Play all audios:
ABSTRACT In clinical practice, human sleep is classified into stages, each associated with different levels of muscular activity and marked by characteristic patterns in the EEG signals. It
is however unclear whether this subdivision into discrete stages with sharply defined boundaries is truly reflecting the dynamics of human sleep. To address this question, we consider
one-channel EEG signals as heterogeneous random walks: stochastic processes controlled by hyper-parameters that are themselves time-dependent. We first demonstrate the heterogeneity of the
random process by showing that each sleep stage has a characteristic distribution and temporal correlation function of the raw EEG signals. Next, we perform a super-statistical analysis by
computing hyper-parameters, such as the standard deviation, kurtosis, and skewness of the raw signal distributions, within subsequent 30-second epochs. It turns out that also the
hyper-parameters have characteristic, sleep-stage-dependent distributions, which can be exploited for a simple Bayesian sleep stage detection. Moreover, we find that the hyper-parameters are
not piece-wise constant, as the traditional hypnograms would suggest, but show rising or falling trends within and across sleep stages, pointing to an underlying continuous rather than
sub-divided process that controls human sleep. Based on the hyper-parameters, we finally perform a pairwise similarity analysis between the different sleep stages, using a quantitative
measure for the separability of data clusters in multi-dimensional spaces. SIMILAR CONTENT BEING VIEWED BY OTHERS SLOW WAVE SYNCHRONIZATION AND SLEEP STATE TRANSITIONS Article Open access 06
May 2022 A SET OF COMPOSITE, NON-REDUNDANT EEG MEASURES OF NREM SLEEP BASED ON THE POWER LAW SCALING OF THE FOURIER SPECTRUM Article Open access 21 January 2021 OVERNIGHT DYNAMICS IN
SCALE-FREE AND OSCILLATORY SPECTRAL PARAMETERS OF NREM SLEEP EEG Article Open access 01 November 2022 INTRODUCTION Many complex systems, such as the earth’s crust, the weather, biological
organisms, or the stock market, show continuous fluctuations of their internal state variables, even in the absence of external perturbations. The underlying processes can often be
quantified in the form of multivariate time series and a mathematical analysis of the time series can be used to predict future states of the system, or simply to better understand its
internal dynamics1,2,3. Although in simple physical systems, state variables fluctuate around a fixed mean value and with a fixed variance (as in the case of local pressure variations in a
gas at equilibrium), complex systems often have multiple dynamical attractors4, i.e., a set of qualitatively different modes of behavior, between which the system will occasionally switch.
Such transitions typically show up in the time series by a sudden (or gradual) change of the statistical properties of the fluctuating state variables. A typical example of such
mode-switching behavior is the sleep cycle in humans and other mammals, where the brain is passing through a sequence of seemingly distinct sleep stages5. In this case, multi-channel
electroencephalographic (EEG) recordings offer a convenient way to quantify the ongoing changes in the brain over long periods, but also with high temporal resolution6,7. The momentary
amplitudes of an N-channel EEG recording represent a point in an N-dimensional state space and the ongoing time series of vectorial amplitudes defines a random walk within this
high-dimensional space. It is then a natural hypothesis that each sleep stage corresponds to a different cluster within EEG state space, and that the trajectory of the random walk moves to
the corresponding cluster whenever a new sleep stage is entered. Indeed, we have confirmed this hypothesis in former work8, where we applied a previously developed method for analyzing and
comparing spatiotemporal cortical activation patterns9. In this context, we have also analyzed the micro-structure of cortical activity during sleep and found that it reflects respiratory
events and the state of daytime vigilance10. Moreover, we have developed a general method to quantify the separability of point clusters in high-dimensional state spaces11. In principle, the
existence of sleep-stage-related clusters within the EEG state space could be exploited for an automatic detection of these stages, based only on the momentary multi-channel amplitudes, or
on short-time averages of those. However, modern methods of automatic sleep-stage detection are usually based on sliding time windows of a larger width, so that the algorithm can also make
use of temporal features in the EEG data that are characteristic for different sleep stages (such as sleep spindles or K-complexes)12,13. In this case, it is not even necessary to record a
large number of EEG channels. Indeed, we have shown that reliable sleep-stage detection is even possible based on a single channel14, thanks to the remarkable ability of machine learning
systems to extract those features from the data that are most relevant for the classification task. In this work, we continue our investigation of single-channel EEG data during human sleep.
However, our present focus is not on the further improvement of automatic sleep-stage detection, but on a more fundamental description of the statistical properties of EEG data, seen as a
temporally heterogeneous random walk. In particular, we investigate how the random walks momentary statistical properties, also called hyper-parameters, are changing during and across sleep
stages. Our approach is based on the method of super-statistical analysis15,16,17, which we have originally developed to analyze the random migration patterns of individual cancer cells18,
revealing that their average migration speed, the directional persistence of the cell trajectories, and other hyper-parameters are time-dependent, reflecting internal mode changes such as
the cell cycle. In subsequent work, we have demonstrated that the method can also be used to extract and model gradual or abrupt hyper-parameter changes in other complex dynamical systems,
such as the climate or the stock market19. In the present study, we apply a simplified version of super-statistical analysis to a set of full-night EEG recordings. Each 30 s epoch of these
recordings has been visually scored by a sleep specialist, according to the AASM (American Academy of Sleep Medicine) rules, so that the data is categorized into four different sleep stages
(REM, N1, N2, and N3) and the wake state. For each sleep-stage-labeled epoch, we compute from the single-channel recordings certain statistical hyper-parameters, such as the standard
deviation (STD), the kurtosis (KUR), and the skewness (SKE) of the EEG amplitude distributions. We show that also these hyper-parameters have characteristic, stage-dependent distributions,
which can be used for a simple Bayesian sleep-stage detection. Moreover, we find that the hyper-parameters are not piece-wise constant, as the traditional hypnograms would suggest.
Interestingly, they show rising or falling trends also within each of the sleep stages, pointing to an underlying continuous neural process that controls human sleep. RESULTS The results
presented throughout this study are based on 68 independent EEG data sets from sleeping human subjects, each recorded during a full night in the sleep lab of the University Hospital
Erlangen. The signals from the three EEG channels can be analyzed, in principle, on at least three different time scales: The shortest scale corresponds to the individual time steps _t_ of
the raw signals, which in our case have been recorded with a rate of 256 values per second and channel. The medium scale is that of epochs _n_, each with a duration of 30 s, corresponding to
7680 successive EEG values per channel. A sleep stage label \({S}_{n}\in
\left\{{{{{\mathrm{Wake}}}}},{{{{{\mathrm{REM}}}}}},{{{{{\mathrm{N1}}}}}},{{{{{\mathrm{N2}}}}}},{{{{{\mathrm{N3}}}}}}\right\}\) has been assigned to each of these epochs by a specialist. It
is noteworthy that, for simplicity, we include Wake to the list of sleep stages. Finally, the longest scale corresponds to sleep phases _J_, which we define as the largest non-interrupted
series of subsequent epochs where the subject remains in the same sleep stage _s_. It is noteworthy that, in contrast to time steps and epochs, sleep phases are time periods with a variable
duration. Our first goal was to establish that single-channel EEG signals _y__k_(_t_) during sleep can be considered as heterogeneous random walks, i.e., as random processes in which the
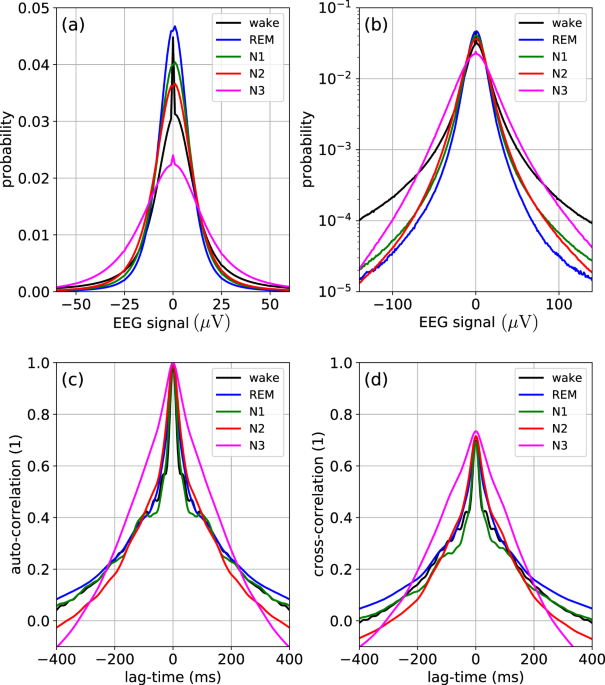
statistical properties change over time, and in particular differ between sleep stages. We therefore analyze, for each sleep stage _s_, the probability distributions _p__s_(_y_) of the raw
EEG amplitudes (Fig. 1a, b). The distributions are computed independently for each epoch and each channel, and finally all distributions with the same sleep stage label are pooled and
averaged. We find that the amplitude distributions _p__s_(_y_) are non-Gaussian and clearly leptocurtic for all sleep stages—an anomaly that is frequently found in complex systems with
super-statistical parameter changes18. In our case, due to the rather extreme tails, the excess KUR of these distributions is unusually large (KURwake ≈ 55, KURREM ≈ 234, KURN1 ≈ 154, KURN2
≈ 141, and KURN3 ≈ 46). Although the distributions for sleep stages REM, N1, and N2 are relatively similar to each other, the wake and N3 stages are considerably broader, which reflects the
heterogeneity of the underlying random process. Furthermore, we compute the autocorrelation function (ACF) _A__n_,_k_(Δ_t_) and the cross-correlation function _C__n_,1,2(Δ_t_) between
channels F4-M1 and C4-M1 (Fig. 1c, d). Here, too, the correlation functions are first computed independently for each epoch and later averaged. For all sleep stages, the EEG amplitudes show
positive temporal auto-correlations up to lag times of 300 ms and very similar results are found for the cross-correlation functions. Interestingly, the N3 stage again differs from the other
sleep stages, in that it has significantly stronger correlations for lag times shorter than about 200 ms. Having thus established the heterogeneous character of the raw EEG signals during
sleep, we turn to a super-statistical analysis and compute certain statistical hyper-parameters from each epoch of these raw signals. In particular, we consider as hyper-parameters the STD
of the amplitude distribution _p__s_(_y_), its excess KUR, its SKE, as well as the value of the ACF at specific lag time Δ_t_ = 300 ms, which yields relatively large differences between the
sleep stages. As a preliminary step, we compute the distributions of the STDs (STE), in the different sleep stages, for individual sleeping subjects (results for the first 4 of our 68 data
sets are shown in Fig. 2). In contrast to a stationary, temporally homogeneous random walk, where the parameter STE could be regarded as fixed (apart from weak sampling fluctuations), we
find in our case rather wide distributions that clearly differ between the sleep stages. The large fluctuation width of STE within the same sleep stage is pointing to dynamical changes of
brain activity that are going on continuously, rather than happening only at the transition points to new sleep stages. Although there is a large degree of variability among different
individuals (heterogeneity of the ensemble), the sleep-stage-specific characteristics of the distributions (temporal heterogeneity) are approximately conserved. A similar behavior is found
for all considered hyper-parameters (STD, KUR, SKE, and ACF) and we therefore have pooled the data over all individuals (Fig. 3). Here we find that for some of the hyper-parameters (KUR,
SKE, and ACF), the sleep-stage-specific differences are mainly visible in the tails of the distributions, correponding to the statistics of extreme values. In the next step, we inspect the
temporal evolution of the hyper-parameters (Fig. 4). We repeatedly observe extreme bursts that exceed the normal range of fluctuations (see shaded area (2) of Fig. 4). Moreover, we
frequently find that certain hyper-parameters rise or fall consistently within and also across sleep phases (see the evolution of the STD in the shaded areas (1) and (3) of Fig. 4). In order
to substantiate these anecdotal observations, we divide the 68 data sets into contiguous sleep phases, i.e., into the longest possible sequences of epochs where a subject remains within the
same sleep stage. In Table 1, we further distinguish between sleep stages in the falling and rising part of the sleep cycle. In each sleep phase, the sequence of hyper-parameters is
least-square-fitted by a linear function and the slope of this function, quantifying the overall trend of the hyper-parameter evolution, is extracted. Finally, we compute the mean slope and
its associated error, for each of the eight sleep stages along the cycle. Although the error often exceeds 50% of the mean, we find that some hyper-parameters have clear positive or negative
trends that are characteristic for each sleep stage. In the next step, we consider sleep as a random walk through the discrete state space of the five sleep stages. In this context, we have
computed the transition probabilities between subsequent sleep phases and between subsequent epochs, which are presented in the form of 5 × 5 transition matrices in Fig. 5a, b. The
resulting elements of the phase-to-phase transition matrix (a) show that every stage has a preferred successor stage (i.e., each row in the matrix has a single, clearly dominating entry).
This leads to the emergence of a default sequence of stages: Wake → N1 → N2. After this, an ongoing oscillation N2 ↔ N1 is most probable, followed by a final descent to N3, and eventually
the subject will again rise up towards the next wake state. In the case of the epoch-to-epoch transition matrix (b), all diagonal elements are close to one, reflecting the strong temporal
persistence of each sleep stage. The epoch-to-epoch transition matrix can be directly used to construct a first-order Markov model for the stochastic succession of sleep stages, as has
already been demonstrated before20. Using such a model, an arbitrary number of simulated hypnograms can be sampled and a typical example is shown in Fig. 5c. By comparing certain
higher-level features of simulated hypnograms (such as the number of oscillations between the non-REM sleep stages) with those of actual data, it may be possible to test the validity of the
first-order Markov process as a model for sleep in future work. Moreover, it may be possible to define a quantitative measure of sleep quality, based on the 25 entries of an individuals
epoch-to-epoch transition matrix, compared to the corresponding values in a reference group of healthy sleepers. The fact that each sleep stage has a specific distribution of
hyper-parameters (compare again Fig. 3) does not only confirm the heterogeneous, non-stationary character of the full-night EEG signals, but it can also be exploited for an automated sleep
stage detection. As a proof-of-concept, we have implemented a simple Bayesian sleep stage detector, which uses the epoch-to-epoch transition matrix as a prior and the three hyper-parameter
distributions of STD, KUR, and SKE as likelihood factors. The detector takes as input an arbitrary 30 s epoch of raw single-channel EEG signal and then computes as an output the posterior
probabilities of the five sleep stages for this epoch. Although it is readily possible to extract the most probable sleep stage from these five continuous values, the distribution as a whole
provides important information about the trustworthiness of the prediction, as sometimes several sleep stages can have simultaneously large probabilities. Although in the present
implementation the selection of hyper-parameters is arbitrary and the detector has not been optimized in any way, the predictions of the detector are in some cases very close to the ground
truth of the human somnologist (Fig. 6a, b). Moreover, we have computed the distribution of prediction accuracies (defined as the fraction of correctly classified epochs), including all our
68 independent data sets. The distributions systematically shift to larger values, i.e., prediction performance becomes better, when more hyper-parameters are included into the Bayesian
likelihood (Fig. 7). In our super-statistical approach, each epoch of the original EEG signals is mapped onto a small tuple of _K_ hyper-parameters. This corresponds to a strong
dimensionality reduction (in our case from 7680 subsequent EEG values down to only _K_ = 3 hyper-parameters) and it is therefore interesting how much information can be preserved in this
data compression process. Ideally, all epochs from the same sleep-state should be mapped to the same cluster of points in the _K_-dimensional embedding space and the achievable accuracy of
the Bayesian detector is fundamentally limited by the degree to which these clusters overlap. We thus perform a quantitative analysis of the separability of the five sleep-stage-specific
clusters in the space of the three hyper-parameters STD, KUR, and SKE. For this purpose, we use two independent measures of cluster separability, the well-established General Discrimination
Value (GDV)11, as well as a more sophisticated measure, called the Cluster Separation Index (CSI) (cf. “Methods” section). These quantities are used to compute the pairwise distances, i.e.,
dissimilarities, between the sleep stages (Fig. 8). In both measures, the minimum distance is found between REM and N1, which means that these sleep stages are most similar. In contrast, the
maximum distance was found between REM and N3, indicating that these sleep stages are most dissimilar to each other. These observations fit very well to the known physiology underlying the
respective sleep stages. Stage N3, also called _δ_-sleep or slow-wave sleep, respectively, represents deep sleep and is physiologically characterized by highly synchronized, low-frequency,
and large-amplitude cortical activity21,22. In contrast, both stages REM and N1 are dominated by asynchronous, low-amplitude, and high-frequency cortical activity21,22. These sleep stages
resemble wakefulness and thus represent the physiological opposite of N3. DISCUSSION Traditionally, the analysis of EEG recordings has mainly focused on the oscillatory features of the
signals, such as _α_-, _β_-, _δ_-, and _θ_-frequency bands, and in the context of sleep also on wavelet-like features, i.e., grapho-elements, such as sleep spindles or K-complexes. Just
recently, it became clear that also the aperiodic component of an EEG signal, in particular the ubiquitous background noise with a _f_−_β_-like power spectrum, contains valuable information
about the physiological state of the subject23,24. Indeed, these aperiodic, scale-free fluctuations have been shown to systematically change with age and with the tasks to be performed25.
Moreover, they offer a novel way to asses the level of arousal26. An alternative approach is to focus neither on oscillatory features, nor on the global power spectrum, but to treat each
single-channel EEG signal simply as a random walk. As has been found very early on27,28, the changes between two subsequent EEG values (the steps of the walk) are not always normally
distributed, and later studies have revealed further anomalous properties of EEG random walks29,30,31. In this work, we treat the signal as a non-stationary, heterogeneous random walk,
generated by a stochastic system with parameters that change over time, depending on the physiological state of the subject. In particular, this random walk has different statistical
properties in each of the five sleep (or, more precisely, vigilance) stages and these differences can be exploited for a simple automated Bayesian sleep stage detection. Although several
methods of automatic sleep-stage detection are already available32,33,34, we have implemented, as a proof-of-concept, a first version of a Bayesian, hyper-parameter-based detector. In
contrast to sleep-stage detectors based on deep neural networks, which suffer from the black box problem35, our Bayesian approach is completely transparent and explainable, as the features
used to distinguish between sleep stages (i.e., the distributions of hyper-parameters) are explicit. Once these hyper-parameter distributions are extracted from the raw data and included
into the likelihood, the Bayesian detector can immediately be applied without any training or further optimization. In contrast, most deep learning applications require extensive training
and are data hungry36. Furthermore, although the posterior probabilities of the momentary sleep stages are mathematically well-defined in the Bayesian approach, it is not clear if the
typical softmax outputs of a deep neural network can actually be interpreted as probabilities, or if they are just a list of scores that sum up to one. Finally, we have shown that the
accuracy of the Bayesian sleep stage detector can be systematically improved, simply by including additional hyper-parameter distributions as factors in the likelihood. In principle, the
number of these factors could be made arbitrarily large by using hyper-parameters such as \({\left\langle {({y}_{t}-\overline{y})}^{m}\right\rangle }_{t\in n}\), the _m_-th central moments
of the fluctuating EEG signal _y__t_ within each 30 s epoch _n_, or \({\left|{\left\langle {y}_{t}{e}^{i{\omega }_{k}t}\right\rangle }_{t\in n}\right|}^{2}\), the magnitude squared of
momentary Fourier components for different frequencies _ω__k_. Besides the probability distributions of the hyper-parameters, we have also studied their gradual evolution over time. Some of
the hyper-parameters show consistent rising or falling trends within and across the phases in which the subject is scored to be in a constant sleep stage. For example, we have observed a
case where the STD of the EEG signal is continuously increasing for about 30 min, whereas the subject is passing from the REM state through N1, to N2, and finally to the N3 state (compare
Fig. 4(3)). Such a gradual buildup of EEG amplitude points to a continuous mechanism in the brain that is regulating the sleep cycle, a phenomenon similar to the change of hyper-parameters
that we have observed in migrating cells during the cell cycle18. We speculate that, rather than sub-dividing sleep into discrete stages, it might be useful to introduce a continuous master
variable \(\phi (t)\in \left[0,2\pi \right]\), roughly resembling the mathematical phase of a sinusoidal oscillation, which tends to increase about linearly with time and which reflects the
momentary position of the subject within the sleep cycle. In principle, it may then be possible to design a simple stochastic first-level model, such as an auto-regressive process of low
order, with coefficients that are not constant but which are top-down controlled (from a second model level) by the master variable _ϕ_(_t_). As we have demonstrated in other contexts18,19,
such super-statistical two-level models are often capable to reproduce the anomalous time-dependent statistics of biological and other complex systems (typically involving non-normally
distributed, long-time-correlated signals) in a particular simple way. In future work, one could therefore attempt to reproduce the sleep-stage-dependent properties of the EEG raw signals
(Fig. 1) and of the various hyper-parameters (Fig. 3) with such a two-level model. Indeed, it may even be possible to relate the phase variable _ϕ_(_t_) to existing models of
sleep37,38,39,40,41. An obvious candidate would be the famous two-process model42,43, in which sleep is controlled by the nonlinear interplay between the circadian propensity for sleep,
governed by an intrinsic circadian oscillator, and a homeostatic drive for sleep that continuously increases during the waking state and dissipates during sleep. In this case, the circadian
and homeostatic signals may directly represent the second-level control signals of a two-level super-statistical model. METHODS HETEROGENEOUS RANDOM WALKS AND SUPERSTATISTICS In this work,
the term superstatistics does not refer to the superposition of different statistical models, as originally studied by Beck and Cohen15,16,17, but more specifically to a method for the
analysis of heterogeneous, time-discrete random walks, as first introduced in refs. 18,19. We define random walks in the broadest sense as time series of momentary values _y_(_t_ = 0, 1, 2,
…), which are assumed to be generated by a stochastic process. In particular, the discrete steps Δ_y_(_t_) = _y_(_t_) − _y_(_t_ − 1) of such a general random walk need not to be normally
distributed and there may exist linear correlations between subsequent momentary values (correlated random walk) or even more complex dependencies between values many time points appart.
Moreover, the underlying stochastic process may also have some deterministic components. A random walk is called heterogeneous if its statistical properties (such as the distribution of
steps _p_(Δ_y_) or the degree of temporal correlations) change over time. As has been shown in refs. 18,19 and elsewhere, this can lead to anomaleous statistical properties of the random
walk as a whole (such as non-Gaussian, fat-tailed step distributions _p_(Δ_y_), or long-time correlations that can be approximated by powerlaw autocorrelation functions), although each
sufficiently small time interval can be described by a regular random walk (often even with Gaussian step distributions and approximately constant statistical parameters). The method of
super-statistical analysis, in its simplest implementation, therefore sub-divides the random walk into small non-overlapping time intervals (windows) and computes relevant statistical
parameters (such as the _n_th-order moments of the momentary distribution function _p_(_y_)) independently for each of these time windows. In the case of a heterogeneous random walk, the
resulting statistical parameters will fluctuate around their mean values much more strongly than expected from sampling statistics only. The fluctuations of the parameters can be described
by (super-statistical) distribution functions, which represent characteristic properties of the heterogeneous random walk. We therefore refer to such strongly fluctuating parameters as
hyper-parameters. GENERATION OF DATA SETS This work is based on 68 independent data sets, each containing 1 full-night 3-channel EEG recording (channels F4-M1, C4-M1, and O2-M1) from a
different human subject during sleep, recorded with a sampling rate of 256 Hz. For most of the following analysis, each of the three channels was treated as a different (sub-)data set and
evaluated separately, except for the computation of the cross-correlation functions (see below). The participants of the study included 46 males and 22 females, with an age range between 21
and 80 years. Exclusion criteria were a positive history of misuse of sedatives, alcohol or addictive drugs, as well as untreated sleep disorders. The study was conducted in the Department
of Otorhinolaryngology, Head Neck Surgery, of the Friedrich-Alexander University Erlangen-Nürnberg, following approval by the local Ethics Committee (323-16 Bc). Written informed consent was
obtained from the participants before the cardiorespiratory polysomnography. After recording, the raw EEG data were analyzed by a sleep specialist accredited by the German Sleep Society,
who removed typical artifacts44 from the data and visually identified the sleep stages in subsequent 30 s epochs, according to the AASM criteria (Version 2.1, 2014)45,46. The resulting,
labeled raw data were then used for our standard statistical and super-statistical analysis, and also as a ground truth to test the performance of the Bayesian sleep-stage classification.
SLEEP-STAGE-SPECIFIC STATISTICAL PROPERTIES OF RAW EEG DATA In a first step, each individual epoch _n_ and channel _k_ was statistically analyzed by computing the probability density
distribution _p__n_,_k_(_y_) of the momentary EEG signal amplitudes _y__n_,_k_(_t_), their temporal ACF $${A}_{n,k}({{\Delta }}t)=\frac{\left\langle
\left({y}_{n,k}(t)-{\overline{y}}_{n,k}\right)\cdot \left({y}_{n,k}(t\,+\,{{\Delta }}t)-{\overline{y}}_{n,k}\right)\right\rangle }{{\sigma }_{n,k}^{2}},$$ (1) as well as the
cross-correlation function between channels 1 and 2 $${C}_{n,1,2}({{\Delta }}t)=\frac{\left\langle \left({y}_{n,1}(t)-{\overline{y}}_{n,1}\right)\cdot \left({y}_{n,2}(t\,+\,{{\Delta
}}t)-{\overline{y}}_{n,2}\right)\right\rangle }{{\sigma }_{n,1}\cdot {\sigma }_{n,2}},$$ (2) where \({\overline{y}}_{n,k}={\left\langle {y}_{n,k}(t)\right\rangle }_{t}\) is the temporal
average of channel _k_s amplitude _y__n_,_k_(_t_) within epoch _n_ and \({\sigma }_{n,k}=\sqrt{{\left\langle {\left({y}_{n,k}(t)-{\overline{y}}_{n,k}\right)}^{2}\right\rangle }_{t}}\) is the
corresponding STD. It is noteworthy that in this case, the STD is equivalent to the root-mean-squared amplitude values that we used in previous studies8,9. In a second step, we have pooled
and averaged _p__n_,_k_(_y_), _A__n_,_k_(Δ_t_), and _C__n_,1,2(Δ_t_) over all epochs that belong to the same sleep stage _s_. The quantities _p__n_,_k_(_y_) and _A__n_,_k_(Δ_t_) were
additionally pooled and averaged over all channels _k_. As a result, we obtain the statistical properties _p__s_(_y_), _A__s_(Δ_t_), and _C__s_,1,2(Δ_t_) that are characteristic for each
sleep stage _s_ and which are shown in Fig. 1. EXTRACTION AND STATISTICAL ANALYSIS OF HYPER-PARAMETERS Based on the raw data _y__n_,_k_(_t_), we have computed for each channel _k_ and epoch
_n_ a set of hyper-parameters, namely the STD $${{{\mbox{STD}}}}_{n,k}=\sqrt{{\left\langle {\left({y}_{n,k}(t)-{\overline{y}}_{n,k}\right)}^{2}\right\rangle }_{t}},$$ (3) the excess curtosis
$${{{\mbox{KUR}}}}_{n,k}={\left\langle {\left(\frac{{y}_{n,k}(t)-{\overline{y}}_{n,k}}{{\sigma }_{n,k}}\right)}^{4}\right\rangle }_{t}-3,$$ (4) the SKE
$${{{\mbox{SKE}}}}_{n,k}={\left\langle {\left(\frac{{y}_{n,k}(t)-{\overline{y}}_{n,k}}{{\sigma }_{n,k}}\right)}^{3}\right\rangle }_{t}$$ (5) and the value of the ACF at the specific lag time
of 300 ms, where ACF differences between the sleep stages are relatively large: $${{{\mbox{ACF}}}}_{n,k}={A}_{n,k}({{\Delta }}t\,=\,300\,{{{{{\mathrm{ms}}}}}}).$$ (6) As these
hyper-parameters are strongly fluctuating themselves, we have pooled them over all epochs and channels and computed their sleep-stage specific distribution functions _p__s_(STD),
_p__s_(KUR), _p__s_(SKE), and _p__s_(ACF), which are shown in Fig. 3. TEMPORAL TREND ANALYSIS OF HYPER-PARAMETERS For a temporal trend analysis of the hyper-parameters, we no longer
partition the EEG time series _y__n_,_k_(_t_) into 30 s epochs, but into longer, contiguous sleep phases: within a given full-night recording, the sleep phases _J_ are defined as the longest
possible continuous time periods \(\left[{T}_{J,{{{{{\mathrm{beg}}}}}}},{T}_{J,{{{{{\mathrm{end}}}}}}}\right]\), in which the subject was scored to be in the same constant sleep stage _s_ =
_s_(_J_). Typically, each sleep phase _J_ contains a large number of subsequent epochs _n_. The hyper-parameters STD_n_,_k_, KUR_n_,_k_, … perform a higher-order random walk within each
sleep phase _J_, and visual inspection reveals that some of these random walks have rising and falling trends (Fig. 4). To evaluate these trends, we approximate the time series of the
hyper-parameters within each contiguous sleep phase by a linear function, _f__h__y__p_,_J_(_n_) ≈ _a__J_ × _n_ + _b__J_, using least-square fits. The slopes _a__J_ of these linear fits are
then pooled and averaged over all sleep phases _J_ with the same sleep stage _s_. The results are shown in Table 1. It is noteworthy that here we have sub-divided the sleep stages REM, N1,
and N2 into the falling and the rising part of the oscillatory motion between the two extreme stages of Wake and N3. EVALUATION OF TRANSITION PROBABILITIES BETWEEN SLEEP STAGES The sequence
of human-scored sleep stage labels _s__n_ for each subsequent epoch _n_ can be regarded as a random walk in a discrete state space \({s}_{n}\in
\left\{{{{{{\mathrm{Wake}}}}}},{{{{{\mathrm{REM}}}}}},{{{{{\mathrm{N1}}}}}},{{{{{\mathrm{N2}}}}}},{{{{{\mathrm{N3}}}}}}\right\}\). As this discrete random walk shows clear temporal
correlations, we have evaluated the (normalized) transition probabilities _p_(_s__J_+1∣_s__J_) between subsequent sleep phases, as well as the transition probabilities _p_(_s__n_+1∣_s__n_)
between subsequent epochs. The resulting transition matrices are shown in Fig. 5a, b. It is worth noting that, by definition, the diagonal elements of the phase-to-phase transition matrix
are zero. By contrast, the diagonal elements of the epoch-to-epoch transition matrix are relatively close to one, as each sleep stage has a high degree of persistence. The epoch-to-epoch
transition matrix defines a Markov random process of first order. After defining the starting stage _s__n_=0, the transition matrix can be used to simulate an arbitrarily long sequence of
sleep stages. An example is shown in the hypnogram of Fig. 5c. BAYESIAN SLEEP STAGE PREDICTION We have implemented a simple Bayesian model that predicts the probabilities _P_(_s__n_) of the
sleep labels \({s}_{n}\in \left\{{{{{{\mathrm{Wake}}}}}},{{{{{\mathrm{REM}}}}}},{{{{{\mathrm{N1}}}}}},{{{{{\mathrm{N2}}}}}},{{{{{\mathrm{N3}}}}}}\right\}\) from the raw EEG data _D__n_ in
each 30 s epoch _n_ (note that _D__n_ here stands for the complete set of 30 × 256 successive EEG values corresponding to the given epoch _n_). The prediction is based on the momentary
values _h__k__n_ of selected statistical hyper-parameters (in our case, the STD _h_1_n_, the KUR _h_2_n_, and the SKE _h_3_n_), which are calculated directly from the data _D__n_, and which
have different likelihoods _q_(_h__k__n_∣_s__n_) in the various sleep stages _s__n_. Furthermore, we take into account the prior probability Π(_s__n_) of the momentary sleep stage, which
depends on the prediction _P_(_s__n_−1) from the last epoch and on the known transition probability _M_(_s__n_∣_s__n_−1). The prediction for the current epoch is then given by
$$P({s}_{n})=\frac{Q({D}_{n}| {s}_{n})\cdot {{\Pi }}({s}_{n})}{{\sum }_{{s}_{n}^{\prime}}Q({D}_{n}| {s}_{n}^{\prime})\cdot {{\Pi }}({s}_{n}^{\prime})}\ .$$ (7) Here, the global likelihood
_Q_(_D__n_∣_s__n_) of the current data epoch _D__n_ is given as the product over the individual likelihoods of the different hyper-parameters _h__k__n_: $$Q({D}_{n}|
{s}_{n})=\mathop{\prod}\limits_{k={{{{{\mathrm{STD}}}}}},\ldots }q({h}_{kn}| {s}_{n})=q({h}_{{{{{{\mathrm{STD}}}}}},n}| {s}_{n})\cdot q({h}_{{{{{{\mathrm{KUR}}}}}},n}| {s}_{n})\cdot \ldots \
.$$ (8) We have numerically implemented these likelihood distribution as continuous spline-extrapolations that were pre-computed from empirical histograms with discrete bins. In this way,
also new data can be handled with extreme values of the hyper-parameters that are outside of the empirical histograms. Another possible implementation would be via kernel density
distributions. The (normalized) prior probability is computed as $${{\Pi }}({s}_{n})=\frac{\ \ \ \ \ \ {\sum }_{{s}_{n-1}}M({s}_{n}| {s}_{n-1})\cdot P({s}_{n-1})}{{\sum
}_{{s}_{n}^{\prime}}{\sum }_{{s}_{n-1}}M({s}_{n}^{\prime}| {s}_{n-1})\cdot P({s}_{n-1})}.$$ (9) In the initial epoch _n_0, we assume for simplicity that the subject is in the wake state. For
occasional epochs in which the raw EEG data are not reliable due to obvious measurement artifacts, Bayesian updating proceeds only on the basis of the prior Π. SEPARABILITY OF SLEEP STAGES
The accuracy of automatic sleep-stage detection depends on how well data clusters from different stages separate in the embedding space, which in our case corresponds to the
three-dimensional space of the hyper-parameters STD, KUR, and SKE. In order to assess this mutual separability of sleep stages in a quantitative way, we use two related measures: the
GDV8,9,11 and the CSI. Both measures take as an input a list of _N_ labeled _D_-dimensional data vectors (points), belonging to _L_ distinct classes (clusters) and produce as an output a
single number that characterizes the degree of separability of these classes. Also, both measures consider two classes as well, separable if the Euclidean distance of data points between the
two classes is typically much larger than the distance of points within the same class. GENERALIZED DISCRIMINATION VALUE We consider _N_ points XN=1..N = (_x__n_,1, ⋯ , _x__n_,_D_),
distributed within _D_-dimensional space. A label _l__n_ assigns each point to one of _L_ distinct classes _C__l_=1.._L_. In order to become invariant against scaling and translation, each
dimension is separately _z_-scored and, for later convenience, multiplied with \(\frac{1}{2}\): $${s}_{n,d}=\frac{1}{2}\cdot \frac{{x}_{n,d}-{\mu }_{d}}{{\sigma }_{d}}.$$ (10) Here, \({\mu
}_{d}=\frac{1}{N}{\sum }_{n = 1}^{N}{x}_{n,d}\) denotes the mean and \({\sigma }_{d}=\sqrt{\frac{1}{N}{\sum}_{n = 1}^{N}{({x}_{n,d}-{\mu}_{d})}^{2}}\) the STD of dimension _d_. Based on the
re-scaled data points SN = (_s__n_,1, ⋯ , _s__n_,_D_), we calculate the mean intra-class distances for each class _C__l_ $$\bar{d}({C}_{l})=\frac{2}{{N}_{l}({N}_{l}\,-\,1)}\mathop{\sum
}\limits_{i=1}^{{N}_{l}-1}\mathop{\sum }\limits_{j=i+1}^{{N}_{l}}d({{{{{{{{\bf{s}}}}}}}}}_{i}^{(l)},{{{{{{{{\bf{s}}}}}}}}}_{j}^{(l)}),$$ (11) and the mean inter-class distances for each pair
of classes _C__l_ and _C__m_ $$\bar{d}({C}_{l},{C}_{m})=\frac{1}{{N}_{l}{N}_{m}}\mathop{\sum }\limits_{i=1}^{{N}_{l}}\mathop{\sum
}\limits_{j=1}^{{N}_{m}}d({{{{{{{{\bf{s}}}}}}}}}_{i}^{(l)},{{{{{{{{\bf{s}}}}}}}}}_{j}^{(m)}).$$ (12) Here, _N__k_ is the number of points in class _k_ and
\({{{{{{{{\bf{s}}}}}}}}}_{i}^{(k)}\) is the _i_th point of class _k_. The quantity _d_(A, B) is the euklidean distance between A and B. Finally, the GDV is calculated from the mean
intra-class and inter-class distances as follows: $$\,{{\mbox{GDV}}}\,=\frac{1}{\sqrt{D}}\left[\frac{1}{L}\mathop{\sum }\limits_{l=1}^{L}\bar{d}({C}_{l})\ -\ \frac{2}{L(L\,-\,1)}\mathop{\sum
}\limits_{l = 1}^{L-1}\mathop{\sum }\limits_{m=l+1}^{L}\bar{d}({C}_{l},{C}_{m})\right]$$ (13) whereas the factor \(\frac{1}{\sqrt{D}}\) is introduced for dimensionality invariance of the
GDV with _D_ as the number of dimensions. In the case of two Gaussian distributed point clusters, the resulting discrimination value becomes −1.0 if the clusters are located such that the
mean inter cluster distance is two times the STD of the clusters. CLUSTER SEPARATION INDEX The basic idea of the GDV is to compare the distance between two clusters with the size of each
individual cluster. However, as cluster size is computed as an average over all point-to-point distances, this quantity can become relatively large in highly non-spherical clusters, e.g.,
when the points are distributed linearly along a straight or curved line. For this reason, the GDV may consider two parallel, line-like clusters 1 and 2 as not well separated, even if each
point in 1 is much closer to some adjacent point of 1 than to any point in 2. To resolve this problem, we have defined an alternative measure of class separability, the CSI, which is based
on nearest-neighbor distance relations and which resembles a quantity used before for margin-based feature selection47. In order to determine the CSI of a labeled set of _N_ data points in
_D_-dimensional space, we compute for each data point _n_ the Euclidean distance \({d}_{n}^{\ ({{{{{\mathrm{min}}}}}},S)}\) to its nearest neighbor within the same class, as well as the
distance \({d}_{n}^{\ ({{{{{\mathrm{min}}}}}},O)}\) to its nearest neighbor among all the other classes. The CSI is then defined as the logarithm of the ratio of these two distances,
averaged over all data points in all classes: $$\,{{\mbox{CSI}}}\,={\left\langle
{{{{{{\mathrm{ln}}}}}}}\,\left({d}_{n}^{({{{{{\mathrm{min}}}}}},O)}/{d}_{n}^{({{{{{\mathrm{min}}}}}},S)}\right)\right\rangle }_{n}$$ (14) Here, it is assumed that all point-to-point
distances in the data are non-zero. As the CSI is based on the ratio of Euclidean distances, it is invariant against translation and scaling. According to the CSI, two parallel line-like
clusters are considered as well separated, provided that the density of points within each line is sufficiently large. It is noteworthy that both the GDV and the CSI produce values around
zero when clusters are not separable. However, as separability increases, the GDV becomes more negative and the CSI more positive. To make both measures better comparable, we are considering
the magnitude ∣_G__D__V_∣ in Fig. 8, where the mutual distances between hyper-parameter clusters from different sleep stages are presented. REPORTING SUMMARY Further information on research
design is available in the Nature Research Reporting Summary linked to this article. DATA AVAILABILITY In the online repository figshare (https://doi.org/10.6084/m9.figshare.17113664), we
provide all required data (with associated Python 3.9.5 scripts) to reproduce the 8 figures of the paper (“Supplementary Data 1.zip”). CODE AVAILABILITY In the online repository figshare
(https://doi.org/10.6084/m9.figshare.17113700), we provide a Python 3.9.5 program (including test data) to run the Bayesian sleep stage detector (“Supplementary Software 1.zip”). REFERENCES
* Pedrycz, W. & Chen, S. _Time Series Analysis, Modeling and Applications. A Computational Intelligence Perspective_ (e-book Google, 2013). * Zumbach, G. _Discrete Time Series,
Processes, and Applications in Finance_ (Springer Science & Business Media, 2012). * Kirchgässner, G., Wolters, J., & Hassler, U. _Introduction to Modern Time Series Analysis_
(Springer Science & Business Media, 2012). * Cambel, A. B. _Applied Chaos Theory: A Paradigm for Complexity_ (Elsevier, 1993). * Baker, T. L. Introduction to sleep and sleep disorders.
_Med. Clin. North Am._ 69, 1123–1152 (1985). Article CAS PubMed Google Scholar * Geyer, J. D., Talathi, S. & Carney, P. R. _Introduction to Sleep and Polysomnography. Clinical Sleep
Disorders_ 265–266 (Lippincott Williams & Wilkins, 2009). * Barlow, J. S. _The Electroencephalogram: Its Patterns and Origins_ (MIT, 1993). * Krauss, P. et al. Analysis of multichannel
eeg patterns during human sleep: a novel approach. _Front. Hum. Neurosci._ 12, 121 (2018). Article PubMed PubMed Central Google Scholar * Krauss, P. et al. A statistical method for
analyzing and comparing spatiotemporal cortical activation patterns. _Sci. Rep._ 8, 1–9 (2018). Article Google Scholar * Traxdorf, M., Krauss, P., Schilling, A., Schulze, H. &
Tziridis, K. Microstructure of cortical activity during sleep reflects respiratory events and state of daytime vigilance. _Somnologie_ 23, 72–79 (2019). Article Google Scholar * Schilling,
A., Maier, A., Gerum, R., Metzner, C. & Krauss, P. Quantifying the separability of data classes in neural networks. _Neural Netw._ 139, 278–293 (2021). Article PubMed Google Scholar
* Vaughn, B. V. & Giallanza, P. Technical review of polysomnography. _Chest_ 134, 1310–1319 (2008). Article PubMed Google Scholar * Burns, J. W., Crofford, L. J. & Chervin, R. D.
Sleep stage dynamics in fibromyalgia patients and controls. _Sleep. Med._ 9, 689–696 (2008). Article PubMed Google Scholar * Krauss, P. et al. Analysis and visualization of sleep stages
based on deep neural networks. _Neurobiol. Sleep. Circad. Rhythms_ 10, 100064 (2021). Article Google Scholar * Beck, C. & GD. Cohen, E. Superstatistics. _Phys. A Stat. Mech. Appl._
322, 267–275 (2003). Article Google Scholar * Cohen, E. G. D. Superstatistics. _Phys. D Nonlinear Phenom._ 193, 35–52 (2004). Article Google Scholar * Beck, C. Generalized statistical
mechanics for superstatistical systems. _Philos. Trans. R. Soc. A Math. Phys. Eng. Sci._ 369, 453–465 (2011). Article Google Scholar * Metzner, C. et al. Superstatistical analysis and
modelling of heterogeneous random walks. _Nat. Commun._ 6, 1–8 (2015). Article Google Scholar * Mark, C. et al. Bayesian model selection for complex dynamic systems. _Nat. Commun._ 9, 1–12
(2018). Article CAS Google Scholar * Kemp, B. & AC. Kamphuisen, H. Simulation of human hypnograms using a markov chain model. _Sleep_ 9, 405–414 (1986). Article CAS PubMed Google
Scholar * Parmeggiani, P. L. & Velluti, R. A. _The Physiologic Nature of Sleep_ (World Scientific, 2005). * Silber, M. H. et al. The visual scoring of sleep in adults. _J. Clin. Sleep.
Med._ 3, 121–131 (2007). Article PubMed Google Scholar * He, B. J., Zempel, J. M., Snyder, A. Z. & Raichle, M. E. The temporal structures and functional significance of scale-free
brain activity. _Neuron_ 66, 353–369 (2010). Article CAS PubMed PubMed Central Google Scholar * He, B. J. Scale-free brain activity: past, present, and future. _Trends Cogn. Sci._ 18,
480–487 (2014). Article PubMed PubMed Central Google Scholar * Donoghue, T. et al. Parameterizing neural power spectra into periodic and aperiodic components. _Nat. Neurosci._ 23,
1655–1665 (2020). Article CAS PubMed PubMed Central Google Scholar * Lendner, J. D. et al. An electrophysiological marker of arousal level in humans. _Elife_ 9, e55092 (2020). Article
CAS PubMed PubMed Central Google Scholar * Elul, R. Gaussian behavior of the electroencephalogram: changes during performance of mental task. _Science_ 164, 328–331 (1969). Article CAS
PubMed Google Scholar * Weiss, M. S. Non-gaussian properties of the eeg during sleep. _Electroencephalogr. Clin. Neurophysiol._ 34, 200–202 (1973). Article CAS PubMed Google Scholar
* Linkenkaer-Hansen, K., Nikouline, V. V., Matias Palva, J. & Ilmoniemi, R. J. Long-range temporal correlations and scaling behavior in human brain oscillations. _J. Neurosci._ 21,
1370–1377 (2001). Article CAS PubMed PubMed Central Google Scholar * Lo, C.-C. et al. Dynamics of sleep-wake transitions during sleep. _EPL (Europhys. Lett.)_ 57, 625 (2002). Article
CAS Google Scholar * Dvir, H. et al. A biased diffusion approach to sleep dynamics reveals neuronal characteristics. _Biophys. J._ 117, 987–997 (2019). Article CAS PubMed PubMed Central
Google Scholar * Boostani, R., Karimzadeh, F. & Nami, M. A comparative review on sleep stage classification methods in patients and healthy individuals. _Comput. Methods Prog.
Biomed._ 140, 77–91 (2017). Article Google Scholar * Faust, O., Razaghi, H., Barika, R., Ciaccio, E. J. & Rajendra Acharya, U. A review of automated sleep stage scoring based on
physiological signals for the new millennia. _Comput. Methods Prog. Biomed._ 176, 81–91 (2019). Article Google Scholar * Chriskos, P. et al. A review on current trends in automatic sleep
staging through bio-signal recordings and future challenges. _Sleep. Med. Rev._ 55, 101377 (2021). Article PubMed Google Scholar * Castelvecchi, D. Can we open the black box of AI?
_Nature_ 538, 20 (2016). Article CAS PubMed Google Scholar * Marcus, G. Deep learning: a critical appraisal. Preprint at https://arXiv.org/1801.00631, (2018). * Saper, C. B., Scammell,
T. E. & Lu, J. Hypothalamic regulation of sleep and circadian rhythms. _Nature_ 437, 1257–1263 (2005). Article CAS PubMed Google Scholar * Abel, J. H., Lecamwasam, K., St Hilaire, M.
A. & Klerman, E. B. Recent advances in modeling sleep: from the clinic to society and disease. _Curr. Opin. Physiol._ 15, 37–46 (2020). Article PubMed Google Scholar * Lo, C.-C.,
Bartsch, R. P. & Ch. Ivanov, P. Asymmetry and basic pathways in sleep-stage transitions. _EPL (Europhys. Lett.)_ 102, 10008 (2013). Article CAS Google Scholar * Dvir, H. et al.
Neuronal noise as an origin of sleep arousals and its role in sudden infant death syndrome. _Sci. Adv._ 4, eaar6277 (2018). Article PubMed PubMed Central Google Scholar * Lin, A., K.L.
Liu, K., Bartsch, R. P. & Ch. Ivanov, P. Dynamic network interactions among distinct brain rhythms as a hallmark of physiologic state and function. _Commun. Biol._ 3, 1–11 (2020). PubMed
PubMed Central Google Scholar * Borbély, A. A. A two process model of sleep regulation. _Hum. Neurobiol._ 1, 195–204 (1982). PubMed Google Scholar * Daan, S., Beersma, D. G. & A
Borbély, A. Timing of human sleep: recovery process gated by a circadian pacemaker. _Am. J. Physiol. Regul. Integr. Comp. Physiol._ 246, R161–R183 (1984). Article CAS Google Scholar *
Tatum, W. O., Dworetzky, B. A. & Schomer, D. L. Artifact and recording concepts in eeg. _J. Clin. Neurophysiol._ 28, 252–263 (2011). Article PubMed Google Scholar * Iber, C. _The AASM
Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specification_ (American Academy of Sleep Medicine, 2007). * American Academy of Sleep Medicine, et
al. _The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, Version 2.0_ (American Academy of Sleep Medicine, 2012). *
Gilad-Bachrach, R., Navot, A. & Tishby, N. Margin based feature selection-theory and algorithms. In _Proc. 21st Int. Conference on Machine learning_, 43 (ICML, 2004). Download references
ACKNOWLEDGEMENTS This work was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation): grant to H.S. and M.T. (project number 455908056), grant to P.K. (project
number 436456810), and grant to A.S. (project number 451810794). All material used in the paper are the intellectual property of the authors. FUNDING Open Access funding enabled and
organized by Projekt DEAL. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Neuroscience Lab, Experimental Otolaryngology, University Hospital Erlangen, Erlangen, Germany Claus Metzner, Achim
Schilling, Holger Schulze & Patrick Krauss * Laboratory of Sensory and Cognitive Neuroscience, Aix-Marseille University, Marseille, France Achim Schilling * Cognitive Computational
Neuroscience Group, Friedrich-Alexander University Erlangen-Nuremberg, Nuremberg, Germany Achim Schilling & Patrick Krauss * Department of Otorhinolaryngology, Paracelsus Medical
University, Nuremberg, Germany Maximilian Traxdorf * Pattern Recognition Lab, Friedrich-Alexander University Erlangen-Nuremberg, Nuremberg, Germany Patrick Krauss Authors * Claus Metzner
View author publications You can also search for this author inPubMed Google Scholar * Achim Schilling View author publications You can also search for this author inPubMed Google Scholar *
Maximilian Traxdorf View author publications You can also search for this author inPubMed Google Scholar * Holger Schulze View author publications You can also search for this author
inPubMed Google Scholar * Patrick Krauss View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS C.M. has conceived of applying super-statistical
analysis to sleep data, implemented the methods, evaluated the data, and wrote the paper. P.K. designed the study, discussed the results, and wrote the paper. A.S. discussed the results.
M.T. provided access to resources and wrote the paper. H.S. provided access to resources and wrote the paper. CORRESPONDING AUTHOR Correspondence to Claus Metzner. ETHICS DECLARATIONS
COMPETING INTERESTS The authors declare no competing interests. ETHICS The study was conducted in the Department of Otorhinolaryngology, Head Neck Surgery, of the Friedrich-Alexander
University Erlangen-Nürnberg (FAU), following approval by the local Ethics Committee (323 - 16 Bc). Written informed consent was obtained from the participants before the cardiorespiratory
polysomnography (PSG). PEER REVIEW INFORMATION _Communications Biology_ thanks Joan Rué Queralt and the other, anonymous, reviewers for their contribution to the peer review of this work.
Primary Handling Editors: Karli Montague-Cardoso. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. SUPPLEMENTARY INFORMATION DESCRIPTION OF ADDITIONAL SUPPLEMENTARY FILES SUPPLEMENTARY SOFTWARE 1 SUPPLEMENTARY DATA 1 REPORTING SUMMARY RIGHTS AND PERMISSIONS
OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or
format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or
other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in
the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the
copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Metzner, C., Schilling, A.,
Traxdorf, M. _et al._ Sleep as a random walk: a super-statistical analysis of EEG data across sleep stages. _Commun Biol_ 4, 1385 (2021). https://doi.org/10.1038/s42003-021-02912-6 Download
citation * Received: 10 September 2021 * Accepted: 23 November 2021 * Published: 10 December 2021 * DOI: https://doi.org/10.1038/s42003-021-02912-6 SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative