Play all audios:
ABSTRACT Despite recent advances in cancer immunotherapy, pancreatic ductal adenocarcinoma (PDAC) remains unresponsive due to an immunosuppressive tumor microenvironment, which is
characterized by the abundance of cancer-associated fibroblasts (CAFs). Once identified, CAF-mediated immune inhibitory mechanisms could be exploited for cancer immunotherapy. Siglec
receptors are increasingly recognized as immune checkpoints, and their ligands, sialic acids, are known to be overexpressed by cancer cells. Here, we unveil a previously unrecognized role of
sialic acid-containing glycans on PDAC CAFs as crucial modulators of myeloid cells. Using multiplex immunohistochemistry and transcriptomics, we show that PDAC stroma is enriched in sialic
acid-containing glycans compared to tumor cells and normal fibroblasts, and characterized by ST3GAL4 expression. We demonstrate that sialic acids on CAF cell lines serve as ligands for
Siglec-7, -9, -10 and -15, distinct from the ligands on tumor cells, and that these receptors are found on myeloid cells in the stroma of PDAC biopsies. Furthermore, we show that CAFs drive
the differentiation of monocytes to immunosuppressive tumor-associated macrophages in vitro, and that CAF sialylation plays a dominant role in this process compared to tumor cell
sialylation. Collectively, our findings unravel sialic acids as a mechanism of CAF-mediated immunomodulation, which may provide targets for immunotherapy in PDAC. SIMILAR CONTENT BEING
VIEWED BY OTHERS SIALIC ACIDS IN PANCREATIC CANCER CELLS DRIVE TUMOUR-ASSOCIATED MACROPHAGE DIFFERENTIATION VIA THE SIGLEC RECEPTORS SIGLEC-7 AND SIGLEC-9 Article Open access 24 February
2021 MACROPHAGES AND FIBROBLASTS AS REGULATORS OF THE IMMUNE RESPONSE IN PANCREATIC CANCER Article 22 April 2025 CGAS-STING SIGNALING ENCOURAGES IMMUNE CELL OVERCOMING OF FIBROBLAST
BARRICADES IN PANCREATIC CANCER Article Open access 30 June 2022 INTRODUCTION Pancreatic Ductal Adenocarcinoma (PDAC) is one of the most aggressive types of cancer, with a 5-year survival of
around 11%1. This poor prognosis is mainly attributed to late diagnosis and limited treatment possibilities. While immunotherapy such as immune checkpoint blockade (ICB) has improved the
survival of patients in a range of human cancers, immunotherapy remains unsuccessful in PDAC due to a relatively low mutational burden and various immunosuppressive mechanisms2.
Understanding the driving factors of immunosuppression is essential to improve treatment possibilities for PDAC. The PDAC tumor microenvironment (TME) is unique in its abundance of dense
fibrotic stroma and suppressive immune cells. The stroma in PDAC can constitute up to 80% of the tumor mass and comprises of extracellular matrix and specialized connective-tissue cells,
including cancer-associated fibroblasts (CAFs)3. CAFs are highly heterogeneous in their phenotypes, origins and functions, including both tumor-promoting and tumor-inhibiting
properties4,5,6,7,8,9. Advances in single-cell technologies have led to the identification of several CAF subsets in PDAC that include inflammatory CAFs (iCAF), myofibroblastic CAFs (myCAF)
and antigen-presenting CAFs (apCAF)10,11,12,13,14. Immune cells in the TME are mainly of the myeloid lineage, the majority being tumor-associated macrophages (TAMs)15,16. Accumulation of
TAMs correlates with poor prognosis of PDAC16,17,18. TAMs are chronically polarized by the tumor and show a mixed phenotype of both anti-tumoral and pro-tumoral activation states, marked by
the expression of HLA-DR and CD86 or CD163, CD206 and PD-L1, respectively15,19. Besides the role of TAMs in tissue remodeling and inflammation, TAMs induce immunosuppression in the TME by
recruiting Tregs and inhibiting CD8+ T and NK cell cytotoxicity through secretion of cytokines such as TGF-β and interleukin (IL)-10 15,20,21,22,23,24,25. Accumulating evidence shows that
the crosstalk between CAFs and immune cells leads to inhibition of anti-tumor immune responses and can hamper immunotherapy 26. In breast, prostate, skin and colorectal cancer, CAFs can
recruit monocytes through secretion of CCL2 and enhance their polarization to immunosuppressive TAMs26. In addition, co-injection of Panc02 PDAC tumor cells with stellate cells increased the
accumulation of myeloid cells, including pro-tumoral TAMs in the tumor27. Moreover, depletion of fibroblast activating protein (FAP)+ CAFs in murine PDAC improved the efficacy of ICB and
reduced tumor growth28. On the other hand, depletion of alpha-smooth muscle actin (α-SMA)+ CAFs accelerated tumor growth but when combined with ICB prolonged the survival of mice6. These
studies highlight a role of CAFs in immunosuppression potentially via myeloid cells. Yet, it is still unclear whether PDAC CAFs can directly induce TAMs with immunosuppressive properties and
what are the mechanisms behind this. A better understanding of CAF-mediated myeloid suppression can contribute to developing more effective immunotherapeutic strategies in PDAC. Changes in
metabolic processes such as glycosylation are a hallmark of cancer and have been shown to alter immune responses via binding to lectin receptors29,30,31. One of the most observed glycan
alterations in cancer is the overexpression of sialic acids29,31,32. The synthesis of sialylated glycans occurs in the Golgi by sialyltransferase enzymes (ST) that utilize the sialic acid
donor cytidine monophosphate _N_-acetylneuraminic acid (CMP-Neu5Ac) generated by the Cytidine Monophosphate _N_-Acetylneuraminic Acid Synthetase (CMAS) in the nucleus33. Tumor cells
overexpress sialic acids to evade immune clearance and create an immunosuppressive environment29,31,32,34. Sialic acids are therefore considered a target for immunotherapy34. Sialic acids
can induce immunosuppression by binding to ITIM-containing Sialic acid-binding immunoglobulin-like lectins (Siglecs) expressed on innate and adaptive immune cells32,35. In cancer, Siglec-7,
-9, -10 and -15 play a key role in facilitating immune evasion36,37,38,39,40,41. The binding of Siglecs to sialic acid is complex and dependent on multiple factors including, sialic acid
linkage, underlying glycan structure and on the protein or lipid it is attached to41,42. Increasing evidence from mouse models highlights the immunosuppressive role of tumor
sialylation43,44,45,46,47. Tumor sialylation suppresses CD8+ T cell and NK cell cytotoxicity and polarizes macrophages to immunosuppressive macrophages, both supporting tumor
growth43,44,45,46,47,48. Targeting tumor sialylation, either through sialic acid mimetics or sialidase-coupled antibodies, improves survival of mice and synergizes with ICB, which is
dependent on Siglec-E, the murine orthologs of Siglec-7 and Siglec-945,46,47,48. Our previous study showed that overexpression of sialic acids in PDAC cells contributes to an
immunosuppressive microenvironment by promoting TAM differentiation via the interaction with Siglec-7 and Siglec-936. These studies highlight the potential of targeting tumor sialylation for
cancer immunotherapy. However, there is a limited understanding of the glycosylation and sialic acid expression on PDAC CAFs, and how this can mediate Siglec-dependent immunosuppression.
Here we study sialylation of CAFs in relation to tumor sialylation, and its role in immune modulation of myeloid cells in PDAC. We report increased sialylation of CAFs in PDAC compared to
tumor cells and normal fibroblasts. PDAC CAFs induce the differentiation of monocytes to immunosuppressive TAMs, a process in which CAF derived sialic acids plays a significant role. These
data, coupled with the finding that the majority ( ~ 90%) of myeloid cells reside in the stroma, highlight that CAF sialylation plays a dominant role in TAM-differentiation when compared to
tumor cell sialylation. RESULTS PDAC STROMA EXPRESSES SIALYLATED GLYCAN STRUCTURES We and others have previously demonstrated that PDAC tumor sialylation drives myeloid suppression through
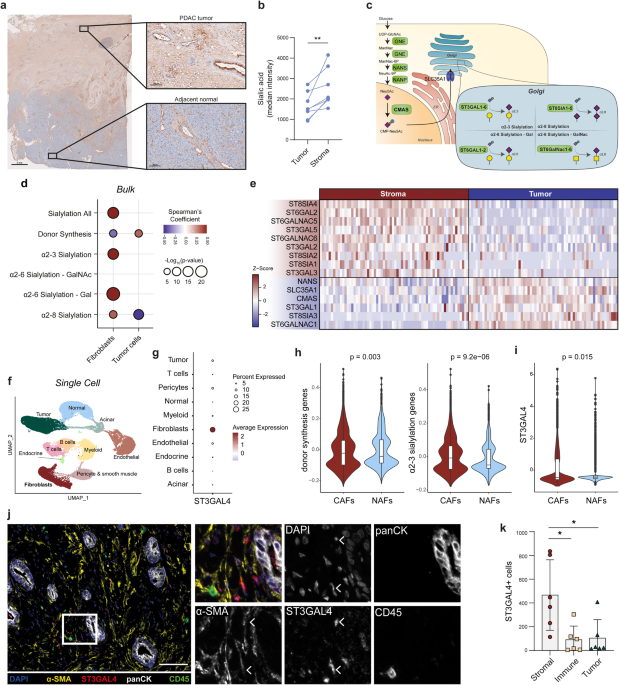
interactions with Siglec-7 and Siglec-936,39,40. Interestingly, DAB staining for sialic acid in PDAC biopsies revealed its presence not only in tumor cells but also in the stromal
compartment (Fig. 1a, Supplementary Fig. 1a). The presence of sialic acids was assessed by staining with an inactivated neuraminidase (Lectenz), that has specificity for all α2-3, α2-6 and
α2-8 sialic acid linkages (pan-Lectenz). The specificity for sialylated structures was confirmed by pre-treating the tissue with neuraminidase, an enzyme that hydrolyses terminal sialic
acids (Supplementary Fig. 1b). To quantify sialic acid levels, PDAC biopsies were co-stained with the tumor marker panCK and pan-Lectenz, allowing for the segmentation of tumor and stromal
areas. Interestingly, the median staining intensity of pan-Lectenz was significantly higher in stromal regions than on the panCK+ tumor cells, underscoring the abundance of sialic acids in
the cancer stroma (Fig. 1b). Given the prevalence of CAFs in PDAC stroma, we hypothesized that CAFs express sialic acids which could drive Siglec-dependent immune modulation. To explore this
hypothesis, we analyzed several publicly available transcriptomic datasets of PDAC, focusing on genes involved in sialic acid-containing glycan production (Fig. 1c). These genes include
those responsible for donor synthesis and transportation (_GNE, NANS, NANP, CMAS, SLC35A1_) and sialyltranferases expressed in the Golgi. Sialyltransferases are categorized based on acceptor
sugar, which is a galactose (Gal), N-acetylgalactosamine (GalNAc) or another sialic acid (Sia), and the linkage (α2-3, α2-6, α2-8) in which they add sialic acids (Fig. 1c). Correlating gene
signatures of different sialylation pathways with CAFs and tumor cell scores in the TCGA bulk RNA sequencing dataset revealed a positive correlation between the overall sialylation-related
gene set and the CAF-gene signature score, while the tumor cell score showed no significant correlation (Fig. 1d). Additionally, pathways involving α2-3 or α2-6 sialylation positively
correlated with CAFs, but not with tumor cells, which instead showed a positive correlation only with donor synthesis genes (Fig. 1d). Using a publicly available RNA sequencing dataset of
microdissected tumor and stroma49, we identified individual sialylation genes associated with stromal sialylation. Tumor cells highly expressed donor synthesis genes (_NANS, CMAS_, and the
transporter _SLC35A1_), while the stroma showed enriched expression of sialyltransferases involved in α2-3, α2-6 and α2-8 sialylation (_ST3GAL2, ST3GAL5, ST6GAL2, ST6GALNAC5, ST6GALNAC6,
ST8SIA1_ and _ST8SIA2_) (Fig. 1e). These findings imply that stromal cells can produce sialylated glycans as they express the genes involved in sialylation. To assess the expression of
sialylation-related genes within distinct TME cell subsets, including CAFs, we utilized publicly available scRNA-Seq data from Peng et al.50. Cell clusters were identified, and the
expression of sialylation genes was analyzed for each cluster (Fig. 1f, Supplementary Fig. 1c, d). Interestingly, we identified the sialyltransferase _ST3GAL4_ to be highly expressed in the
fibroblast cell cluster compared to other cell clusters (Fig. 1g, Supplementary Fig. 1d). ST3GAL4 is one of the six enzymes that transfers sialic acids in a α2-3 linkage to terminal
galactopyranosyl (Gal). ST3GAL4 is involved in generation of the sialyl Lewis X antigen, often upregulated in tumor cells to facilitate invasion, and is described to generate the ligands for
Siglec-936,51. Another sialylation enzyme enriched in stromal cells was ST6GALNAC6, but this enzyme was mainly expressed in smooth muscle cells or pericytes (Supplementary Fig. 1d, e).
Further examination revealed several fibroblast subsets, that we annotated as normal-fibroblast (NAFs), given their presence in normal pancreatic tissue, transitional-CAFs, myofibroblastic
CAFs (myCAFs) and inflammatory-CAFs (iCAFs) (Supplementary Fig. 1f, g). Interestingly, both donor synthesis and α2-3 sialylation genes were significantly enriched in CAFs compared to NAFs
(Fig. 1h). We did not identify significant differences in α2-3 sialylation genes between transitional CAFs, iCAFs or myCAFs, suggesting that sialylation is an overall signature of all CAF
subsets (Supplementary Fig. 1h). _ST3GAL4_ was upregulated in CAFs compared to NAFs and was significantly higher expressed in myCAFs compared to iCAFs (Fig. 1i, Supplementary Fig. 1h). To
validate ST3GAL4 expression in CAFs, PDAC biopsies were co-stained for the CAF marker α-SMA and ST3GAL4. Indeed, ST3GAL4 was expressed in several α-SMA+ cells in the PDAC tissues (Fig. 1j).
Quantification of the ST3GAL4 staining confirmed that ST3GAL4 was predominantly expressed in stromal cells (Fig. 1k). In summary, these data demonstrate that PDAC CAFs express sialic acids
and are characterized by the expression of the sialyltransferase ST3GAL4. SIALIC ACIDS ON CAF CELL LINES SERVE AS LIGANDS FOR SIGLEC RECEPTORS EXPRESSED ON MYELOID CELLS IN THE TME To
further study the sialylation of CAFs, we utilized three fibroblast cell lines (M1 CAFs, T1 CAFs, and PS-1) to model pancreatic CAFs (Supplementary Fig. 2a). The cell lines were
characterized by the lab of origin, showing lack of cancer driver mutations in M1 and T1 CAFs14,52. Although pancreatic stellate cells are typically quiescent cells in homeostasis, they are
known to acquire an activated myofibroblast phenotype when cultured in monolayer or when activated in the TME, and therefore can be used as a model for CAFs4,14. Indeed, the PS-1 cell line
expressed the myofibroblast marker α-SMA, lost the quiescent marker GFAP, and displayed an activated phenotype, and will therefore be referred to as PS-1 CAF (Supplementary Fig. 2b–d). The
mesenchymal origin of these CAF cell lines was confirmed with vimentin staining, and all cell lines expressed the CAF markers α-SMA, FAP and CD90 (Fig. 2a, Supplementary Fig. 2b, e, f)13.
Nevertheless, the three CAF cell lines showed distinct properties and activation states. Firstly, The M1 CAFs and PS-1 CAFs displayed an elongated, aligned phenotype compared to T1 CAFs
(Fig. 2a). Additionally, the PS-1 CAFs were significantly more activated compared to the M1 and T1 CAFs, and several fibroblast markers were differentially expressed in the cell lines
(Supplementary Fig. 2c, d, f). Given the differential phenotype and activation state of the cells, we next aimed to evaluate whether the cells resemble iCAF or myCAFs. Therefore, we analyzed
a set of fibroblast markers and compared their expression to the expression in CAF subsets in scRNA-seq data, as well as their expression on the CAF cell lines after treatment with IL-1β or
TGF-β, inducing an iCAF or myCAF phenotype respectively. While PDGFRα has been described as an iCAF marker13, our analysis associated it with both iCAFs and normal fibroblasts, suggesting
that this marker may not be specific to iCAFs. In line with this, treatment of the cell lines with IL-1β, a cytokine described to induce an iCAF phenotype53, slightly decreased PDGFRα
expression (Supplementary Fig. 2f). PDGFRα was expressed in M1 and T1 CAFs, but not in PS-1 CAFs. Additionally, the cytokine secretion profiles of the CAFs were analyzed, as iCAFs are
characterized by secretion of IL-6, CCL2 and CXCL1213,53 (Supplementary Fig. 2g). All CAF cells secreted high levels of IL-6, IL-8, CCL2 (>5000 pg/mL), suggesting an iCAF-like phenotype
in all cell lines (Supplementary Fig. 2g). Expression of IL-6 and IL-8 were over 2-fold higher in the PS-1 CAFs compared to the M1 and T1 CAFs, suggesting a more iCAF-like phenotype in the
PS-1. In contrast, all cell lines expressed the myCAF marker α-SMA, with the PS-1 being most myCAF-like given its high myofibroblastic, activated phenotype in the gel contraction assay
(Supplementary Fig. 2b, d). Furthermore, all cell lines expressed PD-L1 and did not represent apCAFs, as evidenced by the lack of HLA-DR (Supplementary Fig. 2h). Together, the phenotyping of
the cell lines indicates that the fibroblast cell lines are suitable for in vitro modeling of CAFs in PDAC, and show characteristics of both iCAFs and myCAFs. We next examined the sialic
acid expression in fibroblast and tumor cell lines using various biotinylated probes. Staining with pan-Lectenz or α2-3-specific-Lectenz revealed that all fibroblast cell lines exhibited
equal or higher sialic acid levels than BxPC3 and MiaPaca-2 tumor cell lines (Fig. 2b, Supplementary Fig. 2i). Additionally, staining with plant lectin derived from _Maackia amurensis_
(MAAII) or _Sambucus nigra_ (SNA), with specificity for α2-3 and α2-6 linked sialic acids respectively, demonstrated sialic acid expression in the cell lines, with higher binding to α2-3
linked sialic acids compared to α2-6 linked sialic acids in the M1 and T1 CAFs (Fig. 2c, Supplementary Fig. 2i). Sialic acids have been widely described as immunosuppressive carbohydrates,
acting as ligands for inhibitory Siglec receptors on immune cells. To investigate which Siglec receptors could potentially bind CAF sialic acids, we stained the CAF cell lines with different
Siglec-Fc chimeras. We have demonstrated before that tumor sialic acids interact with Siglec-7 and Siglec-936. Here we show that among the tested Siglecs, Siglec-7, -9, -10 and -15
recognized CAF sialic acids (Fig. 2d). The binding of these Siglec-Fcs was sialic acid specific, as treatment with the enzyme neuraminidase abolished Siglec-Fc binding (Fig. 2d). Other
Siglec receptors, including Siglec-3, -5, -6, -8, -11 and -14, did not bind CAF sialic acids (Supplementary Fig. 2j). Taken together, pancreatic CAFs express sialylated glycans in a variety
of linkages, with α2-3 linked being the most predominant, and these sialic acids serve as ligands for Siglec-7, -9, -10 and -15. Siglecs are present on various immune cells, such as Siglec-7
and -9 on NK cells54,55, Siglec-9 on a subset of T cells56,57, and Siglec-7, -9, -10 and -15 on tumor-associated macrophages (TAMs)36,37,38. Given that myeloid cells can express Siglec-7,
-9, -10 and -1532,36, and are a dominating immune subset in the TME of PDAC58, we investigated the presence of these Siglecs on CD14+ myeloid cells in the TME. Multiplex immunohistochemistry
revealed the existence of CD14+ myeloid cells expressing Siglec-7, -9, -10 or -15 in the PDAC TME (Fig. 2e). Interestingly, most CD14+ cells were located in the stroma, suggesting potential
interactions between stromal cells and myeloid cells (Fig. 2f). Across the examined PDAC biopsies, Siglec-9 emerged as the most abundantly expressed Siglec within the CD14+ cells, followed
by Siglec-10, Siglec-7 and Siglec-15 (Fig. 2g). These data indicate the expression of Siglec-7, -9, -10 and -15 on myeloid cells in PDAC, potentially facilitating interactions with sialic
acids on CAFs. To evaluate whether these receptors show differential expression among distinct monocyte-derived cells, including different macrophage activation and polarization states, we
generated monocyte-derived macrophages (moMACs) and monocyte-derived dendritic cells (moDCs) in vitro. MoMACs were generated by differentiating monocytes with M-CSF, creating non-polarized
moMACs (M0), and were polarized to M1-moMACs using LPS and IFNγ or M2-moMACs using IL-4 and IL-6. MoDCs were generated by addition of GM-CSF and IL-4. Among these cells, Siglec-7, -9 and to
some extend Siglec-10 were expressed on undifferentiated monocytes, moMACs and moDCs. Interestingly, Siglec-15 was expressed on M0- and M2-moMACs, but not on undifferentiated monocytes,
M1-moMACs or moDCs (Supplementary Fig. 3a). DETAILED CHARACTERIZATION OF GLYCAN PROFILES IN PDAC CAF CELL LINES Because of a limited understanding of specific glycans present on CAFs, we
conducted a comprehensive glycan analysis using mass spectrometry, encompassing _O_-glycosylation, _N_-glycosylation, and glycosphingolipids glycosylation (Fig. 3a, Supplementary Data 1).
_O_- and _N_-glycosylation is the process of covalently attaching glycans to proteins at serine/threonine or asparagine residues, respectively59,60. Glycosphingolipids on the other hand, are
the major class of glycolipids61. This analysis revealed that the most abundantly expressed glycans were present in all CAF cell lines, and include gangliosides GM3 and GM2, several
sialylated complex _N_-glycans, and the _O_-glycans disialyl-T and sialyl-T antigen (also called (di)sialyl core 1). However, notable differences were observed, particularly in the PS-1 CAFs
that exhibited a different profile than the M1 and T1 CAFs. The PS-1 CAFs showed a restricted pattern in glycosphingolipids, including only GM3 and GM2 gangliosides. In contrast, the M1 and
T1 CAFs displayed a more diverse ganglioside pattern, expressing additional gangliosides such as GM1 and GD1a, along with sialylated (neo-) lacto series glycosphingolipids (nsGSLs) (Fig.
3a, Supplementary Data 1). Similarly, the _N-_glycosylation and _O_-glycosylation patterns for PS-1 CAFs were more limited than those for M1 and T1 CAFs (Fig. 3a, Supplementary Data 1). To
determine whether the Siglec ligands on CAFs differ from those on tumor cells, the cell lines were treated with inhibitors for _O_-glycosylation, _N_-glycosylation, and glycosphingolipid
synthesis. We previously reported that Siglec-7 ligands on tumor cells are mainly expressed on O-glycosylated proteins. Similarly, treating CAFs with an _O_-glycosylation inhibitor
significantly reduced Siglec-7 ligands (Supplementary Fig. 3b). In contrast, while the _O_-glycosylation inhibitor significantly reduced Siglec-9 ligands on BxPC3 cells, PS-1 CAFs exhibited
a significant reduction in response to the N-glycosylation inhibitor (Fig. 3b). The Siglec-9 ligands on M1 CAFs and T1 CAFs were reduced in response to both _O-_glycosylation and
_N-_glycosylation inhibitors, although this reduction was modest and did not reach statistical significance (Fig. 3b). These results indicate that the Siglec-9 ligands on CAFs, and
particularly those on PS-1 CAFs, differ from those on tumor cells. Furthermore, these results suggest variations in Siglec-9 ligands between PS-1 CAFs and M1 and T1 CAFs, with redundancy of
Siglec-9 ligands in M1 and T1 CAFs. To further investigate the glycosylation pathways in tumor cells and CAFs, we analyzed the gene scores for _O_-glycosylation, _N_-glycosylation and
glycosphingolipids glycosylation synthesis pathways within the scRNA-seq dataset from Peng et al.50 (Fig. 1f). This analysis revealed that tumor cells exhibited a significantly enriched
_O_-glycosylation score, while fibroblasts demonstrated a significantly higher _N-_glycosylation and glycosphingolipids glycosylation synthesis score (Fig. 3c). These results suggest that
_N_-linked glycans and glycosphingolipid glycans are the most important sialic acid-containing glycoconjugates in CAFs. CAFS DIFFERENTIATE MONOCYTES TO IMMUNOSUPPRESSIVE MACROPHAGES
RESEMBLING TAMS Infiltrating monocytes are a source of TAMs in PDAC62. We have previously shown that tumor cells can differentiate monocytes to TAMs36. To evaluate whether CAFs may also
contribute to monocyte to TAM differentiation, we co-cultured a panel of fibroblasts and tumor cells with human monocytes isolated from fresh PBMCs and compared their phenotype using
specific macrophage markers with flow cytometry (Fig. 4a). As controls, monocytes were differentiated into moMACs and moDCs as described above, or co-cultured with stellate cells isolated
from pancreatitis (iHPSC). To analyse the phenotype of the differentiated monocytes we pre-gated on CD45+ cells and performed dimensional reduction analysis (Fig. 4b). The tSNE presented
distinct clusters of M1- and M2-moMACs and moDCs, while the macrophages from the co-culture with fibroblasts or PDAC tumor cell lines clustered together between monocytes and
M-CSF-differentiated moMACs (Fig. 4b). The co-clustering of monocytes co-cultured with PDAC tumor cells or fibroblasts, indicates that fibroblasts can also differentiate monocytes to
macrophages with a TAM phenotype, characterized by expression of CD163 and CD206, markers known to be associated to pro-tumoral immunosuppressive TAMs63,64 (Fig. 4b, c, Supplementary Fig.
4a). Surprisingly, CAFs induced significantly more differentiation to CD163+CD206+ TAMs than PDAC tumor cells (BxPC3) (Fig. 4d). Stellate cells isolated from pancreatitis (iHPSC) did not
show this enhanced capacity to differentiate monocytes to CD163+CD206+ macrophages (Fig. 4d). The co-culture of CAFs and monocytes also contained increased IL-10 levels (Fig. 4e), and
compared to the co-culture with PDAC tumor cells, the macrophages showed a trend toward enhanced expression of PD-L1 (Fig. 4f). In addition, CAF-induced TAMs express CD86 and HLA-DR
(Supplementary Fig. 4b). We also analyzed Siglec expression on TAMs after the co-culture with tumor cells or CAFs. All co-cultures resulted in TAMs expressing Siglec-7 and -9 receptors but
showed minimal expression of Siglec-10 (Fig. 4g). Siglec-15 was expressed on approximately 50% of the CAF-differentiated TAMs (Fig. 4g). To further investigate the functional characteristics
of CAF-differentiated TAMs, we differentiated monocytes in the presence of a CAF-conditioned medium (CM) and co-cultured the macrophages with autologous CD8+ T cells. Addition of CAF-CM
during monocyte differentiation significantly increased the percentage of CD163highCD206high macrophages, indicating that secreted product from CAF can drive TAM differentiation
(Supplementary Fig. 4c). Furthermore, while the control M1-moMACs were potent stimulators of CD8+ T cell proliferation, CAF-conditioned macrophages induced T cell proliferation to a similar
extent as M2-moMACs (Fig. 4h). These results demonstrate that CAFs are potent inducers of monocyte-to-macrophage differentiation, driving their differentiation toward an immunosuppressive
phenotype. CAF-DERIVED SIALIC ACIDS INSTRUCT TAM DIFFERENTIATION VIA SIGLEC-9 Macrophage differentiation and polarization towards a tumor-promoting phenotype involve multifaceted processes
influenced by various factors. Sialic acid-containing glycans, known regulators of macrophage behavior through interactions with Siglec receptors, contribute to the instruction of
immunosuppressive macrophages36,39. Given the prevalence of sialylated glycans in CAFs, we hypothesized a role for the sialic acid-Siglec axis in CAF-mediated TAM differentiation.
Underscoring the relevance of N-glycosylation in CAFs (Fig. 3c), our focus directed towards exploring the impact of CAF sialylation on TAM differentiation in the context of PS-1 CAFs, where
N-glycans play a significant role in Siglec-9 binding. To understand how the sialic acid-Siglec axis is involved in PS-1 CAF-mediated TAM differentiation, we first interfered with the
overall sialylation through treatment with a sialyltransferase inhibitor (SI), which led to a substantial reduction in sialic acid positive cells without affecting cell viability (i.e., from
80 to 100% to <10% positive cells, Supplementary Fig. 5a, b). To use the SI in a co-culture setting, the SI should be washed away before addition of monocytes to prevent a direct effect
of SI on the monocytes. Removal of SI from the culture led to partial recovery of sialic acid expression after 3 days in PS-1 CAFs (Supplementary Fig. 5c). Next, SI-treated PS-1 CAFs were
co-cultured with monocytes to investigate the role of CAF-derived sialic acids in TAM differentiation (Fig. 5a). Abrogation of sialic acid expression in the PS-1 CAFs significantly reduced
the differentiation to CD163+CD206+ TAMs, and increased the inflammatory marker CD86 (Fig. 5a, b). As a second approach to study the role of CAF sialic acids on TAM differentiation, we
generated a CMAS KO cell line to deplete sialic acids from the cell surface. The CMAS enzyme is responsible for generating the activated sialic acid sugar donor, which is subsequently
transported into the Golgi for glycoprotein attachment (Fig. 1c). CMAS KO led to complete depletion of sialic acids from the surface of the PS-1 CAFs, including all the ligands for Siglec-7,
-9, -10 and -15 (Supplementary Fig. 5f). In line with the effect of the SI, removal of sialic acids after knocking out the CMAS enzyme in the PS-1 CAFs decreased the differentiation to
CD163+CD206+ TAMs (Fig. 5c). In addition, removal of CAF sialic acids reduced PD-L1 and HLA-DR expression on TAMs (Fig. 5d, Supplementary Fig. 5g). Together, these data show that sialic
acids on CAFs contribute to TAM differentiation in the context of the PS-1 CAFs. We identified ST3GAL4 as the sialyltransferase enzyme associated with CAFs (Fig. 1g, i). To study whether
ST3GAL4 plays a role in CAF-mediated TAM differentiation, we knocked down ST3GAL4 using siRNA. In line with previous results, ST3GAL4 knockdown in PS-1 CAFs resulted in reduced TAM
differentiation (Fig. 5e). Given that ST3GAL4 is involved in generating sialylated glycan ligands for Siglec-936,65, we knocked-out Siglec-9 in primary CD14+ monocytes to assess whether this
receptor contributes to the CAF-mediated TAM differentiation. Siglec-9 was significantly reduced in CD14+ macrophages following transfection with the Siglec-9 KO plasmid, without affecting
monocyte viability (Fig. 5f, Supplementary Fig. 5j). Interestingly, Siglec-9 KO in monocytes reduced CAF-mediated TAM differentiation in co-culture with PS-1 CAFs (Fig. 5G). The PS-1 CAFs
mediated this effect, as Siglec-9 KO did not affect cytokine-induced differentiation of moMACs (Supplementary Fig. 5k). Thus, our results indicate that Siglec-9 is responsible for sensing
the CAF sialic acids and mediates the CAF-driven TAM differentiation in the context of the PS-1 CAFs. The effect of M1 and T1 CAF sialylation on TAM differentiation was also analyzed, using
SI treatment, siRNA-mediated KD of ST3GAL4, and in Siglec-9 KO monocytes. Technical challenges prevented the generation of CMAS KO in M1 and T1 CAFs. Sialylinhibitor treatment ablated sialic
acids from the M1 and T1 CAFs, without compromising cell viability, an effect retained in the T1 CAFs after SI removal (Supplementary Fig. 5a–c). SI treatment on M1 CAFs did not influence
CAF-driven TAM differentiation (Supplementary Fig. 5d). However, sialic acid removal from M1 CAFs led to reduced CD163 expression on TAMs, suggesting a nuanced effect of M1 CAF sialylation
on TAM differentiation (Supplementary Fig. 5e). Conversely, in T1 CAFs, SI treatment increased differentiation towards CD163+CD206+ TAMs (Supplementary Fig. 5d). Neither ST3GAL4 knockdown in
CAFs nor Siglec-9 KO in monocytes affected the differentiation to CD163+CD206+ TAMs in co-cultures with M1 or T1 CAFs (Supplementary Fig. 5i, m). These results contrast with the impact of
PS-1 CAF sialylation on TAM differentiation, highlighting the context-dependent role of CAF sialylation in differentiating monocytes towards CD163+CD206+ TAMs. The diversity in sialylated
glycan profiles between CAF cell lines (Fig. 3a, b) may underly the differential biological effects of CAF glycosylation on monocyte differentiation. RELATIVE INFLUENCE OF TUMOR CELLS AND
CAFS IN SIALIC ACID-MEDIATED TAM DIFFERENTIATION Given that PS-1 CAF-derived sialic acids contributed to TAM differentiation similarly to tumor sialylation36, we investigated the individual
contributions of tumor- and CAF-derived sialylation in this process. In a co-culture setup of BxPC3 and PS-1 CAFs at a 1:4 ratio, BxPC3 cells proliferated faster, forming islands surrounded
by PS-1 CAFs after 4 days (Fig. 6a). Before co-culturing BxPC3, PS-1 CAFs and monocytes, tumor cells and CAFs were treated separately with SI or vehicle (DMSO). Interestingly, when
exclusively pre-treating PS-1 CAFs with SI before co-culture, a trend towards diminished differentiation into CD163+CD206+ TAMs emerged, accompanied by increased expression of CD86, and a
trend towards elevated HLA-DR levels (Fig. 6b). Surprisingly, exclusive pre-treatment of BxPC3 with SI did not impact the differentiation into CD163+CD206+ TAMs (Fig. 6b), despite SI
treatment of BxPC3 significantly reducing the expression of CD163, CD206, HLA-DR, and PD-L1 on monocytes when PS-1 CAFs were absent in the co-culture (Supplementary Fig. 6a). Only when both
BxPC3 and PS-1 CAFs were treated with SI, a significant reduction in TAM differentiation was observed, and the TAMs expressed increased CD86 and HLA-DR levels (Fig. 6a). These results
demonstrate the essential requirement for losing both tumor and CAF sialylation to reduce TAM differentiation. Importantly, these findings indicate a more prominent role for CAF sialylation
in this process. In an alternative approach to discern the relative contributions of tumor and CAF sialylation, monocyte differentiation was induced in the presence of CM obtained from BxPC3
tumor cells or PS-1 CAFs treated with vehicle control (PS-1CM-DMSO and BxPC3CM-DMSO) or treated with SI (PS-1CM-SI and BxPC3CM-SI). This approach prevented potential confounding effects of
proliferation differences between BxPC3 and PS-1 CAFs. TAM differentiation was reduced in the presence of PS-1CM-SI, but not with BxPC3CM-SI, compared to control (Fig. 6c). The expression of
HLA-DR and PD-L1 on TAMs increased when differentiated in the presence of PS-1CM-SI or BxPC3CM-SI (Supplementary Fig. 6b, c). These findings implicate that secreted sialylated products from
CAFs, more so than from tumor cells, are involved in TAM differentiation. Subsequently, equal amounts of PS-1CM and BxPC3CM were added simultaneously. When monocytes were differentiated in
the presence of both PS-1CM and BxPC3CM in equal ratio, derived from SI or vehicle-treated cells, a reduction in differentiation towards CD163highCD206high TAMs was observed only with
PS-1CM-SI (Fig. 6d). Additionally, the largest increase in PD-L1 and HLA-DR expression was induced by PS-1CM-SI (Fig. 6d). These results suggest that while tumor and CAF sialylation are
essential for contact-dependent TAM differentiation, CAF-derived sialylation plays a more dominant role in TAM differentiation through secreted products. DISCUSSION This study aimed to
evaluate the sialic acid expression in CAFs and its role in immune modulation. Our results demonstrate elevated sialic acid levels in CAFs compared to tumor cells, with ST3GAL4 identified as
the key regulatory enzyme in CAF sialylation. Unlike PDAC tumor cell sialic acids binding Siglec-7 and -936, sialic acids on CAFs serve as ligands for Siglec-7, -9, -10 and -15.
Importantly, we show that CAF-derived sialic acids influence the differentiation of monocytes to CD163+CD206+ macrophages, a phenotype associated with tumor-promoting immunosuppressive TAMs.
This work uncovers CAF-immune crosstalk dependent on CAF glycosylation and its interaction with suppressive receptors on immune cells. We show that CAF-derived sialic acids interacted with
the immune suppressive receptors Siglec-7, -9, -10 and -15. Of these four Siglecs, Siglec-9 was most abundant within the PDAC TME and played a significant role in monocyte-to-TAM
differentiation by CAFs. Yet, we cannot exclude the involvement of other Siglec receptors in this process. Siglec-7, -10 and -15 can also modulate myeloid cells in cancer. Siglec-10 binds
CD24 on breast cancer cells and prevents macrophage-mediated phagocytosis, indicating that Siglec-10 may also suppress macrophage functioning37. Siglec-7 is involved in TAM differentiation
by PDAC tumor cells and Siglec-15 has been described as target for normalizing immunotherapy, with its expression on macrophages and its link with suppressing T cell proliferation36,38. We
found that blood-isolated monocytes did not express Siglec-15, but gained Siglec-15 expression upon differentiation to M0- or M2-moMACs in vitro. We therefore speculate that Siglec-15 could
play a role in macrophage polarization at later stages rather than in the early steps of macrophage differentiation. Our data shows that PS-1 CAF-derived sialic acids contribute to TAM
differentiation and polarization towards an immunosuppressive phenotype, at least in part via Siglec-9. These results align with previous reports showing that engagement of Siglec-9 by
sialylated dendrimers, or by sialylated MUC1 induces immunosuppressive macrophages with a TAM-like phenotype36,39. In addition, PDAC tumor sialylation stimulates TAM differentiation via
Siglec-7 and Siglec-936. Even though similar effects on TAM differentiation can be observed between these studies, there is a key difference in the Siglec ligands between tumor cells and
CAFs (Fig. 3b). Importantly, loss of both tumor and CAF sialylation was required to reduce TAM differentiation. We recently showed that ablation of sialic acid on PDAC tumor cells in vivo
reversed the T cell excluded phenotype and synergized with immunotherapy66. Loss of sialic acid on tumor cells alone did not impact tumor growth in this mouse model, and mice were not cured
with immunotherapy66. Therefore, removing both tumor and CAF sialylation may be necessary to overcome the barriers for immunotherapy efficacy in PDAC. We observed contrasting effects of
sialylation on TAM differentiation among the three CAF cell lines. While SI treatment of PS-1 CAFs reduced TAM differentiation, it enhanced TAM differentiation in context of T1 CAFs. Their
glycosylation profiles exhibited important differences, particularly in PS-1 cells compared to M1 and T1 CAFs. Siglec-9 binding to glycans also differed, with N-glycosylated proteins being
the primary ligands in PS-1 cells, and ligands in M1 and T1 CAF cells being modestly reduced by both O- and N-glycan inhibitors (Fig. 3b). These findings suggest that the specific context of
glycoproteins may influence the biological outcome of Siglec interaction. Supporting this notion, we observed differential expression of several highly glycosylated proteins in the cell
lines (PDPN, CD106, CD146, Supplementary Fig. 2f). Further research is needed to investigate specific Siglec glycoprotein ligands on CAFs and to understand the contexts in which CAF
sialylation promotes or inhibits immunosuppression. A limitation of the current study is the use of immortalized CAF cell lines in vitro, to study the biological implications of CAF
sialylation on myeloid cells. While some CAF characteristics are maintained in culture, some traits are likely not. Although the PS-1 are activated, lost their stellate cell quiescent
phenotype and express several CAF markers and characteristics, they are originally isolated from heatlhy pancreas and may not fully recapitulate CAFs in vivo. Furthermore, cell lines will
not represent the full heterogeneity and plasticity observed in vivo. All three CAF cell lines displayed markers of myCAFs, including α-SMA, but also secreted IL-6, associated with an iCAF
phenotype53. These data underscore the challenge of distinguishing between iCAF and myCAF phenotypes in human fibroblasts in vitro, implicating either the absence of definitive markers or
the inability of in vitro fibroblasts to recapitulate the iCAF/myCAF distinction observed in transcriptomic data. To capture the full complexity and heterogeneity of CAF subsets and their
interaction with immune cells, future studies should evaluate the role of CAF-derived sialic acids on tumor progression and immune modulation in vivo. Although our study focused on the
effect of CAF sialylation on macrophage during their differentiation and polarization, sialic acids can also modify other immune cells through Siglec engagement. A recent study, in
particular, demonstrated that stromal sialylation can impede T cell proliferation67. Furthermore, the engagement of Siglec-9 on CD8+ T cells has been shown to diminish TCR signaling and
effector function56,57. Sialic acids can also affect effector functions of DCs by impacting their maturation, cross-presentation and T-cell priming ability68,69,70,71. Compared to other cell
types in the PDAC TME, the sialyltransferase enzyme ST3GAL4 was highly expressed in CAFs. ST3GAL4 is involved in the synthesis of Siglec-9 ligands on PDAC tumor cells and is associated with
decreased survival36,65. In addition, ST3GAL4 is related to an increased invasive phenotype in tumor cells as it generates the glycan sialyl-Lewis X, which facilitates cell adhesion72,73.
Interestingly, recent studies also observed increased ST3GAL4 expression in the stroma in other cancer types, including colorectal, lung, cervical and esophageal cancer67,74. Stromal
sialylation therefore likely plays a role in other cancer types as well. Over the past decade, multiple strategies have been developed to target the sialic acid-Siglec axis, including
tumor-targeted degradation of sialic acids, sialyltransferase inhibitors, and Siglec blocking antibodies38,45,46,75. Blocking Siglec-7, -9, -10, -15 simultaneously on monocytes is
challenging, and commercially available blocking antibodies do not sufficiently block these receptors. Given the potential involvement of multiple Siglec receptors in both tumor and CAF
immune crosstalk, interference with the ligand, sialic acid, would be the preferred approach, which we accomplished in CAFs by treatment with a sialyltransferase inhibitor or genetic KO of
sialylation enzymes. Interestingly, a phase 1/2 clinical trial, which includes PDAC patients, is currently investigating the safety and potential of sialidase treatment (NCT05259696)76. An
intriguing avenue for further investigation is the potential impact of this treatment on CAF sialylation. In conclusion, CAF sialic acids form ligands for multiple Siglec receptors, such as
Siglec-7, -9, -10 and -15 on immune cells and can modulate TAM differentiation. Therapeutic interventions targeting the sialic acid-Siglec axis are currently focussed on tumor sialylation.
We propose that CAF sialylation should also be considered in the development of sialic acid-based therapies. Future research is necessary to reveal the role of CAF sialylation in modulation
of other immune cells, such as NK cells, T cells and dendritic cells, and how CAF sialylation relates to tumor progression and immune evasion in vivo. MATERIALS & METHODS PATIENT
MATERIAL Formalin-fixed paraffin embedded (FFPE) tissue from PDAC patients was obtained from the Pathology Department of the Amsterdam UMC, location VUMC, with the approval from the Medical
Ethical committee from the Amsterdam UMC, location VUMC. Written consent was obtained from all the patients. All ethical regulations relevant to human research participants were followed.
Stage of lesions can be found in supplementary Table 1. CELL LINES For this project, several human fibroblast cell lines and PDAC tumor cell lines were used (Supplementary Fig. 2a). The
fibroblasts M1 CAFs and T1 CAFs were generated in the laboratory of Dr. Prof. Tuveson and are derived from metastatic lung and primary human pancreatic ductal adenocarcinoma14. The human
pancreatic stellate cell line PS-1 was a kind gift from Dr. Prof. H. Kocher52. PDAC tumor cell line MIA PaCa-2 was acquired via ATCC, BxPC3 was a kind gift from Dr. A. Frampton (Imperial
College, London, UK). All cell lines were cultured in RPMI 1640 (Gibco) containing 10% Fetal Calf Serum (Biowest), 2 mM L-Glutamine (Gibco) and 1000 U per mL Penicillin-Streptomycin (Gibco),
referred to as complete RPMI. All cell lines were routinely tested for mycoplasma using PCR. COLLAGEN GEL CONTRACTION ASSAY Rat-tail collagen type I was reconstituted in 0.1% acetic acid (4
mg per ml). Cells were diluted in complete RPMI and seeded in the collagen solution at 2 × 105 cells per ml and 1 ml hydrogel was poured per well of 12-well plates. Hydrogels were
polymerized for 2 h at 37 oC. Hydrogels were detached from the well surface to allow contraction and culture medium was added. Three times per week the culture medium was refreshed and
pictures of gels were taken using a Sony WX500 camera during a total culture period of two weeks. Hydrogel surface area was measured using ImageJ software. MONOCYTE ISOLATION AND STIMULATION
Healthy donor buffy coats were collected by Sanquin, the Netherlands, from which peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation with
Ficoll-Paque PLUS (GE Healthcare). Using CD14 Microbeads (Miltenyi) human CD14+ monocytes were isolated. Monocytes were cultured in RPMI 1640 (Gibco) containing 10% Fetal Calf Serum
(Biowest), 2 mM L-Glutamine (Gibco) and 1000 U per mL Penicillin-Streptomycin (Gibco). Monocytes were differentiated to monocyte-derived macrophages (moMACs) by the addition of 50 ng/mL
M-CSF. To polarize moMACs, 10 ng/mL LPS, 20 ng/mL IFNγ, 20 ng/mL IL-4 and/or 20 ng/mL IL-6 was added on day 3. Monocyte-derived dendritic cells (moDCs) were generated with 20 ng/mL GM-CSF
and 20 ng/mL IL-4. Differentiation of monocytes in the presence of fibroblast-conditioned medium (CM) was done with 25 ng/mL M-CSF. Conditioned media was generated over a 24-hour period from
cells treated with the Sialyltransferase Inhibitor 3Fax-Peracetyl Neu5Ac (SI) (Calbiochem, Sigma Aldrich). The cells underwent a 3-day incubation with 200 µM SI or vehicle (DMSO), followed
by three washes with PBS before CM collection. CO-CULTURES To study the effect of pancreatic fibroblast and tumor cell lines on monocyte differentiation, cell lines were plated in 24-well
plates (50.000 cells per well) and co-cultured with monocytes (200.000 cells per well) for 4 days. After 4 days, supernatant was collected to analyze the cytokines with ELISA. Cells were
harvested and analyzed by flow cytometry. Dimensional reduction analysis and visualization of co-cultures was done using OMIQ software from Dotmatics. To evaluate the effect of macrophages
on CD8+ T cell proliferation, CD8+ T cells were isolated from PBL’s using negative isolation kit (Miltenyi, Cat#130-096-495), and frozen until use. After thawing, CD8+ T cells were labeled
with CellTrace™ Violet (Invitrogen, Cat#C34557), for 7 min at 37 °C while shaking. Autologous CD8+ T cells were added to the macrophages in 4:1 ratio and cultured for 3 days in the presence
of Ultra-LEAF™ Purified anti-human CD3 (clone OKT3, Biolegend). In experiments using SI treatment, fibroblast cell lines were incubated with 200 µM SI or vehicle (DMSO) for 4 days, after
which the cells were washed, re-plated and co-cultured with monocytes. CRISPR-CAS9 GENE KNOCKOUT Generation of a Siglec-9 knockout (KO) in monocytes was done using nucleofection of freshly
isolated CD14+ monocytes, as reported previously77. First, 6 µL of 160 µM crRNA (Dharmacon, CM-012842-01-0010) and 6 µL of 160 µM tracrRNA (Dharmacon, U-002005-50) were mixed and incubated
at 37 °C to form 12 µL of gRNA:tracrRNA duplex specific for Siglec-9. Subsequently, 12 µL of 40 µM Cas9-NLS protein (Horizon Discoveries, CAS12206) was added, after which the sample was
incubated for 15 minutes at 37 °C to form CRISPR-Cas9 ribonucleoproteins (crRNP). The crRNPs were stored at –70 °C until use. Freshly isolated CD14+ monocytes were nucleofected with crRNP
using the P3 Primary Cell 4D-NucleofectorTM X Kit L (Lonza, V4XP-3034) according to the manufacturer’s protocol. The mock transfection was performed by exposure to the nucleofection process
without crRNPs present. Per condition, 12.5 µL crRNP and 5 × 106 CD14+ monocytes in 100 µL P3 Primary Cell NucleofectorTM solution were added to the NucleovetteTM vessel. After nucleofection
(pulse code DK-100), cells were resuspended in pre-warmed complete RPMI and incubated for 30 minutes at 37 °C. After incubation, cells were harvested from the NucleovetteTM vessel, counted,
and used for co-culture experiments. The generation of CMAS KO in the PS-1 cell line was performed as reported previously36. Briefly, sgRNA strands for human _CMAS_ gene (top strand
CACCGATATCTGAACAGTGTAT; bottom strand AAACATACACTGTTCAGATATC.) were phosphorylated and annealed to clone it in the pSpCas9(BB)-2A-Puro plasmid, a gift from Feng Zhang (Addgene#62988). PS-1
cells were transfected using Lipofectamine™ LTX with PLUS™ Reagent (Invitrogen), selected with puromycin and sorted based on negative staining of α2-3-Lectenz (Lectenz Bio) using BD
FACSAria™ Fusion FACS sorter. The control PS-1 mock was transfected with pSpCas9(BB)-2A-Puro plasmid without guide RNA. FLOW CYTOMETRY All stainings with plant lectins (Vector Laboratories),
Lectenz (Lectenz Bio) or Siglec-Fc chimeras (R&D systems) were performed in HBSS containing magnesium and calcium (Gibco) supplemented with 0.5% fatty-acid free BSA (Sigma). Siglec-Fc
chimeras were pre-incubated at 1 μg/mL with anti-human IgG Fc (Biolegend, clone: HP6017) for 15 minutes at room temperature, after which they were added to the cells. Similarly, 1 μg/mL
lectins and Lectenz reagents were pre-incubated with streptavidin-APC before addition to the cells. Macrophages in Fig. 4 were analyzed with a 14-color antibody panel (supplementary Table 2)
on the Cytek Aurora and analyzed with FlowJo v10 and OMIQ. Fibroblast markers PD-L1, HLA-DR and α-SMA were measured on Cytek Aurora, other experiments were analyzed using the Fortessa™ X‐20
and analyzed with FlowJo v10 (list of antibodies in supplementary Table 2). In indicated experiments, cells were treated at 37 °C for 30 minutes with neuraminidase from _Arthrobacter
ureafaciens_ (Roche Diagnostics, diluted 1:100). To assess the presence of sialylated structures in _N_-glycans, _O_-glycans, or glycolipids, cells were treated for 3 days with 10 μg/mL
Kifunensine, 0.8 mM Benzyl-GalNAc, or 5 μM PPMP, respectively. TISSUE STAININGS Immunohistochemical (IHC) staining of tissues was performed on FFPE sections (5 μm). Tissue slides were
stained with Hematoxylin & Eosin (HE) to verify tumor histology by a pathologist. After deparaffinization and antigen retrieval (DAKO, Tris-EDTA pH9 buffer), endogenous peroxidase
activity was blocked by peroxidase-blocking solution (DAKO) and tissues were blocked with Carbo-Free Blocking Buffer (CFBB) (Vector Labs). Slides were stained with 2 μg/mL pan-Lectenz
(Lectenz Bio), which was pre-complexed with 1 μg/mL streptavidin-HRP for 30 min before adding to the slides. For ST6GALNAC6 stainings, slides were incubated with ST6GALNAC6 antibody (Sigma
Aldrich, HPA018890, 1:10) for two hours at 37 °C. After incubation with the primary antibody (Supplementary Table 2), slides were incubated with BrightVision Poly-HRP-Anti Mouse/Rabbit IgG
Biotin-free (Immunologic, VWRKDPVO55HRP) for 30 minutes. IHC reactions were detected using DAB (3,3’-diaminobenzidine) and slides were counterstained with hematoxylin. After rehydration,
slides were mounted with Entallan and scanned using Vectra Polaris (Akoya Biosciences). If applicable, neuraminidase from _Arthrobacter ureafaciens_ (1:10 Roche Diagnostics) was applied
after endogenous peroxidase blockade for one hour at 37 °C after which the protocol was continued. Multiplex IHC stainings were performed using an Opal multiplex IHC kit (Akoya Biosciences,
NEL821001KT), with detailed information about antibodies listed in supplementary Table 2. Staining was performed according to manufacturer’s procedures. After staining, slides were
counterstained with DAPI and mounted with Fluormount-G (ITK, 0100-01). Slides were scanned using the Vectra Polaris Automated Quantitative Pathology Imaging System (Akoya Biosciences),
software version 1.0.13. First, multiplex-stained slides were scanned using a ×20 magnification with multispectral slide scan bands in order to annotate regions for tumor content. These
regions were imaged in a second round using a 40x magnification with multispectral field bands. Obtained multispectral images were acquired and unmixed using inForm® Tissue Analysis Software
version 2.6.0 with a spectral library build using single stained samples. Quantification of pan-Lectenz intensity was done in QuPath version 0.2.278. After tissue segmentation between tumor
and stroma was applied, annotations were exported to ImageJ version 1.53a and quantified for median intensity of pan-Lectenz staining. Image quantification of ST3GAL4 and Siglec expression
was done using NIS-Elements (version 5.42.04). ST3GAL4 expression on stromal cells was quantified as ST3GAL4+ cells in panCK- areas, lacking CD45. MICROSCOPY OF CELL LINES For the
immunofluorescent staining of fibroblasts, cell lines were grown on 8 well ibidi µ-slide (ibidi) for two days at 37 °C and 5% CO2. After that time, medium was removed and cells were fixed
with 4% paraformaldehyde (PFA) for 15 minutes at room temperature (RT), permeabilized with 0.1% Triton X-100 in PBS for 10 min at RT and blocked using 10% Normal mouse serum (NMS) in PBS
containing 0.1% Tween 20. Cells were incubated overnight at 4 °C with anti-Vimentin Alexa Fluor 594 (Biolegend, dilution 1:200) in PBS containing 2% NMS and 0.1% Tween 20. Next, cells were
washed four times with 0.1% Tween 20 in PBS and counterstained with Alexa Fluor 647 Phalloidin (Invitrogen, dilution 1:400) and DAPI (Invitrogen). Images were acquired using SP8 confocal
microscope (Leica). GLYCAN PROFILING Analysis of glycosphingolipid glycans, _N_-glycans and _O_-glycans of fibroblast cell lines was performed by PGC nano-LC-ESI-MS/MS in negative mode, as
described previously66. CYTOKINE ANALYSIS Cytokines in the supernatant of cell lines were measured using LEGENDplex Human Essential Immune Response Panel kit (Biolegend) and LEGENDplex Human
HSC Myeloid Panel kit (Biolegend) according to manufacturer’s instructions. Briefly, cell lines were plated and grown till 70% confluence, after which the culture media was refreshed. After
24 hours, cytokines were measured with the LEGENDPlex kits. IL-10 levels from co-cultures were measured using ELISA (supplementary Table 2). SIRNA KNOCKDOWN The fibroblast cell lines were
plated in a 6-wells plate and grown over night. Next, siRNA mediated knockdown of ST3GAL4 was achieved with DharmaFECT2 Transfection Reagent (Dharmacon) according to the manufacturer’s
instructions. A non-targeting siRNA (Dharmacon) was taken along as a control. TRANSCRIPTOMIC ANALYSIS The single-cell RNA sequencing (scRNA-Seq) data previously published by Peng et al. was
downloaded from the Genome Sequence Archive project PRJCA001063 as pre-processed row data and imported into the package _Seurat_ (v4) for downstream analysis as described previously36,50.
The dataset included tissues from 24 PDAC patients and 11 normal pancreas, for a total of 56601 cells. The function _FindMarkers_ was used for the generation of gene sets corresponding to
cancer cells and fibroblasts, selecting the significant genes that present a fold change equal or higher than 2 (Supplementary Table 3). For analysis of fibroblast subsets, clusters
containing cells expressing the gene LUM were selected, renormalized using the function SCTransform (regressing out the percentage of mitochondrial genes) and batch effect was corrected
using the package Harmony with default settings. Only samples containing 100 or more cells were used in this analysis, resulting in 6887 cells. We proceed to cluster cells using the
functions FindNeighbors and FindClusters with the top 20 hermony dimensions and a resolution of 0.5. To clean the data, we removed cluster of cells based on the expression of markers
specific for other cell types (PTPRC for immune cells, KRT19 for epithelial cells, or PRSS1 for acinar cells) or low-quality cells (defined by the high content of ribosomal and/or
mitochondrial genes). This resulted in a total of 5344 fibroblasts, derived from 11 PDAC patients and 4 normal samples. For identification of CAF subtypes, cells were re-clustered as
described before but using a resolution of 0.25. The function AddModuleScore was employed to generate gene scores for different glycosylation pathways using genesets previously described74
(supplementary Table 3). The scripts used in this manuscript for the analysis of scRNA-Seq can be found in https://github.com/MolecularCellBiologyImmunology/Sialylation_CAF. Any additional
information is available from the authors upon request. The data from the PAAD project of the TCGA was obtained from the Broad Institute GDAC Firehose (https://gdac.broadinstitut.org)79. The
package _GSVA_ was used for the generation scores evaluating the expression of the different gene sets (corresponding sialylation pathways, fibroblasts and cancer cells, supplementary Table
3) and their association was evaluated using Spearman correlation. Transcriptomic data from microdissected samples was downloaded from the NCBI Gene expression Omnibus (GEO) using the
accession number GSE9332649. The package _limma_ was used for the analysis of the differential gene expression, with FDR correction for multiple comparisons. STATISTICS AND REPRODUCIBILITY
Statistical analysis was performed in Graphpad Prism 9.3.1. Comparison between two groups was done using the two-tailed paired Student _t_ test, unless stated otherwise in the Figure legend.
A _p_-value of <0.05 was considered statistically significant (*_P_ ≤ 0.05, **_P_ ≤ 0.01, ***_P_ ≤ 0.001). All bars in graphs represent the mean and error bars represent the standard
deviation (SD). All data points displayed in graphs in this paper represent biological replicates, indicated in Figure legends by _n_. DATA AVAILABILITY The single-cell RNA sequencing
(scRNA-Seq) data from Peng et al. was downloaded from the Genome Sequence Archive project PRJCA00106350. The scripts used in this manuscript for the analysis of scRNA-Seq can be found in
https://github.com/MolecularCellBiologyImmunology/Sialylation_CAF. The data from the PAAD project of the TCGA was obtained from the Broad Institute GDAC Firehose
(https://gdac.broadinstitut.org)79. Transcriptomic data from microdissected samples was downloaded from the NCBI Gene expression Omnibus (GEO) using the accession number GSE9332649. The
remaining data are available within the Article, Supplementary files or available from the authors upon request. The source data are available in Supplementary Data 1. REFERENCES * Siegel,
R. L., Miller, K. D., Fuchs, H. E. & Jemal, A. Cancer statistics, 2022. _CA Cancer J Clin_ 72, 7–33 (2022). Article PubMed Google Scholar * Hegde, P. S. & Chen, D. S. Top 10
Challenges in Cancer Immunotherapy. _Immunity_ 52, 17–35 (2020). Article CAS PubMed Google Scholar * Valkenburg, K. C., de Groot, A. E. & Pienta, K. J. Targeting the tumour stroma to
improve cancer therapy. _Nat Rev Clin Oncol_ 15, 366–381 (2018). Article PubMed PubMed Central Google Scholar * Sahai, E. et al. A framework for advancing our understanding of
cancer-associated fibroblasts. _Nat Rev Cancer_ 20, 174–186 (2020). Article CAS PubMed PubMed Central Google Scholar * Liu, L. et al. Stromal myofibroblasts are associated with poor
prognosis in solid cancers: A meta-analysis of published studies. _PLoS One_ 11, e0159947 (2016). Article PubMed PubMed Central Google Scholar * Ozdemir, B. C. et al. Depletion of
carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. _Cancer Cell_ 28, 831–833 (2015). Article CAS PubMed Google
Scholar * Rhim, A. D. et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. _Cancer Cell_ 25, 735–747 (2014). Article CAS PubMed PubMed Central
Google Scholar * Hutton, C. et al. Single-cell analysis defines a pancreatic fibroblast lineage that supports anti-tumor immunity. _Cancer Cell_ 39, 1227–1244 e1220 (2021). Article CAS
PubMed PubMed Central Google Scholar * Chen, Y. et al. Type I collagen deletion in αSMA+ myofibroblasts augments immune suppression and accelerates progression of pancreatic cancer.
_Cancer Cell_ 39, 548–565.e546 (2021). Article CAS PubMed PubMed Central Google Scholar * Lavie, D., Ben-Shmuel, A., Erez, N. & Scherz-Shouval, R. Cancer-associated fibroblasts in
the single-cell era. _Nat Cancer_ 3, 793–807 (2022). Article PubMed PubMed Central Google Scholar * Dominguez, C. X. et al. Single-cell RNA sequencing reveals stromal evolution into
LRRC15(+) myofibroblasts as a determinant of patient response to cancer immunotherapy. _Cancer Discov_ 10, 232–253 (2020). Article CAS PubMed Google Scholar * Huang, H. et al.
Mesothelial cell-derived antigen-presenting cancer-associated fibroblasts induce expansion of regulatory T cells in pancreatic cancer. _Cancer Cell_ 40, 656–673 e657 (2022). Article CAS
PubMed PubMed Central Google Scholar * Elyada, E. et al. Cross-species single-cell analysis of pancreatic ductal adenocarcinoma reveals antigen-presenting cancer-associated fibroblasts.
_Cancer Discov_ 9, 1102–1123 (2019). Article CAS PubMed PubMed Central Google Scholar * Ohlund, D. et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in
pancreatic cancer. _J Exp Med_ 214, 579–596 (2017). Article CAS PubMed PubMed Central Google Scholar * Noy, R. & Pollard, J. W. Tumor-associated macrophages: From mechanisms to
therapy. _Immunity_ 41, 49–61 (2014). Article CAS PubMed PubMed Central Google Scholar * Vayrynen, S. A. et al. Composition, spatial characteristics, and prognostic significance of
myeloid cell infiltration in pancreatic cancer. _Clin. Cancer Res._ 27, 1069–1081 (2021). Article CAS PubMed Google Scholar * Ino, Y. et al. Immune cell infiltration as an indicator of
the immune microenvironment of pancreatic cancer. _Br J. Cancer_ 108, 914–923 (2013). Article CAS PubMed PubMed Central Google Scholar * Knudsen, E. S. et al. Stratification of
pancreatic ductal adenocarcinoma: Combinatorial genetic, stromal, and immunologic markers. _Clin. Cancer Res_. 23, 4429–4440 (2017). Article CAS PubMed PubMed Central Google Scholar *
Tekin, C., Aberson, H. L., Bijlsma, M. F. & Spek, C. A. Early macrophage infiltrates impair pancreatic cancer cell growth by TNF-alpha secretion. _BMC Cancer_ 20, 1183 (2020). Article
CAS PubMed PubMed Central Google Scholar * Kiss, M., Van Gassen, S., Movahedi, K., Saeys, Y. & Laoui, D. Myeloid cell heterogeneity in cancer: Not a single cell alike. _Cell Immunol_
330, 188–201 (2018). Article CAS PubMed Google Scholar * Helm, O. et al. Tumor-associated macrophages exhibit pro- and anti-inflammatory properties by which they impact on pancreatic
tumorigenesis. _Int. J. Cancer_ 135, 843–861 (2014). Article CAS PubMed Google Scholar * Candido, J. B. et al. CSF1R(+) macrophages sustain pancreatic tumor growth through T cell
suppression and maintenance of key gene programs that define the squamous subtype. _Cell Rep._ 23, 1448–1460 (2018). Article CAS PubMed PubMed Central Google Scholar * Doedens, A. L. et
al. Macrophage expression of hypoxia-inducible factor-1 alpha suppresses T-cell function and promotes tumor progression. _Cancer Res._ 70, 7465–7475 (2010). Article CAS PubMed PubMed
Central Google Scholar * Chen, W. et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. _J. Exp.
Med._ 198, 1875–1886 (2003). Article CAS PubMed PubMed Central Google Scholar * Thomas, D. A. & Massague, J. TGF-beta directly targets cytotoxic T cell functions during tumor
evasion of immune surveillance. _Cancer Cell_ 8, 369–380 (2005). Article CAS PubMed Google Scholar * Mao, X. et al. Crosstalk between cancer-associated fibroblasts and immune cells in
the tumor microenvironment: new findings and future perspectives. _Mol Cancer_ 20, 131 (2021). Article CAS PubMed PubMed Central Google Scholar * Li, C. et al. Pancreatic stellate cells
promote tumor progression by promoting an immunosuppressive microenvironment in murine models of pancreatic cancer. _Pancreas_ 49, 120–127 (2020). Article CAS PubMed Google Scholar *
Feig, C. et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. _Proc. Natl. Acad. Sci. USA_ 110,
20212–20217 (2013). Article CAS PubMed PubMed Central Google Scholar * Pinho, S. S. & Reis, C. A. Glycosylation in cancer: mechanisms and clinical implications. _Nat. Rev. Cancer_
15, 540–555 (2015). Article CAS PubMed Google Scholar * Rodriguez, E. et al. Analysis of the glyco-code in pancreatic ductal adenocarcinoma identifies glycan-mediated immune regulatory
circuits. _Commun Biol_. 5, 41 (2022). Article CAS PubMed PubMed Central Google Scholar * Bellis, S. L., Reis, C. A., Varki, A., Kannagi, R. & Stanley, P. In _Essentials of
Glycobiology_ (eds th et al.) 631-644 (2022). * Lubbers, J., Rodriguez, E. & van Kooyk, Y. Modulation of Immune Tolerance via Siglec-Sialic Acid Interactions. _Front Immunol_ 9, 2807
(2018). Article PubMed PubMed Central Google Scholar * Lewis, A. L., Chen, X., Schnaar, R. L. & Varki, A. In _Essentials of Glycobiology_ (eds th et al.) 185-204 (2022). * RodrIguez,
E., Schetters, S. T. T. & van Kooyk, Y. The tumour glyco-code as a novel immune checkpoint for immunotherapy. _Nat Rev Immunol_ 18, 204–211 (2018). Article CAS PubMed Google Scholar
* Angata, T., von Gunten, S., Schnaar, R. L. & Varki, A. In _Essentials of Glycobiology_ (eds th et al.) 475-490 (2022). * Rodriguez, E. et al. Sialic acids in pancreatic cancer cells
drive tumour-associated macrophage differentiation via the Siglec receptors Siglec-7 and Siglec-9. _Nat Commun_ 12, 1270 (2021). Article CAS PubMed PubMed Central Google Scholar *
Barkal, A. A. et al. CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. _Nature_ 572, 392–396 (2019). Article CAS PubMed PubMed Central Google Scholar *
Wang, J. et al. Siglec-15 as an immune suppressor and potential target for normalization cancer immunotherapy. _Nat. Med._ 25, 656–666 (2019). Article CAS PubMed PubMed Central Google
Scholar * Beatson, R. et al. The mucin MUC1 modulates the tumor immunological microenvironment through engagement of the lectin Siglec-9. _Nat Immunol_ 17, 1273–1281 (2016). Article CAS
PubMed PubMed Central Google Scholar * Ibarlucea-Benitez, I., Weitzenfeld, P., Smith, P. & Ravetch, J. V. Siglecs-7/9 function as inhibitory immune checkpoints in vivo and can be
targeted to enhance therapeutic antitumor immunity. _Proc. Natl. Acad. Sci. USA_ 118 (2021). ARTN e2107424118 https://doi.org/10.1073/pnas.2107424118. * Boelaars, K. & van Kooyk, Y.
Targeting myeloid cells for cancer immunotherapy: Siglec-7/9/10/15 and their ligands. _Trends in Cancer_ https://doi.org/10.1016/j.trecan.2023.11.009. * van Houtum, E. J. H., Bull, C.,
Cornelissen, L. A. M. & Adema, G. J. Siglec signaling in the tumor microenvironment. _Front Immunol_ 12, 790317 (2021). Article PubMed PubMed Central Google Scholar * Perdicchio, M.
et al. Tumor sialylation impedes T cell mediated anti-tumor responses while promoting tumor associated-regulatory T cells. _Oncotarget_ 7, 8771–8782 (2016). Article PubMed PubMed Central
Google Scholar * Friedman, D. J. et al. ST8Sia6 promotes tumor growth in mice by inhibiting immune responses. _Cancer Immunol Res._ 9, 952–966 (2021). Article CAS PubMed PubMed Central
Google Scholar * Bull, C. et al. Sialic acid blockade suppresses tumor growth by enhancing T-cell-mediated tumor immunity. _Cancer Res._ 78, 3574–3588 (2018). Article CAS PubMed Google
Scholar * Gray, M. A. et al. Targeted glycan degradation potentiates the anticancer immune response in vivo. _Nat. Chem. Biol_. 16, 1376–1384 (2020). Article CAS PubMed PubMed Central
Google Scholar * Stanczak, M. A. et al. Targeting cancer glycosylation repolarizes tumor-associated macrophages allowing effective immune checkpoint blockade. _Sci. Transl. Med._ 14,
eabj1270 (2022). Article CAS PubMed PubMed Central Google Scholar * Xiao, H., Woods, E. C., Vukojicic, P. & Bertozzi, C. R. Precision glycocalyx editing as a strategy for cancer
immunotherapy. _Proc. Natl. Acad. Sci. USA_ 113, 10304–10309 (2016). Article CAS PubMed PubMed Central Google Scholar * Maurer, C. et al. Experimental microdissection enables functional
harmonisation of pancreatic cancer subtypes. _Gut_ 68, 1034–1043 (2019). Article CAS PubMed Google Scholar * Peng, J. et al. Single-cell RNA-seq highlights intra-tumoral heterogeneity
and malignant progression in pancreatic ductal adenocarcinoma. _Cell Res._ 29, 725–738 (2019). Article CAS PubMed PubMed Central Google Scholar * Perez-Garay, M. et al.
alpha2,3-Sialyltransferase ST3Gal IV promotes migration and metastasis in pancreatic adenocarcinoma cells and tends to be highly expressed in pancreatic adenocarcinoma tissues. _Int. J.
Biochem. Cell Biol._ 45, 1748–1757 (2013). Article CAS PubMed Google Scholar * Froeling, F. E. et al. Organotypic culture model of pancreatic cancer demonstrates that stromal cells
modulate E-cadherin, beta-catenin, and Ezrin expression in tumor cells. _Am. J. Pathol_ 175, 636–648 (2009). Article CAS PubMed PubMed Central Google Scholar * Biffi, G. et al.
IL1-induced JAK/STAT signaling is antagonized by TGFbeta to shape CAF heterogeneity in pancreatic ductal adenocarcinoma. _Cancer Discov._ 9, 282–301 (2019). Article PubMed Google Scholar
* Jandus, C. et al. Interactions between Siglec-7/9 receptors and ligands influence NK cell-dependent tumor immunosurveillance. _J. Clin. Invest_ 124, 1810–1820 (2014). Article CAS PubMed
PubMed Central Google Scholar * Hudak, J. E., Canham, S. M. & Bertozzi, C. R. Glycocalyx engineering reveals a Siglec-based mechanism for NK cell immunoevasion. _Nat. Chem. Biol_.
10, 69–75 (2014). Article CAS PubMed Google Scholar * Haas, Q. et al. Siglec-9 regulates an effector memory CD8(+) T-cell subset that congregates in the melanoma tumor microenvironment.
_Cancer Immunol Res._ 7, 707–718 (2019). Article CAS PubMed Google Scholar * Stanczak, M. A. et al. Self-associated molecular patterns mediate cancer immune evasion by engaging Siglecs
on T cells. _J. Clin. Invest_ 128, 4912–4923 (2018). Article PubMed PubMed Central Google Scholar * Clark, C. E. et al. Dynamics of the immune reaction to pancreatic cancer from
inception to invasion. _Cancer Res_. 67, 9518–9527 (2007). Article CAS PubMed Google Scholar * Stanley, P., Moremen, K. W., Lewis, N. E., Taniguchi, N. & Aebi, M. In _Essentials of
Glycobiology_ (eds A. Varki, A. et al.) 103-116 (2022). * Brockhausen, I., Wandall, H. H., Hagen, K. G. T. & Stanley, P. In _Essentials of Glycobiology_ (eds Varki, A. et al.) 117–128
(2022). * Schnaar, R. L., Sandhoff, R., Tiemeyer, M. & Kinoshita, T. In _Essentials of Glycobiology_ (eds Varki et al.) 129-140 (2022). * Zhu, Y. et al. Tissue-resident macrophages in
pancreatic ductal adenocarcinoma originate from embryonic hematopoiesis and promote tumor progression. _Immunity_ 47, 597 (2017). Article CAS PubMed Google Scholar * Ma, R. Y., Black, A.
& Qian, B. Z. Macrophage diversity in cancer revisited in the era of single-cell omics. _Trends Immunol_ 43, 546–563 (2022). Article CAS PubMed Google Scholar * Mantovani, A.,
Allavena, P., Marchesi, F. & Garlanda, C. Macrophages as tools and targets in cancer therapy. _Nat. Rev. Drug Discov._ 21, 799–820 (2022). Article CAS PubMed PubMed Central Google
Scholar * Bull, C. et al. Probing the binding specificities of human Siglecs by cell-based glycan arrays. _Proc. Natl. Acad. Sci. USA_ 118 (2021). https://doi.org/10.1073/pnas.2026102118. *
Boelaars, K. et al. Unraveling the impact of sialic acids on the immune landscape and immunotherapy efficacy in pancreatic cancer. _J. Immunother Cancer_ 11 (2023).
https://doi.org/10.1136/jitc-2023-007805. * Egan, H. et al. Targeting stromal cell sialylation reverses T cell-mediated immunosuppression in the tumor microenvironment. _Cell Rep_. 42,
112475 (2023). Article CAS PubMed Google Scholar * Ding, Y. et al. The lectin Siglec-G inhibits dendritic cell cross-presentation by impairing MHC class I-peptide complex formation. _Nat
Immunol_ 17, 1167–1175 (2016). Article CAS PubMed Google Scholar * Wang, J. et al. Siglec receptors modulate dendritic cell activation and antigen presentation to T cells in cancer.
_Front Cell Dev Biol._ 10, 828916 (2022). Article PubMed PubMed Central Google Scholar * Rughetti, A. et al. Recombinant tumor-associated MUC1 glycoprotein impairs the differentiation
and function of dendritic cells. _J. Immunol. (Baltimore, Md. : 1950)_ 174, 7764–7772 (2005). Article CAS Google Scholar * Ohta, M. et al. Immunomodulation of monocyte-derived dendritic
cells through ligation of tumor-produced mucins to Siglec-9. _Biochem. Biophys. Res. Commun._ 402, 663–669 (2010). Article CAS PubMed Google Scholar * Gomes, C. et al. Expression of
ST3GAL4 leads to SLe(x) expression and induces c-Met activation and an invasive phenotype in gastric carcinoma cells. _PLoS One_ 8, e66737 (2013). Article CAS PubMed PubMed Central
Google Scholar * Takada, A. et al. Adhesion of human cancer cells to vascular endothelium mediated by a carbohydrate antigen, sialyl Lewis A. _Biochem. Biophys. Res. Commun._ 179, 713–719
(1991). Article CAS PubMed Google Scholar * Rodriguez, E., Lindijer, D., Vliet, S. J. V., Vallejo, J. J. G. & Kooyk, Y. V. The transcriptional landscape of glycosylation-related
genes in cancer. _bioRxiv_, 2022.2009.2028.509853 (2022). https://doi.org/10.1101/2022.09.28.509853. * Stanczak, M. A. & Laubli, H. Siglec receptors as new immune checkpoints in cancer.
_Mol. Aspects Med_. 101112 (2022). https://doi.org/10.1016/j.mam.2022.101112. * Luke, J. J. et al. Abstract CT034: GLIMMER-01: initial results from a phase 1 dose escalation trial of a
first-in-class bi-sialidase (E-602) in solid tumors. _Cancer Res._ 83, CT034–CT034 (2023). Article Google Scholar * Hiatt, J. et al. Efficient generation of isogenic primary human myeloid
cells using CRISPR-Cas9 ribonucleoproteins. _Cell Rep._ 35, 109105 (2021). Article CAS PubMed PubMed Central Google Scholar * Bankhead, P. et al. QuPath: Open source software for
digital pathology image analysis. _Sci. Rep._ 7, 16878 (2017). Article PubMed PubMed Central Google Scholar * Cancer Genome Atlas Research Network. Electronic address, a. a. d. h. e.
& Cancer Genome Atlas Research, N. Integrated Genomic Characterization of Pancreatic Ductal Adenocarcinoma. _Cancer Cell_ 32, 185–203 e113 (2017). Article Google Scholar Download
references ACKNOWLEDGEMENTS We would like to acknowledge the Microscopy and Cytometry Core Facility at the Amsterdam UMC-Location VUmc for providing assistance in cytometry experiments. This
work is financially supported by KWF VU2014-7200 to K.B.; SPINOZANWO SPI-93-538 to K.B., E.R., T.E. and Y.K.; KWF 12789 to K.B., B.O.S., D.L.; LSH-TKI project DC4Balance LSM1806-SGF to AH
and China Scholarship Council no. 202006940010 to C.L. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Amsterdam UMC location Vrije Universiteit Amsterdam, Molecular Cell Biology and
Immunology, De Boelelaan, 1117, Amsterdam, Netherlands Kelly Boelaars, Ernesto Rodriguez, Zowi R. Huinen, Chang Liu, Babet O. Springer, Katarzyna Olesek, Laura Goossens-Kruijssen, Thomas van
Ee, Dimitri Lindijer, Willemijn Tak, Aram de Haas, Laetitia Wehry, Joline P. Nugteren-Boogaard, Aleksandra Mikula, Charlotte M. de Winde, Reina E. Mebius, Sandra J. van Vliet & Yvette
van Kooyk * Cancer Center Amsterdam, Cancer Biology and Immunology, Amsterdam, The Netherlands Kelly Boelaars, Ernesto Rodriguez, Zowi R. Huinen, Chang Liu, Babet O. Springer, Katarzyna
Olesek, Laura Goossens-Kruijssen, Thomas van Ee, Dimitri Lindijer, Willemijn Tak, Aram de Haas, Laetitia Wehry, Aleksandra Mikula, Charlotte M. de Winde, Reina E. Mebius, Elisa Giovannetti,
Maarten F. Bijlsma, Sandra J. van Vliet & Yvette van Kooyk * Amsterdam Institute for Infection and Immunity, Cancer Immunology, Amsterdam, The Netherlands Kelly Boelaars, Ernesto
Rodriguez, Zowi R. Huinen, Chang Liu, Babet O. Springer, Katarzyna Olesek, Laura Goossens-Kruijssen, Thomas van Ee, Dimitri Lindijer, Willemijn Tak, Aram de Haas, Laetitia Wehry, Joline P.
Nugteren-Boogaard, Aleksandra Mikula, Charlotte M. de Winde, Reina E. Mebius, Sandra J. van Vliet & Yvette van Kooyk * Amsterdam UMC location Vrije Universiteit Amsterdam, Pulmonary
Medicine, De Boelelaan, 1117, Amsterdam, the Netherlands Chang Liu * Leiden University Medical Center, Center for Proteomics and Metabolomics, Albinusdreef 2, 2333 ZA, Leiden, the
Netherlands Di Wang & Manfred Wuhrer * Laboratory, Cold Spring Harbor, New York, USA David A. Tuveson * Amsterdam UMC location Vrije Universiteit Amsterdam, Medical Oncology, De
Boelelaan, 1117, Amsterdam, Netherlands Elisa Giovannetti * Cancer Pharmacology Lab, AIRC Start-Up Unit, Fondazione Pisana per la Scienza, Pisa, Italy Elisa Giovannetti * Amsterdam UMC,
location University of Amsterdam, Center for Experimental and Molecular Medicine, Laboratory for Experimental Oncology and Radiobiology, Meibergdreef 9, 1105AZ, Amsterdam, The Netherlands
Maarten F. Bijlsma * Oncode Institute, Amsterdam, The Netherlands Maarten F. Bijlsma Authors * Kelly Boelaars View author publications You can also search for this author inPubMed Google
Scholar * Ernesto Rodriguez View author publications You can also search for this author inPubMed Google Scholar * Zowi R. Huinen View author publications You can also search for this author
inPubMed Google Scholar * Chang Liu View author publications You can also search for this author inPubMed Google Scholar * Di Wang View author publications You can also search for this
author inPubMed Google Scholar * Babet O. Springer View author publications You can also search for this author inPubMed Google Scholar * Katarzyna Olesek View author publications You can
also search for this author inPubMed Google Scholar * Laura Goossens-Kruijssen View author publications You can also search for this author inPubMed Google Scholar * Thomas van Ee View
author publications You can also search for this author inPubMed Google Scholar * Dimitri Lindijer View author publications You can also search for this author inPubMed Google Scholar *
Willemijn Tak View author publications You can also search for this author inPubMed Google Scholar * Aram de Haas View author publications You can also search for this author inPubMed Google
Scholar * Laetitia Wehry View author publications You can also search for this author inPubMed Google Scholar * Joline P. Nugteren-Boogaard View author publications You can also search for
this author inPubMed Google Scholar * Aleksandra Mikula View author publications You can also search for this author inPubMed Google Scholar * Charlotte M. de Winde View author publications
You can also search for this author inPubMed Google Scholar * Reina E. Mebius View author publications You can also search for this author inPubMed Google Scholar * David A. Tuveson View
author publications You can also search for this author inPubMed Google Scholar * Elisa Giovannetti View author publications You can also search for this author inPubMed Google Scholar *
Maarten F. Bijlsma View author publications You can also search for this author inPubMed Google Scholar * Manfred Wuhrer View author publications You can also search for this author inPubMed
Google Scholar * Sandra J. van Vliet View author publications You can also search for this author inPubMed Google Scholar * Yvette van Kooyk View author publications You can also search for
this author inPubMed Google Scholar CONTRIBUTIONS K.B. and Y.v.K conceived the study. K.B., E.R., Z.H., C.L., D.W., B.O.S., K.O., T.v.E., A.d.H., J.P.N., D.A.T., E.G., M.F.B., M.W.,
S.J.v.V, Y.v.K designed experiments or provided essential technical support. K.B., E.R., Z.H., C.L., D.W., B.O.S., K.O., L.G., T.v.E., D.L., W.T., A.d.H., L.W., J.P.N.,A.M. and C.M.d.W.
Carried out experiments and acquired experimental data. D.W. and M.W. performed glycan profiling. K.B., E.R., Z.H., C.L., D.W., B.O.S., K.O., T.v.E. and A.M. analyzed data. E.R. Performed
transcriptomic analysis. K.B. and Y.v.K drafted the manuscript. K.B., E.R., D.W., B.O.S., K.O., L.G., T.v.E., D.L., W.T., A.d.H., L.W., J.P.N., A.M., C.M.d.W., R.E.M., D.A.T., E.G., M.F.B.,
M.W., S.J.v.V and Y.v.K provided critical intellectual content. Y.v.K Supervised the study. CORRESPONDING AUTHOR Correspondence to Yvette van Kooyk. ETHICS DECLARATIONS COMPETING INTERESTS
The authors declare no competing interests PEER REVIEW PEER REVIEW INFORMATION : _Communications Biology_ thanks Heinz Läubli, Julia M Houthuijzen and the other, anonymous, reviewer(s) for
their contribution to the peer review of this work. Primary Handling Editor: Christina Karlsson Rosenthal. A peer review file is available. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer
Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION PEER REVIEW FILE SUPPLEMENTARY INFORMATION DESCRIPTION
OF ADDITIONAL SUPPLEMENTARY FILES SUPPLEMENTARY DATA 1 RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which
permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to
the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless
indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or
exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints
and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Boelaars, K., Rodriguez, E., Huinen, Z.R. _et al._ Pancreatic cancer-associated fibroblasts modulate macrophage differentiation via
sialic acid-Siglec interactions. _Commun Biol_ 7, 430 (2024). https://doi.org/10.1038/s42003-024-06087-8 Download citation * Received: 09 February 2023 * Accepted: 21 March 2024 * Published:
09 April 2024 * DOI: https://doi.org/10.1038/s42003-024-06087-8 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative