Play all audios:
ABSTRACT Treatment of pneumococcal infections is limited by antibiotic resistance and exacerbation of disease by bacterial lysis releasing pneumolysin toxin and other inflammatory factors.
We identified a previously uncharacterized peptide in the _Klebsiella pneumoniae_ secretome, which enters _Streptococcus pneumoniae_ via its AmiA-AliA/AliB permease. Subsequent
downregulation of genes for amino acid biosynthesis and peptide uptake was associated with reduction of pneumococcal growth in defined medium and human cerebrospinal fluid, irregular cell
shape, decreased chain length and decreased genetic transformation. The bacteriostatic effect was specific to _S. pneumoniae_ and _Streptococcus pseudopneumoniae_ with no effect on
_Streptococcus mitis, Haemophilus influenzae, Staphylococcus aureus_ or _K. pneumoniae_. Peptide sequence and length were crucial to growth suppression. The peptide reduced pneumococcal
adherence to primary human airway epithelial cell cultures and colonization of rat nasopharynx, without toxicity. We identified a peptide with potential as a therapeutic for pneumococcal
diseases suppressing growth of multiple clinical isolates, including antibiotic resistant strains, while avoiding bacterial lysis and dysbiosis. SIMILAR CONTENT BEING VIEWED BY OTHERS AMIA
AND ALIA PEPTIDE LIGANDS, FOUND IN _KLEBSIELLA PNEUMONIAE_, ARE IMPORTED INTO PNEUMOCOCCI AND ALTER THE TRANSCRIPTOME Article Open access 30 May 2024 AMIA AND ALIA PEPTIDE LIGANDS ARE
SECRETED BY _KLEBSIELLA PNEUMONIAE_ AND INHIBIT GROWTH OF _STREPTOCOCCUS PNEUMONIAE_ Article Open access 23 December 2022 PNEUMOCOCCAL SURFACE PROTEIN A (PSPA) PREVENTS KILLING OF
_STREPTOCOCCUS PNEUMONIAE_ BY INDOLICIDIN Article Open access 09 October 2024 INTRODUCTION Antibiotic resistance is one of the biggest threats to society, with the current trajectory
predicted to lead to 10 million deaths due to antibiotic resistance per year by 20501. The economic impact is also significant, with the cost of antibiotic failure estimated to be €1.5
billion in Europe annually2. Amongst the priority pathogens listed by the World Health Organization is penicillin-non-susceptible _Streptococcus pneumoniae_ (pneumococcus)3,4. These
Gram-positive bacteria are responsible for diseases including meningitis, pneumonia, and septicaemia with significant morbidity and mortality, particularly in young children, the elderly,
and the immunocompromised5. The pneumococcus is the most common cause of community-acquired pneumonia globally5, with antibiotic resistance a growing concern6 and so new treatment strategies
are needed. Prior to the potential invasion, _S. pneumoniae_ must colonize the human nasopharyngeal niche alongside other members of the microbiota. Some commensal microbiota species such
as _Dolosigranulum pigrum_, _Corynebacterium_7,8,9, _Streptococcus salivarius_10, and _Prevotella melaninogenica_11 protect from colonization by _S. pneumoniae_, for example, by modulating
the innate immune response or by l-lactic acid production. _S. pneumoniae_ is also proposed to sense the environment via its transporter Ami-AliA/AliB permease, which takes up oligopeptides,
resulting in gene expression modulation12 and affecting nasopharyngeal colonization13. We previously expressed this transporter’s oligopeptide binding proteins AmiA, AliA, and AliB as
recombinant proteins and identified their peptide ligands from the nasal wash of children. Depending on the peptide, they affected phenotypes such as growth14,15. Some of the peptides had
sequences that matched ribosomal proteins of _Klebsiella pneumoniae_, another resident of the respiratory tract with the potential to cause pneumonia, and were found in the secretome of _K.
pneumoniae_16. It is unknown whether a functional Ami-AliA/AliB permease is required for the effect of such _K. pneumoniae_ peptides, whether peptide uptake is necessary, and whether other
_K. pneumoniae_ secretome peptides that hijack the permease remain to be discovered. To address this, we harvested the extracellular peptidome of actively growing _K. pneumoniae_ in a
peptide-free medium and analysed it by mass spectrometry. We identified a previously uncharacterized peptide of 11 amino acid length, which inhibited the growth of a wide range of clinical
pneumococcal isolates, including those resistant to antibiotics, affected cell shape, decreased chain length, and transformation efficiency. We tested its effects not only in a culture
medium but also in human cerebrospinal fluid (hCSF), at the air interface of primary human airway epithelial cell cultures and in vivo in the rat nasopharynx in the presence of a microbiota.
Following uptake via the Ami permease, the pneumococcal transcriptome and proteome were altered, and pneumococcal colonization decreased. We propose that this interspecies peptide has
therapeutic potential to treat pneumococcal diseases. RESULTS _KLEBSIELLA PNEUMONIAE_ RELEASES A PREVIOUSLY UNCHARACTERIZED RIBOSOMAL PEPTIDE We extracted peptides secreted by live _K.
pneumoniae_ into a chemically defined medium (CDM) by performing solid-phase extraction and identified the peptides by LC–MS/MS. Along with previously identified AmiA and AliA peptide
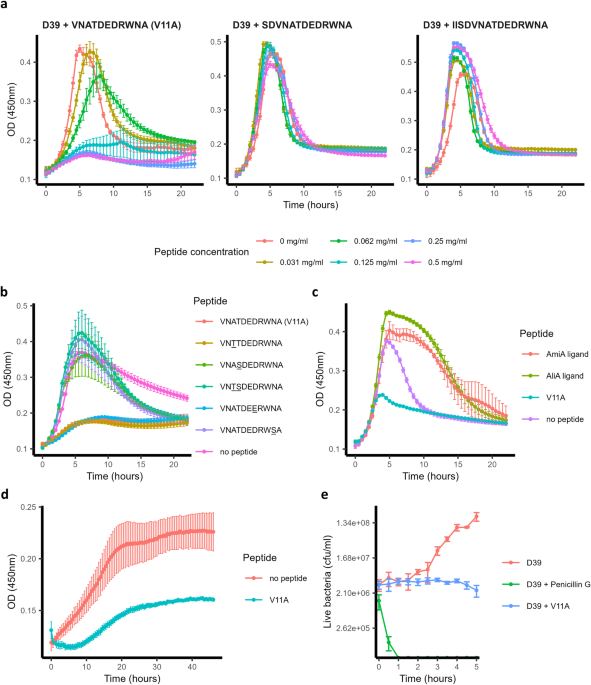
ligands16, we found another ribosomal peptide of similar amino acid length. Three peptides were present, which included the sequence VNATDEDRWNA, found in ribosomal protein S14: 11-amino
acid VNATDEDRWNA, 13-amino acid SDVNATDEDRWNA, and 15-amino acid IISDVNATDEDRWNA. The 11-amino acid peptide is abbreviated here as V11A. PEPTIDE V11A SUPPRESSES PNEUMOCOCCAL GROWTH We tested
the effect of peptide V11A and the 13- and 15-amino acid versions on the growth of _S. pneumoniae_ laboratory strain D39 in CDM. V11A, but not the longer peptides, suppressed growth in a
dose-dependent manner (Fig. 1a). By performing BLAST with the sequence of peptide V11A, we found amino acid differences at positions 3, 4, 7, and/or 10 in various species. For example,
serine, instead of threonine, at position 4 was found in _Escherichia coli, Enterobacter hormaechei_, and _Haemophilus parainfluenzae_ strains. We therefore tested whether these differences
influence the growth-inhibiting effect of peptide V11A on _S. pneumoniae_. We found that peptides with substitutions at positions 4 or 10 did not suppress pneumococcal growth, whereas
substitutions at positions 3 or 7 had no effect (Fig. 1b). Since peptide V11A inhibited the growth of _S. pneumoniae_ laboratory strain D39 (Fig. 1a) we tested its effect on diverse clinical
isolates. We found that peptide V11A inhibited the growth of a wide range of isolates, regardless of serotype or antibiotic resistance (Table 1 and Supplementary Fig. 1). In contrast to
previously identified _K. pneumoniae_ ribosomal peptides, AmiA ligand (AKTIKITQTR) and AliA ligand (FNEMQPIVDRQ), V11A suppressed the growth of a trimethoprim-sulfamethoxazole resistant
isolate 1154.75 (serotype 23F) (Fig. 1c). Growth of this isolate (1154.75) was also suppressed by V11A in human cerebrospinal fluid (hCSF) from five different donors, mimicking the
environment of _S. pneumoniae_ during pneumococcal meningitis (Fig. 1d, Supplementary Fig. 2). To test the specificity of growth inhibition by peptide V11A, we tested its effect on growth of
other common bacterial colonizers of the nasopharynx. None were inhibited by V11A, except _Streptococcus pseudopneumoniae_, a very close relative of _S. pneumoniae_ (Table 1 and
Supplementary Fig. 1). There were two pneumococcal strains that were not inhibited by V11A: South African strain 17619 and laboratory strain R6, which we have shown previously both have
mutated AmiA and whose growth is also not suppressed by the AmiA and AliA peptides16. A BLAST search of the AmiA sequence covering the relevant mutation was performed to determine its
frequency: YGYVYTADPETLDYLISRK (mutation in bold and underlined). Of the 5000 sequences examined, only 4 (0.08%) had this mutation. A time-kill assay showed that V11A had a bacteriostatic
effect on pneumococcus in contrast to the bactericidal antibiotic penicillin G, used as a control (Fig. 1e). In summary, peptide V11A suppressed growth of a range of pneumococcal isolates in
CDM and suppressed growth of a clinical pneumococcal isolate in hCSF, the effect was bacteriostatic, species-specific and the peptide length and sequence were critical. PNEUMOCOCCAL GROWTH
INHIBITION BY V11A REQUIRES UPTAKE VIA AMI-ALIA/ALIB PERMEASE As peptide V11A did not inhibit two pneumococcal strains with mutated AmiA we hypothesized that the Ami-AliA/AliB oligopeptide
permease is involved in the response to V11A. We confirmed this with a mutant in which AmiC, one of the two permease subunits, was disrupted. In the Δ_amiC_ mutant the growth suppression
effect of V11A was lost (Fig. 2a). To determine whether peptide V11A is taken up into _S. pneumoniae_ D39 and whether a functional Ami-AliA/AliB oligopeptide permease is necessary for this,
we incubated D39 and the Δ_amiC_ mutant for 5 min with FITC-labelled peptide V11A and performed epifluorescence microscopy. We found clear homogeneous FITC staining intracellularly only in
the D39 strain with a functional permease and greatly reduced uptake in the Δ_amiC_ mutant (Fig. 2b). In the orthogonal view of a z-stack, we see the FITC staining inside the bacterial cell
and not on the membrane (Supplementary Fig. 3). For versions of peptide V11A with amino acid differences that did not suppress pneumococcal growth (positions 4 or 10, Fig. 1b), we found
greatly reduced uptake of FITC-labelled peptide in the cytoplasm of either D39 or its ∆_amiC_ mutant (Supplementary Fig. 4). In conclusion, we found that pneumococcal growth suppression by
peptide V11A requires uptake of the peptide via a functional Ami-AliA/AliB oligopeptide permease. PEPTIDE V11A AFFECTS PNEUMOCOCCAL MORPHOLOGY, CHAIN LENGTH AND TRANSFORMATION The antibiotic
aztreonam and β-lactamase inhibitor clavulanic acid have been shown to increase pneumococcal chain length and also induce competence17, which can allow the pneumococcus to take up and
incorporate exogenous DNA, supporting the rapid spread of antimicrobial resistance. We, therefore, analysed the effect of peptide V11A on pneumococcal cell morphology, chain length and
transformation rate. We grew three different pneumococcal strains with or without peptide V11A until the mid-log phase and performed microscopy at 40x and 100x magnification. Untreated
bacteria within the same chain had homogenous size and shape. In the V11A peptide-treated samples, bacteria within the same chain had heterogenous shapes and sizes, with some cells enlarged
and rounder than in the untreated sample (Fig. 3a). For all three pneumococcal strains, peptide V11A also significantly decreased chain length (Fig. 3b, Supplementary Fig. 5). We tested the
effect of peptide V11A on competence-stimulating peptide (CSP-1)-mediated transformation rate in strain D39. V11A, but not the control peptide (V11A with an amino acid difference at position
4), reduced the mean transformation rate from 1.44% to 0.169%, which equals an 8.5-fold reduction (Fig. 3c). In summary, we found that V11A changes pneumococcal cell morphology, decreases
the chain length and transformation rate. PEPTIDE V11A DOWNREGULATES GENES INVOLVED IN AMINO ACID AND PROTEIN METABOLISM To decipher the mechanism by which V11A caused the phenotypic changes
described above, we identified differentially expressed genes in pneumococcal strain D39 treated with peptide V11A compared to the untreated control. Upon V11A treatment, more genes were
downregulated than upregulated, as seen in the volcano plot in Supplementary Fig. 6a. Eighty-nine genes underwent a significant change in expression equal or greater than 1.25 log2 fold
change (log2FC), of which 64 were downregulated and 25 were upregulated. With these down- and upregulated genes, we performed Gene Ontology (GO) enrichment analysis. For upregulated genes,
we did not find biological processes with statistically significant enrichment compared to the genome background. We found several biological processes enriched in the downregulated genes,
mainly amino acid biosynthesis, import and transport, but also peptide and protein transport (Supplementary Fig. 6b). With the same cutoffs, we made a gene interaction network (Fig. 4). We
see that downregulated genes cluster to the following: AmiA permease (_ami_ genes and _aliA_), branched-chain amino acid biosynthesis (_ilv_ genes), branched-chain amino acid transporter
(_liv_ genes) and riboflavin synthesis (_rib_ genes). One interesting gene cluster of upregulated genes is fatty acid biosynthesis (_fab_ genes). At the level of single genes, the most
downregulated gene was SPD_0161, a Mn2+/Fe2+ transporter and the most upregulated gene was SPD_1524 (transcriptional regulator, _gntR_). We confirmed significant downregulation of _aliA,
ilvB_, _livJ_ and significant upregulation of _gntR_ by real-time RT-PCR (Supplementary Fig. 6c). We found _codY_, a global regulator of protein metabolism, downregulated −1.2 log2FC. We did
not find any _com_ genes differentially expressed, however, we found downregulation of other genes potentially involved in competence18,19,20: _ssb_ with −0.5 log2FC, _hrcA_ with −1.1
log2FC and _grpE_ with −1.2 log2FC. We found _dnaJ_ and _dnaK_ downregulated with −1.6 log2FC and −1.3 log2FC, respectively. _Lic_ genes (_licC, licD1-D3, licT_) were downregulated by −0.3
to −0.7 log2FC, _phtD_, _phtE_ downregulated −1.1 and −1.9 log2FC, respectively. _CbpA_ was downregulated −0.5 log2FC and _nanA _-1 log2FC. To summarize, we found that V11A altered the
pneumococcal transcriptome, mainly downregulating genes involved in amino acid and protein metabolism, but also transcriptional regulators. PEPTIDE V11A ALTERS THE PNEUMOCOCCAL PROTEOME We
also analysed the effect of peptide V11A on the proteome of _S. pneumoniae_ strain D39. We identified some proteins only detected in the sample without peptide treatment as “off” and
proteins only detected in the peptide treatment sample as “on” in Table 2 for _S. pneumoniae_ strain D39 and in Supplementary Tables 1a and 1b for strains 106.66 and 208.41. We found
choline-binding protein D to be switched off by V11A treatment and dehydratase FabZ to be turned on in strain D39. In strain 106.66, we found the permease protein of the branched-chain amino
acid ABC transporter (encoded by the _livH_ gene) and riboflavin biosynthesis protein RibBA to be turned off. Thus, we found alteration of the proteome by V11A with some proteins turned on
or off in line with the transcriptomic changes. NO TOXIC EFFECT WAS DETECTED FOR PEPTIDE V11A TO HUMAN AIRWAY EPITHELIAL CELL CULTURES OR ZEBRAFISH LARVAE Well-differentiated airway
epithelial cell (AEC) cultures are organotypic cell cultures with air–liquid interfaces and recapitulate many aspects of the respiratory tract, including airway cell heterogeneity, mucus
production and ciliary beating activity21. To evaluate whether peptide V11A is cytotoxic to primary human AEC (hAEC) cultures, we incubated the hAEC cultures together with peptide V11A for
30 h. We collected the medium from the apical and basolateral side and determined cytotoxicity calculated as %LDH release using the formula of Rayamajhi et al.22. The LDH release was the
same and low in media collected from hAEC cultures treated with and without peptide V11A. Therefore no cytotoxicity was detected (Supplementary Fig. 7a). Immunofluorescence staining also
shows the same cell morphology with intact tight junctions and ciliated cells present in all samples (Supplementary Fig. 7b). To test whether the peptide is toxic in vivo, zebrafish larvae
were exposed to 0.5 mg/ml or 0.25 mg/ml V11A and their swimming behaviour determined in light and dark conditions. This light–dark locomotion test is a well-established metric for toxicity
measurements that can reveal, for example, reactivity and muscular weakness23,24. The presence of the peptide did not affect the swimming behaviour (Supplementary Fig. 7c). In conclusion, no
toxic effect of peptide V11A was detected in vitro to hAEC cultures or in vivo to zebrafish larvae. PEPTIDE V11A INHIBITS PNEUMOCOCCAL ADHERENCE TO HUMAN AIRWAY EPITHELIAL CELL (HAEC)
CULTURES AND COLONIZATION OF THE RAT NASOPHARYNX Increased chain length has been associated with increased adherence of pneumococci to epithelial cells25. As peptide V11A decreased
pneumococcal chain length (Fig. 3b, Supplementary Fig. 5) and was not toxic to hAEC cultures (Supplementary Fig. 7), we tested its effect on pneumococcal adherence in vitro using the same
model. We exposed hAEC cultures from three different donors to _S. pneumoniae_ strain D39 and quantified the number of adhered bacteria on the apical surface after removing non-adhered
bacteria 24 h post-inoculation. Peptide V11A, but not the control peptide (V11A with an amino acid difference at position 10), decreased bacterial adherence to 22% of that of the control
without peptide (Fig. 5a). The control peptide had no effect on adherence. To test whether peptide V11A had an effect in vivo in the presence of the microbiota, we inoculated rat pups
intranasally with _S. pneumoniae_ strain 1154.75 (serotype 23F) with or without peptide V11A and quantified bacterial load in nasal wash and nasopharynx 24 and 48 h later. At 24 h,
pneumococci were detected in the nasopharynx of 9 out of 11 rats (81.81%) in the absence of V11A and in 3 out of 11 (27.27%) in the nasopharynx of rats that had received V11A (Chi-squared
test: _p_ = 0.03228). Bacterial loads at 24 h are shown in Fig. 5b and were significantly lower in the nasopharynx of rats that had received V11A (_p_ = 0.01367). Fewer bacteria in the
nasopharynx of the peptide-treated rats than controls were also seen at 48 h (not significant, _p_ = 0.30, Supplementary Fig. 8a). Bacterial load in the nasal wash was also reduced
significantly in presence of V11A at 24 h (_p_ = 0.01989) and 48 h (_p_ = 0.01704) (Supplementary Fig. 8b). Therefore, peptide V11A inhibited pneumococcal adherence in vitro to hAEC cultures
and colonization in vivo of the rat nasopharynx. DISCUSSION We found a previously uncharacterized peptide, V11A, in the secretome of _K. pneumoniae_, which modulates pneumococcal
phenotypes, particularly growth and colonization. For the first time we show that an interspecies peptide suppresses pneumococcal growth not only in a defined medium but in human
cerebrospinal fluid, a site of infection in pneumococcal meningitis. Growth suppression is achieved following uptake via the Ami-AliA/AliB permease leading to transcriptional and proteomic
changes. V11A inhibited pneumococcal growth in a dose-dependent manner, with the effect lost for versions of the peptide longer than 11 amino acids or with differences at positions 4 or 10.
These changes presumably prevent the peptide from binding to its target on the pneumococcus. V11A suppressed the growth of genetically diverse clinical isolates of pneumococcus, which bodes
well for its use as a therapeutic agent. Two strains unaffected by V11A are known to have mutations in the protein AmiA which led us to speculate that AmiA is required for the effect of
V11A16. The role of the Ami-AliA/AliB oligopeptide permease in the uptake of V11A was confirmed by the observation that deletion of one of the permease subunits, AmiC, abrogated the effect
of the peptide on growth and that fluorescently labelled V11A was taken up by the D39 parent strain, but not the ∆_amiC_ mutant. V11A did not affect the growth of other bacterial colonizers
of the nasopharynx tested: _S. mitis, H. influenzae, S. aureus_ or _K. pneumoniae_. Another proteinaceous interspecies molecule from the genus _Lysinibacillus_ was found previously, which
depends on the Ami permease for growth inhibition of not only _S. pneumoniae_ but also _S. mitis_26. V11A therefore appears to have a more species-specific effect. This specificity towards
pneumococci is an attractive feature and an advantage over antibiotics which affect multiple species and may lead to dysbiosis of the microbiota. This is also an advantage of V11A over
antimicrobial peptides (AMPs) of the host innate immune response, which tend to have a broad effect27. In contrast to β-lactam lytic antibiotics, such as penicillin, used to treat
pneumococcal infections, V11A had a bacteriostatic effect. By reducing the number of bacteria but not causing their lysis, little pneumolysin should be released compared to treatment with a
β-lactam antibiotic. This would be a significant advantage in treatment, particularly of meningitis where antibiotic-induced bacterial lysis causes the release of pneumolysin, which
exacerbates the inflammatory response, neuronal death, and therefore disease28,29. The host cytokine response to bacterial lysis can reduce the chance of survival and increase neurological
sequelae such as hearing loss and learning difficulties in patients who do survive30. We can associate many of the phenotype changes observed with changes in the transcriptome or proteome
caused by peptide V11A. The genes most downregulated by peptide V11A were all associated with transport, energy or the synthesis and transport of branched-chain amino acids. Efficient
acquisition of branched-chain amino acids is associated with nasopharyngeal colonization31. The top-upregulated gene was _gntR_, which has a role in sensing environmental and nutritional
cues32. The proteins FabK, FabG and FabF, which are encoded in the _fabKDGF-accB-fabZ-accCDA_ operon, participate in enzymatic reactions of fatty acid biosynthesis by adding
malonyl-coenzyme-A molecules to the growing hydrocarbon chain33. We found FabZ protein expression switched on in the presence of peptide V11A along with heterogeneous cell sizes and
decreased chain length. _Fab_ and _acc_ genes were also upregulated in the transcriptome following peptide exposure. Another study found that a competence-associated quorum peptide, BriC,
altered fatty acid biosynthesis in _S. pneumoniae_ and also promoted nasopharyngeal colonization34. We found modulation of fatty acid biosynthesis by interspecies peptide V11A. This,
together with observed phenotype changes, is in agreement with a study using a CRISPRi screen where repression of _fabD_, _fabK, fabZ, accB_ and _accD_ led to irregular cell shapes and
longer chains35. Cell chaining is associated with a longer competence and transformation time window as cell chains retain, rather than diffuse the CSP peptide17. This is compatible with our
finding that in addition to decreased chain length, we also found that peptide V11A decreased the transformation efficiency of _S. pneumoniae_. Competence contributes to the astonishing
genomic plasticity of _S. pneumoniae_, which can facilitate antigenic variation, vaccine escape and the acquisition of antibiotic resistance19. For a potential therapeutic agent, decreasing
the ability of the pneumococcus to acquire antibiotic resistance is of advantage. We did not find any _com_ genes, which are involved in regulating and developing competence for genetic
transformation36, differentially expressed upon peptide V11A treatment to explain the reduction of transformation efficiency. However, we found downregulation of other genes, namely _ssb_,
_hrcA_, _grpE_, _dnaK_ and _dnaJ_, which have been associated with regulating competence18,20. The _ssbB_ gene is regulated by the alternative sigma factor ComX18 and therefore expressed
uniquely during competence for genetic transformation. It encodes an alternative single-stranded DNA-binding protein that might increase the likelihood of multiple transformation events in
the same cell37. The pneumococcal competence response exhibits a broad phenotype, combining chaperone and protease production with genetic recombination, which includes induction of such
stress response proteases and chaperones38. Furthermore, in the proteomics data, CbpD was turned off by V11A peptide treatment. CbpD is a competence-specific murein hydrolase that lyses a
subfraction of the pneumococcal population or close relatives in order to release DNA for uptake39,40 and is produced exclusively by competent cells41. One study found that deletion of CbpD
reduced transformation efficiency in _Streptococcus thermophilus_42. The same has been observed in _Streptococcus sanguinis_ lacking LytF43 and _Streptococcus suis_, lacking CrfP44, which
are both functional analogues of CbpD. The CodY regulon is involved in the regulation of genes involved in amino acid metabolism, carbon metabolism and iron uptake and among genes described
as differentially expressed in D39∆_codY_ were also _aliA, ilv_ and _liv_ genes45. In our transcriptomics data _aliA, ilv_ and _liv_ genes and _codY_ are differentially expressed upon
peptide V11A treatment. Colonization, a prerequisite for nasopharyngeal carriage of _S. pneumoniae_, allows pneumococcus to spread between hosts and to cause invasive disease. Adherence to
host cells is one of the main features facilitating colonization46. The Ami permease has been suggested previously to modulate pneumococcal adherence to epithelial cells by modulating the
expression of adhesins on the pneumococcal surface during the first stages of colonization47. Increased bacterial chain length has been associated with enhanced adherence of pneumococci to
epithelial cells25. In accordance with this, peptide V11A, which decreased chain length, inhibited pneumococcal adherence to human airway epithelial cell cultures. V11A downregulated _phtD_,
_phtE_, c_bpA_ and _nanA_. Pneumococcal surface and histidine triad proteins PhtD and PhtE play a role in adherence to respiratory mucosa and anti-PhtD antibodies can inhibit bacterial
attachment48,49. CbpA binds the host proteins factor H and vitronectin46, which might facilitate adherence to host cells and NanA has been suggested to function as an adhesin50. Furthermore,
V11A downregulated _lic_ genes, including _licC_, _licD1-D3_, _licT_. LicD2 mutants showed decreased transformation competence, decreased adherence, reduced nasopharyngeal colonization and
reduced virulence in another study51. To have potential as a therapeutic, the peptide must have an effect in vivo. We found that V11A significantly reduced the percentage of rats colonized
and bacterial load in the nasopharynx and nasal wash of infant rats, indicating its effectiveness in a nutritionally complex environment in the presence of a microbiota. V11A also reduced
the growth of isolate 1154.75 (serotype 23F), which the AmiA and AliA ligands did not. Furthermore, AmiA and AliA peptides increased the chain length of bacteria and did not affect adherence
to epithelial cells15, while V11A decreased both chain length and adherence. While the AmiA and AliA peptides reduced the transformation rate by 3-fold15, V11A caused an 8.5-fold reduction.
The limitations of the study include that we used high doses of peptides, but optimization, for example, of delivery, could reduce effective doses. Future studies would also need to test
whether resistance to peptides develops over time, as happens with antibiotics. Alterations to the genes encoding penicillin-binding proteins, involved in cell wall construction, allow
bacteria to escape the action of the β-lactam antibiotics52,53. However, the peptide acts by specific binding to the substrate-binding protein of an ABC transporter which has a role in
sensing peptides in the environment. Mutation would mean the loss of this function. Therefore, we predict this is less likely to occur. This is supported by the fact that a BLAST search of
AmiA and AliA proteins indicates that they are highly conserved16 and the mutation in AmiA of strain R6, associated with lack of response to V11A, was found in fewer than 0.08% of
pneumococcal genomes. A limitation of the colonization study is that peptide was administered simultaneously with the bacteria, in contrast to the situation of treatment when
colonization/infection would be established then peptide administered. However, the experiment established proof of concept that peptides can be effective in vivo. We have shown that
interspecies peptide V11A produced by _K. pneumoniae_ modulated phenotypes of _S. pneumoniae_, particularly suppressing its growth in the medium and in human cerebrospinal fluid. We
speculate that the import of the peptide via the pneumococcal AmiA-AliA/AliB permease may be advantageous to _K. pneumoniae_ in the shared niche of the nasopharynx. Uptake of peptide V11A
led to the downregulation of genes for amino acid biosynthesis, caused irregular cell shape, decreased chain length and decreased genetic transformation. The bacteriostatic effect was
species-specific and dependet on peptide sequence and length. The peptide reduced pneumococcal adherence to hAEC cultures and colonization of rat nasopharynx, without apparent toxicity. We,
therefore, propose that peptide V11A has potential as a therapeutic agent against pneumococcal diseases with the advantages of effectiveness against antibiotic-resistant strains, avoiding
bacterial lysis and the subsequent damaging inflammatory response and specificity of action, avoiding dysbiosis of the microbiota. METHODS BACTERIAL STRAINS AND CULTURE CONDITIONS Bacterial
strains and culture conditions were used as described previously16. Briefly, the bacterial strains were stored at -80 °C in the Protect Microorganism Preservation System (Technical Service
Consultants Ltd.). As previously described, _S. pneumoniae_ was streaked out on CSBA plates, _H. influenzae_ on CHOC plates and both grown overnight at 37 °C and 5% CO2; _K. pneumoniae_ and
_S. aureus_ were streaked out on CSBA plates and grown overnight at 37 °C and atmospheric CO2 concentration. Swiss clinical _S. pneumoniae_ strains originated from a strain collection
obtained from nationwide surveillance for nasopharyngeal _S. pneumoniae_ isolates from children with respiratory infection54 or were kindly provided by Carlo Casanova, Institute for
Infectious Diseases, Bern. Strains used, including reference strains, are shown in Table 1. The D39 parent strain (ds865, D39 (∆_comAB::erm_)) and its mutant ∆_amiC_ (ds1039, D39,
∆_amiC::Janus_) were used in Fig. 226. Swiss clinical isolates of commensal streptococci _S. pseudopneumoniae_ (410.05) and _S. mitis_ (3308.46) were also used16. As previously, chemically
defined media (CDM)16, BHI medium and PBS were made in-house. IDENTIFICATION OF PEPTIDES IN THE SECRETOME Peptides secreted by _K. pneumoniae_ were identified as described previously by mass
spectrometry16. PEPTIDES Synthetic peptides (Genscript, Thermo Fisher Scientific) were ordered with ≥95% purity. Peptides for fluorescent microscopy were labelled at the C-terminus with
Lys(FITC). For human airway epithelial cell cultures and in vivo experiments (zebrafish larvae, rats), peptides were endotoxin-free and with TFA removal (salt form: acetate). HUMAN
CEREBROSPINAL FLUID Residual human cerebrospinal fluid (hCSF) from five anonymized patients undergoing lumbar puncture in 2023 due to non-inflammatory conditions was used for growth assay.
GROWTH ASSAY Growth assays were done as described previously16 and plotted in R with error bars representing SEM of 3 independent biological replicates. For growth in hCSF, bacteria were
inoculated as described elsewhere55 with twice the inoculum in PBS compared to the growth assay in CDM due to anticipated slower growth in hCSF. TIME-KILL ASSAY Following overnight culture
on agar plates, bacteria were sub-cultured in BHI medium overnight for approximately 7 h until the cultures reached OD600nm = 0.5 in mid-log phase. 500 µl of the overnight culture were
sub-cultured in 5 ml of fresh BHI until OD600nm = 0.5, centrifuged at 3000×_g_ for 7 min and the pellet resuspended in 5 ml CDM. The absorbance was adjusted in CDM to OD600nm = 0.05, then 3
ml of this bacterial suspension was mixed with 7 ml CDM to reach ca. 5 × 106 cfu (colony forming units)/ml. Penicillin G and peptide were resuspended to 0.5 mg/ml with the bacterial
suspension. In Eppendorf tubes, 1 ml cultures without or with Penicillin G or peptide were grown in a water bath at 37 °C and every 30 min for 5 h, 10 µl were removed to plate out dilutions
onto CSBA plates. Viable bacteria counts were determined after overnight incubation of the CSBA plates at 37 °C and 5% CO2. EPIFLUORESCENCE MICROSCOPY Following overnight culture on agar
plates, _S. pneumoniae_ D39 parent strain and D39∆_amiC_ were grown in BHI to OD600nm = 0.1–0.2, 1 ml culture centrifuged at 10,000×_g_ for 1 min and the pellet resuspended in 100 µl BHI and
50 µl of 1 mg/ml fluorescently labelled peptide. After 5 min incubation time, the bacteria were washed in 1 ml PBS three times. After the last wash, the pellet was resuspended in 40 µl of
PBS and 1.5 µl was spotted onto an acrylamide pad in a gene-frame on a microscope slide. We prepared acrylamide slides by sticking a gene-frame (Thermo Scientific, AB0578) onto a glass slide
and mixing the following in a tube per acrylamide slide: 370 µl PBS, 125 µl 40% acrylamide/bis-acrylamide 29:1 (Sigma-Aldrich, A7802), 0.5 µl TEMED (Sigma-Aldrich, T9281), 5 µl freshly
prepared 10% APS (Sigma-Aldrich, A3678). From this mix, we poured 500 µl into the gene-frame and added another glass slide to close it. After 30 min at room temperature, we gently slid off
one glass slide, cut the acrylamide pads into small pieces and incubated them in PBS for at least 30 min before use. Microscopy was performed using a Zeiss Axio Imager M1 fluorescence
microscope with a ×100 oil immersion objective (EC Plan-Neufluoar 100x/1.30 Oil M27), filter with 493 nm excitation and 517 nm emission wavelength for the FITC channel and images
photographed by a Zeiss Axiocam 712 mono camera. We edited pictures for Fig. 2b with the same settings for all samples to maintain comparability between samples and intensity of fluorescence
using the Software Zeiss ZEN 3.8: denoising the brightfield channel picture with setting real wavelets to 1.0, followed by adjusting the histogram upper white limit in the brightfield
channel to 6000 and in the FITC channel to 5000. BACTERIAL SHAPE CHARACTERIZATION Bacterial culturing was done as described for the growth assay for 2.5 h then 800 µl were centrifuged at
3000×_g_ for 7 min. Most of the supernatant was discarded and the pellet resuspended in the remaining volume of CDM (ca. 30 µl). A bacterial suspension of 10 µl was mixed with 2 µl
fluorescein isothiocyanate (FITC)-Dextran solution (Sigma-Aldrich, resuspended to 10 mg/ml in water). Of this mixture, 10 µl were pipetted onto a microscope slide and the coverslip was
applied firmly. The slides were viewed using a Zeiss Axio Imager M1 fluorescence microscope with a ×100 oil immersion objective and brightfield images photographed by a Zeiss AxioCam HRc
camera. CHAIN FORMATION Bacteria were cultured as described above and 8 µl were pipetted onto a microscope slide, air-dried, heat-fixed by flame and Gram-stained using the Aerospray TB Slide
Stainer (ELITechGroup). Pictures were taken with ×40 objectives in the Cytation 5 multimode reader (Biotek) with the Gen5 Software (Biotek). Microscopy image analysis of chain length was
done using MicrobeJ56. Results for each sample represent measurements of >1200 bacterial chains from at least 12 pictures of 3 independent biological replicates. Bacteria chain detection
settings (=objects) were set to exclude objects on the picture edge, objects with branching and only recognized as objects with a pixel width of 5-15. TRANSFORMATION ASSAY The protocol was
adapted from elsewhere57 to the following: _S. pneumoniae_ strain D39 was grown in BHI + 5% FCS to OD600nm = 0.5, then 150 µl sub-cultured in 9.5 ml of fresh BHI + 5% FCS until OD600nm =
0.1. After centrifugation at 3000×_g_ for 7 min the pellet was resuspended in the same volume of CDM. One ml of the bacterial suspension with 100 ng/ml CSP-1 (EMRLSKFFRDFILQRKK) with and
without 0.5 mg/ml peptide V11A was incubated for 12 min at 37 °C. After 100 µl were transferred into a new prewarmed tube and 1 µg chromosomal DNA from streptomycin-resistant strain 104.37
was added, and the culture was incubated for 20 min at 30 °C, then 900 µl prewarmed CDM was added, and the culture incubated for 90 min at 37 °C. Serial dilutions in PBS were plated onto
CSBA plates with and without 200 µg/ml streptomycin. The number of colonies was counted after overnight incubation and the transformation rate was calculated. GENE EXPRESSION ANALYSIS BY
RNA-SEQ AND RT-QPCR Following overnight culture on agar plates, _S. pneumoniae_ strain D39 was grown in BHI + 5% FCS to OD600nm = 0.5 and 100 µl of the overnight culture was sub-cultured in
10 ml of fresh CDM until OD600nm = 0.2. The culture was split into two 5 ml cultures and 0.5 mg/ml peptide was added to one sample. After 15 min incubation at 37 °C, transcription was
stopped with RNAprotect bacteria reagent (Qiagen) and total RNA was isolated with the RNeasy kit (Qiagen) according to the manufacturer’s manual. RNA concentration and integrity were
assessed with Nanodrop (Thermo Scientific) and Agilent Biolanalyzer (Agilent) before submitting 6 biological replicates of each sample to the Next Generation Sequencing Platform (University
of Bern), where quality control assessments, rRNA depletion, generation of libraries and sequencing were carried out. At the Next Generation Sequencing Platform (University of Bern), the
quantity and quality of the purified total RNA were assessed using a Qubit 4.0 fluorometer (Thermo Fisher Scientific) with the Qubit RNA BR Assay Kit (Thermo Fisher Scientific, Q10211) and
an Advanced Analytical Fragment Analyser System using a Fragment Analyser RNA Kit (Agilent, DNF-471), respectively. Thereafter, 150 ng of input RNA was depleted of ribosomal RNA using a
RiboCop rRNA Depletion Kit for Gram-Positive Bacteria (G+) (Lexogen, SKU 127) following the Lexogen User guide 125UG246V0102. Next, cDNA libraries were generated using a CORALL RNA-Seq V2
Library Prep Kit with UDI 12 nt Set A3 (Lexogen, SKU 173) according to the RTL protocol and 15 PCR cycles (Lexogen User Guide 171UG394V0100). The resulting cDNA libraries were evaluated
using a Qubit 4.0 fluorometer with the Qubit dsDNA HS Assay Kit (Thermo Fisher Scientific, Q32854) and an Agilent Fragment Analyser with an HS NGS Fragment Kit (Agilent, DNF-474),
respectively. Pooled cDNA libraries were sequenced paired-end using a NextSeq 1000/2000 P2 Reagents v3 (200 cycles; Illumina, 20046812) on an Illumina NextSeq 1000 instrument. The run
produced, on average, 26 million reads/library. The quality of the sequencing run was assessed using Illumina Sequencing Analysis Viewer (Illumina version 2.4.7) and all base call files were
demultiplexed and converted into FASTQ files using Illumina bcl2fastq conversion software v2.20. Lexogen (Vienna, Austria) performed quality control, read processing and differential gene
expression analysis up to the log fold change table: quality control analysis with fastqc, UMI extraction with umi_tools extract, trimming with cutadapt, STAR alignment to the reference
genome of _S. pneumoniae_ D39, umi deduplication with umi_tools dedup and differential gene expression analysis with DESeq2. With the differential gene expression table from Lexogen and by
retrieving a STRING network setting the species to “_Streptococcus pneumoniae_ D39” in Cytoscape 3.8.0, we created a gene association network with genes ≥1.25 log2FC upregulated or ≤−1.25
log2FC downregulated. cDNA was synthesized using SuperScript IV Reverse transcriptase (Invitrogen), including RNaseOUT Recombinant Ribonuclease Inhibitor (Invitrogen), according to the
manufacturer’s protocol. RT-qPCR was done according to the manufacturer’s protocol using TaqMan Fast Advanced Master Mix (Applied Biosystems) and Custom TaqMan Gene Expression Assays
(Applied Biosystems) with sequences in Supplementary Table 2. PROTEOMIC ANALYSIS Following overnight culture on agar plates, _S. pneumoniae_ strain D39 was grown in BHI + 5% FCS to OD600nm =
0.5 and 100 µl of overnight culture was sub-cultured in 10 ml of fresh CDM until OD600nm = 0.2. The culture was split into two 5 ml cultures and 0.5 mg/ml peptide was added to one sample.
After 15 min incubation at 37 °C, both cultures were centrifuged at 4000×_g_ for 5 min at 4 °C. The pellet was washed in 1 ml PBS and transferred to an Eppendorf tube to centrifuge at
4000×_g_ for 5 min. The supernatant was removed and the pellet was snap frozen in liquid nitrogen. Mass spectrometry was performed at the Core Facility Proteomics and Mass Spectrometry
(University of Bern) as described as follows and elsewhere58. Bacterial pellets were lysed in 50 μl buffer containing 8 M urea and 100 mM Tris–HCl pH 8.0 and protease inhibitor cocktail
(cOmplete w/o EDTA, Roche). Protein concentration was determined with Qubit assay (ThermoFisher) and adjusted to 1 mg/ml with lysis buffer and an aliquot corresponding to 10 μg was
processed. Proteins were reduced by the addition of 1/10 volume of 0.1 M DTT and incubation for 30 min at 37 °C, followed by alkylation with a five-fold molar excess of iodoacetamide and
incubation for 30 min at 37 °C in the dark. Iodoacetamide was quenched by 4/10 volume of 0.1 M DTT and urea concentration lowered to 4 M by addition of 20 mM Tris/HCl pH 8.0 with 2 mM CaCl2.
Proteins were digested for 2 h at 37 °C with sequencing grade LysC (Promega) at a protein/protease ratio of 50:1, followed by urea dilution to 1.6 M and overnight digestion at ambient
temperature with 1:50 sequencing grade trypsin (Promega). Digestion was stopped with trifluoroacetic acid (TFA) to a final concentration of 1% (v/v), followed by dilution to 0.1 μg/μl of
protein. Proteome analysis was done by nano-liquid chromatography on a Dionex Ultimate 3000 (Thermo Fisher Scientific) through a CaptiveSpray source coupling to a timsTOF Pro mass
spectrometer (Bruker) with an end-plate offset of 500 V, a drying temperature of 200 °C, and with the capillary voltage fixed at 1.6 kV. A volume of 2 µl (200 ng) from the protein digest was
loaded onto a pre-column (PepMap 100 C18, 5 µm, 100 A, 300 µm diameter × 5 mm length, ThermoFisher) at a flow rate of 10 µl/min with 0.05% (v/v) TFA in water/acetonitrile 98:2. After
loading, peptides were eluted in back flush mode onto a home-made C18 CSH Waters column (1.7 µm, 130 Å, 75 µm × 20 cm) by applying a 90 min gradient of 5% acetonitrile to 40% in water/0.1%
formic acid, at a flow rate of 250 nl/min and data was acquired in data-dependent acquisition mode using the parallel acquisition serial fragmentation (PASEF) option. The mass range was set
between 100 and 1700 _m_/_z_, with 10 PASEF scans between 0.7 and 1.4 V s/cm2. The accumulation time was set to 2 ms and the ramp time to 100 ms, respectively. Fragmentation was triggered at
20,000 arbitrary units, and peptides (up to a charge of 5) were fragmented using collision-induced dissociation with a spread between 20 and 59 eV. DDA data was interpreted and processed
with MaxQuant (version 2.0.1.0) against the UniprotKB _S. pneumoniae_ D39 strain protein sequences (release February 2021) as described elsewhere58. GENERATION OF HUMAN AIRWAY EPITHELIAL
CELL (HAEC) CULTURES Human AEC cultures were cultured in 6.5 mm Transwells® with 0.4 µm pore polyester membrane insert in flat bottom 24-well plates (Corning, 3470) as described
previously21. We seeded 1 × 105 cells per insert. Experiments were performed once a pseudostratified layer of differentiated airway epithelial cells was formed, including cilia movement and
physiological mucus production. CYTOTOXICITY ASSAY IN HAEC CULTURES Cytotoxicity of 30 h peptide incubation on hAEC cultures was determined by measuring the release of lactate dehydrogenase
(LDH), which is a stable cytosolic enzyme released upon cell lysis, and visualizing the ultrastructure of the epithelium. To the apical side of hAEC cultures, 40 µl of TEER solution (NaCl
0.9%, CaCl2 1.25 mmol/l and HEPES 10 mmol/l dissolved in distilled water) were added as negative control, 40 µl of peptide resuspended in TEER solution to 0.25 mg/ml and 0.5 mg/ml and 40 µl
of 1x LDH+ control solution from the CytoTox® Non-Radioactive Cytotoxicity Assay (Promega) were added to the apical side. After 30 h of incubation, 160 µl TEER solution was added to the
apical side and the medium from the apical and basolateral side were collected and both samples of each culture were used for cytotoxicity determination. Release of LDH was measured to
determine the cytotoxicity of peptide V11A on hAEC cultures using the CytoTox® Non-Radioactive Cytotoxicity Assay (Promega) according to the manufacturer’s instructions. TOXICITY ASSAY IN
ZEBRAFISH LARVAE AB zebrafish eggs were kindly provided by Anna Gliwa, Institute of Anatomy (University of Bern), obtained by natural mating procedures. Zebrafish eggs were collected within
the first hours post fertilization (hpf) and eggs and larvae were maintained at 28.5 °C on a 14 h/10 h light/dark cycle in a dedicated incubator (IPP110plus, Memmert GmbH) and used for
experiments until no later than 5 days post fertilization (dpf). The fertilized eggs were kept for 1 day in E3 embryo medium (60× stock solution contains 34.8 g NaCl, 1.6 g KCl, 5.8 g CaCl2,
9.78 g MgCl2*6 H2O) supplemented with 0.3 mg/ml methylene blue. On the following day, the media was changed to E3 only. Unfertilized eggs and deformed embryos were discarded. Peptides were
resuspended in E3 to 0.5 mg/ml and 0.25 mg/ml. E3 was used as a negative control and 150 µg/ml LPS in E3 as a positive control for toxicity. Healthy larvae of similar developmental phases
were picked 3 dpf and 15 larvae were transferred to each well of a six-well plate. For peptide toxicity testing, 2 wells (i.e. 30 larvae) were used per concentration, and for positive and
negative control, one well each. E3 was replaced by 2 ml of E3 without or with LPS or peptide and larvae were incubated for 48 h at 28.5 °C with light/dark cycle as above. At 48 h a
DanioVision Observation Chamber (Nodus) dark/light protocol59 with 1 h adaptation time, followed by 20 min dark, 10 min light, and 20 min dark, was run to investigate whether peptide
treatment caused swimming behavioural changes with Ethovision XT 15 software. Larvae were euthanized after the experiment with 0.4% Tricaine. ADHERENCE ASSAY ON HAEC CULTURES Two days prior
to infection, the basolateral medium of hAEC cultures was changed to a fresh medium without antibiotics. Bacterial culture of _S. pneumoniae_ strain D39 was prepared as described for the
growth assay. On the day of infection, 500 µl of the overnight culture were sub-cultured in 5 ml of fresh BHI until OD600nm = 0.4, then centrifuged at 3000×_g_ for 7 min and the pellet was
resuspended in 5 ml TEER solution for one wash, before resuspending again in 5 ml fresh TEER solution and diluting the bacterial solution for inoculation of hAEC cultures to 1 × 106 cfu/ml.
AEC cultures were washed with TEER to remove mucus at the time of infection. Peptides were resuspended in bacterial inoculum solution to 0.5 mg/ml and hAEC cultures inoculated with 40 µl of
bacterial inoculum with or without peptides per insert (to give a final inoculum of 4 × 104 cfu/insert). Next, the hAEC cultures were incubated for 24 h at 37 °C in a humidified atmosphere
with 5% CO2 then three washes with 200 µl of TEER solution were performed apically. Human AEC cultures were detached by adding 100 µl TrypLETM Express Enzyme (1x), phenol red (Gibco) for 30
min at 37 °C, 5% CO2 with resuspension after the first 10 min before continuing the incubation time. The trypsinized samples were collected into 100 µl of BHI with 5% FCS and directly
processed by making dilutions and plating them onto CSBA plates to determine cfu after overnight incubation at 37 °C, 5% CO2. RAT COLONIZATION MODEL Bacterial culture of _S. pneumoniae_
strain 1154.75 (serotype 23F) was prepared as described for the growth assay. On the day of inoculation, 500 µl of the overnight culture were sub-cultured in 5 ml of BHI until OD600nm = 0.4,
then centrifuged at 3000×_g_ for 7 min and the pellet washed in 0.85% NaCl solution before resuspending again, in 5 ml 0.85% NaCl solution for an inoculum of 5 × 107 cfu/ml. We performed
two experiments on different occasions: (1) two litters each of 12 (6 male, 6 female) nursing Wistar rats along with their dams (specific pathogen-free, SPF) were obtained from Charles River
(Germany), kept in individually ventilated cages with a 12 h light/dark cycle and constant temperature of 22 ± 2 °C, and provided with tap water and gamma-sterilized pellet diet ad libitum.
Once 14 days old, the rat pups were inoculated intranasally with the bacterial inoculum with or without 0.5 mg/ml peptide V11A by dripping 5 µl of inoculum into each nare, in alternate.
Separate litters of 12 pups were used for no peptide and peptide conditions. Then, 24 h after bacterial inoculation, 6 pups of each litter (3 male, 3 female) were sacrificed by an overdose
of pentobarbital (150 mg/kg, i.p.). Nasal wash was done by flushing through the trachea with 200 µl 0.85% NaCl solution and harvesting from the nostrils, and the nasopharynx was extracted.
For the remaining pups, 0.85% NaCl solution or 0.5 mg/ml V11A in 0.85% NaCl solution was administered again and 48 h after bacterial inoculation, sacrifice, nasal wash and nasopharynx
extraction were done as above. (2) The second experiment was done similarly to the first but with the following changes: one litter of 10 (5 male, 5 female) nursing Wistar rats along with
their dam were obtained and kept in a ventilated cage. Once 14 days old, the rat pups were inoculated intranasally with the bacterial inoculum with or without 0.5 mg/ml peptide V11A by
dripping 5 µl of inoculum into each nare, in alternate. All pups were kept in the same cage. Then, 24 h after bacterial inoculation, all pups were sacrificed and nasal wash and nasopharynx
extraction were performed as above. The extracted nasopharynxes were placed into 2 ml homogenization tubes with beads (2.8 mm diameter ceramic beads, BER0072, Labgene Scientific) containing
300 µl of PBS on ice and homogenized with 3 cycles of 30 s and 4500 rpm and 30 s break with Precellys Evolution homogenizer (Bertin Technologies). The nasal wash and nasopharynx samples were
directly processed by making dilutions and plating them onto CSBA plates with 3 µg/ml trimethoprim and 57 µg/ml sulfamethoxazole to determine cfu after overnight incubation of the plates at
37 °C, 5% CO2. STATISTICS All analyses were done in the R version 4.0.3 within R studio version 1.3.1093. Shapiro–Wilk normality tests were used to evaluate normality. If more than one
sample group was not normally distributed, the Wilcoxon rank sum test was done, otherwise _t_-test was used. As no cell of the expected frequencies in the contingency table was less than 5,
a Chi-squared test was performed to test for the effect of peptide V11A on number of rats colonized. In all analyses, a _p_-value of ≤0.05 was considered statistically significant. ETHICS
All research was performed in accordance with the relevant guidelines and regulations. In accordance with the Swiss Human Research Law, researchers at Bern University Hospital received
anonymized discarded leftovers of human cerebrospinal fluid samples collected in clinical routine. For the human primary airway epithelial cell model the anonymized post-mortem tissue
material was obtained through the Tissue Bank Bern (TBB) in accordance with ethical approval (KEK-BE 1571/2019). Studies with the rats were approved by the Animal Care and Experimentation
Committee of the Canton Bern, Switzerland, and follow the ethical principles and guidelines for experiments on animals as published by the Swiss Federal Veterinary Office, licence number
BE64/2022. REPORTING SUMMARY Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article. DATA AVAILABILITY All data generated or
analysed during this study are included in this published article and supplementary files. The source data underlying the figures and Supplementary figures can be found in Supplementary Data
1. The transcriptomic data for this study has been deposited in the European Nucleotide Archive (ENA) at EMBL-EBI under accession number PRJEB73628 and processed data (normalized counts and
differential gene expression analysis) are available in Supplementary Data 2. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE60
partner repository with the dataset identifier PXD050408 and processed data are available in Supplementary Data 3. REFERENCES * Willyard, C. The drug-resistant bacteria that pose the
greatest health threats. _Nature_ 543, 15 (2017). Article CAS PubMed Google Scholar * Eleraky, N. E., Allam, A., Hassan, S. B. & Omar, M. M. Nanomedicine fight against antibacterial
resistance: an overview of the recent pharmaceutical innovations. _Pharmaceutics_ 12, 142 (2020). Article CAS PubMed PubMed Central Google Scholar * World Health Organization. _WHO
Publishes List of Bacteria for Which New Antibiotics are Urgently Needed_
https://www.who.int/en/news-room/detail/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (2017). * Asokan, G. V., Ramadhan, T., Ahmed, E. & Sanad,
H. WHO Global Priority Pathogens List: a bibliometric analysis of medline-pubmed for knowledge mobilization to infection prevention and control practices in Bahrain. _Oman Med. J._ 34,
184–193 (2019). Article CAS PubMed PubMed Central Google Scholar * van der Poll, T. & Opal, S. M. Pathogenesis, treatment, and prevention of pneumococcal pneumonia. _Lancet_ 374,
1543–1556 (2009). Article PubMed Google Scholar * Cillóniz, C., Garcia-Vidal, C., Ceccato, A. & Torres, A. Antimicrobial resistance among _Streptococcus pneumoniae_ in _Antimicrobial
Resistance in the 21st Century_ (eds. Fong, I., Shlaes, D., Drlica, K.) 13–38 (Springer, 2018). * Brugger, S. D. et al. _Dolosigranulum pigrum_ cooperation and competition in human nasal
microbiota. _mSphere_ 5, e00852–00820 (2020). Article CAS PubMed PubMed Central Google Scholar * Raya Tonetti, F. et al. The respiratory commensal bacterium _Dolosigranulum pigrum_
040417 improves the innate immune response to _Streptococcus pneumoniae_. _Microorganisms_ 9, 1324 (2021). Article PubMed PubMed Central Google Scholar * Horn, K. J. et al.
_Corynebacterium_ species inhibit _Streptococcus pneumoniae_ colonization and infection of the mouse airway. _Front. Microbiol._ 12, 804935 (2021). Article PubMed Google Scholar *
Santagati, M., Scillato, M., Patanè, F., Aiello, C. & Stefani, S. Bacteriocin-producing oral streptococci and inhibition of respiratory pathogens. _FEMS Immunol. Med. Microbiol._ 65,
23–31 (2012). Article CAS PubMed Google Scholar * Horn, K. J., Schopper, M. A., Drigot, Z. G. & Clark, S. E. Airway _Prevotella_ promote TLR2-dependent neutrophil activation and
rapid clearance of _Streptococcus pneumoniae_ from the lung. _Nat. Commun._ 13, 3321 (2022). Article PubMed PubMed Central Google Scholar * Claverys, J. P., Grossiord, B. & Alloing,
G. Is the Ami-AliA/B oligopeptide permease of _Streptococcus pneumoniae_ involved in sensing environmental conditions? _Res. Microbiol._ 151, 457–463 (2000). Article CAS PubMed Google
Scholar * Kerr, A. R. et al. The Ami-AliA/AliB permease of _Streptococcus pneumoniae_ is involved in nasopharyngeal colonization but not in invasive disease. _Infect. Immun._ 72, 3902–3906
(2004). Article CAS PubMed PubMed Central Google Scholar * Nasher, F., Heller, M. & Hathaway, L. J. _Streptococcus pneumoniae_ proteins AmiA, AliA, and AliB bind peptides found in
ribosomal proteins of other bacterial species. _Front. Microbiol._ 8, 2688 (2018). Article PubMed PubMed Central Google Scholar * Nasher, F. et al. Peptide ligands of AmiA, AliA, and
AliB proteins determine pneumococcal phenotype. _Front. Microbiol._ 9, 3013 (2018). Article PubMed PubMed Central Google Scholar * Lux, J. et al. AmiA and AliA peptide ligands are
secreted by _Klebsiella pneumoniae_ and inhibit growth of _Streptococcus pneumoniae_. _Sci. Rep._ 12, 22268 (2022). Article PubMed PubMed Central Google Scholar * Domenech, A., Slager,
J. & Veening, J. W. Antibiotic-induced cell chaining triggers pneumococcal competence by reshaping quorum sensing to autocrine-like signaling. _Cell Rep._ 25, 2390–2400.e2393 (2018).
Article CAS PubMed PubMed Central Google Scholar * Slager, J., Aprianto, R. & Veening, J.-W. Refining the pneumococcal competence regulon by RNA sequencing. _J. Bacteriol._ 201,
e00780–00718 (2019). Article CAS PubMed PubMed Central Google Scholar * Winkler, M. E. & Morrison, D. A. Competence beyond genes: filling in the details of the pneumococcal
competence transcriptome by a systems approach. _J. Bacteriol._ 201, e00238–00219 (2019). Article CAS PubMed PubMed Central Google Scholar * Kwun, M. J., Ion, A. V., Oggioni, M. R.,
Bentley, S. D. & Croucher, N. J. Diverse regulatory pathways modulate bet hedging of competence induction in epigenetically-differentiated phase variants of _Streptococcus pneumoniae_.
_Nucleic Acids Res._ 51, 10375–10394 (2023). Article CAS PubMed PubMed Central Google Scholar * Gultom, M., Laloli, L. & Dijkman, R. Well-differentiated primary mammalian airway
epithelial cell cultures. _Methods Mol. Biol._ 2203, 119–134 (2020). Article CAS PubMed Google Scholar * Rayamajhi, M., Zhang, Y. & Miao, E. A. Detection of pyroptosis by measuring
released lactate dehydrogenase activity. _Methods Mol. Biol._ 1040, 85–90 (2013). Article CAS PubMed PubMed Central Google Scholar * Basnet, R. M., Zizioli, D., Taweedet, S., Finazzi,
D. & Memo, M. Zebrafish larvae as a behavioral model in neuropharmacology. _Biomedicines_ 7, 23 (2019). Article PubMed PubMed Central Google Scholar * Rock, S., Rodenburg, F.,
Schaaf, M. J. M. & Tudorache, C. Detailed analysis of Zebrafish larval behaviour in the light dark challenge assay shows that diel hatching time determines individual variation. _Front.
Physiol._ 13, 827282 (2022). Article PubMed PubMed Central Google Scholar * Rodriguez, J. L., Dalia, A. B. & Weiser, J. N. Increased chain length promotes pneumococcal adherence and
colonization. _Infect. Immun._ 80, 3454–3459 (2012). Article CAS PubMed PubMed Central Google Scholar * Hauge, I. H. et al. A novel proteinaceous molecule produced by _Lysinibacillus_
sp. OF-1 depends on the Ami oligopeptide transporter to kill _Streptococcus pneumoniae_. _Microbiology (Reading)_ 169, 001313 (2023). Article CAS PubMed Google Scholar * Baltzer, S. A.
& Brown, M. H. Antimicrobial peptides: promising alternatives to conventional antibiotics. _J. Mol. Microbiol. Biotechnol._ 20, 228–235 (2011). CAS PubMed Google Scholar * Hirst, R.
A., Kadioglu, A., O’Callaghan, C. & Andrew, P. W. The role of pneumolysin in pneumococcal pneumonia and meningitis. _Clin. Exp. Immunol._ 138, 195–201 (2004). Article CAS PubMed
PubMed Central Google Scholar * Tabusi, M. et al. Neuronal death in pneumococcal meningitis is triggered by pneumolysin and RrgA interactions with β-actin. _PLoS Pathog._ 17, e1009432
(2021). Article CAS PubMed PubMed Central Google Scholar * Leib, S. L. & Täuber, M. G. Pathogenesis of bacterial meningitis. _Infect. Dis. Clin. North Am._ 13, 527–548 (1999).
Article CAS PubMed Google Scholar * Green, A. E. et al. Airway metabolic profiling during _Streptococcus pneumoniae_ infection identifies branched chain amino acids as signatures of
upper airway colonisation. _PLoS Pathog._ 19, e1011630 (2023). Article CAS PubMed PubMed Central Google Scholar * Al-Bayati, F. A. et al. Pneumococcal galactose catabolism is controlled
by multiple regulators acting on pyruvate formate lyase. _Sci. Rep._ 7, 43587 (2017). Article PubMed PubMed Central Google Scholar * Heath, R. J., White, S. W. & Rock, C. O. Lipid
biosynthesis as a target for antibacterial agents. _Prog. Lipid Res._ 40, 467–497 (2001). Article CAS PubMed Google Scholar * Aggarwal, S. D. et al. Competence-associated peptide BriC
alters fatty acid biosynthesis in _Streptococcus pneumoniae_. _mSphere_ 6, e0014521 (2021). Article PubMed Google Scholar * Liu, X. et al. High-throughput CRISPRi phenotyping identifies
new essential genes in _Streptococcus pneumoniae_. _Mol. Syst. Biol._ 13, 931 (2017). Article PubMed PubMed Central Google Scholar * Piotrowski, A., Luo, P. & Morrison, D. A.
Competence for genetic transformation in _Streptococcus pneumoniae_: termination of activity of the alternative sigma factor ComX is independent of proteolysis of ComX and ComW. _J.
Bacteriol._ 191, 3359–3366 (2009). Article CAS PubMed PubMed Central Google Scholar * Attaiech, L. et al. Role of the single-stranded DNA-binding protein SsbB in pneumococcal
transformation: maintenance of a reservoir for genetic plasticity. _PLoS Genet._ 7, e1002156 (2011). Article CAS PubMed PubMed Central Google Scholar * Stevens, K. E., Chang, D., Zwack,
E. E. & Sebert, M. E. Competence in _Streptococcus pneumoniae_ Is regulated by the rate of ribosomal decoding errors. _mBio_ 2, e00071–00011 (2011). Article PubMed PubMed Central
Google Scholar * Kausmally, L., Johnsborg, O., Lunde, M., Knutsen, E. & Havarstein, L. S. Choline-binding protein D (CbpD) in _Streptococcus pneumoniae_ is essential for
competence-induced cell lysis. _J. Bacteriol._ 187, 4338–4345 (2005). Article CAS PubMed PubMed Central Google Scholar * Johnsborg, O., Eldholm, V., Bjørnstad, M. L. & Håvarstein,
L. S. A predatory mechanism dramatically increases the efficiency of lateral gene transfer in _Streptococcus pneumoniae_ and related commensal species. _Mol. Microbiol._ 69, 245–253 (2008).
Article CAS PubMed Google Scholar * Eldholm, V., Johnsborg, O., Haugen, K., Ohnstad, H. S. & Havarstein, L. S. Fratricide in _Streptococcus pneumoniae_: contributions and role of the
cell wall hydrolases CbpD, LytA and LytC. _Microbiology (Reading)_ 155, 2223–2234 (2009). Article CAS PubMed Google Scholar * Biornstad, T. J., Ohnstad, H. S. & Havarstein, L. S.
Deletion of the murein hydrolase CbpD reduces transformation efficiency in _Streptococcus thermophilus_. _Microbiology (Reading)_ 158, 877–885 (2012). Article PubMed Google Scholar *
Cullin, N., Redanz, S., Lampi, K. J., Merritt, J. & Kreth, J. Murein hydrolase LytF of _Streptococcus sanguinis_ and the ecological consequences of competence development. _Appl.
Environ. Microbiol._ 83, e01709–e01717 (2017). Article PubMed PubMed Central Google Scholar * Zhu, Y. et al. CrfP, a fratricide protein, contributes to natural transformation in
_Streptococcus suis_. _Vet. Res._ 52, 50 (2021). Article CAS PubMed PubMed Central Google Scholar * Hendriksen, W. T. et al. CodY of _Streptococcus pneumoniae_: link between nutritional
gene regulation and colonization. _J. Bacteriol._ 190, 590–601 (2008). Article CAS PubMed Google Scholar * Weiser, J. N., Ferreira, D. M. & Paton, J. C. _Streptococcus pneumoniae_:
transmission, colonization and invasion. _Nat. Rev. Microbiol._ 16, 355–367 (2018). Article CAS PubMed PubMed Central Google Scholar * Cundell, D. R., Pearce, B. J., Sandros, J.,
Naughton, A. M. & Masure, H. R. Peptide permeases from _Streptococcus pneumoniae_ affect adherence to eucaryotic cells. _Infect. Immun._ 63, 2493–2498 (1995). Article CAS PubMed
PubMed Central Google Scholar * Kallio, A. et al. Role of Pht proteins in attachment of _Streptococcus pneumoniae_ to respiratory epithelial cells. _Infect. Immun._ 82, 1683–1691 (2014).
Article PubMed PubMed Central Google Scholar * Adamou, J. E. et al. Identification and characterization of a novel family of pneumococcal proteins that are protective against sepsis.
_Infect. Immun._ 69, 949–958 (2001). Article CAS PubMed PubMed Central Google Scholar * Uchiyama, S. et al. The surface-anchored NanA protein promotes pneumococcal brain endothelial
cell invasion. _J. Exp. Med._ 206, 1845–1852 (2009). Article CAS PubMed PubMed Central Google Scholar * Zhang, J. R., Idanpaan-Heikkila, I., Fischer, W. & Tuomanen, E. I.
Pneumococcal licD2 gene is involved in phosphorylcholine metabolism. _Mol. Microbiol._ 31, 1477–1488 (1999). Article CAS PubMed Google Scholar * Engel, H. et al. A low-affinity
penicillin-binding protein 2x variant is required for heteroresistance in _Streptococcus pneumoniae_. _Antimicrob. Agents Chemother._ 58, 3934–3941 (2014). Article PubMed PubMed Central
Google Scholar * Engel, H. et al. Heteroresistance to fosfomycin is predominant in _Streptococcus pneumoniae_ and depends on the murA1 gene. _Antimicrob. Agents Chemother._ 57, 2801–2808
(2013). Article CAS PubMed PubMed Central Google Scholar * Mühlemann, K., Matter, H. C., Täuber, M. G. & Bodmer, T. Nationwide surveillance of nasopharyngeal _Streptococcus
pneumoniae_ isolates from children with respiratory infection, Switzerland, 1998–1999. _J. Infect. Dis._ 187, 589–596 (2003). Article PubMed Google Scholar * Müller, A. et al.
Pneumococcal serotype determines growth and capsule size in human cerebrospinal fluid. _BMC Microbiol._ 20, 16 (2020). Article PubMed PubMed Central Google Scholar * Ducret, A.,
Quardokus, E. M. & Brun, Y. V. MicrobeJ, a tool for high throughput bacterial cell detection and quantitative analysis. _Nat. Microbiol._ 1, 16077 (2016). Article CAS PubMed PubMed
Central Google Scholar * Kjos, M. et al. Expression of _Streptococcus pneumoniae_ bacteriocins is induced by antibiotics via regulatory interplay with the competence system. _PLoS Pathog._
12, e1005422 (2016). Article PubMed PubMed Central Google Scholar * Uldry, A.-C. et al. Effect of sample transportation on the proteome of human circulating blood extracellular
vesicles. _Int. J. Mol. Sci._ 23, 4515 (2022). Article PubMed PubMed Central Google Scholar * Faria, M. et al. Acrylamide acute neurotoxicity in adult zebrafish. _Sci. Rep._ 8, 7918
(2018). Article PubMed PubMed Central Google Scholar * Perez-Riverol, Y. et al. The PRIDE database resources in 2022: a hub for mass spectrometry-based proteomics evidences. _Nucleic
Acids Res._ 50, D543–D552 (2021). Article PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS We are grateful to the following people for providing pneumococcal strains:
Jeffrey Weiser (New York University, USA) for D39, Philippe Moreillon (Centre Hospitalier Universitaire Vaudois, Switzerland) for R6 and Ralf Reinert (National Reference Center for
Pneumococcus, Germany) for reference strains. We thank Carlo Casanova (Institute for Infectious Diseases, University of Bern) for kindly providing strains of _S. pneumoniae, H. influenzae,
S. aureus, M. catarrhalis_ and _K. pneumoniae_. We would like to thank the Next Generation Sequencing Platform, University of Bern, for performing the high-throughput sequencing experiments.
We are grateful to Sabina Berezowska and Irene Ramos-Centeno (Institute of Pathology, University of Bern) for providing the tissues for the hAEC culture establishment via the Tissue Bank
Bern. We thank Anna Gliwa (Institute for Anatomy, University of Bern) for providing zebrafish larvae eggs. We are grateful to Marianne Küffer, Adrian Wegmüller and Linus Rechsteiner
(Institute for Infectious Diseases, University of Bern), Sophie Braga-Lagache and Natasha Buchs (Proteomics and Mass Spectrometry Core Facility, University of Bern) and Zhian Salehian
(Norwegian University of Life Sciences) for their technical support. This work was funded by grant number 192067 from the Swiss National Science Foundation to L.J.H. We gratefully
acknowledge the Swiss National Science Foundation (R’Equip grant no. 316030-189737) and the University of Bern for financing the mass spectrometry equipment. AUTHOR INFORMATION AUTHORS AND
AFFILIATIONS * Faculty of Medicine, Institute for Infectious Diseases, University of Bern, Bern, Switzerland Janine Lux, Hannah Portmann, Lucía Sánchez García, Maria Erhardt, Lalaina
Holivololona, Laura Laloli, Manon F. Licheri, Ronald Dijkman, Denis Grandgirard, Stephen L. Leib & Lucy J. Hathaway * Graduate School for Cellular and Biomedical Sciences, University of
Bern, Bern, Switzerland Janine Lux * Department of Fundamental Microbiology, University of Lausanne, Lausanne, Switzerland Clement Gallay & Jan-Willem Veening * Department of Neurology,
Bern University Hospital and University of Bern, Bern, Switzerland Robert Hoepner * MRC Centre for Global Infectious Disease Analysis, Sir Michael Uren Hub, White City Campus, Imperial
College London, London, UK Nicholas J. Croucher * Faculty of Chemistry, Biotechnology and Food Science, Norwegian University of Life Sciences, 1430, Ås, Norway Daniel Straume * Proteomics
and Mass Spectrometry Core Facility, Department for BioMedical Research (DBMR), University of Bern, Bern, Switzerland Manfred Heller Authors * Janine Lux View author publications You can
also search for this author inPubMed Google Scholar * Hannah Portmann View author publications You can also search for this author inPubMed Google Scholar * Lucía Sánchez García View author
publications You can also search for this author inPubMed Google Scholar * Maria Erhardt View author publications You can also search for this author inPubMed Google Scholar * Lalaina
Holivololona View author publications You can also search for this author inPubMed Google Scholar * Laura Laloli View author publications You can also search for this author inPubMed Google
Scholar * Manon F. Licheri View author publications You can also search for this author inPubMed Google Scholar * Clement Gallay View author publications You can also search for this author
inPubMed Google Scholar * Robert Hoepner View author publications You can also search for this author inPubMed Google Scholar * Nicholas J. Croucher View author publications You can also
search for this author inPubMed Google Scholar * Daniel Straume View author publications You can also search for this author inPubMed Google Scholar * Jan-Willem Veening View author
publications You can also search for this author inPubMed Google Scholar * Ronald Dijkman View author publications You can also search for this author inPubMed Google Scholar * Manfred
Heller View author publications You can also search for this author inPubMed Google Scholar * Denis Grandgirard View author publications You can also search for this author inPubMed Google
Scholar * Stephen L. Leib View author publications You can also search for this author inPubMed Google Scholar * Lucy J. Hathaway View author publications You can also search for this author
inPubMed Google Scholar CONTRIBUTIONS L.J.H. conceived the study, J.L. wrote the first draft of the manuscript. J.L., L.J.H., D.G. and S.L.L. participated in the design of the study. J.L.,
H.P. and L.S.G. did bacterial growth assays. D.S. provided D39 parental strain and D39∆_amiC_ and R.H. provided hCSF. J.L. did epifluorescence microscopy after preliminary experiments and
establishment of a protocol with C.G. and J.W.V. J.L. analysed bacterial cell shape, chain length, transformation rate, RNA-Seq and proteomics. N.J.C. provided support for the analysis of
RNA-Seq data in R. M. He. analysed mass spectrometry data. J.L., L.L., M.F.L. and R.D. performed or advised on hAEC culture assays. L.H. did cytotoxicity assay with zebrafish larvae. J.L.,
M.E. and D.G. performed experiments with infant rats. All authors gave final approval for publication. CORRESPONDING AUTHOR Correspondence to Lucy J. Hathaway. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare the following competing interests: L.J.H. and J.L. are inventors on a patent application pertaining to this work. R.H. received speaker/advisor honorary from
Merck, Novartis, Roche, Biogen, Alexion, Sanofi, Janssen, Bristol-Myers Squibb, Teva/Mepha and Almirall. He received research support within the last 5 years from Roche, Merck, Sanofi,
Biogen, Chiesi, and Bristol-Myers Squibb. He also received research grants from the Swiss MS Society and the SITEM Insel Support Fund and is a member of the Advisory Board of the Swiss and
International MS Society. He also serves as deputy editor-in-chief for the Journal of Central Nervous System Disease. All conflicts are not related to this work. All other authors declare no
competing interests. PEER REVIEW PEER REVIEW INFORMATION _Communications Biology_ thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling
Editors: Tobias Goris. A peer review file is available. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. SUPPLEMENTARY INFORMATION PEER REVIEW FILE SUPPLEMENTARY INFORMATION DESCRIPTION OF ADDITIONAL SUPPLEMENTARY MATERIALS SUPPLEMENTARY DATA 1 SUPPLEMENTARY DATA 2
SUPPLEMENTARY DATA 3 REPORTING SUMMARY RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing,
adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons
licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a
credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted
use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT
THIS ARTICLE CITE THIS ARTICLE Lux, J., Portmann, H., Sánchez García, L. _et al._ _Klebsiella pneumoniae_ peptide hijacks a _Streptococcus pneumoniae_ permease to subvert pneumococcal growth
and colonization. _Commun Biol_ 7, 425 (2024). https://doi.org/10.1038/s42003-024-06113-9 Download citation * Received: 22 November 2023 * Accepted: 26 March 2024 * Published: 08 April 2024
* DOI: https://doi.org/10.1038/s42003-024-06113-9 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link
is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative