Play all audios:
CD47 is a cell surface ligand expressed on all nucleated cells. It is a unique immune checkpoint protein acting as “don’t eat me” signal to prevent phagocytosis and is constitutively
overexpressed in many tumors. However, the underlying mechanism(s) for CD47 overexpression is not clear. Here, we show that irradiation (IR) as well as various other genotoxic agents induce
elevated expression of CD47. This upregulation correlates with the extent of residual double-strand breaks (DSBs) as determined by γH2AX staining. Interestingly, cells lacking mre-11, a
component of the MRE11-RAD50-NBS1 (MRN) complex that plays a central role in DSB repair, or cells treated with the mre-11 inhibitor, mirin, fail to elevate the expression of CD47 upon DNA
damage. On the other hand, both p53 and NF-κB pathways or cell-cycle arrest do not play a role in CD47 upregualtion upon DNA damage. We further show that CD47 expression is upregulated in
livers harvested from mice treated with the DNA-damage inducing agent Diethylnitrosamine (DEN) and in cisplatin-treated mesothelioma tumors. Hence, our results indicate that CD47 is
upregulated following DNA damage in a mre-11-dependent manner. Chronic DNA damage response in cancer cells might contribute to constitutive elevated expression of CD47 and promote immune
evasion.
SIRPα–CD47 pair of molecules has emerged as a novel immune checkpoint (IC) that targets the innate immune system. CD47 (also called integrin-associated protein, IAP) is a cell surface
transmembrane glycoprotein widely expressed on many cells of epithelial and mesenchymal origin and is highly expressed on tumor cells1. SIRPα (also known as CD172a or SHPS-1) is a
transmembrane glycoprotein receptor expressed predominantly on myeloid and neuronal cells and has been linked to cell adhesion2,3. SIRPα ligation by its cognate ligand CD47, used as a marker
of ‘self’, results in a negative signal that inhibits phagocytic activity3,4,5,6, therefore protecting CD47 expressing cells from elimination by engaging SIRPα on phagocytes. CD47 is
consequently overexpressed on various types of tumors, allowing cancer cells to escape macrophage phagocytosis and immune surveillance7,8,9,10,11,12,13. Interestingly, CD47 upregulation was
found to serve as a mechanism for leukemia progenitors to avoid phagocytosis7,14. Moreover, high CD47 expression is associated with poor prognosis in human malignancies15,16.
The inhibitory checkpoint immunotherapy, which has revolutionized cancer treatment over recent years, aims to block these inhibitory signals and reactivate the immune system against the
tumor. Indeed, blocking CD47-SIRPα interaction via anti-CD47 antibodies restored cancer cell phagocytosis and destruction by macrophages11,12,13,14. Surprisingly, CD47 blockade not only
refurbished macrophage phagocytosis but also enabled T cell-mediated antitumor cytotoxic response17,18. Hence, these data suggest that CD47-SIRPα represents a new tumor escape mechanism that
in addition to its ability to inhibit innate immunity also acts to restrain the adaptive immune response. Previously, we uncovered a unique mechanism wherein CD47 inhibits T cells
indirectly by contact-dependent induction of aberrant antigen-presenting cell (APC) maturation. In turn, these CD47-conditioned APCs act as regulatory cells that actively regulate T-cell
activity19,20,21,22. Of note, combination of CD47-SIRPα interaction blockade with ionizing radiation (IR) synergistically inhibits tumor growth23,24,25.
Studies have suggested that cells may lose surface CD47 during apoptosis to enable phagocytic clearance (reviewed in26). Previously we demonstrated that while in apoptotic non-malignant
cells CD47 surface expression is reduced at early stages of apoptosis, CD47 expression is significantly increased upon exposure to sub-lethal ionizing radiation (IR)22. In contrast, Vermeer
et al., reported that irradiation reduced surface CD47 expression in head and neck squamous cell carcinoma in a dose-dependent manner27. Interestingly, in a series of studies David Roberts’
group reported that blocking CD47 signaling induced by the CD47 ligand thrombospondin-1, or suppressing CD47 expression, protects normal tissues from IR injury while sensitizing the tumor
cells28,29,30,31, suggesting CD47 in tumor cells is important for DNA-damage repair. Notably, data implying that loss of CD47 function modulated DNA damage response and improved DNA repair
in non-malignant cells also exists28. Thus, the evidence suggests that CD47 expression is an important factor influencing cellular response to IR and phagocytic clearance. However, the
relationship between IR, DNA damage and CD47 expression, has not been studied yet.
Exposure to ionizing radiation induces DNA damage, specifically DNA double-strand breaks (DSBs)32,33. The Mre11–Rad50–Nbs1 (MRN) and the Ku heterodimer (Ku70-Ku80) complexes are among the
first responders that bind the DSBs independently of other factors. These complexes play important roles in sensing, as well as promoting the repair of DNA ends via both HR and NHEJ
pathways34,35,36,37. MRN and Ku70-80 complexes also have a key role in activating kinases ataxia-telangiectasia mutated (ATM) and DNA-dependent protein kinase catalytic subunit (DNA-PKcs),
respectively38,39,40. In turn, these phosphoinositide-3-kinase (PI3K) family members target other proteins including the histone H2AX (referred to as γH2AX) that is phosphorylated over a
large region surrounding the DSB, forming readily visualized foci, and creating a binding site for other DDR proteins41.
In the present study, we demonstrate that IR as well as various other genotoxic agents to non-malignant and malignant cells induced elevated expression of CD47 in a dose-dependent manner.
This upregulation correlates with the extent of residual DSB as determined by γH2AX staining, that is used as a marker to the extent of DNA damage caused by irradiation or other genotoxic
agents. Interestingly, cells lacking mre-11, a component of the MRN complex that plays a central role in DSB repair, fail to elevate the expression of CD47 upon DNA damage induction. On the
other hand, p53, that directs cellular response to DNA damage, is not required for this upregulation, which occurred in p53−/− cell line and was not affected by MDM-2 inhibitor. In addition,
NFkB pathway or cell-cycle arrest does not account for the increase of CD47 expression. Hence, our data suggest that CD47 is upregulated following DNA damage in an mre-11 dependent-manner
in both malignant and non-malignant cells.
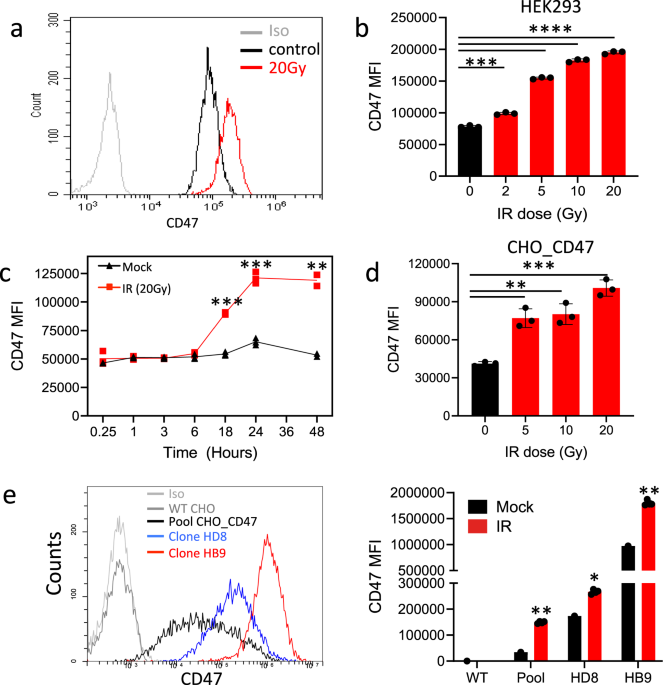
To characterize the effect of IR on CD47 expression, we analyzed the dose and time dependency of CD47 surface expression following γ-irradiation. Using Annexin/PI staining we first confirmed
cell’s viability following the various doses of γ-irradiation (Fig. S1). CD47 expression on live cells, was significantly elevated on HEK-293T cells in response to γ-irradiation in a
dose-dependent manner (Fig. 1a, b). Elevated expression was evident at 6 h following irradiation and peaked at 24 h (Fig. 1c). On the other hand, CD47 expression was not upregulated by other
cell stress conditions, including heat shock, or incubation in serum-free medium (Fig. S2).
a Representative histogram of CD47 staining and flow cytometric analysis before and after γ-irradiation (HEK-293T cells). b Dose-dependent expression of CD47 (MFI) in HEK-293T cells 24 h
after γ-irradiation. c Time-dependent expression of CD47 (MFI) in HEK-293T cells following 20Gy γ-irradiation. d CD47 expression (MFI) in CHO cells transfected with human CD47 24 h after
γ-irradiation. e Representative histogram of CD47 staining of CHO_CD47 of different clones (left panel) and their corresponding CD47 expression (MFI) in response to 20 Gy γ-irradiation
(right panel). Graphs show an average (±STD) of four replicates in each group. One of at least four independent experiments is shown. *p