds were set to a frequency of one (as per above). Based on the features of the network and to reduce the workload, we used the following equation to find the total
number of shortest pathways from the random formula’s vertex to the vertex of one symptom or ‘viral respiratory infection’ in each network:
In the above equation, the number of shortest pathways from the vertex of ith compound to one symptom (or ‘viral respiratory infection’) for the actual LCTE network was obtained with R
package igraph software. After finding the total number of the shortest pathways for each random network, the distribution percentile for the total number of shortest pathways in the
1,000,000 random networks was calculated.
The general effects of LCTE were predicted by KEGG pathway and Disease Ontology (DO) enrichment. The full list of proteins targeted by the LCTE-containing compounds is mapped to these two
databases, and significantly enriched results (with the adjusted P value cutoff set to 0.05) were extracted. The analyses were carried out by the R package clusterProfiler (v3.14.2)
functions enrichKEGG and enrichDO and the dependent R package was installed and activated in advance as instructed.
All data were processed using statistical language R (v3.6.2) unless otherwise specified. Protein enrichment, KEGG pathway enrichment, and DO disease enrichment have been detailed above. The
shortest pathways in the real LCTE formula network were extracted by package igraph, and the shortest pathways for the random control networks were calculated using the equation given
above. The percentiles of the total number of shortest pathways for the random controls were calculated with quantile function of R package stats (v3.6.2). Adjusted P