Play all audios:
ABSTRACT Autism spectrum disorders are more common in males, and have a substantial genetic component. Chromosomal 16p11.2 deletions in particular carry strong genetic risk for autism, yet
their neurobiological impact is poorly characterised, particularly at the integrated systems level. Here we show that mice reproducing this deletion (16p11.2 DEL mice) have reduced GABAergic
interneuron gene expression (decreased parvalbumin mRNA in orbitofrontal cortex, and male-specific decreases in Gad67 mRNA in parietal and insular cortex and medial septum). Metabolic
activity was increased in medial septum, and in its efferent targets: mammillary body and (males only) subiculum. Functional connectivity was altered between orbitofrontal, insular and
auditory cortex, and between septum and hippocampus/subiculum. Consistent with this circuit dysfunction, 16p11.2 DEL mice showed reduced prepulse inhibition, but enhanced performance in the
continuous performance test of attentional ability. Level 1 autistic individuals show similarly heightened performance in the equivalent human test, also associated with parietal,
insular-orbitofrontal and septo-subicular dysfunction. The data implicate cortical and septal GABAergic dysfunction, and resulting connectivity changes, as the cause of pre-attentional and
attentional changes in autism. SIMILAR CONTENT BEING VIEWED BY OTHERS STRUCTURAL AND FUNCTIONAL BRAIN-WIDE ALTERATIONS IN A350V _IQSEC2_ MUTANT MICE DISPLAYING AUTISTIC-LIKE BEHAVIOR Article
Open access 22 March 2021 LINKING HAPLOINSUFFICIENCY OF THE AUTISM- AND SCHIZOPHRENIA-ASSOCIATED GENE _CYFIP1_ WITH STRIATAL-LIMBIC-CORTICAL NETWORK DYSFUNCTION AND COGNITIVE INFLEXIBILITY
Article Open access 14 June 2024 HIGHLY DEMARCATED STRUCTURAL ALTERATIONS IN THE BRAIN AND IMPAIRED SOCIAL INCENTIVE LEARNING IN _TBX1_ HETEROZYGOUS MICE Article Open access 27 October 2024
INTRODUCTION Autism spectrum disorders (ASD) are a heterogeneous group of related neurodevelopmental disorders characterised by social dysfunction, communication difficulties, repetitive
behaviour, restricted interests and altered sensory perception. Interestingly, cognitive abilities range from profound intellectual disability (ID) to enhanced performance in some cognitive
domains1,2. In recent years, an increase in the prevalence of ASD has been observed, with current estimates of ~1 in 100 people affected. Boys are around 4 times more likely to be affected
than girls, although this gap is narrowing3,4,5. There are currently no effective treatments for ASD. There is a strong genetic component to the chance of developing ASD, with heritability
estimates of up to 90%6. Typically, multiple common sequence variations of small effects interact to increase the chance of developing ASD, in combination with environmental risk factors.
However, in addition to these common genetic variants, rare variants also exist that individually have a much greater effect. These rare variants can be genetically reproduced in mice,
providing valuable insight into the neurobiological dysfunction that underlies ASD. If translationally-relevant phenotypes and endophenotypes can be identified, these mouse models will
ultimately provide a means to identify and test novel treatment strategies for aspects of ASD. Carriers of a ~29 gene deletion on chr.16p11.2 are at greatly increased chance (~35 times) of
developing ASD7,8,9,10,11 and related conditions with shared heritability, such as attention deficit hyperactivity disorder (ADHD) and anxiety disorders12. 16p11.2 deletions are one of the
most prevalent causes of syndromic ASD and hence are of great interest for further understanding the neurobiological dysfunction that underlies ASD symptomatology. Given the heterogeneity of
ASD, it is not surprising that structural and functional neuroimaging studies have identified many affected brain regions and networks. These include changes in fronto-temporal and
frontoparietal regions, the amygdala-hippocampal complex, cerebellum, basal ganglia and cingulate cortex13,14,15,16. These regions form part of functional brain networks including the
Default Mode Network (DMN), the Salience Network (SalN) and the Visual Attention Network (VAN). These networks, and the interactions between them, are dysfunctional in ASD. ASD is associated
with both hypoconnectivity and hyperconnectivity of these macroscale brain networks, whereas connectivity in local circuits is increased16,17,18,19. According to current theories, this
altered connectivity sits alongside, and is possibly caused by, GABAergic interneuron and glutamate system dysfunction. Excitation/inhibition (E/I) imbalance in ASD, with compromised
function of local inhibitory GABAergic interneurons in cortical areas, is thought to disrupt the balance of intrinsic and extrinsic regional network activity20. While certain symptoms of ASD
have been broadly correlated with particular networks, hallmark connectivity patterns remain unclear. Adoption of multi-scale and integrative (endo)phenotyping, along with effective forward
and reverse translational approaches, is key to understanding ASD and identifying translational biomarkers for diagnosis, prognosis and treatment validation21. The identification of 16p11.2
deletions as a major risk factor for ASD provides an opportunity to reverse translate into preclinical studies, in order to gain an increased understanding of the neurobiological mechanisms
involved in ASD. In addition, translational data-driven preclinical brain imaging approaches can be used to identify cross-species commonalities in brain dysfunction, as well as reveal
hitherto unknown brain network impairments in ASD22. Here we undertake a multi-scale and integrative characterisation of CNS function in mice reproducing the 16p11.2 deletion, with a focus
on characterising translationally-relevant perturbations. Thus we characterise ASD-relevant alterations in GABAergic interneuron gene expression and related functional brain imaging changes,
in terms of regional metabolism and data-driven functional brain network connectivity changes. Guided by our observations in these endophenotypic measures we prioritised the
characterisation of behaviours dependent on the brain regions and networks we identified as being dysfunctional in 16p11.2 DEL mice. Thus, we characterise the performance of 16p11.2 DEL mice
in behavioural translational measures known to be impacted in ASD – pre-attentional sensorimotor gating, measured using prepulse inhibition (PPI) of the startle reflex, and attentional
performance, in the visual rodent continuous performance task (rCPT) utilising touchscreen apparatus designed to reproduce the widely employed human CPT23. There are conflicting reports on
whether attentional processing is enhanced or impaired in ASD individuals. However, there is emerging evidence to support an enhanced capacity for selective attention in some
contexts1,24,25,26. RESULTS FUNCTIONAL BRAIN IMAGING IDENTIFIES WIDESPREAD DYSFUNCTIONAL INTEGRATION BETWEEN DIVERSE NEURAL SYSTEMS IN 16P11.2 DEL MICE Most CNS regions showed unchanged
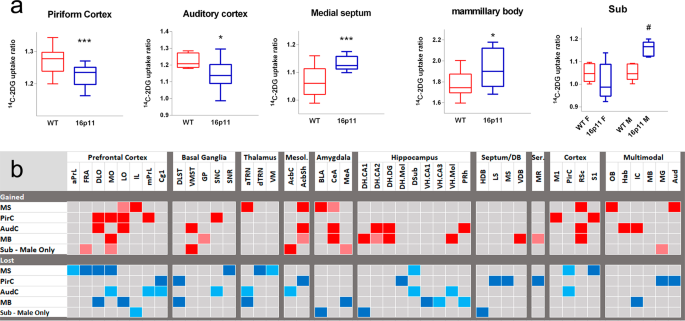
metabolic activity in 16p11.2 DEL mice, as evidenced by unaltered 14C-2-DG metabolism. However, metabolism in the piriform (PirC) and auditory cortex (AudC) was reduced in 16p11.2 DEL mice.
In contrast, metabolic activity was increased in the medial septum (MS) and mammillary body (MB) (Fig. 1a). These effects were not influenced by sex. However, we also found markedly elevated
metabolism in the hippocampal subiculum (Sub) of 16p11.2 DEL mice, but only in males (Fig. 1a). To elucidate the dysfunctional inter-regional connectivity of these regions in 16p11.2 DEL
mice we employed Partial Least Squares Regression (PLSR) analysis (Fig. 1b). We found evidence for both gained and lost inter-regional functional connectivity for each region, supporting
widespread neural systems dysconnectivity in 16p11.2 DEL mice. For the MS, functional connectivity was gained to amygdala (BLA, CeA), retrosplenial (RSC) and AudC. Altered MS functional
connectivity to the prefrontal cortex (PFC) was complex, with gained connectivity to the infralimbic (IL) and lateral orbital (LO) cortical subfields, but lost connectivity to other PFC
subfields (anterior prelimbic (aPrL), frontal association cortex (FRA), DLO, MO) and to the hippocampal Sub. We also found evidence for both gained and lost connectivity with the PFC, on a
subfield-dependent basis, when the AudC and PirC were considered as seed regions in the analysis. This included gained functional connectivity to orbital cortex subfields (DLO, MO, LO) but
lost connectivity to cingulate cortex (Cg1) in 16p11.2 DEL mice. Interestingly, there was also evidence for dysfunctional connectivity between the AudC and hippocampus in 16p11.2 DEL mice,
with gained connectivity to the dorsal hippocampus (DHCA2, DH.DG) but lost connectivity to the Sub of the ventral hippocampus. A similar pattern of hippocampal dysconnectivity was also
observed for the MB, with lost connectivity to ventral hippocampus (VH.CA1, VH.CA3) but enhanced connectivity to dorsal hippocampus (DH.CA1, DH.CA2, DH.DG) in 16p11.2 DEL mice. Given that
hippocampal Sub activity was selectively increased in males, we characterised the alterations in Sub connectivity seen selectively in male 16p11.2 DEL animals. Again we found evidence for
complex dysfunctional connectivity to the PFC, with both gained (FRA, MO) and lost (IL) connectivity supported on a PFC subfield-dependent basis. Overall these data support complex
dysfunctional connectivity between a diverse range of neural systems in 16p11.2 DEL mice, with notably altered prefrontal (PrL, IL, LO, DLO, MO), hippocampal and temporal cortex (PirC, AudC)
functional connectivity. In addition, dysfunctional connectivity with closely-related subcortical areas including the amygdala, thalamus, septum and mammillary body was also found. BRAIN
NETWORK HUB DYSCONNECTIVITY IN 16P11.2 DEL MICE SUPPORTS ALTERED PFC, SEPTUM AND INSULAR CORTEX CONNECTIVITY Given the complex inter-regional dysconnectivity seen in 16p11.2 DEL mice (Fig.
1) we next sought to characterise the alterations in brain network hub functional connectivity in these animals, using centrality analysis. This identified the PFC, FRA and the insular
cortex (Ins) as important functional hubs in the brain networks of WT mice, that lost their hub status in 16p11.2 DEL mice. In addition, the dentate gyrus of the dorsal hippocampus (DH.DG)
lost its hub status, as did the vertical limb of the diagonal band of broca (VDB), part of the septum/DB system and the ventromedial thalamic (VM) nucleus (Table 1). Only one region, the
horizontal limb of the diagonal band of Broca (HDB), also part of the septum/DB, was identified as a region that gained hub status, indicating the increased connectivity of this region in
the brain networks of 16p11.2 DEL mice. The loss of network hubs in frontal and temporal cortex, and the reciprocal changes in septum/DB, suggest a reorganisation of fronto-temporal and
associated subcortical circuit connectivity in 16p11.2 DEL mice. To characterise the altered inter-regional connectivity that underlies the altered hub status of these regions, we employed
PLSR analysis, using the regions showing altered centrality as the seed brain regions in this analysis27,28,29. Again, these results supported dysfunctional PFC and orbitofrontal cortex
(OFC) connectivity in 16p11.2 DEL mice. Lost PFC/OFC-hippocampal (FRA and DH.DG seeds), PFC-septum (FRA and VDB seeds), PFC/OFC-thalamic (VM seed) and PFC-accumbens (FRA seed) functional
connectivity was evident in 16p11.2 DEL mice (Fig. 2a–f). Insular and septum/DB dysconnectivity was further supported in 16p11.2 mice in this analysis. Inter-regional dysconnectivity between
the Ins and the OFC, perirhinal cortex (PRh) and accumbens/striatum was found (Fig. 2c). When the VDB was considered as the seed region, compromised septum-hippocampal and septum-accumbens
connectivity was identified (Fig. 2e). By contrast, when the HDB was considered as the seed region, gained septum-thalamic, septum-OFC and septum-hippocampal connectivity was detected in
16p11.2 DEL mice (Fig. 2f). Interestingly, there was no overlap between the PFC and hippocampal subfields showing lost connectivity to the septum VDB and those showing gained connectivity to
the septum HDB. Overall, these data support a complex, region-specific reorganisation of septum connectivity to the PFC and hippocampus in 16p11.2 DEL mice. GABAERGIC INTERNEURON GENE
EXPRESSION IS ALTERED IN BRAIN REGIONS IMPACTED BY 16P11.2 DELETION Since ASD is associated with GABAergic interneuron dysfunction and alterations in GABAergic interneuron gene
expression30,31, we hypothesised that the perturbations in regional and network connectivity detected in 16p11.2 DEL mice may, in part, reflect localised compromised inhibitory interneuron
function. Indeed, ASD is associated with reductions in GABAergic interneuron markers in cortical areas shown to be dysfunctional in 16p11.2 DEL mice, such as PFC/OFC and parietal cortex
(ParC)32. We, therefore, examined the expression of mRNAs for _Gad1_, encoding the GABA synthetic enzyme Gad67, and the interneuron subtype-specific activity-dependent markers parvalbumin
(_Pvalb_), calbindin (_Calb1_), calretinin (_Calb2_) and somatostatin (_Sst_), in 16p11.2 DEL mice. We observed reduced _Pvalb_ expression in multiple PFC/OFC subfields including the
prelimbic (PrL), infralimbic (IL) and medial orbital (MO) cortex (Fig. 3a, b, e). In contrast, _Calb1_ expression was elevated in the PFC (PrL and IL subfields) (Fig. 3c–e). We found no
evidence that the impact of 16p11.2 DEL on GABAergic gene expression was significantly influenced by sex in these PFC/OFC regions. The connectivity analysis identified altered connectivity
between the OFC and septum/DB system and between the OFC and Ins in 16p11.2 DEL mice. We therefore also examined GABAergic gene expression in the septum and Ins. In the septum, clear
sex-dependent alterations in GABAergic gene expression were detected. Male, but not female, 16p11.2 DEL mice showed reduced _Gad1_ expression in MS and LS (Fig. 4a, b, d, e). By contrast,
female, but not male, 16p11.2 DEL mice showed elevated _Pvalb_ expression in the MS (Fig. 4e, g, i). Conversely, Male-specific _Gad1_ reductions were also observed in the Ins (Fig. 4c),
while _Sst_ and _Pvalb_ expression were unaltered in 16p11.2 DEL mice of both sexes (Fig. 4f, h). Similarly _Gad1_ expression levels were also selectively decreased in the ParC of male
16p11.2 DEL mice (Fig. 5b, c, i). Conversely, _Pvalb_ expression in ParC was increased in 16p11.2 DEL mice, independent of sex (Fig. 5e, f) while _Sst_ expression was unaltered (Fig. 5h, j).
Somewhat surprisingly, no differences in GABAergic gene expression were detected in the AudC or PirC, where metabolic activity was previously identified as being reduced in 16p11.2 DEL mice
(Fig. 1, Supplementary Table 1) The dysfunction of thalamocortical loops is a feature of a number of neurodevelopmental disorders, including ASD33. The thalamic reticular nucleus (TRN) is a
key regulator of all thalamocortical loops34, and of the mediodorsal thalamus—PFC loop in particular35. Given the TRN dysconnectivity found in 16p11.2 DEL mice (Figs. 1 and 2), the
GABAergic dysfunction characterised in other brain regions in these animals (Figs. 3–5) and the high density of GABA interneurons in this structure, we also investigated GABAergic gene
expression in the TRN. Again, there was a male-specific reduction in _Gad1_ expression in the TRN (Fig. 5a, c, i), while _Sst_ expression was reduced in 16p11.2 DEL mice of both sexes (Fig.
5g, i, j) and _Pvalb_ expression levels were unaltered (Fig. 5d, f). 16P11.2 DEL MICE SHOW IMPAIRED PRE-ATTENTIONAL FILTERING AND ENHANCED ATTENTIONAL PROCESSING Our brain connectivity and
GABAergic gene expression studies in 16p11.2 DEL mice highlighted a complex and widespread dysregulation of neuronal circuitry implicated in behavioural changes relevant to ASD, such as
repetitive behaviours36, social difficulties37,38, pre-attentional filtering (PPI) and attentional processing. This includes dysfunction of the PFC/OFC, septum and TRN. PPI involves a
diffuse neural circuit with a core component of AudC, PrL, ParC, Ins, TRN, septum, hippocampus, habenula and inferior colliculi39,40, while increased attentional demands recruit circuitry
centred on Ins, PrL, OFC, cingulate, ParC, MS, Sub and mammillary bodies41,42,43. Therefore, to elucidate the behavioural impact of these molecular and systems-level alterations, and to
assess alignment with ASD-relevant deficits, we tested 16p11.2 DEL mice in the PPI and rCPT translational paradigms. While startle sensitivity was unaffected by the 16p11.2 deletion (Fig.
6a), there was a detriment in PPI in 16p11.2 DEL mice (Fig. 6b), supporting impaired pre-attentional processing in these animals. While the effect of the deletion on PPI appeared to be most
evident at the lower prepulse intensities of 4 and 8 dB, we found no evidence for a significant interaction between genotype and prepulse intensity on PPI, supporting a deficit in PPI across
all prepulse intensities. The rCPT offers a translational opportunity to assess cognitive function in a paradigm close to that used clinically, and in which ASD individuals show altered
performance26. Intriguingly, 16p11.2 DEL mice showed significantly better performance than WT mice during training for the rCPT. For example, over 12 sessions of stage 3 training, where
animals are learning stimulus-specific responding and non-responding, the sensitivity index (SI) was significantly higher in 16p11.2 DEL mice (_p_ = 0.016) (Supplementary Fig. 1). Similarly,
over 10 sessions at stage 4 of training, where animals are faced with increasing complexity of non-reinforced stimuli, perceptual sensitivity (_d_’) was higher for 16p11.2 DEL as compared
to WT mice, and the number of commission errors was lower (Supplementary Fig. 1). The stable performance of animals once the task was fully acquired (stage 6) is shown in Fig. 7. 16p11.2 DEL
mice overall maintain enhanced performance as compared to WT mice when fully trained (Hit rate (HR), _p_ = 0.001) (Fig. 7a–e). This effect was only evident in male 16p11.2 DEL mice (sex x
genotype interaction _p_ < 0.001, Fig. 7a). Males in general had a higher HR (_p_ < 0.001), higher SI (_p_ < 0.001) and higher _d_’ (_p_ = 0.006) when compared to female mice, but
16p11.2 DEL males have higher performance in all these indices as compared to WT males (_p_ < 0.001) (Fig. 7a, c, d). To further probe the enhanced attentional performance seen in 16p11.2
DEL mice we tested performance in the rCPT under conditions of increased attentional load, by reducing and varying the duration of stimulus presentation. As expected, HR and false alarm
rate (FAR) decreased with shorter stimulus presentations (Fig. 8a, b). However, 16p11.2 DEL mice maintained their higher levels of performance compared to WT mice in all indices (Fig. 8a–e).
Again, this mainly reflected the enhanced performance of 16p11.2 male mice (e.g. HR: genotype x sex interaction F(1,69) = 14.84, _p_ < 0.001, DEL M > WT M Tukey _p_ = 0.001). Reaction
times, for both correct and incorrect responses, were unaffected by 16p11.2 genotype, at any stage of the rCPT (Supplementary Table 2) indicating that there was no difference in speed of
processing between the two genotypes. DISCUSSION Chromosomal microdeletions at 16p11.2 are one of the largest known risk factors for ASD and represent a unique opportunity for reverse
translation. Here we report multi-scale ASD-relevant alterations in 16p11.2 DEL mice, including observations at the molecular, brain network and behavioural levels. The greater prevalence of
sporadic ASD in males is also observed in 16p11.2 deletion carriers11,44. We, therefore, predicted that phenotypes would be more overt in male 16p11.2 DEL mice. Indeed, male-specific
effects detected in 16p11.2 DEL mice were evident at all scales analysed. These observations provide integrative insight into the mechanisms by which 16p11.2 deletion increases the risk of
developing ASD, and also provide translational measures against which putative therapeutics can be validated. Increased E/I imbalance is a core feature of ASD45,46,47 and is also observed in
relevant genetic mouse models29,48, with some behavioural deficits corrected by optogenetic modulation of E/I balance within the PFC49. Our findings of reduced _Pvalb_ expression in the PFC
and OFC, together with male-specific reductions in _Gad1_ (septum, Ins and parietal cortices), support the putative loss of localised GABAergic inhibition in these regions. Interestingly,
these molecular changes align with the increased neuronal excitability and decreased GABAergic neurotransmission reported in the PFC of 16p11.2 DUP mice50. Increased E/I balance may be
present in 16p11.2 DEL mice, a suggestion supported by recent in vitro studies in the hippocampus51 and in vivo studies in cerebral cortex48 of these animals. Our data suggest that increased
E/I balance in these animals reflects, in part, GABAergic interneuron dysfunction. However, evidence also supports enhanced glutamatergic neurotransmission in 16p11.2 DEL mice51,52,53.
Notably, our GABA gene expression changes align with findings of reduced cortical GABA interneuron markers in ASD30,31. Decreased _GAD67_ expression in ParC is reported in post-mortem tissue
from autistic individuals (males), and is arguably the most robustly reported neurochemical phenotype. In addition, _Pvalb_ expression in ParC is profoundly susceptible to developmental
factors54, so the altered _Pvalb_ expression seen here is suggestive of compromised neurodevelopmental processes in 16p11.2 DEL mice. One question that our study does not answer is whether
the changes in GABAergic interneuron gene expression reflect changes in the number of interneurons in a given brain region, or altered gene expression levels alone. Either way, these changes
are indicative of compromised GABAergic interneuron function. The cellular basis of these changes certainly warrants further systematic investigation, particularly given the observation
that reduced numbers of Calb2-positive interneurons are seen in some cortical regions in 16p11.2 DEL mice55. 16p11.2 DEL mice show altered connectivity between the PFC and temporal cortical
regions, and altered connectivity between these cortical regions and the septum/DB. Widespread dysconnectivity as a consequence of 16p11.2 deletion is supported, with altered connectivity in
regions that contribute to a number of dysfunctional brain networks implicated in ASD, including the DMN, SalN, VAN and central executive network (CEN). These functional connectivity
changes are accompanied by reduced GABAergic cell markers in cortical regions and the septum, consistent with the idea that locally altered E-I balance contributes to the altered
inter-regional functional connectivity seen in these animals. Human 16p11.2 DEL carriers also have compromised PFC-temporal-parietal connectivity56. In our data-driven analysis of
large-scale brain network connectivity, we found reduced PFC connectivity in 16p11.2 DEL mice, including lost PFC hub connectivity (FrA) and lost PFC inter-regional connectivity with
hippocampus, septum and nucleus accumbens (Figs. 1 and 2). Using rsFMRI in another 16p11.2 DEL mouse model, Bertero et al 201856 also reported reduced long-range PFC connectivity. PFC
hypoconnectivity is also evident in other mouse models relevant to the ASD including _Shank3_ mutant57, _Cntnap_ mutant58, _Nrxn1a_ hemizygous29 and BTBR59 mice. However, in contrast to the
previous report in 16p11.2 DEL mice, we also found PFC subfield-specific increases in connectivity, indicating complex PFC dysconnectivity in these animals. Observations of PFC
dysconnectivity in ASD are also complex, with both increases and decreases reported18,60. Thus 16p11.2 DEL mice seem to replicate the functional complexity of PFC dysconnectivity seen in
ASD, although further work is needed to map these connectivity parallels in detail. We also found profound dysconnectivity between the PFC and hippocampus in 16p11.2 DEL mice (Figs. 1 and
2), paralleling that seen in ASD61. As these functional connectivity changes include the dysconnectivity of regions within the DMN, this supports compromised DMN function in 16p11.2 DEL
mice, consistent with compromised DMN connectivity in ASD 17,62,63 and human 16p11.2 DEL carriers56. Fibres within the uncinate fasciculus (UF) link temporal regions (including the ventral
hippocampus, AudC and PirC) with the frontal cortex (including PrL, IL, LO, DLO and Ins)64,65. One of the most robust structural abnormalities in ASD is compromised UF
integrity19,66,67,68,69,70, and UF abnormalities are associated with impaired social and cognitive function71,72. UF abnormalities are also observed in humans with 16p11.2 deletions73. It is
therefore intriguing that we observed functional dysconnectivity between these regions in 16p11.2 DEL mice (Figs. 1 and 2), which are accompanied by social deficits in these animals74. It
will therefore be important for future work to characterise UF structure in 16p11.2 DEL mice, as a potentially valuable translatable biomarker. We detected overt changes in cerebral
metabolism in 16p11.2 DEL mice in the septum (MS), mammillary bodies (MB) and hippocampal subiculum (males only), and in PirC and AudC. The MB receives substantial afferent innervation from
both subiculum and medial septum, so the data imply a particular impairment in this circuit. In fact, this is the precisely limbic circuitry where cellular morphology changes are reported in
ASD75,76. In addition, decreased AudC activity is observed in individuals with level 1 (previously known as high-functioning) ASD77, paralleling our observations in 16p11.2 DEL mice. The
male-specific elevation in subiculum activity identified here (Fig. 1) is also noteworthy, since ASD in males is associated with altered activity in this region15,78. The core limbic
circuitry affected in male 16p11.2 DEL mice is summarised in Supplementary Fig. 2. Substantial evidence supports Insular dysfunction in ASD, with decreased activity and hypoconnectivity
supported13,14,79, including Insular-OFC hypoconnectivity in individuals with level 1 ASD37. This Insular dysfunction is paralleled in 16p11.2 DEL mice, where its hub status was lost along
with connectivity to OFC, perirhinal cortex, nucleus accumbens and dorsolateral striatum. The Insular has key functions in interoreception, integrating external and internal stimuli and
salience, in part through its actions on SalN connectivity and modulation of the DMN and CEN80. Insular dysconnectivity to SalN/CEN regions in 16p11.2 DEL mice is consistent with the
behavioural changes seen in these animals, and the association of SalN dysconnectivity with ASD symptoms81,82. Impaired interoception is thought to underlie other deficits in ASD, including
impaired theory of mind (ToM) and deficits in social communication83. Thus the social deficits reported in 16p11.2 DEL mice74 may also be related to this Insular dysconnectivity. MS
hyperactivity is accompanied by abnormal (gained) connectivity to amygdala nuclei and the mPFC (IL) in 16p11.2 DEL mice. By contrast, MS connectivity to other PFC/OFC subfields is lost,
indicating a profound rearrangement of the functional connectivity of this region. In parallel GABAergic interneuron gene expression was reduced in the septum of 16p11.2 DEL mice, consistent
with the increased metabolism in this area resulting from local disinhibition. Whilst anatomical evidence supports septum changes in ASD76, to our knowledge this is the first time that
functional dysconnectivity of the septum with other brain regions has been identified in an ASD-relevant rodent model. Interestingly, we have recently reported reduced sociability in 16p11.2
DEL mice74. Since reduced GABAergic inhibition in the LS is sufficient to cause a loss of sociability in juvenile rats84, the reduced _Gad1_ (_GAD67_) expression seen in the LS of male
16p11.2 DEL mice (Fig. 4) may relate to the social deficits reported in these animals, and may be of relevance to social deficits seen in ASD. MS forms part of a network important in
affiliative behaviours, such as social connection. There are anatomical projections from MS to the IL, Sub, mammillary bodies and amygdala in rodents and humans85,86,87. Interestingly, the
direct GABAergic projections from MS to the hippocampus, including the Sub86,87, inhibit subicular inhibitory interneurons, thereby disinhibiting subicular principal cells. Thus the
compromised GABAergic gene expression seen in the MS of male 16p11.2 DEL mice may contribute to the subicular hyperactivity seen in these animals. ASD individuals exhibit impaired PPI88,
with the impairment diminishing to some extent with age. We observe a deficit in PPI in relatively young, 16p11.2 DEL mice. Other studies have reported no change in PPI in 16p11.2 DEL
mice89,90. However, the mice in these earlier studies had a mixed genetic background. The mixed background may increase variability and decrease statistical power, potentially explaining the
discrepancy. Two other studies have reported that PPI is dramatically decreased, to the point of being absent, in a different genetic strain of 16p11.2 DEL mice52,91. However, the strain
used in these studies was deaf, so it is difficult to draw translationally-relevant conclusions from the results. Mechanistically, a complex network involving AudC, PrL, ParC, Ins, TRN,
septum, hippocampus, habenula and inferior colliculi is involved in PPI39,92, with the habenula in particular possessing strong functional connectivity with both AudC and Ins40. Considering
the altered functional connectivity we observe in 16p11.2 DEL mice, and its striking overlap with this circuitry, it is no surprise to find that PPI is impaired. This includes, disrupted
functional connectivity between the habenula and the AudC and Ins in 16p11.2 DEL mice (Figs. 1 and 2), along with disturbed PFC, septum and hippocampal connectivity. Moreover, GABAergic cell
function is strongly implicated in the regulation of PPI, as evidenced by PPI deficits in _Pvalb_ deficient mice93 and in mice with selective _Gad1_ deficiency in Pvalb-expressing
neurons94. Thus the PPI deficit is consistent with the GABAergic deficits seen in 16p11.2 DEL mice. The PPI deficit identified in 16p11.2 DEL mice represent a potentially translational
phenotype, particularly when integrated with the functional neuroimaging and GABAergic cell deficits identified in these animals. Table 2 summarises the relationship between ASD-relevant
phenotypes and brain region function in humans, and the overlap with the dysfunctional brain regions present in 16p11.2 DEL mice. Attentional capabilities in ASD have attracted considerable
interest, with debate over the attentional domains affected, including selective, sustained and shifting attention. Some aspects of attentional processing are known to be enhanced in
autistic individuals24,26,95. In particular, autistic individuals can out-perform control subjects in tests involving visual and auditory search2,24,95,96,97, with debate over whether this
enhanced performance relates to altered attentional or perceptual mechanisms95. Recent evidence suggests that enhanced perceptual capacity might underlie both superior performance in visual
search and some of the deficits seen in other attentional abilities, for example through higher distractibility24. The improved attentional performance of 16p11.2 DEL mice in the visual rCPT
task aligns with these human studies. This was largely due to enhanced performance in male 16p11.2 DEL mice, which is interesting given the greater prevalence of ASD in males. Whilst not
directly comparable to the present data, the performance of 16p11.2 DEL mice has been assessed in other cognitive tasks. 16p11.2 DEL mice are slower in acquiring competency in a pairwise
discrimination and reversal learning touchscreen task91. The difference between the results in this task and our rCPT results may reflect the differing cognitive domains involved (visual
discrimination and intradimensional flexibility versus visual discrimination and attention), or the fact that the mice in the other study had a mixed (C57Bl6/129/CD-1) genetic background.
The mice on the mixed genetic background also show a higher neonatal fatality rate than those used in our study, further suggestive of other genetic factors interacting with the 16p11.2
deletion. In addition, these mixed background mice, being deaf, also lacked the auditory feedback that accompanies correct responses in these touchscreen tests, which may make learning more
difficult. In our study, enhanced rCPT performance in 16p11.2 DEL mice was observed during training, when fully trained, and when the attentional demands of the task were acutely increased.
From a translational perspective, and consistent with these observations, ASD individuals (predominantly male) show increased _d_’ in the CPT, especially under conditions of increased
attentional load24. This supports the translational relevance of the 16p11.2 DEL mouse model in this regard. In terms of the relevant neurocircuitry, it is intriguing that Ins connectivity
to components of the SalN (nucleus accumbens) is altered in 16p11.2 DEL mice (Fig. 2), as the Ins influences interactions between the DMN (“off task”) and CEN/SalN (“on task”) networks41,98.
Interestingly, fMRI studies in ASD support decreased inactivation of the DMN during cognitive tasks, which may relate to Ins dysconnectivity62. For optimal cognitive performance, a
reciprocal antagonistic relationship is required between these networks99. The DMN (e.g. mPFC and posterior cingulate) is typically deactivated during attention-demanding tasks, whereas the
CEN (particularly the Dorsal Attention Network comprising frontoparietal regions) is activated. In ASD there are deficits in the DMN, with both hyper- and hypo-connectivity profiles
reported17. The improved rCPT performance seen in 16p11.2 DEL mice may result from a shift in the reciprocal antagonistic relationship between the DMN and CEN, putatively supported by our
data showing dysfunction in DMN and CEN regions. This certainly warrants further investigation. Some genetic and toxicological mouse models of aspects of ASD show deficits in simple tests of
cognitive function (e.g. novel object recognition, delayed match to position, fear conditioning, Morris water maze). While the translational relevance of some of these tests is in question,
overt impairment has generally been observed, particularly with manipulations associated with more severe ends of the spectrum100,101,102,103,104. There is a relative lack of data from more
sophisticated and translational cognitive tests. However, in models of genetic lesions associated with more severe impairment, including a mouse model of Fragile X syndrome105 and in mice
of the inbred BTBR strain which lacks a corpus callosum106, attentional deficits have been observed. However, enhanced cognitive performance is detected in other mouse models of ASD with
high construct validity. For example, enhanced learning is seen in various mouse models with ASD-related mutations, including _Shank1_−/−107,_Taok2_−/−108, _Ctnbb1_−/– (Pvalb cell
conditional)109, _Nrxn1α +/−_ mice110 and also in _Aspm_−/− mice that show reduced TRN Pvalb expression111. A recent publication reports that mice with an ASD-associated neuroligin-3
mutation also show enhanced ability in the rCPT112. Thus an emerging picture of enhanced performance in specific cognitive domains is evident in mouse models relevant to ASD. We note that
hypofunction in the ERK pathway is associated with cognitive impairment rather than enhancement113. However, mice with _Taok2_ deletion exhibit clearly enhanced performance compared to WTs
in a spatial learning task108. Hence the decreased _Taok2_ gene dosage present in 16p11.2 DEL mice may contribute to the cognitive phenotypes observed in the current study. Interestingly,
increased visual search efficiency in ASD is associated with heightened activation of ParC114. As increased perceptual load, where altered attentional performance in autistic individuals is
most evident (Remington 2012), is associated with greater ParC recruitment115, the decreased GABAergic gene expression seen in ParC in 16p11.2 DEL mice may result in elevated local E/I
balance which, in turn, may contribute to enhanced attentional performance in these animals. We have recently reported an extensive characterisation of CNS dysfunction in mice with the
corresponding duplication (16p11.2 DUP mice)116. Since this strain is on the same genetic background, it is informative to compare the two studies. The changes in brain metabolism and
functional connectivity seen are more marked in 16p11.2 DEL as compared to 16p11.2 DUP mice. This could have been predicted—considering the 50% change in gene dosage in 16p11.2 DEL as
compared to the 33% change in 16p11.2 DUP mice. Interestingly, both 16p11.2 DEL and 16p11.2 DUP mice show impaired OFC connectivity. For 16p11.2 DUP mice this is most pronounced in terms of
connectivity to hippocampus and amygdala116, whereas for 16p11.2 DEL mice this is most pronounced for connectivity to the hippocampus and Ins. Why the OFC-hippocampal axis should be
especially sensitive to abnormal 16p11.2 gene dosage is unclear, but the data focus attention on this interaction as potentially key for the aetiology of 16p11.2-related neurodevelopmental
disorders. PPI is impaired in both 16p11.2 DEL (Fig. 5) and 16p11.2 DUP mice116. Thus either elevated or reduced gene dosage in this region impairs pre-attentional sensorimotor gating. This
is consistent with the fact that both patients with schizophrenia, where risk is associated with 16p11.2 duplication, and autistic individuals, where risk is associated with 16p11.2
deletion, show compromised PPI88. It is also consistent with the observation that both the deletion and duplication impact on neurocircuitry that contributes to PPI in mice. In terms of
attentional processing, we have detected reciprocal phenotypes for the reciprocal CNVs. While 16p11.2 DUP mice show impaired rCPT performance116, 16p11.2 DEL mice show enhanced rCPT
performance (Figs. 7 and 8). This is similar to humans, where individuals carrying the duplication show more impaired working memory and executive function than those carrying the deletion9.
Future work dedicated to directly comparing both duplication and deletion-carrying individuals in the human CPT would therefore be of great translational interest. In this work, we have
undertaken a multi-scale, integrative characterisation of mice carrying the 16p11.2 deletion to elucidate the translational alignment of this mouse model with observations in ASD, and to
further identify the mechanisms through which the 16p11.2 deletion increases the risk of developing neurodevelopmental disorders. Key observations include translationally aligned disruptions
in GABAergic gene expression, putatively contributing to locally altered E-I balance. In addition, we identify perturbed regional metabolism and functional connectivity that aligns with the
impaired pre-attentional sensorimotor gating and superior attentional performance seen in these animals. These findings align with those reported in autistic individuals. Thus we have
established the translational utility of the 16p11.2 DEL mouse model for ASD and highlighted a range of phenotypes against which the validity of future therapeutics can be tested. METHODS
ETHICS APPROVAL AND CONSENT TO PARTICIPATE The study was conducted under UK Home Office regulations, and was approved by the University of Glasgow College of Medical, Veterinary and Life
Sciences Ethics Committee. ANIMALS Male and female mice (Jackson Laboratory, Stock No. 013128) hemizygous for the 0.44 Mb region of mouse chromosome 7, syntenic to the human 16p11.2
deletion, were generated by Mills and colleagues117. Mice were backcrossed onto the C57BL/6 N background using “Max-Bax” SNP-guided back-crossing (Charles River) to generate experimental
mice, so that, in contrast to some other studies using this strain, the mice for this study are effectively congenic74. Primers used for genotyping are listed in Supplementary Table 4.
Genotpyping was performed using a PCR protocol of 94 °C for 2 min, then 10 cycles of 94 °C for 20 s, 65 °C for 15 s and 68 °C for 10 s, followed by 28 cycles of 94 °C for 15 s, 60 °C for 15
s and 72 °C for 10 s. 16p11.2 DEL mice, and littermate wild-type (WT) controls, were group-housed, with food and water ad libitum. All work was approved by the University of Strathclyde and
Lancaster University Animal Welfare and Ethics Review Boards (AWERB) and conducted in accordance with the Home Office (UK) Animals (Scientific Procedures) Act 1986. 14C-2-DEOXYGLUCOSE
FUNCTIONAL BRAIN IMAGING We used our standard protocols27,28 for 14C-2-deoxyglucose (14C-2-DG) metabolic functional brain imaging in mice aged 10–11 weeks old. Autoradiographic signal
intensity was measured using the MCID system, by an operator blind to the experimental identity of the samples. The group sizes: WT, _n_ = 12 (6 male) and 16p11.2 DEL, _n_ = 12 (6 male)
mice, based on our previous experiments with GM mice, were estimated to provide 85% power to detect an effect at _p_ < 0.05 (Minitab). Network science algorithms, using the igraph
package118 in R119, were used to determine functional brain network structure, as described previously27,28,116,120. These algorithms permit the assessment of regional importance and
connectivity in the context of the whole brain network, with highly connected hub brain regions being identified as having high centrality. In this way, we determined altered hub brain
region status on the basis of both degree (ki) and betweenness (Bc) centrality. The significance of group differences in centrality were determined by permutation (55,000 permutations), with
significance set at _p_ < 0.05. PLSR analysis allows the determination of the inter-regional connectivity for a selected seed brain region, thus allowing the characterisation of the
inter-regional connectivity changes that underlie the altered functional hub status of a brain region. Data were analysed with the Pls package121 in R, according to our published
procedures27,28,29. If the lower bound of the 95% confidence interval (CI) of the variable importance to the projection (VIP) statistic (estimated by jack-knifing) exceeded 1.0, then
significant inter-regional connectivity to the seed region was considered to exist within the experimental group. Significantly altered connectivity in 16p11.2 DEL mice was determined by
comparison of the VIP statistic via calculation of the standardised _z_ score, with a _z_ score >1.96 or <−1.96 considered to be significant. Data are reported for two levels of
significance, _z_ = 1.96 (equivalent to _p_ = 0.05) and _z_ = 2.58 (equivalent to _p_ = 0.01). Significantly altered connectivity was confirmed by a 95% CI of the VIP > 1.0 in one
experimental group, but not the other (95% CI lower bound VIP < 1.0). IN SITU HYBRIDISATION (ISH) In situ hybridisation (ISH) was used to assess regional mRNA expression according to our
standard protocol116,122,123 using 35S-labelled 45mer oligonucleotide probes. Sequences of oligonucleotide probes used are listed in Supplementary Table 4. Brains (from mice 9–11 weeks of
age) were sectioned and mounted onto slides in same sex, different genotype pairs. 20 μm frozen cryostat sections, mounted on microscope slides coated with poly-L-Lysine (Sigma-Aldrich, UK),
were fixed for 10 min in 4% paraformaldehyde (Sigma-Aldrich, UK) on ice. Hybridisation between sections and oligonucleotides—3’end-labelled with 35S-dATP and terminal deoxynucleotidyl
transferase (ThermoFisher, UK)—was performed at 42 °C overnight in a humidified chamber. All samples were processed at the same time, for each target mRNA. Sections were then washed in
standard saline citrate buffer (Sigma-Aldrich, UK) for 1 h at 55 °C, before being dehydrated in ethanol. Specificity of labelling was monitored using competition controls (25× excess of
unlabelled oligonucleotide)122,124,125, and ImageJ was used to assess autoradiographic signal intensity, by a researcher blind to the identity of the samples. Eight sections per animal were
analysed. Each microscope slide contained sections from one WT and one 16p11.2 DEL mouse of the same sex. The signal intensity for each region was measured on both sides of the brain in each
section. Similarly, background signal values were taken from adjacent areas lacking specific signal (as assessed by the competition control) on both sides of the brain in each section.
These were then subtracted from the signal in the areas with specific labelling. Both resulting “specific” signal values were included in the statistical analysis, and “side” (left vs right
hemisphere) included as a random factor. We found no evidence that brain hemisphere significantly impacted on gene expression or influenced the impact genotype on the expressions of the gene
targets analysed. The group sizes: WT, _n_ = 12–13 (8–9 male) and 16p11.2 DEL, _n_ = 12 (8 male) mice, were estimated, based on our previous experience, to give 99% power to detect an
effect at _p_ < 0.05 (Minitab). PREPULSE INHIBITION (PPI) Mice were 13–14 weeks of age, and SR-LAB chambers (San Diego Instruments, San Diego, CA) were used as previously
described116,126. Each chamber consisted of a sound attenuated cabinet (inside height: 28.7 cm, inside width: 28.7 cm, inside depth: 30 cm) that was lit and ventilated, with a Plexiglass
cylinder (3.7 cm inner diameter, 12.7 cm long) situated on top of a removable stand in which a piezoelectric accelerometer was attached underneath. An initial startle curve (random
presentation of 60 trials of 65 dB, 69 dB, 73 dB, 77 dB, 85 dB, 90 dB, 100 dB, 110 dB, 120 dB—full spectrum white noise) was obtained. For PPI determination, mice were allowed to acclimatise
to the 65 dB background noise for 5 min, and 5 × 40 ms, 120 dB startling stimuli were then played to partially habituate the animals to the startling stimulus. Mice were then tested over 60
trials. The session consisted of 10 repetitions of each of the following prepulse trials: a 20 ms prepulse of either 4, 6, or 8 dB above background, followed by a 100 ms inter-pulse
interval, then a 40 ms startling stimulus at 120 dB above background. Randomly interspersed between prepulse trials were 10 × 120 dB startling stimuli alone and 10 x “no stimulus” trials in
which movements were recorded but no stimulus was delivered. These trials were presented in random order with inter-trial intervals averaging ~15 s, but were either (randomly) 12, 13, 14,
15, 16, or 17 s long. Movements of the animal were recorded for 40 ms from the beginning of the 120 dB startling stimulus, or, in the case of “no stimulus” trials, from the end of the
inter-trial interval for 10 ms. The session finished with 5 × “120 dB startle only” trials to give an indication of overall habituation to the startle response the mice exhibited when
compared with the first 5 × “120 dB startle only” trials. % PPI was calculated in relation to startle reactivity at 120 dB (Eq. 1). $${{{{{\rm{PPI}}}}}}=\,
({{{{{\rm{startle}}}}}}\,{{{{{\rm{reactivity}}}}}}\,{{{{{\rm{at}}}}}}\,120\,{{{{{\rm{dB}}}}}}\,{{{{{\rm{without}}}}}}\,{{{{{\rm{prepulse}}}}}} -
{{{{{\rm{startle}}}}}}\,{{{{{\rm{reactivity}}}}}}\,{{{{{\rm{at}}}}}}\,120\,{{{{{\rm{dB}}}}}}\,{{{{{\rm{with}}}}}}\,{{{{{\rm{prepulse}}}}}}) \\ \, \times
100/{{{{{\rm{startle}}}}}}\,{{{{{\rm{reactivity}}}}}}\,{{{{{\rm{at}}}}}}\,120\,{{{{{\rm{dB}}}}}}\,{{{{{\rm{without}}}}}}\,{{{{{\rm{prepulse}}}}}}.$$ (1) The group sizes: WT, _n_ = 16 (8
male) and 16p11.2 DEL, _n_ = 16 (8 male) mice, according to our previous experience with this test in GM mice, were estimated to provide 92% power to detect an effect at _p_ < 0.05
(Minitab). RODENT CONTINUOUS PERFORMANCE TEST (RCPT) The rCPT was conducted using Campden instruments mouse touchscreen operant boxes and ABETII touch software. Mice (11–13 weeks of age)
were trained according to Kim et al.,127 and as previously described116. Mice were assessed for attentional performance (hit rate (HR) and false alarm rate (FAR)), along with composite
measures of performance analogous to those used clinically (Sensitivity index—SI, perceptual sensitivity—_d_’ and index of response bias—RI), and measures of processing speed (reaction
time). The task stimuli were presented in the centre panel of three discrete sections of the touchscreen, accessible via a 3 aperture horizontal mask. Correct responses were rewarded with
(YazooTM) strawberry milkshake (70 µl), along with an auditory stimulus and illumination of the food reward hopper. Stimuli were either rewarded (S+) or punished (S−) by a correction phase,
involving repeated stimulus presentation until the response is correctly withheld. Successive stages of basal response learning (stage 1), stimulus-specific responding (stage 2) and
stimulus-specific responding and non-responding (stage 3), were followed by manipulations that increases cognitive load, including an increased number of S- stimuli and reduced stimulus
presentation times (2 sec, 1.5 sec, 1 sec; limited hold 2.5 sec, 2 sec, 1.5 sec) in stages 4–6 of the task. The group sizes: WT, _n_ = 12 (6 male) and 16p11.2 DEL, _n_ = 12 (6 male) mice,
according to our previous experience with GM mice in this task, were estimated to provide 86% power to detect an effect at _p_ < 0.05 (Minitab). The parameters calculated were: Hit Rate
(HR)—the rate at which animals respond to the correct (S+) stimulus (Eq. 2). False Alarm Rate (FAR)—the rate at which animals respond to the incorrect (S−) stimulus (Eq. 3); Sensitivity
Index (SI)—the perceptual discriminability between the correct (S+) and incorrect (S−) stimuli, with higher values indicate better discrimination;127 Perceptual Sensitivity (_d_’) (Eq. 4);
Responsivity Index (RI)—the criterion or willingness to make responses, with conservative responding indicated by low RI values and liberal responding indicated by high RI values127, see
erratum for correct formula. Note that the LnBeta index, which is also widely used, and where low values indicate more liberal responding strategies, is the inverse of this measure.
$${{{{{\rm{HR}}}}}}={{{{{\rm{Hit}}}}}}/({{{{{\rm{Hit}}}}}}+{{{{{\rm{Miss}}}}}});$$ (2)
$${{{{{\rm{FAR}}}}}}={{{{{\rm{False}}}}}}\,{{{{{\rm{alarm}}}}}}/({{{{{\rm{False}}}}}}\,{{{{{\rm{alarm}}}}}}+{{{{{\rm{Correct}}}}}}\,{{{{{\rm{rejection}}}}}})$$ (3)
$${{{{{\rm{d}}}}}}\hbox{'}=\,
{{{{{\rm{z}}}}}}({{{{{\rm{HR}}}}}})-{{{{{\rm{z}}}}}}({{{{{\rm{FAR}}}}}}),{{{{{\rm{where}}}}}}\,{{{{{\rm{z}}}}}}\,{{{{{\rm{scores}}}}}}\,{{{{{\rm{are}}}}}}\,{{{{{\rm{calculated}}}}}}\,{{{{{\rm{based}}}}}}\,{{{{{\rm{on}}}}}}\,{{{{{\rm{performance}}}}}}\\
\, {{{{{\rm{of}}}}}}\,{{{{{\rm{WT}}}}}}\,{{{{{\rm{mice}}}}}}\,{{{{{\rm{under}}}}}}\,{{{{{\rm{standard}}}}}}\,{{{{{\rm{conditons}}}}}};$$ (4) STATISTICS AND REPRODUCIBILITY Analysis was
performed blind to the experimental group wherever possible, as described above. Mice were allocated to test groups in randomised order. The use and analysis of technical replicates in the
in situ hybridisation studies is described above. Group sizes in all studies were determined based on power analysis (>0.8, _p_ < 0.05) using Minitab. Statistical significance was
generally assessed by ANOVA (Minitab/R), with prior Box-Cox normalisation where data deviated substantially from normality. Post hoc comparisons were made using Tukey’s HSD or Mann-Whitney
tests for specific planned comparisons between groups of particular interest. For ISH data analysis, 3-way ANOVA was employed (factors: genotype, sex and slide pairing). Details of
statistical outputs are provided in Supplementary Table 1. REPORTING SUMMARY Further information on research design is available in the Nature Portfolio Reporting Summary linked to this
article. DATA AVAILABILITY The 16p11.2 DEL mice used in this study are available from Jackson laboratories (strain 013128). Data shown in the figures are deposited at Dryad:
https://doi.org/10.5061/dryad.x69p8czp7. REFERENCES * Crespi, B. J. Autism as a disorder of high intelligence. _Front. Neurosci._ 10, 300–300 (2016). Article PubMed PubMed Central Google
Scholar * Caron, M. J., Mottron, L., Rainville, C. & Chouinard, S. Do high functioning persons with autism present superior spatial abilities? _Neuropsychologia_ 42, 467–481 (2004).
Article PubMed Google Scholar * Taylor, B., Jick, H. & MacLaughlin, D. Prevalence and incidence rates of autism in the UK: time trend from 2004–2010 in children aged 8 years. _BMJ
Open_ 3, e003219 (2013). Article PubMed PubMed Central Google Scholar * Sun, X. et al. Autism prevalence in China is comparable to Western prevalence. _Mol. Autism_ 10, 7 (2019). Article
PubMed PubMed Central Google Scholar * Chiarotti F., Venerosi A. Epidemiology of autism spectrum disorders: a review of worldwide prevalence estimates since 2014. _Brain Sci._ 10, 274
(2020). * Sandin, S. et al. The heritability of autism spectrum disorder. _JAMA_ 318, 1182–1184 (2017). Article PubMed PubMed Central Google Scholar * Weiss, L. A. et al. Association
between microdeletion and microduplication at 16p11.2 and autism. _N. Engl. J. Med._ 358, 667–675 (2008). Article CAS PubMed Google Scholar * Cooper, G. M. et al. A copy number variation
morbidity map of developmental delay. _Nat. Genet._ 43, 838–846 (2011). Article CAS PubMed PubMed Central Google Scholar * Stefansson, H. et al. CNVs conferring risk of autism or
schizophrenia affect cognition in controls. _Nature_ 505, 361–366 (2014). Article CAS PubMed Google Scholar * Walsh, K. M. & Bracken, M. B. Copy number variation in the
dosage-sensitive 16p11.2 interval accounts for only a small proportion of autism incidence: a systematic review and meta-analysis. _Genet. Med._ 13, 377–384 (2011). Article PubMed Google
Scholar * Niarchou, M. et al. Psychiatric disorders in children with 16p11.2 deletion and duplication. _Transl. Psychiatry_ 9, 8 (2019). Article PubMed PubMed Central Google Scholar *
Gudmundsson, O. O. et al. Attention-deficit hyperactivity disorder shares copy number variant risk with schizophrenia and autism spectrum disorder. _Transl. Psychiatry_ 9, 258 (2019).
Article PubMed PubMed Central Google Scholar * Di Martino, A. et al. The autism brain imaging data exchange: towards a large-scale evaluation of the intrinsic brain architecture in
autism. _Mol. Psychiatry_ 19, 659–667 (2014). Article PubMed Google Scholar * Kana, R. K., Keller, T. A., Minshew, N. J. & Just, M. A. Inhibitory control in high-functioning autism:
decreased activation and underconnectivity in inhibition networks. _Biol. Psychiatry_ 62, 198–206 (2007). Article PubMed Google Scholar * Monk, C. S. et al. Abnormalities of intrinsic
functional connectivity in autism spectrum disorders. _Neuroimage_ 47, 764–772 (2009). Article PubMed Google Scholar * Jann, K. et al. Altered resting perfusion and functional
connectivity of default mode network in youth with autism spectrum disorder. _Brain Behav._ 5, e00358 (2015). Article PubMed PubMed Central Google Scholar * Padmanabhan, A., Lynch, C.
J., Schaer, M. & Menon, V. The default mode network in autism. _Biol. Psychiatry Cogn. Neurosci. Neuroimaging_ 2, 476–486 (2017). PubMed PubMed Central Google Scholar * Rane, P. et
al. Connectivity in autism: a review of MRI connectivity studies. _Harv. Rev. Psychiatry_ 23, 223–244 (2015). Article PubMed PubMed Central Google Scholar * Ecker, C., Bookheimer, S. Y.
& Murphy, D. G. Neuroimaging in autism spectrum disorder: brain structure and function across the lifespan. _Lancet Neurol._ 14, 1121–1134 (2015). Article PubMed Google Scholar *
Sohal, V. S. & Rubenstein, J. L. R. Excitation-inhibition balance as a framework for investigating mechanisms in neuropsychiatric disorders. _Mol. Psychiatry_ 24, 1248–1257 (2019).
Article PubMed PubMed Central Google Scholar * Pratt, J. & Hall, J. Biomarkers in neuropsychiatry: a prospect for the twenty-first century? _Curr. Top. Behav. Neurosci._ 40, 3–10
(2018). Article PubMed Google Scholar * Dawson, N. et al. Altered functional brain network connectivity and glutamate system function in transgenic mice expressing truncated
disrupted-in-Schizophrenia 1. _Transl. Psychiatry_ 5, e569 (2015). Article CAS PubMed PubMed Central Google Scholar * Mar, A. C. et al. The touchscreen operant platform for assessing
executive function in rats and mice. _Nat. Protoc._ 8, 1985–2005 (2013). Article CAS PubMed PubMed Central Google Scholar * Remington, A. M., Swettenham, J. G. & Lavie, N.
Lightening the load: perceptual load impairs visual detection in typical adults but not in autism. _J. Abnorm. Psychol._ 121, 544–551 (2012). Article PubMed PubMed Central Google Scholar
* Rommelse, N. N., Geurts, H. M., Franke, B., Buitelaar, J. K. & Hartman, C. A. A review on cognitive and brain endophenotypes that may be common in autism spectrum disorder and
attention-deficit/hyperactivity disorder and facilitate the search for pleiotropic genes. _Neurosci. Biobehav Rev._ 35, 1363–1396 (2011). Article CAS PubMed Google Scholar * Allen, G.
& Courchesne, E. Attention function and dysfunction in autism. _Front. Biosci._ 6, D105–D119 (2001). Article CAS PubMed Google Scholar * Dawson, N. et al. Sustained NMDA receptor
hypofunction induces compromised neural systems integration and schizophrenia-like alterations in functional brain networks. _Cereb. Cortex_ 24, 452–464 (2012). Article PubMed Google
Scholar * Dawson, N., Morris, B. J. & Pratt, J. A. Subanaesthetic ketamine treatment alters prefrontal cortex connectivity with thalamus and ascending subcortical systems. _Schizophr.
Bull._ 39, 366–377 (2011). Article PubMed PubMed Central Google Scholar * Hughes, R. et al. Ketamine restores thalamic-prefrontal cortex functional connectivity in a mouse model of
neurodevelopmental disorder-associated 2p16.3 deletion. _Cereb. Cortex_ 30, 2358–2371 (2019). Article Google Scholar * Parikshak, N. N. et al. Genome-wide changes in lncRNA, splicing, and
regional gene expression patterns in autism. _Nature_ 540, 423–427 (2016). Article CAS PubMed PubMed Central Google Scholar * Hashemi, E., Ariza, J., Rogers, H., Noctor, S. C. &
Martínez-Cerdeño, V. The number of parvalbumin-expressing interneurons is decreased in the prefrontal cortex in autism. _Cereb. Cortex_ 27, 1931–1943 (2016). PubMed Central Google Scholar
* Fatemi, S. H. et al. Downregulation of GABAA receptor protein subunits α6, β2, δ, ε, γ2, θ, and ρ2 in superior frontal cortex of subjects with autism. _J. Autism Dev. Disord._ 44,
1833–1845 (2014). Article PubMed Google Scholar * Nair, A., Treiber, J. M., Shukla, D. K., Shih, P. & Müller, R. A. Impaired thalamocortical connectivity in autism spectrum disorder:
a study of functional and anatomical connectivity. _Brain_ 136, 1942–1955 (2013). Article PubMed PubMed Central Google Scholar * Pratt, J. A. & Morris, B. J. The thalamic reticular
nucleus: a functional hub for thalamocortical network dysfunction in schizophrenia and a target for drug discovery. _J. Psychopharmacol._ 29, 127–137 (2015). Article PubMed Google Scholar
* Guillery, R. W., Feig, S. L. & Lozsadi, D. A. Paying attention to the thalamic reticular nucleus. _Trends Neurosci._ 21, 28–32 (1998). Article CAS PubMed Google Scholar * Abbott,
A. E. et al. Repetitive behaviors in autism are linked to imbalance of corticostriatal connectivity: a functional connectivity MRI study. _Soc. Cogn. Affect. Neurosci._ 13, 32–42 (2017).
Article PubMed Central Google Scholar * Ebisch, S. J. et al. Altered intrinsic functional connectivity of anterior and posterior insula regions in high-functioning participants with
autism spectrum disorder. _Hum. Brain Mapp._ 32, 1013–1028 (2011). Article PubMed Google Scholar * Yizhar, O. et al. Neocortical excitation/inhibition balance in information processing
and social dysfunction. _Nature_ 477, 171–178 (2011). Article CAS PubMed PubMed Central Google Scholar * Rohleder, C. et al. The functional networks of prepulse inhibition: neuronal
connectivity analysis based on FDG-PET in awake and unrestrained rats. _Front. Behav. Neurosci._ 10, 148 (2016). Article PubMed PubMed Central Google Scholar * Ely, B. A., Stern, E. R.,
Kim, J. W., Gabbay, V. & Xu, J. Detailed mapping of human habenula resting-state functional connectivity. _Neuroimage_ 200, 621–634 (2019). Article PubMed Google Scholar * Menon, V.
& Uddin, L. Q. Saliency, switching, attention and control: a network model of insula function. _Brain Struct. Funct._ 214, 655–667 (2010). Article PubMed PubMed Central Google Scholar
* Wall, P. M. & Messier, C. The hippocampal formation — orbitomedial prefrontal cortex circuit in the attentional control of active memory. _Behav. Brain Res._ 127, 99–117 (2001).
Article CAS PubMed Google Scholar * Aggleton J.P., Christiansen K. Chapter 4—The subiculum: the heart of the extended hippocampal system. In: Progress in Brain Research (eds. O’Mara S.,
Tsanov M.) (Elsevier, 2015). * Zufferey, F. et al. A 600 kb deletion syndrome at 16p11.2 leads to energy imbalance and neuropsychiatric disorders. _J. Med. Genet._ 49, 660–668 (2012).
Article CAS PubMed Google Scholar * Lee, E., Lee, J. & Kim, E. Excitation/inhibition imbalance in animal models of autism spectrum disorders. _Biol. Psychiatry_ 81, 838–847 (2017).
Article PubMed Google Scholar * Dickinson, A., Jones, M. & Milne, E. Measuring neural excitation and inhibition in autism: different approaches, different findings and different
interpretations. _Brain Res._ 1648, 277–289 (2016). Article CAS PubMed Google Scholar * Horder, J. et al. Reduced subcortical glutamate/glutamine in adults with autism spectrum
disorders: a [(1)H]MRS study. _Transl. Psychiatry_ 3, e279 (2013). Article CAS PubMed PubMed Central Google Scholar * Antoine, M. W., Langberg, T., Schnepel, P. & Feldman, D. E.
Increased excitation-inhibition ratio stabilizes synapse and circuit excitability in four autism mouse models. _Neuron_ 101, 648–661.e644 (2019). Article CAS PubMed PubMed Central Google
Scholar * Selimbeyoglu, A. et al. Modulation of prefrontal cortex excitation/inhibition balance rescues social behavior in CNTNAP2-deficient mice. _Sci. Transl. Med._ 9, eaah6733 (2017).
Article PubMed PubMed Central Google Scholar * Rein, B. et al. Reversal of synaptic and behavioral deficits in a 16p11.2 duplication mouse model via restoration of the GABA synapse
regulator Npas4. _Mol. Psychiatry_ 26, 1967–1979 (2020). Article PubMed PubMed Central Google Scholar * Lu, H.-C., Mills, A. A. & Tian, D. Altered synaptic transmission and
maturation of hippocampal CA1 neurons in a mouse model of human chr16p11.2 microdeletion. _J. Neurophysiol._ 119, 1005–1018 (2018). Article PubMed Google Scholar * Portmann, T. et al.
Behavioral abnormalities and circuit defects in the basal ganglia of a mouse model of 16p11.2 deletion syndrome. _Cell Rep._ 7, 1077–1092 (2014). Article CAS PubMed PubMed Central Google
Scholar * Tian, D. et al. Contribution of mGluR5 to pathophysiology in a mouse model of human chromosome 16p11.2 microdeletion. _Nat. Neurosci._ 18, 182–184 (2015). Article CAS PubMed
PubMed Central Google Scholar * Weisenhorn, D. M. V., Celio, M. R. & Rickmann, M. The onset of parvalbumin-expression in interneurons of the rat parietal cortex depends upon extrinsic
factor(s). _Eur. J. Neurosci._ 10, 1027–1036 (1998). Article Google Scholar * Pucilowska, J. et al. Pharmacological inhibition of ERK signaling rescues pathophysiology and behavioral
phenotype associated with 16p11.2 chromosomal deletion in mice. _J. Neurosci._ 38, 6640–6652 (2018). Article CAS PubMed PubMed Central Google Scholar * Bertero, A. et al.
Autism-associated 16p11.2 microdeletion impairs prefrontal functional connectivity in mouse and human. _Brain_ 141, 2055–2065 (2018). Article PubMed Google Scholar * Pagani, M. et al.
Deletion of autism risk gene Shank3 disrupts prefrontal connectivity. _J. Neurosci._ 39, 5299–5310 (2019). Article CAS PubMed PubMed Central Google Scholar * Liska, A. et al. Homozygous
loss of autism-risk gene CNTNAP2 results in reduced local and long-range prefrontal functional connectivity. _Cereb. Cortex_ 28, 1141–1153 (2018). Article PubMed Google Scholar *
Sforazzini, F. et al. Altered functional connectivity networks in acallosal and socially impaired BTBR mice. _Brain Struct. Funct._ 221, 941–954 (2016). Article PubMed Google Scholar *
Delmonte, S., Gallagher, L., O’Hanlon, E., Mc Grath, J. & Balsters, J. Functional and structural connectivity of frontostriatal circuitry in autism spectrum disorder. _Front. Hum.
Neurosci._ 7, 430 (2013). Article PubMed PubMed Central Google Scholar * Rolls, E. T. et al. Effective connectivity in autism. _Autism Res._ 13, 32–44 (2020). Article PubMed Google
Scholar * Kennedy, D. P., Redcay, E. & Courchesne, E. Failing to deactivate: resting functional abnormalities in autism. _Proc. Natl. Acad. Sci. USA_ 103, 8275–8280 (2006). Article CAS
PubMed PubMed Central Google Scholar * Kennedy, D. P. & Courchesne, E. The intrinsic functional organization of the brain is altered in autism. _Neuroimage_ 39, 1877–1885 (2008).
Article PubMed Google Scholar * Hau, J. et al. Revisiting the human uncinate fasciculus, its subcomponents and asymmetries with stem-based tractography and microdissection validation.
_Brain Struct. Funct._ 222, 1645–1662 (2017). Article PubMed Google Scholar * Kovner, R., Oler, J. A. & Kalin, N. H. Cortico-limbic interactions mediate adaptive and maladaptive
responses relevant to psychopathology. _Am. J. Psychiatry_ 176, 987–999 (2019). Article PubMed PubMed Central Google Scholar * van Duin E.D.A., Zinkstok J., McAlonan G., van Amelsvoort
T. White matter brain structure in Asperger’s syndrome. In: _Comprehensive Guide to Autism_ (eds Patel V.B., Preedy V.R., Martin C.R.). Springer New York (2014). * Poustka, L. et al.
Fronto-temporal disconnectivity and symptom severity in children with autism spectrum disorder. _World J. Biol. Psychiatry_ 13, 269–280 (2012). Article PubMed Google Scholar * Kumar, A.
et al. Alterations in frontal lobe tracts and corpus callosum in young children with autism spectrum disorder. _Cereb. Cortex_ 20, 2103–2113 (2010). Article PubMed Google Scholar *
Catani, M. et al. Frontal networks in adults with autism spectrum disorder. _Brain_ 139, 616–630 (2016). Article PubMed PubMed Central Google Scholar * Pugliese, L. et al. The anatomy of
extended limbic pathways in Asperger syndrome: a preliminary diffusion tensor imaging tractography study. _Neuroimage_ 47, 427–434 (2009). Article PubMed Google Scholar * Pardini, M. et
al. Long-term cognitive and behavioral therapies, combined with augmentative communication, are related to uncinate fasciculus integrity in autism. _J. Autism Dev. Disord._ 42, 585–592
(2012). Article PubMed Google Scholar * Oishi, K. et al. Critical role of the right uncinate fasciculus in emotional empathy. _Ann. Neurol._ 77, 68–74 (2015). Article PubMed Google
Scholar * Ahtam, B., Link, N., Hoff, E., Ellen Grant, P. & Im, K. Altered structural brain connectivity involving the dorsal and ventral language pathways in 16p11.2 deletion syndrome.
_Brain Imaging Behav._ 13, 430–445 (2019). Article PubMed PubMed Central Google Scholar * Mitchell, E. J. et al. Drug-responsive autism phenotypes in the 16p11.2 deletion mouse model: a
central role for gene-environment interactions. _Sci. Rep._ 10, 12303 (2020). Article CAS PubMed PubMed Central Google Scholar * Bauman, M. L. & Kemper, T. L. Neuroanatomic
observations of the brain in autism: a review and future directions. _Int. J. Dev. Neurosci._ 23, 183–187 (2005). Article PubMed Google Scholar * Kemper, T. L. & Bauman, M. L.
Neuropathology of infantile autism. _Mol. Psychiatry_ 7, S12–S13 (2002). Article PubMed Google Scholar * Tietze, F. A. et al. Auditory deficits in audiovisual speech perception in adult
Asperger’s syndrome: fMRI study. _Front. Psychol._ 10, 2286 (2019). Article PubMed PubMed Central Google Scholar * Lee, J. M., Kyeong, S., Kim, E. & Cheon, K.-A. Abnormalities of
inter- and intra-hemispheric functional connectivity in autism spectrum disorders: a study using the autism brain imaging data exchange database. _Front. Neurosci._ 10, 191–191 (2016).
Article PubMed PubMed Central Google Scholar * Uddin, L. Q. Brain mechanisms supporting flexible cognition and behavior in adolescents with autism spectrum disorder. _Biol. Psychiatry_
89, 172–183 (2021). Article CAS PubMed Google Scholar * Li, R. et al. The fronto-insular cortex causally mediates the default-mode and central-executive networks to contribute to
individual cognitive performance in healthy elderly. _Hum. Brain Mapp._ 39, 4302–4311 (2018). Article PubMed PubMed Central Google Scholar * Uddin, L. Q. et al. Salience network-based
classification and prediction of symptom severity in children with autism. _JAMA Psychiatry_ 70, 869–879 (2013). Article PubMed PubMed Central Google Scholar * Abbott, A. E. et al.
Patterns of atypical functional connectivity and behavioral links in autism differ between default, salience, and executive networks. _Cereb. Cortex_ 26, 4034–4045 (2016). Article PubMed
PubMed Central Google Scholar * Quattrocki, E. & Friston, K. Autism, oxytocin and interoception. _Neurosci. Biobehav. Rev._ 47, 410–430 (2014). Article CAS PubMed PubMed Central
Google Scholar * Bredewold, R., Schiavo, J. K., van der Hart, M., Verreij, M. & Veenema, A. H. Dynamic changes in extracellular release of GABA and glutamate in the lateral septum
during social play behavior in juvenile rats: Implications for sex-specific regulation of social play behavior. _Neuroscience_ 307, 117–127 (2015). Article CAS PubMed Google Scholar *
Gaykema, R. P., Luiten, P. G., Nyakas, C. & Traber, J. Cortical projection patterns of the medial septum-diagonal band complex. _J. Comp. Neurol._ 293, 103–124 (1990). Article CAS
PubMed Google Scholar * Unal, G., Joshi, A., Viney, T. J., Kis, V. & Somogyi, P. Synaptic targets of medial septal projections in the hippocampus and extrahippocampal cortices of the
mouse. _J. Neurosci._ 35, 15812–15826 (2015). Article CAS PubMed PubMed Central Google Scholar * Freund, T. F. & Antal, M. GABA-containing neurons in the septum control inhibitory
interneurons in the hippocampus. _Nature_ 336, 170–173 (1988). Article CAS PubMed Google Scholar * Cheng, C. H., Chan, P. S., Hsu, S. C. & Liu, C. Y. Meta-analysis of sensorimotor
gating in patients with autism spectrum disorders. _Psychiatry Res._ 262, 413–419 (2018). Article PubMed Google Scholar * Arbogast, T. et al. Reciprocal effects on neurocognitive and
metabolic phenotypes in mouse models of 16p11.2 deletion and duplication syndromes. _PLoS Genet._ 12, e1005709 (2016). Article PubMed PubMed Central Google Scholar * Brunner, D. et al.
Comprehensive analysis of the 16p11.2 deletion and null Cntnap2 mouse models of autism spectrum disorder. _PLoS One_ 10, e0134572 (2015). Article PubMed PubMed Central Google Scholar *
Yang, M. et al. 16p11.2 deletion syndrome mice display sensory and ultrasonic vocalization deficits during social interactions. _Autism Res._ 8, 507–521 (2015). Article PubMed PubMed
Central Google Scholar * Braff, D. L., Swerdlow, N. R. & Geyer, M. A. Symptom correlates of prepulse inhibition deficits in male schizophrenic patients. _Am. J. Psychiatry_ 156,
596–602 (1999). Article CAS PubMed Google Scholar * Popelář, J., Rybalko, N., Burianová, J., Schwaller, B. & Syka, J. The effect of parvalbumin deficiency on the acoustic startle
response and prepulse inhibition in mice. _Neurosci. Lett._ 553, 216–220 (2013). Article PubMed Google Scholar * Brown, J. A. et al. Inhibition of parvalbumin-expressing interneurons
results in complex behavioral changes. _Mol. Psychiatry_ 20, 1499–1507 (2015). Article CAS PubMed PubMed Central Google Scholar * Kaldy, Z., Giserman, I., Carter, A. S. & Blaser, E.
The mechanisms underlying the ASD advantage in visual search. _J. Autism Dev. Disord._ 46, 1513–1527 (2016). Article PubMed PubMed Central Google Scholar * O’Riordan, M. & Plaisted,
K. Enhanced discrimination in autism. _Q. J. Exp. Psychol. A_ 54, 961–979 (2001). Article PubMed Google Scholar * Remington, A. & Fairnie, J. A sound advantage: Increased auditory
capacity in autism. _Cognition_ 166, 459–465 (2017). Article PubMed Google Scholar * Uddin, L. Q., Kinnison, J., Pessoa, L. & Anderson, M. L. Beyond the tripartite
cognition-emotion-interoception model of the human insular cortex. _J. Cogn. Neurosci._ 26, 16–27 (2014). Article PubMed Google Scholar * Anticevic, A. et al. The role of default network
deactivation in cognition and disease. _Trends Cogn. Sci._ 16, 584–592 (2012). Article PubMed PubMed Central Google Scholar * Verma, V., Paul, A., Amrapali Vishwanath, A., Vaidya, B.
& Clement, J. P. Understanding intellectual disability and autism spectrum disorders from common mouse models: synapses to behaviour. _Open Biol._ 9, 180265 (2019). Article CAS PubMed
PubMed Central Google Scholar * Molosh, A. I. & Shekhar, A. Neurofibromatosis type 1 as a model system to study molecular mechanisms of autism spectrum disorder symptoms. _Prog.
Brain Res._ 241, 37–62 (2018). Article PubMed Google Scholar * Pasciuto, E. et al. Autism spectrum disorders: translating human deficits into mouse behavior. _Neurobiol. Learn Mem._ 124,
71–87 (2015). Article CAS PubMed Google Scholar * Kas, M. J. et al. Assessing behavioural and cognitive domains of autism spectrum disorders in rodents: current status and future
perspectives. _Psychopharmacology_ 231, 1125–1146 (2014). Article CAS PubMed Google Scholar * Grayson, B. et al. Assessment of disease-related cognitive impairments using the novel
object recognition (NOR) task in rodents. _Behav. Brain Res._ 285, 176–193 (2015). Article PubMed Google Scholar * Moon, J. et al. Attentional dysfunction, impulsivity, and resistance to
change in a mouse model of fragile X syndrome. _Behav. Neurosci._ 120, 1367–1379 (2006). Article CAS PubMed Google Scholar * McTighe, S. M., Neal, S. J., Lin, Q., Hughes, Z. A. &
Smith, D. G. The BTBR mouse model of autism spectrum disorders has learning and attentional impairments and alterations in acetylcholine and kynurenic acid in prefrontal cortex. _PLoS One_
8, e62189 (2013). Article CAS PubMed PubMed Central Google Scholar * Hung, A. Y. et al. Smaller dendritic spines, weaker synaptic transmission, but enhanced spatial learning in mice
lacking Shank1. _J. Neurosci._ 28, 1697–1708 (2008). Article CAS PubMed PubMed Central Google Scholar * Richter, M. et al. Altered TAOK2 activity causes autism-related
neurodevelopmental and cognitive abnormalities through RhoA signaling. _Mol. Psychiatry_ 24, 1329–1350 (2019). Article CAS PubMed Google Scholar * Dong, F. et al. Deletion of CTNNB1 in
inhibitory circuitry contributes to autism-associated behavioral defects. _Hum. Mol. Genet._ 25, 2738–2751 (2016). CAS PubMed PubMed Central Google Scholar * Hughes, R. B.,
Whittingham-Dowd, J., Clapcote, S. J., Broughton, S. J. & Dawson, N. Altered medial prefrontal cortex and dorsal raphé activity predict genotype and correlate with abnormal learning
behavior in a mouse model of autism-associated 2p16.3 deletion. _Autism. Res._ 15, 614–627 (2022). Article PubMed PubMed Central Google Scholar * Garrett, L. et al. A truncating Aspm
allele leads to a complex cognitive phenotype and region-specific reductions in parvalbuminergic neurons. _Transl. Psychiatry_ 10, 66 (2020). Article CAS PubMed PubMed Central Google
Scholar * Burrows, E. L. et al. Mice with an autism-associated R451C mutation in neuroligin-3 show a cautious but accurate response style in touchscreen attention tasks. _Genes Brain
Behav._ 21, e12757 (2022). Article CAS PubMed Google Scholar * Samuels, I. S. et al. Deletion of ERK2 mitogen-activated protein kinase identifies its key roles in cortical neurogenesis
and cognitive function. _J. Neurosci._ 28, 6983–6995 (2008). Article CAS PubMed PubMed Central Google Scholar * Keehn, B., Brenner, L., Palmer, E., Lincoln, A. J. & Müller, R. A.
Functional brain organization for visual search in ASD. _J. Int. Neuropsychol. Soc._ 14, 990–1003 (2008). Article PubMed Google Scholar * Wojciulik, E. & Kanwisher, N. The generality
of parietal involvement in visual attention. _Neuron_ 23, 747–764 (1999). Article CAS PubMed Google Scholar * Bristow, G. C. et al. 16p11 duplication disrupts
hippocampal-orbitofrontal-amygdala connectivity, revealing a neural circuit endophenotype for schizophrenia. _Cell Rep._ 31, 107536 (2020). Article CAS PubMed Google Scholar * Horev, G.
et al. Dosage-dependent phenotypes in models of 16p11.2 lesions found in autism. _Proc. Natl. Acad. Sci._ 108, 17076–17081 (2011). Article PubMed PubMed Central Google Scholar * Csárdi
G., Nepusz T. The igraph software package for complex network research. _Int. J. Complex Syst._ 1695, 1–9 (2006). * RCoreTeam. _R: A language and environment for statistical computing._ (R
Foundation for Statistical Computing, Vienna, Austria, 2018). https://www.R-projectorg/. Google Scholar * Dawson, N., Morris, B. J. & Pratt, J. A. Functional brain connectivity
phenotypes for schizophrenia drug discovery. _J. Psychopharmacol._ 29, 169–177 (2015). Article PubMed Google Scholar * Mevik B.-H., Wehrens R., Liland K. Pls: PTRNial least squares and
principle component regression. _R package version 27-1_ https://cran.r-project.org/web/packages/pls/index.html (2019). * Wisden W., Morris B.J. In situ hybridisation protocols for the
brain. (Academic Press, 1994). * Morris, B. J., Reimer, S., Höllt, V. & Herz, A. Regulation of striatal prodynorphin mRNA levels by the raphe-striatal pathway. _Brain Res._ 464, 15–22
(1988). CAS PubMed Google Scholar * Wisden, W. et al. Differential expression of immediate-early genes in the hippocampus and spinal cord. _Neuron_ 4, 603–614 (1990). Article CAS PubMed
Google Scholar * Johnston, H. M. & Morris, B. J. NMDA and nitric oxide increase microtubule-associated protein 2 gene. _J. Neurochem._ 63, 379–382 (1994). Article CAS PubMed Google
Scholar * Openshaw, R. L. et al. Map2k7 haploinsufficiency induces brain imaging endophenotypes and behavioral phenotypes relevant to schizophrenia. _Schizophr. Bull._ 46, 211–223 (2019).
Article PubMed Central Google Scholar * Kim, C. H. et al. The continuous performance test (rCPT) for mice: a novel operant touchscreen test of attentional function. _Psychopharmacology_
232, 3947–3966 (2015). Article CAS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS This research was supported by the Medical Research Council (UK), grant
MR/N012704/1. We are very grateful to John Craig for performing the genotyping for these studies. This research was supported by the Medical Research Council (UK), grant MR/N012704. AUTHOR
INFORMATION Author notes * Greg C. Bristow Present address: School of Pharmacy and Medical Sciences, University of Bradford, Bradford, BD7 1DP, UK * These authors contributed equally:
Rebecca L. Openshaw, David M. Thomson, Greg C. Bristow. * These authors jointly supervised this work: Judith A. Pratt, Brian J. Morris, Neil Dawson. AUTHORS AND AFFILIATIONS * School of
Psychology and Neuroscience, College of Medical, Veterinary and Life Sciences, University of Glasgow, Sir James Black Building, Glasgow, G12 8QQ, UK Rebecca L. Openshaw & Brian J. Morris
* Strathclyde Institute of Pharmacy and Biomedical Sciences, University of Strathclyde, Glasgow, G4 0RE, UK David M. Thomson, Emma J. Mitchell & Judith A. Pratt * Department of
Biomedical and Life Sciences, Lancaster University, Lancaster, LA1 4YW, UK Greg C. Bristow & Neil Dawson Authors * Rebecca L. Openshaw View author publications You can also search for
this author inPubMed Google Scholar * David M. Thomson View author publications You can also search for this author inPubMed Google Scholar * Greg C. Bristow View author publications You can
also search for this author inPubMed Google Scholar * Emma J. Mitchell View author publications You can also search for this author inPubMed Google Scholar * Judith A. Pratt View author
publications You can also search for this author inPubMed Google Scholar * Brian J. Morris View author publications You can also search for this author inPubMed Google Scholar * Neil Dawson
View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS D.M.T., R.L.O., G.B. and E.J.M. performed the experiments, conducted most of the data
analysis and contributed to writing the manuscript. J.A.P., N.D. and B.J.M. conceived the study, provided guidance on experimental design and input to methodological optimisation,
contributed to the interpretation of results and wrote most of the manuscript. CORRESPONDING AUTHORS Correspondence to Brian J. Morris or Neil Dawson. ETHICS DECLARATIONS COMPETING INTERESTS
The authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Communications Biology_ thanks the anonymous reviewers for their contribution to the peer review of this
work. Primary Handling Editors: Christoph Anacker and George Inglis. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published
maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION REPORTING SUMMARY RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative
Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the
original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in
the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended
use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Openshaw, R.L., Thomson, D.M., Bristow, G.C. _et al._ 16p11.2 deletion mice exhibit
compromised fronto-temporal connectivity, GABAergic dysfunction, and enhanced attentional ability. _Commun Biol_ 6, 557 (2023). https://doi.org/10.1038/s42003-023-04891-2 Download citation
* Received: 06 December 2022 * Accepted: 01 May 2023 * Published: 24 May 2023 * DOI: https://doi.org/10.1038/s42003-023-04891-2 SHARE THIS ARTICLE Anyone you share the following link with
will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative