Play all audios:
Download PDF Perspective Open access Published: 04 November 2022 Reassessing a cryptic history of early trilobite evolution James D. Holmes ORCID: orcid.org/0000-0001-8804-21491 & Graham E.
Budd ORCID: orcid.org/0000-0001-9007-43691 Communications Biology volume 5, Article number: 1177 (2022) Cite this article
8298 Accesses
10 Citations
42 Altmetric
Metrics details
Subjects Evolutionary theoryPalaeontology AbstractTrilobites are an iconic Paleozoic group of biomineralizing marine euarthropods that appear abruptly in the fossil record (c. 521 million years ago) during the Cambrian ‘explosion’. This
sudden appearance has proven controversial ever since Darwin puzzled over the lack of pre-trilobitic fossils in the Origin of Species, and it has generally been assumed that trilobites must
have an unobserved cryptic evolutionary history reaching back into the Precambrian. Here we review the assumptions behind this model, and suggest that a cryptic history creates significant
difficulties, including the invocation of rampant convergent evolution of biomineralized structures and the abandonment of the synapomorphies uniting the clade. We show that a vicariance
explanation for early Cambrian trilobite palaeobiogeographic patterns is inconsistent with factors controlling extant marine invertebrate distributions, including the increasingly-recognized
importance of long-distance dispersal. We suggest that survivorship bias may explain the initial rapid diversification of trilobites, and conclude that the group’s appearance at c. 521 Ma
closely reflects their evolutionary origins.
Similar content being viewed by others Combining palaeontological and neontological data shows a delayed diversification burst ofcarcharhiniform sharks likely mediated by environmental change Article Open access 19 December 2022 Cenozoic history of the tropical marine biodiversity hotspot Article Open access 26 June
2024 Contrasting Early Ordovician assembly patterns highlight the complex initial stages of the Ordovician Radiation Article Open access 09 March 2022 Introduction
The timing of early animal evolution is a controversial problem, with opinion generally divided between two major schools of thought. One view holds that the fossil record is relatively
accurate, and that the appearance of the major animal groups during the Cambrian radiation closely reflects their evolutionary origins1,2,3. The opposing view, based largely on divergence
dates estimated by molecular clocks, suggests a much deeper evolutionary history4,5,6,7. In recent years, differences between these views have reduced8, with recognition of certain members
of the Ediacara biota as probable eumetazoans9,10 and more refined molecular divergence estimates coming closer in-line with the fossil record6,11. Nevertheless, there remains a mismatch
between these views, particularly as to whether the classic ‘explosion’ of taxa (including trace fossils) across the Cambrian Terreneuvian Series represents the true radiation of bilaterian
crown groups, or whether the origins of these are to be found earlier, in the Ediacaran.
Trilobites are a clade of total-group euarthropods whose first appearance datum (FAD) marks the boundary between the Terreneuvian and provisional Cambrian Series 2 (currently dated to c. 521
Ma)12,13. They are one of the largest and most successful Paleozoic groups, persisting for some 270 million years, and represented by over 22,000 described species14,15. This excellent
fossil record—a result of their easily-preserved, biomineralized exoskeleton that was molted many times during life—can be used to address important questions concerning early animal
evolution16,17. Trilobites have been viewed as exemplary for the argument of deep bilaterian (and therefore metazoan) divergence dates, and formed an important part of the argument for early
proponents of this view4,18,19. One reason for this is that trilobites supposedly show substantial provincialism when they appear in the fossil record, being separated into two major
biogeographic areas in the early Cambrian: the ‘olenelline’ province (e.g., Laurentia, Baltica) and the ‘redlichiine’ province of Gondwana (including Antarctica, Australia, China and India,
amongst other regions), with a transitional zone (sometimes referred to as the ‘bigotinid’ province) occurring in areas such as West Gondwana and Siberia20,21,22 (Fig. 1; we use suborders
here23). It has generally been assumed that trilobites must have a cryptic evolutionary history for such a pattern to be produced, and that observed distributions of taxa are a result of
vicariance, in this case resulting from supercontinent breakup and the subsequent isolation of certain paleocontinents. These patterns have been linked to either the breakup of Rodinia (c.
700–800 Ma)19 or the ephemeral Pannotia (c. 550–600 Ma)24,25,26, although the refinement of molecular clock estimates suggests that the former in particular is unlikely. Given the accepted
position of trilobites as total-group euarthropods27, linking of these biogeographic patterns to supercontinent breakup in the Neoproterozoic has been used to support the argument for a
deep, cryptic history of arthropod evolution, and early animal evolution more generally4,18,19,24,25. However, this reasoning is based on two major assumptions: (a) that the earliest
trilobites already show established biogeographic provincialism and phylogenetic diversity; and (b) that observed biogeographic patterns result from vicariance rather than dispersal. It also
raises questions about the early trilobite fossil record that are not easily answered. For example, if a biomineralized exoskeleton and associated traits are synapomorphies of the group,
why are Terreneuvian trilobites absent from the fossil record despite an adequate shelly record across the same period?
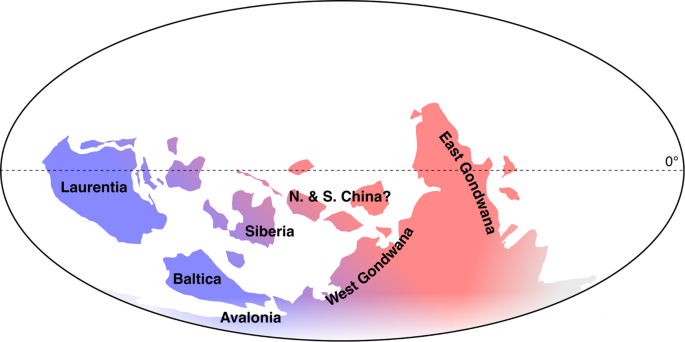
Fig. 1: Global palaeogeographical reconstruction at c. 520 Mashowing hypothetical locations of palaeocontinents and trilobite faunal provinces.
Colors show the approximate areas of the so-called ‘olenelline’ (blue) and ‘redlichiine’ (red) early Cambrian trilobite faunal provinces, and the transitional (purple) area between these.
Importantly, this provincialism is not strongly evident in the earliest trilobite faunas, which are known from the transitional areas of Siberia and West Gondwana, as well as Laurentia (see
main text for details). Note that the positions of North (N.) and South (S.) China are consistently uncertain in Cambrian geographic reconstructions and are marked as such (?). Map based on
fig. 2.7 of Torsvik & Cocks92.
Full size imageHere we review the evidence for a cryptic history of early trilobite evolution. We also review the assumptions behind the supporting vicariance model, in light of >30 years of work that has
greatly improved our understanding of the factors responsible for modern biogeographic patterns, including the relative importance of vicariance and dispersal.
The biomineralizationproblem
Although alternative views have been proposed28,29, trilobites (probably excluding agnostines30) are now almost universally accepted to form a clade defined by several synapomorphies,
including calcification of the dorsal exoskeleton, and eyes with calcified lenses and circumocular sutures31,32,33. Dorsal facial sutures may also be a synapomorphy of the group, either
excluding or including olenellines depending on whether their absence in this group represents the ancestral state or secondary loss respectively (dorsal facial sutures may also be
independently derived in eodiscids16). As such, any ancestral trilobite that lived prior to their FAD (at c. 521 Ma) should possess a biomineralized exoskeleton (unless secondarily lost);
however, no Terreneuvian trilobite has been found despite the presence of diverse shelly faunas across much of this period. Any argument that Terreneuvian (or Precambrian) trilobites are
absent from the record because they were non-mineralized implies that all of the major trilobite lineages must have diverged sometime during this earlier period, and then all independently
evolved a calcitic exoskeleton at about c. 521 Ma or shortly thereafter. This argument effectively discards the defining synapomorphies of the clade, implying that trilobites are
polyphyletic as currently conceived—a view that most workers would reject. Arguments have been made for such rampant convergence, e.g., changes in seawater chemistry triggering the
development of calcitic skeletons in several bilaterian lineages around this time16. However, it remains highly improbable that the characters associated with biomineralization mentioned
above were developed independently in all lineages hypothesized to cross this boundary. For example, based on the recent Cambrian trilobite phylogenetic analysis of Paterson et al.16 this
implies eleven independent acquisitions of a calcified exoskeleton and associated eye structures, and seven acquisitions of dorsal facial sutures (based on their multi-epoch clock model and
ignoring divergences from 521 to 522 Ma to allow for uncertainty in the record; see their fig. 116). In addition, if trilobites as currently conceived really did independently evolve these
traits, this implies the presence of a large number of non-mineralized trilobite sister groups at this time, and one would expect to see some of these in the various early Cambrian
Lagerstätten. Yet, there are no examples from these deposits that can be considered a ‘non-mineralized trilobite’ (note that any such taxon would have to be more closely related to a
particular trilobite group than to other trilobites). It seems highly unlikely that all of these suddenly went extinct in the interval between c. 521 Ma and the oldest of these deposits
(e.g., the Chengjiang biota), or for some reason are not preserved.
In the absence of any other compelling reason to doubt the accuracy of the trilobite fossil record, it is thus much more parsimonious to assume that the characters discussed above represent
true synapomorphies, and that trilobites arose close to their FAD. It should be noted that although the position of agnostines does not greatly affect the arguments presented here, the
exclusion of this group from Trilobita suggests a possible independent acquisition of a calcified dorsal exoskeleton.
Arthropod traces in the TerreneuvianTrace fossils have also been used to argue for a cryptic history of trilobite evolution. Arthropod traces from the Terreneuvian such as Rusophycus have been attributed to trilobites34
(despite appearing c. 10–15 million years prior to the first appearance of the group35), although some authors have since suggested that these could have been produced by other
arthropods36,37. Trilobites are members of a much larger diversity of Cambrian euarthropods, the great majority of which are non-mineralized. Many of these (like trilobites) exhibit a series
of biramous (‘two-branched’), gnathobase-bearing appendages along the anterior-posterior axis38,39,40. In particular, the Artiopoda (a large clade of trilobite-like euarthropods, including
trilobites) generally have very similar appendages38, and this is clearly a primitive trait of the group. Many artiopodans also exhibit comparable overall morphologies to trilobites, and
some of these likely filled similar ecological niches. Thus, it might be expected that such taxa produced similar traces across the early history of artiopodans, which must have occurred
prior to the FAD of trilobites (Fig. 2). It is even possible that more basal non-mineralized stem-euarthropods (e.g., fuxianhuiids41 and Parapeytoia42, which exhibit the same basic appendage
structure) could produce similar traces. The recent interpretation of Cambrian Series 2 (Stage 4) Rusophycus from Canada as being produced by a non-mineralized crustacean-like arthropod43
supports the idea that these early traces could be produced by non-trilobites. It has also been pointed out that although trace fossils like Rusophycus and Cruziana occur after the Permian
mass extinction (e.g., in the Triassic44,45), this is not considered evidence of post-Permian trilobites46 (these are also attributed to crustacean-like taxa). Why should we consider the
presence of these traces prior to c. 521 Ma in a different light, when other obvious candidates for producing them are present? Based on the above, a more literal reading of the trilobite
fossil record is not incongruent with the trace fossil record. Rather, it supports the interpretation of these traces representing the early diversification of total-group euarthropods
starting in the early Terreneuvian (Fortunian), more derived artiopodan-type taxa later in the Terreneuvian (e.g., the more ‘typical’ Rusophycus occurring in Stage 235) and allows additional
time for the evolution of trilobites before their FAD at c. 521 Ma.
Fig. 2: Simplified phylogeny of the Artiopoda.A uniting feature of this group are their similar biramous appendages and it is likely that some of the non-trilobite artiopodans produced the same kinds of traces as trilobites (e.g.,
Rusophyscus, Cruziana). If trilobites arose close to when they appear in the fossil record (c. 521 Ma) there must have been a substantial earlier history of artiopodans (and other more
distantly related taxa), thus obviating the requirement to suggest trilobites produced these traces in the Terreneuvian. Topology based on fig. 6b of Ortega-Hernández et al.38.
Full sizeimageThe vicariance assumption
Distributions of related terrestrial and freshwater taxa separated by ocean basins can be explained by either vicariance resulting from continental separation, or oceanic dispersal (i.e.,
long-distance dispersal across oceans). In the Origin of Species47, Darwin argued that occasional dispersal events—rare but occurring regularly across vast periods of geological time—could
explain such distributions, and undertook various experiments to show that certain plant seeds can remain viable after long periods immersed in salt water, or in the crops and digestive
tracts of birds. He essentially argued against vicariance-only explanations by suggesting that dispersal of successful organisms likely explained the observation that fossil faunas were most
similar within the same intervals of geological time (even across great distances), and that this was supported by the marine faunas of different continents being more similar than
terrestrial faunas47. Darwin also noted that organisms on volcanic islands must have arrived by dispersal, and this remains a strong argument today—the important of such events in populating
oceanic islands on geological timescales cannot be denied48,49. However, the validation of plate tectonics and the rise of cladistics from about the 1960s onwards provided a compelling
model of vicariance by continental fragmentation testable with phylogenetic hypotheses, and vicariance (or cladistic) biogeography became the dominant paradigm50. In contrast, hypotheses of
oceanic (or what Darwin referred to as ‘accidental’) dispersal were considered unscientific as they could not be falsified, and essentially every pattern could be explained by invoking some
series of dispersal events50 (although as Darwin pointed out, factors controlling dispersal such as ocean currents and prevailing winds are not ‘accidental’47). Nevertheless, it was under
the general assumption that vicariance must be responsible that such explanations for trilobite biogeographic patterns emerged.
Over the last 25 years or so, it has become clear that oceanic dispersal plays a much greater role in explaining modern biogeographic distributions than previously thought50,51,52, and
methods of vicariance biogeography that a priori exclude dispersal as a factor have been widely criticized53,54,55. Molecular dating has shown that phylogenetic splits within many groups
thought to represent instances of vicariance by continental separation occurred much too recently for this to be the case50. Disjunct distributions will often be controlled to some extent by
tectonic movements, but this recent work suggests that vicariant signal is often overlaid to a greater-or-lesser extent by instances of oceanic dispersal, creating a complex web of patterns
that varies in different groups56. This is true even for groups once considered to show classic Gondwanan vicariant distributions (i.e., related to the breakup of Africa, Antarctica,
Australia, India and South America), including cichlids and other freshwater fish57,58, and various plant groups including Araucaria and Nothofagus56,59. This does not mean that vicariance
is an insignificant factor in explaining biogeographic distributions, and it is still suggested to be the dominant factor in some cases (e.g., in Southern Hemisphere terrestrial animals56).
However, it is now clear that we can no longer simply assume that vicariance is the major factor controlling such distributions.
Early work proposing vicariance as the major factor in explaining early Cambrian trilobite biogeographical patterns4,18,19 stated that provincialism shown by the earliest trilobites
essentially proved an “earlier phase of vicariance unrecorded in the fossil record” (Fortey et al.19, p. 18). Coupled with the implicit assumption in this reasoning that vicariance must be
the cause of such patterns (rather than dispersal), the extremely deep divergence dates being proposed at this time by early molecular clocks suggested that trilobites may have a cryptic
history extending back several hundred million years, and that vicariance associated with the breakup of Rodinia might be responsible for the observed biogeographic patterns.
More recently, several studies that used a modified version of Brooks Parsimony Analysis have suggested that trilobite biogeographic patterns may have resulted from the breakup of the more
recent, ephemeral (and controversial60,61) supercontinent Pannotia in the late Neoproterozoic24,26, in line with more recent molecular clock estimates6,11. These methods involve replacing
terminal taxa and internal nodes in phylogenies with biogeographic distributions, and coding the resulting information from these in a separate character matrix, where the ‘taxa’ represent
biogeographic regions62. This matrix is then subjected to a parsimony analysis and the resulting tree topologies are interpreted to reflect histories of faunal connections (in this case
between palaeocontinents). ‘Vicariance’ trees are meant to reflect branching associated with the erection of barriers between areas, whereas ‘geo-dispersal’ trees reflect the removal of
barriers24,62. As such, it is important to note that geo-dispersal represents a completely different concept to oceanic dispersal. In fact, there is no mechanism within these models that
allows for true oceanic dispersal, in that any relationships resolved are interpreted to result from isolation or amalgamation of areas due to the erection or removal of barriers between
these (e.g., related to tectonic movements or sea-level fluctuations). Therefore, such models can produce well-resolved ‘vicariance’ trees that are not necessarily the result of vicariance.
For example, similar histories of connections in different groups can be produced by dispersal influenced by factors such as relative distances between continents and ocean currents50, which
are primary controls on modern marine animal distributions. As mentioned above, there are many modern cases where molecular dating supports explanations of oceanic dispersal, despite
phylogenetic relationships apparently being consistent with vicariant hypotheses50. Clearly then, timing of divergences is a key issue when interpreting biogeographic patterns in light of
dispersal and vicariance. Recent morphological clock estimates that suggest trilobites most likely arose in the Fortunian16 (likely overestimates; see below) represent a situation analogous
to these modern cases, with the age of the group apparently post-dating estimates of continental separation. As with these modern examples, if a vicariance explanation is shown to be
inconsistent due to a mismatch in timing between tectonic events and taxon divergence estimates, then oceanic dispersal is likely to be responsible.
Dispersal: a major factor inexplaining marine invertebrate distributions
The argument over the importance of vicariance (by continental separation) versus oceanic dispersal in explaining modern biogeographic distributions may be largely irrelevant to the case of
trilobites, as this form of vicariance has generally only been used to explain distributions of terrestrial and freshwater taxa50. In modern marine animals—including taxa restricted to
continental shelves as most early trilobites were—instances of vicariance almost always involve the splitting of populations by land (rather than by seaways as invoked for Cambrian
trilobites)63,64,65. Today, marine invertebrate distributions—including differences in faunas separated by deep ocean basins (e.g., Atlantic, Pacific)—are controlled by varying dispersal
ability in relation to a complex series of factors, including ocean currents, physical barriers and latitudinal/temperature gradients, rather than relictual vicariant patterns from when
these seaways first opened66,67. Thus, invoking continental separation as the major factor in explaining trilobite distributions is inconsistent with what is known for the modern marine
fauna.
Rapid dispersal (geologically speaking) has been considered less likely in explaining Cambrian trilobite biogeographic patterns than a cryptic history requiring invocations of rampant
convergence or rarity16. In fact, marine invertebrates can generally disperse rapidly across large distances66, either by planktonic larval dispersal, or other measures almost certainly
present during the Cambrian, such as transport on semi-submerged substrates such as floating macroalgal or pumice rafts68,69. For example, even in the last few hundred years, pumice rafts
have occurred frequently in all major oceans, and have been shown to develop diverse assemblages and transfer organisms vast distances (e.g., in a recent incident >80 species and >5000
km70). Southern Hemisphere coastal communities are also biologically linked by frequent, long-distance dispersal on macroalgal rafts69. Such rafts have helped to confirm that marine
invertebrate distributions are strongly controlled by ocean currents70,71,72. Importantly, although the majority of recruitment to such vectors is by taxa with pelagic larvae, benthic marine
invertebrates lacking such stages are also transported regularly on both pumice and macroalgal rafts68,73,74. It is reasonable to assume that trilobites (particularly small individuals)
could be as well, and that dispersal was a major driver of marine invertebrate distributions in the Cambrian, as it is today.
(A lack of) provincialism and phylogenetic diversity in theearliest trilobites
It has been stated repeatedly that, even in their earliest history, trilobites show marked biogeographic provincialism and phylogenetic diversity4,16,18,19,20,75, being separated into the
‘olenelline’ and ‘redlichiine’ provinces discussed previously. If indeed trilobites show distinct provincialism from the point they appear in the fossil record, then this might be considered
a strong argument for a cryptic history. However, even a brief examination of the data shows that this view is not well supported.
It seems clear that the earliest trilobites appear at a similar time in Laurentia, Siberia and West Gondwana76,77, and initially consist of olenelline fallotaspidoids from the families
Archaespididae and Fallotaspididae, and redlichiines from the Family Bigotinidae78,79,80,81,82 (Fig. 3a), which have been suggested to be closely related80. Despite the argument for
provincialism, similar fallotaspidoids are present in all three of these regions, while bigotinids are apparently absent from Laurentia. The oldest trilobites in China and East Gondwana are
primitive redlichioids such as Parabadiella, the ages of which are somewhat controversial76,77,83. In Australia, it has recently been suggested that these appear at about the same time as
the oldest trilobites from the regions discussed above;84,85 however, these East Gondwanan trilobites show similarities to redlichioids found slightly higher in the West Gondwanan
successions86. Regardless, these clearly represent primitive forms likely closely related to bigotinids16,80. In Baltica and Avalonia, the first trilobites to appear are derived olenelline
holmiids generally thought to be somewhat younger than the taxa discussed above76.
Fig. 3: Relationships between early trilobites in the Order Redlichiida.a Higher-level taxonomy of the trilobite Order Redlichida, largely after Adrain23. Note that this does not represent an exhaustive list of taxa within the various groups shown, only those
discussed in the text or shown in b. b Phylogeny of early Cambrian trilobites (modified from fig. 2 of Paterson et al.16; see this publication for full species names and dating references).
Tips represent ages of included species, but although we have attempted to retain approximate relative internal branch lengths (while increasing rates towards the base of the tree), these
are not precisely to scale. The tree is rooted between the suborders Olenellina and Redlichiina (see main text) with a divergence date of 521.5 Ma, to illustrate the scenario of a relatively
accurate fossil record. Comparison between the early redlichiine bigotinid Bigotina bivallata (c, d) and the early olenelline fallotaspidoid Profallotaspis tyusserica (e, f). These taxa
show considerable morphological similarities, supporting a close relationship between these groups. Modified with permission from Bushuev et al.78 and Geyer;80 scale bars 1 mm.
Full sizeimage
Based on this, it is clear that faunas occurring within the lowermost several trilobite biozones (representing perhaps several million years) in regions where trilobites first appear are
similar, and contain primitive forms that are more-or-less closely related—in direct contradiction to the claim of ‘established provincialism’ in the earliest trilobites. Only after this
time did the relatively distinct faunas of the two major biogeographic provinces become established, essentially involving a radiation of redlichiines in Gondwana (and elsewhere), and a
continued dominance of olenellines in Laurentia. However, there is clear overlap of these high-level taxa, with sutured trilobites appearing rapidly in all regions. It has been suggested
previously that such patterns may result from the continued rifting seen in the early Cambrian (following Neoproterozoic breakup), with dispersal between palaeocontinents initially being
easier, and faunas becoming more provincial later as continental separation continued87. This illustrates how distributions can reflect tectonic relationships without requiring strict
vicariance by continental fragmentation. Worldwide trilobite biogeographic patterns in the early Cambrian have not actually been explored in great detail (although see Álvaro et al.75) and
should be more thoroughly investigated. For example, overlap of elements within the ‘distinct’ faunas discussed above suggests that such patterns might be better explained by a more
continuous longitudinal gradient, ranging from Laurentia in the west to East Gondwana in the east (Fig. 1).
The claim that the earliest trilobites show established phylogenetic diversity also seems to have little basis. As mentioned above, the earliest trilobites to appear are olenelline
fallotaspidoids and redlichiine bigotinids, and despite these groups being classified in different suborders based largely on the presence (Redlichiina) or absence (Olenellina) of dorsal
facial sutures, these groups show marked morphological similarities. For example, a comparison of the bigotinid Bigotina bivallata (Fig. 3c, d) and the fallotaspidoid Profallotaspis
tyusserica (Fig. 3e, f) shows comparable overall proportions, and considerable similarities in the shape and orientation of the eye ridges, and how these extend from the anterior of the
glabella—this seems to be a primitive trait of the earliest trilobites80. It has generally been assumed that early olenellines lacking dorsal facial sutures (such as fallotaspidoids)
represent the ancestral state. We agree that this is the most parsimonious (although not the only) alternative, and the presence of anterior facial sutures in the olenelline P. tyusserica
(Fig. 3e, f) may provide a clue as to how dorsal sutures first evolved in trilobites78. Whilst posterior facial sutures appear to be absent in this species, opening of the anterior sutures
(linking the circumocular and perrostral/marginal sutures) would have substantially widened the ecdysial gape, and also made it much easier for the ‘free’ cheeks to break off during molting,
as appears to have happened in the specimen refigured here (Fig. 3e, f). The presence of anterior facial sutures in P. tyusserica, as well as the very similar overall form of this species
and bigotinids such as B. bivallata, suggests that these morphologies may be close to the split between the sutured redlichiines and unsutured olenellines, with the earliest representatives
of both groups essentially showing continuous variation through these forms. Thus, the separation of the earliest redlichiines and olenellines into different suborders based largely on the
presence/absence of dorsal facial sutures substantially overstates the amount of morphological variation present in the earliest trilobites. This illustrates the unavoidable limitations of
the taxonomic system when splitting high-level taxa close to their origins (we must ‘draw the line’ somewhere).
Diversification through time and morphospaceAn important consideration of a more literal interpretation of the trilobite fossil record is the consistency of this model with hypothesized phylogenetic relationships and observed patterns
of diversity through time. Figure 3b shows a simplified version of a recent Cambrian trilobite phylogeny (based on the ‘relaxed clock’ model of Paterson et al.16), with a (modified)
divergence date of 521.5 Ma—illustrating a scenario of a relatively accurate fossil record. Under this assumption, and given the likely primitive absence of dorsal facial sutures, the
rooting of the original tree on the Fallotaspididae is reasonable. However, given that the oldest fallotaspidoids (not included in the analysis, e.g., Profallotaspis) tend to show
morphologies more intermediate with bigotinids, an argument could be made to root the tree closer to the split between these groups, and this is what is shown in Fig. 3b. This makes little
difference to the arguments presented here, although it does illustrate a very early split between the Olenellina and Redlichiina, as might be expected based on the fossil record. In either
case, the Fallotaspidoidea are suggested to be paraphyletic (darker branches at the base of the tree), giving rise to a polyphyletic Olenelloidea. This supports the idea of a
fallotaspidoid-type morphology being a primitive trait, present across the base of the tree. It also supports the early divergence of primitive redlichioids (e.g., bigotinids, abadiellids)
from a fallotaspidoid-type morphology (we note that under the ‘epoch clock’ model16 these are suggested to be slightly more derived).
If trilobites have a substantial cryptic history, we might expect to see different morphologies appearing randomly in time and morphospace, as previously invisible lineages appear in the
fossil record. However, in general, it seems that stratigraphic appearance closely reflects phylogeny, suggesting that this is not the case. Initially, we see a very small number of related
families, followed by the appearance of more derived groups interpreted as diversifying from these older taxa. For example, there is a clear gradient of morphologies from fallotaspidoids,
through bigotinids80 and early redlichioids such as Lemdadella88 and Parabadiella83, to younger, more derived forms such as Eoredlichia89 and Redlichia39. The same is true for olenellines:
from early fallotaspidoids, through later examples like Nevadia and Judomia, to younger olenelloids like Olenellus and Holmia82 (Fig. 3b). Other early groups like the ellipsocephaloids are
likely to have evolved from early redlichioid or bigotinid-type morphologies (the Bigotinidae are sometimes included in this group23). Such patterns of appearance and diversification through
time suggest that we are observing a real radiation, and that there is no need to invoke an extended cryptic evolutionary history to explain trilobite biogeographic distributions or
phylogenetic relationships in the early Cambrian.
The push of the pastRecent studies90,91 have emphasized that successful clades that survive for long periods of time are statistically likely to have high initial rates of diversification, an effect termed the
push of the past (POTPa). Such clades have a higher chance of surviving for any given length of time compared to those with low initial diversification rates. The size of the POTPa is
controlled by turnover rates; i.e., for a given rate of diversification (speciation-extinction rate), a higher POTPa is implied for a high extinction rate. Such an effect is likely to
significantly shorten the cryptic history predicted by recent morphological clocks16, which suggested that trilobites most likely arose in the Fortunian. These authors noted that their
projections were necessarily based on rates within the visible part of the tree (after 519 Ma, the age of the oldest trilobites included in their analysis), and that if rates were higher
prior to this then the estimated divergence dates would be overestimates16. They also showed that enforcing a divergence date of 522 Ma would require elevated rates at the base of the trees
(as expected by the POTPa), but this had little effect on their overall results; rates remained relatively homogeneous across the Cambrian16. This suggests any reversion to the background
rate occurred quickly, and that the slightly higher rates they observed in the early Cambrian may result from a short period of rapid diversification close to when trilobites appear. As
such, we suggest that the mismatch between their divergence estimates (within error bars as young as c. 526 Ma) and an even more literal reading of the trilobite fossil record are
potentially explained by effects such as the POTPa (Fig. 4). It should also be noted that the POTPa suggests that it is unlikely for the absence of trilobites in the Terreneuvian to be
explained by rarity or low diversity, as such groups are highly unlikely to persist for long without going extinct90,91. In fact, the effect of the POTPa predicts that diversity will
initially increase rapidly, meaning that a fossil group like trilobites with high preservation potential should appear in the record soon after it arises.
Fig. 4: An illustration of thelikely effect of the push of the past (POTPa) on the early radiation of trilobites.
The solid blue line shows a scenario with a substantial POTPa, as might be expected for an extremely diverse and successful clade like Trilobita. The diversification rate (the slope of the
line) is initially very high before falling to the background rate. The dashed red line shows a projection of trilobite diversity into the unobserved early history of the group based on the
‘visible’ part of the tree—in this case after 519 Ma (shown by the vertical dashed line), similar to the Cambrian trilobite phylogenies and morphological clock divergence estimates of
Paterson et al.16. Survivorship biases like the POTPa may explain the discrepancies between such estimates and the trilobite fossil record.
Full size imageConclusionsThe suggestion of non-mineralized trilobites in the Terreneuvian or earlier is shown to be highly unparsimonious, implying rampant convergence of structures associated with exoskeletal
biomineralization in all major early trilobite lineages, and abandonment of the synapomorphies uniting the clade. This suggests that no credible reason has been proposed for the absence of
Terreneuvian trilobites in the fossil record, given the assumption of a substantial cryptic evolutionary history. Despite previous statements to the contrary, when trilobites appear in the
fossil record they show limited provincialism and relatively low phylogenetic diversity. Even when more distinct faunas develop across the remainder of Cambrian Series 2 there is
considerable overlap between these, and patterns of diversification suggest this is occurring in real time (rather than resulting from divergence prior to the FAD of trilobites). Given the
change in our understanding of the relative importance of vicariance and dispersal in explaining modern biogeographic patterns over the last several decades—and the general observation that
modern marine invertebrate faunas do not show vicariant patterns resulting from continental separation—trilobite biogeographic patterns are unlikely to result from this form of vicariance.
The mismatch between recent morphological clock estimates (that suggest trilobites probably emerged in the Fortunian) and an even more literal reading of the fossil record can be explained
by effects such as the push of the past, which anticipates higher rates of diversification during the initial radiation of clades—particularly in the case of very long-lived and successful
groups like trilobites. We conclude that the FAD of trilobites closely reflects their evolutionary origins, and that there is no compelling evidence to suggest an extended cryptic
evolutionary history for this group.
Reporting summaryFurther information on experimental design is available in the Nature Research Reporting Summary linked to this paper.
References Budd, G. E. The Cambrian fossil record and the origin of the phyla. Integr. Comp. Biol. 43, 157–165 (2003).
Article PubMed Google Scholar
Budd, G. E. & Jensen, S. A critical reappraisal of the fossil record of the bilaterian phyla. Biol. Rev. 75, 253–295 (2000).
Article CAS PubMed Google Scholar
Budd, G. E. & Mann, R. P. The dynamics of stem and crown groups. Sci. Adv. 6, eaaz1626 (2020).
Article PubMed PubMed Central Google Scholar
Cooper, A. & Fortey, R. Evolutionary explosions and the phylogenetic fuse. Trends Ecol. Evol. 13, 151–156 (1998).
Article CAS PubMed Google Scholar
Erwin, D. H. The origin of animal body plans: A view from fossil evidence and the regulatory genome. Development 147, dev182899 (2020).
Article CAS PubMed Google Scholar
Erwin, D. H. et al. The Cambrian Conundrum: Early divergence and later ecological success in the early history of animals. Science 334, 1091–1097 (2011).
Article CAS PubMed Google Scholar
Sperling, E. A. & Stockey, R. G. The temporal and environmental context of early animal evolution: Considering all the ingredients of an “explosion”. Integr. Comp. Biol. 58, 605–622 (2018).
Article CAS PubMed Google Scholar
Cunningham, J. A., Liu, A. G., Bengtson, S. & Donoghue, P. C. J. The origin of animals: Can molecular clocks and the fossil record be reconciled? BioEssays 39, e201600120 (2017).
Article Google Scholar
Dunn, F. S., Liu, A. G. & Donoghue, P. C. J. Ediacaran developmental biology. Biol. Rev. 93, 914–932 (2018).
Article PubMed Google Scholar
Evans, S. D., Hughes, I. V., Gehling, J. G. & Droser, M. L. Discovery of the oldest bilaterian from the Ediacaran of South Australia. Proc. Natl Acad. Sci. USA 117, 7845–7850 (2020).
Article CAS PubMed PubMed Central Google Scholar
Lee, M. S. Y., Soubrier, J. & Edgecombe, G. D. Rates of phenotypic and genomic evolution during the Cambrian explosion. Curr. Biol. 23, 1889–1895 (2013).
Article CAS PubMed Google Scholar
Maloof, A. C. et al. The earliest Cambrian record of animals and ocean geochemical change. Geol. l Soc. Am. Bull. 122, 1731–1774 (2010).
Article CAS Google Scholar
Maloof, A. C. et al. Constraints on early Cambrian carbon cycling from the duration of the Nemakit-Daldynian–Tommotian boundary δ13C shift, Morocco. Geology 38, 623–626 (2010).
Article CAS Google Scholar
Adrain, J. M. A synopsis of Ordovician trilobite distribution and diversity. In ‘Early Palaeozoic Biogeography and Palaeogeography’ (Eds D. A. T. Harper, T. Servais). Geol. Soc. Lond. Mem.
38, 297–336 (2013).
Paterson, J. R. The trouble with trilobites: classification, phylogeny and the cryptogenesis problem. Geol. Mag. 157, 35–46 (2020).
Article CAS Google Scholar
Paterson, J. R., Edgecombe, G. D. & Lee, M. S. Y. Trilobite evolutionary rates constrain the duration of the Cambrian explosion. Proc. Natl Acad. Sci. USA 116, 4394–4399 (2019).
Article PubMed PubMed Central Google Scholar
Holmes, J. D., Paterson, J. R. & García-Bellido, D. C. Complex axial growth patterns in an early Cambrian trilobite from South Australia. Proc. R. Soc. B. 288, 20212131 (2021).
Article PubMed PubMed Central Google Scholar
Fortey, R. A., Briggs, D. E. G. & Wills, M. A. The Cambrian evolutionary ‘explosion’ recalibrated. Bioessays 19, 429–434 (1997).
Article Google Scholar
Fortey, R. A., Briggs, D. E. G. & Wills, M. A. The Cambrian evolutionary ‘explosion’: decoupling cladogenesis from morphological disparity. Biol. J. Linn. Soc. Lon. 57, 13–33 (1996).
Google Scholar
Fortey, R. A. & Owens, R. M. Major Evolutionary Radiations 139–164 (Clarendon Press, 1990).
Pillola, G. L. Trilobites du Cambrien inférieur du SW de la Sardaigne, Italie. Palaeontogr. Ital. 78, 1–173 (1991).
Google Scholar
Kobayashi, T. Three faunal provinces in the Early Cambrian Period. Proc. Jpn. Acad. 48, 242–247 (1972).
Article Google Scholar
Adrain, J. M. Class Trilobita Walch, 1771. Zootaxa 3148, 104–109 (2011).
Article Google Scholar
Lieberman, B. S. Taking the pulse of the Cambrian radiation. Integr. Comp. Biol. 43, 229–237 (2003).
Article PubMed Google Scholar
Lieberman, B. S. Phylogenetic analysis of some basal early Cambrian trilobites, the biogeographic origins of the Eutrilobita, and the timing of the Cambrian radiation. J. Paleontol. 76, 692
(2002).
Article Google Scholar
Meert, J. G. & Lieberman, B. S. A palaeomagnetic and palaeobiogeography perspective on the latest Neoproterozoic and early Cambrian tectonic events. J. Geol. Soc. Lond. 161, 477–487 (2004).
Article Google Scholar
Hughes, N. C. The evolution of trilobite body patterning. Annu. Rev. Earth Planet. Sci. 35, 401–434 (2007).
Article CAS Google Scholar
Lauterbach, K.-E. Schlüsselereignisse in der Evolution des Grundplans der Arachnata (Arthropoda). Abh. Verh. Naturwiss. Ver. Hambg. 23, 163–327 (1980).
Google Scholar
Lauterbach, K.-E. Synapomorphien zwischen Trilobiten- und Cheliceratenzweig der Arachnata. Zool. Anz. 210, 213–238 (1983).
Google Scholar
Moysiuk, J. & Caron, J.-B. Burgess Shale fossils shed light on the agnostid problem. Proc. R. Soc. B. 286, 20182314 (2019).
Article CAS PubMed PubMed Central Google Scholar
Edgecombe, G. D. & Ramsköld, L. Relationships of Cambrian Arachnata and the systematic position of Trilobita. J. Paleontol. 73, 263–287 (1999).
Article Google Scholar
Ramsköld, L. & Edgecombe, G. D. Trilobite monophyly revisited. Hist. Biol. 4, 267–283 (1991).
Fortey, R. A. & Whittington, H. B. The Trilobita as a natural group. Hist. Biol. 2, 125–138 (1989).
Article Google Scholar
Crimes, T. P. Trace fossils and correlation of late Precambrian and early Cambrian strata. Geol. Mag. 124, 97–119 (1987).
Article Google Scholar
Budd, G. E. & Jackson, I. S. C. Ecological innovations in the Cambrian and the origins of the crown group phyla. Philos. Trans. R. Soc. B 371, 20150287 (2016).
Article Google Scholar
Budd, G. E. & Telford, M. J. The origin and evolution of arthropods. Nature 457, 812–817 (2009).
Article CAS PubMed Google Scholar
Stein, M., Budd, G. E., Peel, J. S. & Harper, D. A. T. Arthroaspis n. gen., a common element of the Sirius Passet Lagerstatte (Cambrian, North Greenland), sheds light on trilobite ancestry.
BMC Evol. Biol. 13, 99 (2013).
Article PubMed PubMed Central Google Scholar
Ortega-Hernández, J., Legg, D. A. & Braddy, S. J. The phylogeny of aglaspidid arthropods and the internal relationships within Artiopoda. Cladistics 29, 15–45 (2013).
Article PubMed Google Scholar
Holmes, J. D., Paterson, J. R. & García-Bellido, D. C. The trilobite Redlichia from the lower Cambrian Emu Bay Shale Konservat-Lagerstätte of South Australia: Systematics, ontogeny and
soft-part anatomy. J. Syst. Palaeontol. 18, 295–334 (2020).
Article Google Scholar
Bicknell, R. D. C. et al. Biomechanical analyses of Cambrian euarthropod limbs reveal their effectiveness in mastication and durophagy. Proc. R. Soc. B. 288, 20202075 (2021).
Article PubMed PubMed Central Google Scholar
Yang, J. et al. Early Cambrian fuxianhuiids from China reveal origin of the gnathobasic protopodite in euarthropods. Nat. Commun. 9, 470 (2018).
Article PubMed PubMed Central Google Scholar
Budd, G. E. The origin and evolution of the euarthropod labrum. Arthropod Struct. Dev. 62, 101048 (2021).
Article PubMed Google Scholar
Pratt, B. R. Lower Cambrian Rusophycus from Ellesmere Island, Arctic Canada: Ichnofossil of a predatory, non-trilobite arthropod. PALAIOS 37, 165–184 (2022).
Article Google Scholar
Zonneveld, J.-P., Pemberton, S. G., Saunders, T. D. A. & Pickerill, R. K. Large, robust Cruziana from the Middle Triassic of Northeastern British Columbia: Ethologic, biostratigraphic, and
paleobiologic significance. PALAIOS 17, 435–448 (2002).
Article Google Scholar
Bromley, R. & Asgaard, U. Triassic freshwater ichnocoenoses from Carlsberg Fjord, East Greenland. Palaeogeogr. Palaeoclimatol. Palaeoecol. 28, 39–80 (1979).
Article Google Scholar
Donovan, S. K. Cruziana and Rusophycus: Trace fossils produced by trilobites … in some cases? Lethaia 43, 283–284 (2010).
Article Google Scholar
Darwin, C. On the Origin of Species by Means of Natural Selection (John Murray, 1859).
Quammen, D. The Song of the Dodo: Island Biogeography in an Age of Extinctions (Hutchinson, 1996).
Cowie, R. H. & Holland, B. S. Dispersal is fundamental to biogeography and the evolution of biodiversity on oceanic islands. J. Biogeogr. 33, 193–198 (2006).
Article Google Scholar
de Queiroz, A. The resurrection of oceanic dispersal in historical biogeography. Trends Ecol. Evol. 20, 68–73 (2005).
Article PubMed Google Scholar
Gillespie, R. G. et al. Long-distance dispersal: A framework for hypothesis testing. Trends Ecol. Evol. 27, 47–56 (2012).
Article PubMed Google Scholar
de Queiroz, A. The Monkey’s Voyage: How Improbable Journeys Shaped the History of Life (Basic Books, 2014).
Waters, J. M. et al. Biogeography off the tracks. Syst. Biol. 62, 494–498 (2013).
Article PubMed Google Scholar
Matzke, N. J. Model selection in historical biogeography reveals that founder-event speciation is a crucial process in island clades. Syst. Biol. 63, 951–970 (2014).
Article PubMed Google Scholar
Briggs, J. C. Panbiogeography: Its origin, metamorphosis and decline. Russ. J. Mar. Biol. 33, 273–277 (2007).
Article Google Scholar
Sanmartín, I. & Ronquist, F. Southern Hemisphere biogeography inferred by event-based models: Plant versus animal patterns. Syst. Biol. 53, 216–243 (2004).
Article PubMed Google Scholar
Matschiner, M. Gondwanan vicariance or trans-Atlantic dispersal of cichlid fishes: A review of the molecular evidence. Hydrobiologia 832, 9–37 (2019).
Article Google Scholar
Capobianco, A. & Friedman, M. Vicariance and dispersal in southern hemisphere freshwater fish clades: A palaeontological perspective: Vicariance and dispersal in freshwater fishes. Biol.
Rev. 94, 662–699 (2019).
Article PubMed Google Scholar
Noben, S. et al. Biogeography of the Gondwanan tree fern family Dicksoniaceae—A tale of vicariance, dispersal and extinction. J. Biogeogr. 44, 2648–2659 (2017).
Article Google Scholar
Murphy, J. B. et al. Pannotia: In defence of its existence and geodynamic significance. Geol. Soc. Spec. Publ. 503, 13–39 (2021).
Article Google Scholar
Evans, D. A. D. Pannotia under prosecution. Geol. Soc. Spec. Publ. 503, 63–81 (2021).
Article Google Scholar
Lieberman, B. S. & Eldredge, N. Trilobite biogeography in the Middle Devonian: Geological processes and analytical methods. Paleobiology 22, 66–79 (1996).
Article Google Scholar
Laakkonen, H. M., Hardman, M., Strelkov, P. & Väinölä, R. Cycles of trans‐Arctic dispersal and vicariance, and diversification of the amphi‐boreal marine fauna. J. Evol. Biol. 34, 73–96
(2021).
Article PubMed Google Scholar
Bernardi, G., Findley, L. & Rocha-Olivares, A. Vicariance and dispersal across Baja California in disjunct marine fish populations. Evolution 57, 1599–1609 (2003).
Article PubMed Google Scholar
Mirams, A. G. K., Treml, E. A., Shields, J. L., Liggins, L. & Riginos, C. Vicariance and dispersal across an intermittent barrier: Population genetic structure of marine animals across the
Torres Strait land bridge. Coral Reefs 30, 937–949 (2011).
Article Google Scholar
Palumbi, S. R. Genetic divergence, reproductive isolation, and marine speciation. Annu. Rev. Ecol. Syst. 25, 547–572 (1994).
Article Google Scholar
Rosenblatt, R. H. & Waples, R. S. A genetic comparison of allopatric populations of shore fish species from the eastern and central Pacific Ocean: Dispersal or vicariance? Copeia 1986,
275–284 (1986).
Article Google Scholar
Winston, J. E. Dispersal in Marine Organisms without a pelagic larval phase. Integr. Comp. Biol. 52, 447–457 (2012).
Article PubMed Google Scholar
Fraser, C. I. et al. Southern Hemisphere coasts are biologically connected by frequent, long-distance rafting events. Curr. Biol. 32, 3154–3160.e3 (2022).
Article CAS PubMed Google Scholar
Bryan, S. E. et al. Rapid, long-distance dispersal by pumice rafting. PLoS One 7, e40583 (2012).
Article CAS PubMed PubMed Central Google Scholar
Bryan, S. E. et al. Pumice rafting and faunal dispersion during 2001–2002 in the Southwest Pacific: record of a dacitic submarine explosive eruption from Tonga. Earth Planet. Sci. Lett. 227,
135–154 (2004).
Article CAS Google Scholar
Jokiel, P. L. & Cox, E. F. Drift pumice at Christmas Island and Hawaii: evidence of oceanic dispersal patterns. Mar. Geol. 202, 121–133 (2003).
Article Google Scholar
Fraser, C. I., Nikula, R. & Waters, J. M. Oceanic rafting by a coastal community. Proc. R. Soc. B. 278, 649–655 (2011).
Article PubMed Google Scholar
Nikula, R., Spencer, H. G. & Waters, J. M. Passive rafting is a powerful driver of transoceanic gene flow. Biol. Lett. 9, 20120821 (2013).
Article PubMed PubMed Central Google Scholar
Álvaro, J. J. et al. Global Cambrian trilobite palaeobiogeography assessed using parsimony analysis of endemicity. In ‘Early Palaeozoic Biogeography and Palaeogeography’ (Eds D. A. T.
Harper, T. Servais). Geol. Soc. Lond. Mem. 38, 273–296 (2013).
Landing, E., Geyer, G., Brasier, M. D. & Bowring, S. A. Cambrian evolutionary radiation: Context, correlation, and chronostratigraphy—Overcoming deficiencies of the first appearance datum
(FAD) concept. Earth-Sci. Rev. 123, 133–172 (2013).
Article CAS Google Scholar
Zhang, X. et al. Challenges in defining the base of Cambrian Series 2 and Stage 3. Earth-Sci. Rev. 172, 124–139 (2017).
Article CAS Google Scholar
Bushuev, E., Goryaeva, I. & Pereladov, V. New discoveries of the oldest trilobites Profallotaspis and Nevadella in the northeastern Siberian Platform, Russia. Bull. Geosci. 89, 347–364
(2014).
Hollingsworth, J. S. Lithostratigraphy and biostratigraphy of Cambrian Stage 3 in western Nevada and eastern California. Mus. North. Ariz. Bull. 67, 26–42 (2011).
Google Scholar
Geyer, G. The earliest known West Gondwanan trilobites from the Anti-Atlas of Morocco, with a revision of the Family Bigotinidae Hupé, 1953. Foss. Strat. 64, 55–153 (2019).
Article Google Scholar
Geyer, G. The Moroccan fallotaspidid trilobites revisited. Beringeria 18, 89–199 (1996).
Google Scholar
Palmer, A. R. & Repina, L. N. Through a glass darkly: Taxonomy, phylogeny, and biostratigraphy of the Olenellina. Univ. Kans. Paleontol. Contrib. 3, 1–35 (1993).
Google Scholar
Bengtson, S., Conway Morris, S., Cooper, B. J., Jell, P. A. & Runnegar, B. N. Early Cambrian fossils from South Australia. Mem. Assoc. Australas. Palaeontol. 9, 1–364 (1990).
Google Scholar
Betts, M. J. et al. Early Cambrian chronostratigraphy and geochronology of South Australia. Earth-Sci. Rev. 185, 498–543 (2018).
Article CAS Google Scholar
Betts, M. J. et al. Global correlation of the early Cambrian of South Australia: Shelly fauna of the Dailyatia odyssei Zone. Gondwana Res. 46, 240–279 (2017).
Article Google Scholar
Hupé, P. Contribution à l’étude du Cambrien inférieur et du Précambrien III de l’Anti-Atlas Marocain. Direction de. la Prod. Industrielle et. des. Mines, Div. des. Mines et. de. la Géologie,
Serv. Géologique, Notes et. Mémoires 103, 1–402 (1953).
Google Scholar
Budd, G. E. & Jensen, S. The limitations of the fossil record and the dating of the origin of the Bilateria. Syst. Assoc. Spec. Publ. 66, 170–193 (2003).
Google Scholar
Palmer, A. R. & Rowell, A. J. Early Cambrian Trilobites from the Shackleton limestone of the central transantarctic mountains. Paleontol. Soc. Mem. 45, 1–28 (1995).
Google Scholar
Dai, T. & Zhang, X. Ontogeny of the redlichiid trilobite Eoredlichia intermediata from the Chengjiang Lagerstätte, lower Cambrian, southwest China. Lethaia 46, 262–273 (2013).
Article Google Scholar
Budd, G. E. & Mann, R. P. Survival and selection biases in early animal evolution and a source of systematic overestimation in molecular clocks. Interface Focus. 10, 20190110 (2020).
Article PubMed PubMed Central Google Scholar
Budd, G. E. & Mann, R. P. History is written by the victors: The effect of the push of the past on the fossil record. Evolution 72, 2276–2291 (2018).
Article PubMed PubMed Central Google Scholar
Torsvik, T. H. & Cocks, L. R. M. New global palaeogeographical reconstructions for the Early Palaeozoic and their generation. In ‘Early Palaeozoic Biogeography and Palaeogeography’ (Eds D.
A. T. Harper, T. Servais). Geol. Soc. Lond. Mem. 38, 5–24 (2013).
Download references
AcknowledgementsWe thank Nigel Hughes and Greg Edgecombe for useful comments that substantially improved the manuscript. We also thank Marissa Betts for comments on an early version of this work, and Ben
Slater for insightful discussions.
FundingOpen access funding provided by Uppsala University.
Author informationAuthors and Affiliations Department of Earth Sciences, Palaeobiology, Uppsala University, Villavägen 16, Uppsala, 752 36, Sweden
James D. Holmes & Graham E. Budd
AuthorsJames D. HolmesView author publications You can also search for this author inPubMed Google Scholar
Graham E. BuddView author publications You can also search for this author inPubMed Google Scholar
ContributionsJ.D.H. conceived the study and wrote the manuscript with input from G.E.B.
Corresponding author Correspondence to James D. Holmes.
Ethics declarations Competing interestsThe authors declare no competing interests.
Peer review Peer review informationCommunications Biology thanks Nigel Hughes and Gregory Edgecombe for their contribution to the peer review of this work. Primary Handling Editor: Luke R. Grinham. Peer reviewer reports are
available.
Additional informationPublisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary informationPeer Review FileReportingSummary-NewRights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or
format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or
other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in
the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the
copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and permissions
About this articleCite this article Holmes, J.D., Budd, G.E. Reassessing a cryptic history of early trilobite evolution. Commun Biol 5, 1177 (2022).
https://doi.org/10.1038/s42003-022-04146-6
Download citation
Received: 05 July 2022
Accepted: 20 October 2022
Published: 04 November 2022
DOI: https://doi.org/10.1038/s42003-022-04146-6
Share this article Anyone you share the following link with will be able to read this content:
Get shareable link Sorry, a shareable link is not currently available for this article.
Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative