Play all audios:
ABSTRACT Bioapplication is an emerging field of metal-organic frameworks (MOF) utilization, but biocompatible MOFs with permanent porosity are still a rarity in the field. In addition,
biocompatibility of MOF constituents is often overlooked when designing bioMOF systems, intended for drug delivery. Herein, we present the a Zn(II) bioMOF based on vitamin C as an
independent ligand (bioNICS-1) forming a three-dimensional chiral framework with permanent microporosity. Comprehensive study of structure stability in biorelavant media in static and
dynamic conditions demonstrates relatively high structure resistivity, retaining a high degree of its parent specific surface area. Robustness of the 3D framework enables a slow degradation
process, resulting in controllable release of bioactive components, as confirmed by kinetic studies. BioNICS-1 can thus be considered as a suitable candidate for the design of a small drug
molecule delivery system, which was demonstrated by successful loading and release of urea—a model drug for topical application—within and from the MOF pores. SIMILAR CONTENT BEING VIEWED BY
OTHERS APPLICATION OF NOVEL NANOMAGNETIC METAL–ORGANIC FRAMEWORKS AS A CATALYST FOR THE SYNTHESIS OF NEW PYRIDINES AND 1,4-DIHYDROPYRIDINES VIA A COOPERATIVE VINYLOGOUS ANOMERIC BASED
OXIDATION Article Open access 05 March 2021 NICKEL FERRITE NANOPARTICLES DOPED ON HOLLOW CARBON MICROSPHERES AS A NOVEL REUSABLE CATALYST FOR SYNTHESIS OF _N_-SUBSTITUTED PYRROLE DERIVATIVES
Article Open access 05 July 2023 CREATING HIERARCHICAL PORES IN METAL–ORGANIC FRAMEWORKS VIA POSTSYNTHETIC REACTIONS Article 28 October 2022 INTRODUCTION Metal-organic frameworks (MOFs) are
crystalline and generally porous materials consisting of metal ions coordinated to organic molecules, capable of forming 2D and 3D structures with specific structural features. Large pore
volume, high surface-to-volume ratio, the possibility of pore functionalization and the use of active constituents, are properties commonly attributed to these materials. MOFs are already
well established in the various fields of science, such as catalysis, gas storage and separation, sensing or electrochemistry and they are increasingly gaining interest in the biomedicine
applications as well1,2,3,4,5. The compositional variety of these materials allows us to create systems with an agreeable toxicological profile for the use of drug delivery, contrast agents
and theranostics6,7,8,9,10. Such MOFs are, if constructed from at least one biologically active unit, further on referred to as bioMOFs11,12,13. From the perspective of MOF design, Zn(II) is
probably one of the most frequently used cations. Its electronic configuration namely enables a variety of coordination geometries ranging from four- to sixfold polyhedra and thus the
formation of many different secondary building units (SBUs), most commonly found either as discrete clusters or infinite rod-like shapes14,15. However, 3D infinite SBUs forming inorganic
skeletons, which have been observed for Mn-, Ni- or Mg-based MOFs for instance, are to the best of our knowledge not yet known for the Zn-based frameworks16,17,18. From the biological
perspective, Zn(II) is an essential element with recommended daily intake (RDI) value of ~10 mg and a crucial component of a large number of enzymes and transcriptional factors19,20. It has
a significant role in response to oxidative stress, DNA replication and damage repair, as well as immune response, apoptosis, aging and synthesis of proteins, for example, collagen21. Zinc’s
toxicity has also been well studied and oral lethal dose (represented by LD50 value) was determined to be close to 3 g kg−1 of body weight in humans22,23. In contrast to Fe(III) being most
frequently used in the design of bioMOFs, Zn with lower RDI than Fe does not have the tendency to bio-accumulate in the body caused by the lack of active physiological excretion
pathways21,24. Moreover, Zn is regarded as an antioxidant mineral, is redox inactive and as a cation (within RDI values) does not promote the generation of undesirable reactive oxygen
species (ROS)19,21. Its antimicrobial activity is depended on concentration and time of contact. Zinc’s effect is a consequence of (i) direct interaction with the microbial membrane leading
to its destabilization and (ii) interaction with nucleic acids and deactivation of the respiratory system25. Therefore, Zn can present a suitable candidate in constructing bioMOF systems.
When designing a bioMOF by using a desired drug or active molecule as a linker, achieving permanent porosity, although desirable, is not always a priority. This is well exampled in a
non-porous bioMIL-5 where both constituents, azelaic acid and Zn(II) are used for the antibacterial treatment of skin disorders and are released in a controllable manner26. Permanent
porosity, with the pores accessible for hosting molecules after solvent removal, would however offer additional bioapplicative opportunities. The lack of permanent porosity or
inaccessibility of the pores for drug molecules in bioMOF structures, when using small endogenous molecules as ligands, is considered to be the main drawback in their development27. There
are however a few examples of porous bioMOFs with therapeutic linkers worth mentioning—(i) analogue Mg-MOF-74, where olsalazine (prodrug of the anti-inflammatory 5-aminosalicylic acid)28
replaced 2,5-dihydroxybenzene-1,4-dicarboxylate (DBDC) as a linker; (ii) medi-MOF-129, which utilizes curcumin (anti-oxidative, anti-inflammatory, anticancer activity) and (iii) bioMIL-330,
which employs 3′3,5′5-azobenzenetetracarboxylate (antimicrobial) as a linker, however, this framework selectively adsorbs CO2 and NO and is not otherwise considered porous. Besides
pharmaceutically active ingredients, there are different groups of organic bio-molecules with abilities to coordinatively bind with metal cations or SBUs, such as amino acids, nucleobasis,
porphyrins or sugars31,32,33,34,35,36,37,38. Vitamins with natural abundance and a variety of biomedical applications have the ability to bind metals through multiple coordination sites as
well. In spite of the apparent advantages that this group of biomolecules offers, vitamin B3 (niacin/nicotinic acid) is, to the best of our knowledge, the only example used as a ligand in
designing bioMOFs39,40,41. In that manner, vitamin C (_L_-ascorbic acid—ASC) also presents a suitable, biologically active linker. Just as vitamin B3, ASC is also water-soluble and has
anti-inflammatory and antioxidant properties however, their pharmacology is very different. Vitamin C is present in plasma and tissues, and presents an essential cofactor in numerous
enzymatic reactions (biosynthesis of collagen, carnitine, and neuropeptides)42. Together with other vitamins and trace elements such as Zn improves immune functions21. Vitamin C is a weak
sugar acid (pKa1 = 4.25 and pKa2 = 11.79) that forms a monoanion (HA) at pH 4–5 and dianion (A) at pH 11–12. Various materials where metal cations are combined with ascorbic acid are known,
yet they possess molecular or 1D chain-like structures or use ascorbic acid only as a sacrificial agent for sensing applications43,44,45,46,47,48,49,50,51. Therefore, a three-dimensional
bioMOF framework composed of ascorbic acid as an independent ligand was not known up until now. Herein we present Zn-based ascorbate MOF (bioNICS-1; bioMOF material of National Institute of
Chemistry, Slovenia), possessing a unique structure with 3D infinite inorganic building unit inter-connected through ascorbate ligands. BioNICS-1 presents, to the best of our knowledge, the
first example of ascorbate with permanent microporosity, enabling the encapsulation of small drug molecules. Insight into the degradation of Zn-ascorbate structure in biorelavant media shows
great potential for controlled drug release. RESULTS AND DISCUSSION BioNICS-1 crystallizes from solvothermal conditions using only ethanol as a solvent. Ethanol was chosen for the synthesis
as it is technologically safe with a relatively low impact on human health and the environment, and is so considered as a green solvent52,53,54. Furthermore, ethanol acts as a weak ligand
and thus has low tendency to coordinatively participate in the formation of framework structure. Consequently, its removal to activate the MOF framework is typically easier than in the case
of the use of stronger-ligand solvents such as water or dimethylformamide. STRUCTURE PROPERTIES A broader size scale of crystals can be achieved either by using modulator (acetic acid)
resulting in the formation of individual crystallites with the size up to 2 µm (bioNICS-1-aa), or the choice of the heating source (microwave) for the synthesis of nanoparticles
(bioNICS-1-mw) with dimensions up to 50 nm. The details of the synthesis procedures are described in the “Methods” section. The particle size of differently prepared bioNICS-1 products was
comparatively estimated by SEM (Supplementary Fig. 1) and Scherrer calculations (Supplementary Table 1). The results of dynamic light scattering (DLS) measurements were not satisfactory for
proper particle size distribution analysis due to the agglomeration tendencies of the nanocrystals55, which can be explained by the low surface charge deducted by Zeta potential measurements
(Supplementary Fig. 2). Upon stirring the suspension of bioNICS-1 in water media, sedimented material does re-disperse—whether or not these are original agglomerates or newly formed ones
remains unclear. Agglomeration of nanoparticles in liquid media is a common occurrence and could be later addressed with surface modifications to provide either greater surface charge or
steric interference56. The crystal structure of bioNICS-1 was determined by using 3D electron diffraction (3DED) (Supplementary Fig. 3, Supplementary Table 2) and further refined with PXRD
(Supplementary Fig. 4, Supplementary Table 3). For electron diffraction study bioNICS-1-aa was used, whereas all the remaining investigations were performed on bioNICS-1 product synthesized
under conventional conditions followed by the activation described in the Supplementary methodes. Results of PXRD, TGA and N2 isotherms deemed these two materials to be the same
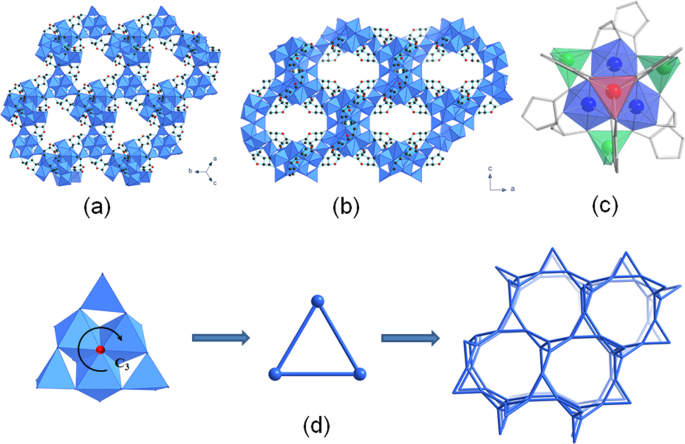
(Supplementary Fig. 5). The crystal structure contains infinite three-dimensional inorganic building unit bridged through the ascorbate moieties, with Zn(II) occurring in three different
coordination environments i.e. octahedral, trigonal prismatic and tetrahedral (Fig. 1c). Such coordination diversity of Zn(II) cation within one framework structure is a rarity among MOFs
and is a consequence of the multi-dentate property of ascorbic acid being fully deprotonated with all four hydroxyl and additional carbonyl O atom participating in coordination with Zn(II)
centres (Supplementary Fig. 6). An additional O atom that shares a common vertex of three ZnO6 octahedra is subjected to _C3_ symmetry (Fig. 1d) and belongs to the independent hydroxyl
group, yielding the overall chemical formula of Zn3(ASC)(OH). The chirality of ascorbic acid results in a chiral framework, as bioNICS-1 crystallizes in the space group _I_213. The
arrangement of Zn(II)-centred polyhedra can be simplified as triangular motifs with tetrahedra on their corners, resulting LCV topology (Fig. 1d)57. Even though the ascorbate ligands are not
part of the topological description, their role in linking of the cations is crucial for the formation of the framework, since the absence of ascorbate does not yield any precipitate nor is
this structure possible to obtain with a different linker. A similar arrangement of inorganic units can be found for example in MIL-77(Ni) structure17, but for Zn-based SBUs this is
unprecedented. A complex system of polyhedral inorganic chains forms two types of intersecting channels with type A running along each unit cell axis and type B running along [111] defined
by eight-membered and six-membered rings, having diameters of 6.5 and 5.5 Å respectively (Fig. 1a, b). The open framework structure of bioNICS-1 was confirmed by N2 sorption isothermal
measurements. Type I isotherm classified by IUPAC indicates permanent microporosity with BET surface area of 553 m2 g−1 (Fig. 2a, Supplementary Figs. 7 and 8) and micropore volume of 0.22
cm3 g−1. Pore size distribution using NLDFT method shows a bimodal distribution of micropores with the peaks centred at 5.6 and 6.6 Å belonging to B and A channel types respectively, already
described above. BioNICS-1 so represents the first case of vitamin C-based MOF material with proven permanent microporosity. Moreover, the high density of Zn(II) sites within bioNICS-1
structure suggests the acid nature of the MOF framework. Indeed, results of temperature-programmed desorption (TPD) of ammonia shown in Fig. 2b and Supplementary Fig. 9 confirms that
approximately 1/3 of the total Zn(II) cations (2.6 mmol g−1) that builds the structure represent mostly weak acid sites that are potentially accessible for therapeutic gasotransmitters, such
as nitric oxide (NO), hydrogen sulfide (H2S) or carbon monoxide (CO)58. STRUCTURE SOLUBILITY IN AQUEOUS MEDIA Due to the open structure, bioNICS-1 is considered as a potential drug carrier.
In addition, because of the nature of building units, the framework itself can represent a source of bioactive components, which can have, with suitable dosing, a therapeutic effect. The
material was first tested for structure thermal stability (Supplementary Figs. 11 and 12) and solubility in water media (see Supplementary Note 1 for details) in order to investigate the
release of the material’s constituents. To approximate bioNICS-1 behaviour to realistic conditions of human organism-environment, the tests included the influence of three different
parameters: (a) the possible impact of pH. For that purpose, water and saline solutions were adapted to pH values of 3.5, 7.4 and 9.0; (b) the presence of ionic species that are most
commonly present in body fluids. For that purpose, saline (0.9% NaCl) and phosphate buffer solution (PBS), in addition to the demineralized water, were used; and (c) the influence of
environment dynamics on the framework. Therefore, the stability tests were performed in steady state and under stirring. Structural stability of material was studied with PXRD and
BET-specific surface measurements of recovered products in comparison to the untreated material. The rate of released Zn(II) cations within the supernatant was also measured by atomic
absorption spectrometry (AAS) analysis in order to better correlate with the degradation, assuming that the detected concentration of released Zn(II) is directly linked with the rate of
framework degradation. In all cases, the majority of the material remains undissolved in water, saline and PBS solutions after 24 h, with its structural integrity remaining generally
unchanged after treatment. However, diffraction peak broadening and decrease of their heights occur due to the decrease in crystallite size. The additional appearance of Zn-oxalate phase,
which is formed as a consequence of partial degradation of ascorbic to oxalic acid, can be observed as well (Fig. 3a and Supplementary Fig. 12). (a) pH of the media does not have a marginal
effect on the material’s degree of solubility or degradation. The pH values of the supernatant settle at around 6 after 24 h, regardless of the initial pH value, type of media and dynamics.
This is however confirmed to be normal, since ascorbic acid being released into the supernatant acts as an efficient buffer in wide range of pH values59. (b) Evidently, the porosity is
preserved to a large extent (up to 68 %) for the products exposed to demineralized water (Supplementary Figs. 14, 16 and Supplementary Table 4). The decrease of the surface area is most
probably affected by the presence of Zn-oxalate phase within the bulk material. The presence of additional ionic species affects the pore accessibility even further. That is, by inducing the
solubility of the parent MOF due to the increase of the ionic strength, as in the case of saline solution, or by provoking progressive degradation of the MOF framework due to the anion
replacement with the ligand, which can take place in phosphate buffer solution60,61. As expected, the framework degrades to a larger extent in saline solutions, which is consistent with the
trend of BET surface area values and 24 h the degradation rate (release of zinc). Modification of micropores during the degradation tests (Supplementary Fig. 15) follows the same trend as a
decrease in BET specific surface area however; average pore size is not significantly affected by dissolution media. The amount of released Zn(II), however, remains under 10% in all cases
(Fig. 3b and Supplementary Fig. 13). Zinc release in PBS solution at dynamic conditions is comparable to the one where the material was exposed to water. On the other side, bioNICS-1 show
profoundly higher insolubility (below 1%) in PBS when leaving the mixture undisturbed. STEM-EDXS elemental mapping revealed that Zn phosphate-based shell with a thickness of ~10 nm is formed
in such conditions (Fig. 3c) which apparently decelerates subsequent dissolution of the Zn-ascorbate framework. (c) This recrystallization process is apparently more pronounced when the
material is subjected to dynamic as opposed to static conditions. Nevertheless, relatively high stability of bioNICS-1, even in acidic or basic starting conditions, is unusual for Zn-based
MOF systems and can be attributed to the sum of specific structural features such as high connectivity of the pentadentate ascorbic acid molecule to Zn(II) centres, the high dimensionality
of inorganic building units and the rigidity of the framework62. The most pronounced effect of dynamics is observed when stirring is applied in PBS solution where ~5% of Zn(II) is released
from the framework (Fig. 3b and Supplementary Fig. 13) and recrystallization to oxalate is most notable (Fig. 3a), resulting in a decrease of microporosity to about 28 % of the parent value
(Supplementary Figs. 14, 16 and Supplementary Table 4). These results however are reassuring, because they imply that the framework can be readily dissolved in conditions that are (out of
tested ones), most closely related to the body’s environment. Therefore, future encapsulated drugs are going to be released and constituents of bioMOF further metabolized. Additional
information of the degradation process was investigated by the release of organic (ligand) constituents from bioNICS-1 framework by liquid NMR (Supplementary Fig. 17). The released ascorbic
acid in aqueous media (water or saline) in all cases additionally degrades into threonic acid representing a common metabolite of a vitamin C63. However, 13C NMR spectra of the bioNICS-1
degradation in PBS solutions indicate the presence of two additional unknown C4 molecular species, which most probably occur as a result of threonic acid esterification with phosphate
anions. KINETICS AND MECHANISM OF STRUCTURE DEGRADATION In a further attempt to investigate the degradation mechanism of bioNICS-1 under different aqueous conditions, the release kinetics
was studied (Fig. 4a). For demineralized water, saline and phosphate buffer solutions the pH was adjusted to 7.4. Zn(II) concentrations were measured by AAS from liquid solutions sampled
after specified times of stirring, ranging from 5 min to 7 days. The release of Zn(II) shows similar trends for all used media, which cannot be described with any specific kinetic model
established for drug release64,65. The Zn(II) cation release is entirely governed by the framework dissolution rate, rather than diffusion through the inert matrix, as presumed by the afore
mentioned models. In the case of bioNICS-1, the release profile shows two stages. Initial dissolution of the bioNICS-1 framework within the first 6 h appears as a slight burst release of
Zn(II) cations followed by a relatively slow dissolution process in a controllable manner, which follows a concentration-independent zero-order rate profile. The framework dissolution is
most likely inhibited by the formation of insoluble Zn(II)-oxo—based species due to the ligand exchange in the shell domains of the crystallite, which significantly slows down the subsequent
dissolution of the framework (Fig. 4d). The dissolution rate is, as expected, the fastest in saline solutions and slowest in PBS solutions, releasing 18 and 5% of Zn2+ from the bioMOF
framework, respectively. The mechanism was confirmed in the case of bioNICS-1 degradation in PBS solution, representing the most biologically relevant medium. The formation of
phosphate-based nanodomains on the bioNICS-1 crystallites has already been proven by TEM elemental mapping as described earlier in the text. On the other hand, the XRD patterns of the
recovered solids from PBS revealed the gradual formation of additional Zn-oxalate phase, as a result of partial degradation of ascorbic acid to oxalic acid (Fig. 4c). Both phases cannot be
complementarily detected using diffraction and microscopic methods. Zn-phosphate domains are of amorphous nature and therefore not detectable by XRD, on the other hand, Zn-oxalate is not
reliably distinguishable from Zn-ascorbate phase by TEM elemental mapping due to the similar zinc content in both investigated phases. The formation of both types of insoluble domains nicely
coincides with the release kinetic profile. Initial dissolution of bioNICS-1 framework is reflected in slight burst release of Zn(II) cations within the first 6 h of treatment. Afterwards,
the release process stabilizes for the remaining time to PBS exposure in linear progression, due to the formation of phosphate- and oxalate-based phases on the shell domains of the
crystallites. In addition, the relative crystallinity of the bioNICS-1 framework deteriorates simultaneously with the recrystallization process of bioNICS-1 to Zn-oxalate (Fig. 4b, and
Supplementary Fig. 18). It can be concluded that the release of Zn(II) cations is subjected to a controllable manner for most of the treatment time and apparently becomes governed by the
solubility of Zn-oxalate rather than bioNICS-1 itself with increasing time of exposure in the solution. The proposed mechanism can be easily projected to the bioNICS-1 degradation in
demineralized water and saline solution as well. The Zn(II) release rates in such conditions are faster as in the case of treatment in PBS solution where the presence of phosphate-based
shell domains additionally inhibits the dissolution process. DRUG LOADING As the intended application of the material is drug delivery and to prove that small-molecule drugs can be
encapsulated and subsequently released from the microporous framework, bioNICS-1 was loaded with urea which is a model drug for topical application and cosmetic agent9,66,67. Namely, the
size of the pores between 5.5. and 6.5 Å, hydrophilic nature of the framework with accessible acid sites as confirmed by ammonia TPD and suitable structural stability in water media, makes
the bioNICS-1 suitable host for urea loading. Other—small—therapeutic molecules such as hydroxycarbamide, dimethyl fumarate, phosphonomethanoic acid and penicillamine68 could also be loaded
into the pores. Drug loading was done via a simple impregnation method where activated material was dispersed in an ethanol solution of urea for 48 h. The TG/DTG curve of the urea-loaded and
dried material (bioNICS-1@urea) show a weight loss of 11.5% in the temperature region between 100 and 220 °C, which is not observed in the TG curve of the pristine bioNICS-1 (Fig. 5a and
Supplementary Fig. 20). The efficient encapsulation of urea drug within the micropores of bioNICS-1 is also indicated by the significant decrease of BET specific surface area from 553 to 150
m2/g for the pristine and bioNICS-1@urea materials respectively as deduced from the N2 sorption isotherms (Fig. 5b). The absence of the urea-corresponded peaks in the XRD pattern of loaded
material additionally confirms that the drug is confined within the micropores rather than recrystallized separately or on the surface of the material (Supplementary Fig. 19). The capability
of drug release was demonstrated by the immersion of bioNICS-1@urea in demineralized water for 12 h. The urea drug was identified in the supernatant by liquid 1H NMR analysis (inset in Fig.
5a and Supplementary Fig. 21). Quantitative evaluation of the NMR spectra showed that 9–10 wt.% of the loaded material corresponds to the released urea. With other words, 80–90% of the
loaded drug is released in an aqueous medium within 12 h, which is triggered by the partial degradation of bioNICS-1 framework as described in details above. CONCLUSION A new MOF containing
bio-compatible Zn(II) cations and ascorbic acid as the main constituents were developed using facile solvothermal synthesis with the use of non-toxic solvent ethanol. The first reported case
of Zn-ascorbate MOF (bioNICS-1) is excelled by several unique structural features—diverse Zn(II) coordination geometry forming an infinite three-dimensional inorganic building unit bridged
through ascorbate ligands, resulting in three-dimensional ascorbate-based framework structure with permanent microporosity and accessible acid metal sites. BioNICS-1 was thoroughly examined
for structural stability and solubility in different aqueous conditions using water, saline and PBS solutions adjusted at pH values from 3.5 to 9 under stirring or static conditions.
Framework rigidity and a high degree of Zn(II) binding via all five hydroxyl-type oxygen atoms provides relatively high structure stability and so enable controllable release of bioactive
components. The highest solubility rate is achieved in saline solutions, where ~9% of the framework’s Zn(II) cations were released and about 40% of its initial porosity was preserved after
24 h of stirring. On the other hand, phosphate ions in PBS solution significantly inhibit the framework dissolution due to the formation of less soluble oxalate and phosphate-based domains
on the surface of crystallites as evidenced by XRD, TEM and NMR analysis. BioNICS-1 solubility is almost pH-independent due to the strong buffering effect of the released ascorbic acid
settling the pH of the media at an approximate value of 6. Kinetic studies showed that regardless of the media type, the framework dissolution undergoes two regimes. A slight burst effect is
observed within the first 6 h, followed by a slow dissolution described in zero-order kinetics, releasing 5 and 18% of Zn2+ from bioNICS-1 in PBS and saline solution respectively, within 7
days. The proposed dissolution of the framework is suppressed by the formation of Zn-oxalate shell domains on the surface of the bioNICS-1 crystallites. The accessibility of the micropores
was confirmed by successful impregnation with model drug—urea and its efficient release triggered by the partial framework degradation in aqueous media. Overall, the slow dissolution and
recrystallization process of bioNICS-1 with a burst effect, which could no dubitably be well managed with suitable shaping, makes the material an exceptional and highly promising candidate
for a controlled small drug delivery system provided by its framework microporosity. METHODS SYNTHETIC APPROACHES BioNICS-1 was prepared using EtOH as a solvent with Zn/ASC ratio of 2:1
using conventional heating. Typically 0.752 g of zinc(II) acetate dehydrate (Sigma Aldrich) and 0.3 g of _L_-ascorbic acid (Sigma Aldrich) was added to 10 ml of ethanol (Sigma Aldrich). The
reaction mixture was heated at 120 °C for 1 day in Teflon-lined Stainless-steel autoclaves. Larger independent crystals for the structural analysis were prepared with the addition of acetic
acid (Alfa Aesar). The optimum EtOH/acetic acid volume ratio was 10:1 (1 ml of acetic acid in 10 ml of ethanol). CHARACTERIZATION METHODS X-ray powder diffraction data of the samples were
collected on a PANalytical X’Pert PRO high-resolution diffractometer with CuKa radiation (_λ_ = 1.5406 Å) in the range from 5 to 60° (2θ) with the step of 0.034° per 100 s using fully opened
100 channel X’Celerator detector. For the purposes of Rietveld refinement of the crystal structure model, the XRD powder data was collected on the same equipment using transmission mode in
the range from 5 to 90° 2θ with the step of 0.016°/300 s. Prior to the measurement, the bioNICS-1 sample was degassed at 150 °C overnight and sealed in glass capillary. Morphological
properties and size estimation of the samples was observed by scanning electron microscopy measurements (SEM) on Zeiss Supra™ 3VP field-emission gun microscope. Elemental analysis was
performed by energy dispersive X-ray analysis with an INCA Energy system attached to the above described microscope and by Perkin Elmer 2400 Series II CHNS analyser. The structure of
bioNICS-1 was solved from 3DED data, collected using a JEOL JEM-2100 LaB6 transmission electron microscope. Details of the structure analysis are provided in the Supplementary Methods
section. The thermal analysis (TG/DTG) was performed on a Q5000 IR thermogravimeter (TA Instruments, Inc.). The measurements were carried out in air flow of 10 ml/min, by heating samples
from 25 to 700 °C at the rate of 10 °C min−1. Temperature-programmed X-ray powder diffraction pattern of samples was recorded also on the PANalyticalX’Pert PRO diffractometer additionally
equipped with a high-temperature sample cell, from room temperature to 500 °C in steps of 50 °C in static air. N2 sorption isotherms measurements were performed on Quantachrome AUTOSORB iQ3.
The specific surface areas were determined by Brunauer–Emmett–Teller (BET) method based on the N2 sorption isotherms measured at 77 K in p/po relative pressure range between 4 × 10−2 and 6
× 10−3 selected according to Roquerol plots. Before the measurement, samples were activated under vacuum at 150 °C for 15 h. The temperature-programmed desorption (TPD) experiments were
performed using the Micromeritics AutoChem II 2920 apparatus. The sample (120 mg) was positioned inside a U-shaped quartz reactor and pre-treated in a flow Ar at 150 °C for 180 min. After
pre-treatment, the latter was cooled to 50 °C and saturated with 10% NH3 in helium for 30 min. Weakly adsorbed NH3 was removed in a flow of pure He for 60 min. The samples were heated to 800
°C at 20 °C/min and NH3 desorption was monitored by a mass spectrometer (Pfeiffer Vacuum Thermostar) following the characteristic _m/z_ fragments. Pulses of 0.5 mL 10% NH3 in He (Messer)
were used as an external standard for NH3-TPD signal calibration. STEM-EDXS elemental mapping was performed on a probe Cs-corrected Jeol ARM 200CF Scanning Transmission Electron Microscope
equipped with a Centurio Energy Dispersive X-ray Spectroscopy (EDXS) system with a 100 mm2 SDD detector was used for imaging and acquisition of elemental mappings. THE AQUEOUS STABILITY TEST
250 mg of activated bioNICS-1 was suspended in 25 mL of purified water and saline solution, pH was regulated with HCl and NaOH to reach the values of 3.5; 7.4 and 9.0. The test was done in
parallels; one of them was left undisturbed, the other exposed to dynamic conditions on a circular plane mixer for 24 h. Afterwards the samples were left to settle down before separately
collecting the supernatant and the sediment for further analysis. The filtered sediment was left to dry on air over night and its identity was checked and confirmed with PXRD and N2
isotherms analysis. The same procedure was repeated with phosphate buffer solution at pH 7.4. Supernatants were collected and analysed for the presence of organic compounds by NMR
spectroscopy. 10% D2O was added to 0.5 ml of each sample. The samples were transferred to 5 mm NMR tubes and 1H NMR spectra were recorded on a Bruker Avance Neo 600 MHz NMR spectrometer with
a QCI cryo probe at 25 °C using excitation sculpting to suppress the water signal. In addition, 13C, 1H-13C HSQC and 1H-13C HMBC NMR spectra were recorded on the sample of supernatant after
treatment in phosphate buffer solution under dynamic conditions to obtain more detail about the nature of compounds in the sample. Chemical shifts were referenced externally to the chemical
shift of NaTMSP (δH 0 ppm, δC 0 ppm). ZN(II) RELEASE KINETICS The test was a kinetic modification of the previously described procedure presented by Howarth et al. Briefly, 250 mg of
activated bioNICS-1 was suspended in 25 mL of 3 different media (purified water, saline and phosphate buffer solution), which represented about 5 mg of Zn/1 mL media. Solutions had pH of 7.4
which was regulated with HCl and/or NaOH. Samples of release medium (5 mL) were collected for analysis at set time intervals (1, 2, 4, 6, 12, 24, 48, 72, 96 and 168 h) using a syringe with
a 0.80 µm filter attached, and supplemented with the same volume of fresh media. This way the volume of the release medium stayed constant throughout the test, and _sink_ conditions were
maintained. The concentration of Zn(II) cations within the investigated solutions were determined by PerkinElmer AAnalyst 200 flame atomic absorption spectrometer (AAS). The concentration of
the released Zn(II) was calculated using Eq. 1, where Ct(corr.) is the corrected concentration at the time t, Ct is the apparent concentration at the time t, v is the volume of the sample
taken and V is the total volume of the dissolution medium.
$${{{{{{\rm{C}}}}}}}_{{{{{{\rm{t}}}}}}}({{{{{\rm{corr}}}}}}.)={{{{{{\rm{C}}}}}}}_{{{{{{\rm{t}}}}}}}+\frac{{{{{{\rm{v}}}}}}}{{{{{{\rm{V}}}}}}}\mathop{\sum
}\limits_{0}^{{{{{{\rm{t}}}}}}-1}{{{{{{\rm{C}}}}}}}_{{{{{{\rm{t}}}}}}}$$ (1) FRAMEWORK DEGRADATION KINETICS IN PBS 85 mg of activated bioNICS-1 was suspended in 8,5 mL of PBS pH 7.4 and
immediately exposed to dynamic conditions on a circular plane mixer. At a set time point (5 min, 10 min, 20 min, 40 min, 1 h, 2 h, 4 h, 8 h, 12 h, 24 h, 48 h, 72 h, 96 h) individual samples
were filtered, dried on air over night and sediments identity checked with PXRD. The relative crystallinity of the bioNICS-1 material recovered from PBS solution after different times of
exposure was estimated by comparison of peak intensities corresponding to 011 reflection occurring at 6.0° 2θ. The weight contribution of Zn-oxalate which is formed during the above
mentioned process was calculated from Rietveld quantification analysis using TOPAS Academic V6 software package69. DRUG LOADING AND RELEASE EXPERIMENTS Activated bioNICS-1 (200 mg) was
suspended in EtOH solution of urea (25 mg mL−1) for 48 h under constant stirring. Impregnation was done in two parallels, one at room temperature and one at 40 °C. Both residues were
filtered and washed with EtOH, then dried on air over night before proceeding with further tests. The recovered product was immersed in demineralized water for 12 h. After that the
supernatant was qualitatively and quantitatively evaluated by liquid 1H NMR. DATA AVAILABILITY The data generated during this study are included in the manuscript and the Supplementary
Information. REFERENCES * Furukawa, H., Cordova, K. E., O’Keeffe, M. & Yaghi, O. M. The chemistry and applications of metal-organic frameworks. _Science_ 341, 1230444 (2013). Article
PubMed Google Scholar * Li, S. L. & Xu, Q. Metal-organic frameworks as platforms for clean energy. _Energy Environ. Sci._ 6, 1656–1683 (2013). Article CAS Google Scholar * Li, J.
R., Kuppler, R. J. & Zhou, H. C. Selective gas adsorption and separation in metal-organic frameworks. _Chem. Soc. Rev._ 38, 1477–1504 (2009). Article CAS PubMed Google Scholar *
Baumann, A. E., Burns, D. A., Liu, B. & Thoi, V. S. Metal-organic framework functionalization and design strategies for advanced electrochemical energy storage devices. _Commun. Chem._
2, 1–14 (2019). Article Google Scholar * Morozan, A. & Jaouen, F. Metal organic frameworks for electrochemical applications. _Energy Environ. Sci._ 5, 9269 (2012). Article CAS Google
Scholar * Horcajada, P. et al. Metal–organic frameworks in biomedicine. _Chem. Rev._ 112, 1232–1268 (2012). Article CAS PubMed Google Scholar * Horcajada, P. et al. Metal–organic
frameworks as efficient materials for drug delivery. _Angew. Chem._ 118, 6120–6124 (2006). Article Google Scholar * Rieter, W. J., Taylor, K. M. L., An, H., Lin, W. & Lin, W. Nanoscale
metal−organic frameworks as potential multimodal contrast enhancing agents. _J. Am. Chem. Soc._ 128, 9024–9025 (2006). Article CAS PubMed PubMed Central Google Scholar * Horcajada, P.
et al. Porous metal–organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. _Nat. Mater._ 9, 172–178 (2010). Article CAS PubMed Google Scholar *
Rojas, S., Arenas-Vivo, A. & Horcajada, P. Metal-organic frameworks: a novel platform for combined advanced therapies. _Coord. Chem. Rev._ 388, 202–226 (2019). Article CAS Google
Scholar * An, J. et al. Metal-adeninate vertices for the construction of an exceptionally porous metal-organic framework. _Nat. Commun._ 3, 604 (2012). Article PubMed Google Scholar *
An, J. & Rosi, N. L. Tuning MOF CO2 adsorption properties via cation exchange. _J. Am. Chem. Soc._ 132, 5578–5579 (2010). Article CAS PubMed Google Scholar * Li, T. et al. Systematic
modulation and enhancement of CO2: N2selectivity and water stability in an isoreticular series of bio-MOF-11 analogue. _Chem. Sci._ 4, 1746–1755 (2013). Article CAS Google Scholar *
Tranchemontagne, D. J., Tranchemontagne, J. L., O’keeffe, M. & Yaghi, O. M. Secondary building units, nets and bonding in the chemistry of metal–organic frameworks. _Chem. Soc. Rev._ 38,
1257–1283 (2009). Article CAS PubMed Google Scholar * Schoedel, A., Li, M., Li, D., O’Keeffe, M. & Yaghi, O. M. Structures of metal–organic frameworks with rod secondary building
units. _Chem. Rev._ 116, 12466–12535 (2016). Article CAS PubMed Google Scholar * Dybtsev, D. N., Chun, H., Yoon, S. H., Kim, D. & Kim, K. Microporous Manganese formate: a simple
metal-organic porous material with high framework stability and highly selective gas sorption properties. _J. Am. Chem. Soc._ 126, 32–33 (2004). Article CAS PubMed Google Scholar *
Guillou, N., Livage, C., Drillon, M. & Férey, G. The chirality, porosity, and ferromagnetism of a 3D nickel glutarate with intersecting 20-membered ring channels. _Angew. Chem. Int. Ed._
42, 5314–5317 (2003). Article CAS Google Scholar * Rood, J. A., Noll, B. C. & Henderson, K. W. Synthesis, structural characterization, gas sorption and guest-exchange studies of the
lightweight, porous metal-organic framework α-[Mg 3(O2CH)6]. _Inorg. Chem._ 45, 5521–5528 (2006). Article CAS PubMed Google Scholar * Shankar, A. H. & Prasad, A. S. Zinc and immune
function: the biological basis of altered resistance to infection. _Am. J. Clin. Nutr._ 68, 447S–463S (1998). Article CAS PubMed Google Scholar * Trumbo, P., Yates, A. A., Schlicker, S.
& Poos, M. Dietary reference intakes. _J. Am. Diet. Assoc._ 101, 294–301 (2001). Article CAS PubMed Google Scholar * Chasapis, C. T., Ntoupa, P. S. A., Spiliopoulou, C. A. &
Stefanidou, M. E. Recent aspects of the effects of zinc on human health. _Arch. Toxicol._ 94, 1443–1460 (2020). Article CAS PubMed Google Scholar * Roney, N. et al. ATSDR evaluation of
the health effects of zinc and relevance to public health. _Toxicol. Ind. Health_ 22, 423–493 (2006). Article CAS PubMed Google Scholar * Plum, L. M., Rink, L. & Hajo, H. The
essential toxin: impact of zinc on human health. _Int. J. Environ. Res. Public Health_ 7, 1342–1365 (2010). Article CAS PubMed PubMed Central Google Scholar * Delahaut, V. et al.
Toxicity and bioaccumulation of Cadmium, Copper and Zinc in a direct comparison at equitoxic concentrations in common carp (Cyprinus carpio) juveniles. _PLoS One_ 15, e0220485 (2020).
Article CAS PubMed PubMed Central Google Scholar * Pasquet, J. et al. The contribution of zinc ions to the antimicrobial activity of zinc oxide. _Colloids Surf. A Physicochem. Eng.
Asp._ 457, 263–274 (2014). Article CAS Google Scholar * Tamames-Tabar, C. et al. A Zn azelate MOF: combining antibacterial effect. _CrystEngComm_ 17, 456–462 (2015). Article CAS Google
Scholar * Horcajada, P. et al. Metal-organic frameworks in biomedicine. _Chem. Rev._ 112, 1232–1268 (2012). Article CAS PubMed Google Scholar * Levine, D. J. et al. Olsalazine-based
metal-organic frameworks as biocompatible platforms for H2 adsorption and drug delivery. _J. Am. Chem. Soc._ 138, 10143–10150 (2016). Article CAS PubMed Google Scholar * Su, H. et al. A
highly porous medical metal-organic framework constructed from bioactive curcumin. _Chem. Commun._ 51, 5774–5777 (2015). Article CAS Google Scholar * Miller, S. R. et al. A rare example
of a porous Ca-MOF for the controlled release of biologically active NO. _Chem. Commun._ 49, 7773–7775 (2013). Article CAS Google Scholar * Rojas, S., Devic, T. & Horcajada, P. Metal
organic frameworks based on bioactive components. _J. Mater. Chem. B_ 5, 2560–2573 (2017). Article CAS PubMed Google Scholar * Gould, J. A., Jones, J. T. A., Bacsa, J., Khimyak, Y. Z.
& Rosseinsky, M. J. A homochiral three-dimensional zinc aspartate framework that displays multiple coordination modes and geometries. _Chem. Commun._ 46, 2793 (2010). Article CAS
Google Scholar * Lin, W. et al. A porphyrin-based metal–organic framework as a pH-responsive drug carrier. _J. Solid State Chem._ 237, 307–312 (2016). Article CAS Google Scholar * Lu,
K., He, C. & Lin, W. Nanoscale metal–organic framework for highly effective photodynamic therapy of resistant head and neck cancer. _J. Am. Chem. Soc._ 136, 16712–16715 (2014). Article
CAS PubMed PubMed Central Google Scholar * Lu, K., He, C. & Lin, W. A chlorin-based nanoscale metal–organic framework for photodynamic therapy of colon cancers. _J. Am. Chem. Soc._
137, 7600–7603 (2015). Article CAS PubMed PubMed Central Google Scholar * Abrahams, B. F., Moylan, M., Orchard, S. D. & Robson, R. Zinc saccharate: a robust, 3D coordination network
with two types of isolated, parallel channels, one hydrophilic and the other hydrophobic. _Angew. Chem. Int. Ed._ 42, 1848–1851 (2003). Article CAS Google Scholar * Sha, J. et al.
Unprecedented α-cyclodextrin metal-organic frameworks with chirality: structure and drug adsorptions. _Polyhedron_ 127, 396–402 (2017). Article CAS Google Scholar * Rajkumar, T., Kukkar,
D., Kim, K.-H., Sohn, J. R. & Deep, A. Cyclodextrin-metal–organic framework (CD-MOF): from synthesis to applications. _J. Ind. Eng. Chem._ 72, 50–66 (2019). Article CAS Google Scholar
* Pinto, R. V. et al. Vitamin B3metal-organic frameworks as potential delivery vehicles for therapeutic nitric oxide. _Acta Biomater._ 51, 66–74 (2017). Article CAS PubMed Google
Scholar * Lu, J. Y. & Babb, A. M. A new 3-D neutral framework coordination polymer constructed via square pyramidal binuclear Cu(II) and nicotinato ligand. _Inorg. Chem. Commun._ 4,
716–718 (2001). Article CAS Google Scholar * Miller, S. R. et al. Biodegradable therapeutic MOFs for the delivery of bioactive molecules. _Chem. Commun._ 46, 4526–4528 (2010). Article
CAS Google Scholar * Marriott, B., Birt, D., Stalling, V. & Yates, A. ‘Vitamin C’ Present Knowledge in Nutrition Basic Nutrition and Metabolism. in _Present Knowledge in Nutrition
Basic Nutrition and Metabolism_ 155–170 (Elsevier Inc., 2020). https://doi.org/10.1016/C2018-0-02422-6 * Hearn, R. A. & Bugg, C. E. Calcium binding to carbohydrates: crystal structure of
calcium ascorbate dihydrate. _Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem._ 30, 2705–2711 (1974). Article Google Scholar * Hvoslef, J. Changes in conformation and bonding
of ascorbic acid by ionization. The crystal structure of sodium ascorbate. _Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem._ 25, 2214–2223 (1969). Article CAS Google Scholar *
Hollis, L. S., Amundsen, A. R. & Stern, E. W. Synthesis, structure, and antitumor properties of platinum complexes of vitamin C. _J. Am. Chem. Soc._ 107, 274–276 (1985). Article CAS
Google Scholar * Hughes, D. L. Crystal structure of thallium(I)L-ascorbate. _J. Chem. Soc. Dalt. Trans_. https://doi.org/10.1039/dt9730002209 (1973). * Stahl, K., Andersen, J. E. T. &
Christgau, S. Strontium diibuprofenate dihydrate, strontium malonate sesquihydrate, strontium diascorbate dihydrate and strontium 2-oxidobenzoate hydrate at 120 K. _Acta Crystallogr. Sect. C
Cryst. Struct. Commun_. 62, (2006). * Nath, M., Jairath, R., Eng, G., Song, X. & Kumar, A. New organotin(IV) ascorbates: synthesis, spectral characterization, biological and
potentiometric studies. _Spectrochim. Acta—Part A Mol. Biomol. Spectrosc._ 61, 77–86 (2005). Article Google Scholar * Dong, J., Zhao, D., Lu, Y. & Sun, W.-Y. Photoluminescent
metal–organic frameworks and their application for sensing biomolecules. _J. Mater. Chem. A_ 7, 22744–22767 (2019). Article CAS Google Scholar * Yue, D. et al. A two-dimensional
metal-organic framework as a fluorescent probe for ascorbic acid sensing. _Eur. J. Inorg. Chem._ 2018, 173–177 (2018). Article CAS Google Scholar * Das, G. S., Shim, J. P., Bhatnagar, A.,
Tripathi, K. M. & Kim, T. Y. Biomass-derived carbon quantum dots for visible-light-induced photocatalysis and label-free detection of Fe(III) and ascorbic acid. 9, 15084 (2019). *
Kumar, S. et al. Green synthesis of metal–organic frameworks: a state-of-the-art review of potential environmental and medical applications. _Coord. Chem. Rev._ 420, 213407 (2020). Article
CAS Google Scholar * Alder, C. M. et al. Updating and further expanding GSK’s solvent sustainability guide. _Green. Chem._ 18, 3879–3890 (2016). Article CAS Google Scholar * Byrne, F.
P. et al. Tools and techniques for solvent selection: green solvent selection guides. _Sustain. Chem. Process._ 4, 1–24 (2016). Article Google Scholar * Starsich, F. H. L., Herrmann, I. K.
& Pratsinis, S. E. Nanoparticles for Biomedicine: coagulation during synthesis and applications. _Annu. Rev. Chem. Biomol. Eng_. 10, 155–174 (2019). * Aulton, M. & Taylor, K.
_Aulton’s Pharmaceutics The Design and Manufacture of Medicines_. (Elsevier, 2017). * Friedrichs, O. D., O’Keeffe, M. & Yaghi, O. M. Three-periodic nets and tilings: semiregular nets.
_Acta Crystallogr. Sect. A Found. Crystallogr._ 59, 515–525 (2003). Article Google Scholar * Zhang, M., Qiao, R. & Hu, J. Engineering metal–organic frameworks (MOFs) for controlled
delivery of physiological gaseous transmitters. _Nanomaterials_ 10, 1134 (2020). Article CAS PubMed Central Google Scholar * Liu, S., Ellars, C. E. & Edwards, D. S. Ascorbic acid:
useful as a buffer agent and radiolytic stabilizer for metalloradiopharmaceuticals. _Bioconjug. Chem._ 14, 1052–1056 (2003). Article CAS PubMed Google Scholar * Li, X. et al. New
insights into the degradation mechanism of metal-organic frameworks drug carriers. _Sci. Rep._ 7, 13142 (2017). Article CAS PubMed PubMed Central Google Scholar * Bellido, E. et al.
Understanding the colloidal stability of the mesoporous MIL-100(Fe) nanoparticles in physiological media. _Langmuir_ 30, 5911–5920 (2014). Article CAS PubMed Google Scholar * Qadir, N.
U., Said, S. A. M. & Bahaidarah, H. M. Structural stability of metal organic frameworks in aqueous media—Controlling factors and methods to improve hydrostability and hydrothermal cyclic
stability. _Microporous Mesoporous Mater._ 201, 61–90 (2015). Article CAS Google Scholar * Englard, S. & Seifter, S. The biochemical functions of ascorbic acid. _Annu. Rev. Nutr._ 6,
365–406 (1986). Article CAS PubMed Google Scholar * Al Haydar, M., Abid, H. R., Sunderland, B. & Wang, S. Metal organic frameworks as a drug delivery system for flurbiprofen. _Drug
Des. Devel. Ther._ 11, 2685–2695 (2017). Article CAS PubMed PubMed Central Google Scholar * Cunha, D. et al. Rationale of drug encapsulation and release from biocompatible porous
metal-organic frameworks. _Chem. Mater._ 25, 2767–2776 (2013). Article CAS Google Scholar * Verzì, A. E., Musumeci, M. L., Lacarrubba, F. & Micali, G. History of urea as a
dermatological agent in clinical practice. _Int. J. Clin. Pract_. 74 (Suppl 187), e13621 (2020). * [Urea as an antimicrobial and dehydration agent for the local treatment of suppurative
surgical infection]—PubMed. https://pubmed.ncbi.nlm.nih.gov/6382770/. (Accessed 21 October 2021). * Lowe, D. The Smallest Drugs | Science | AAAS.
https://www.science.org/content/blog-post/smallest-drugs (2014). * Coelho, A. A. TOPAS and TOPAS-Academic: an optimization program integrating computer algebra and crystallographic objects
written in C++. _J. Appl. Crystallogr._ 51, 210–218 (2018). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We would like to thank Dr. Janvit Teržan from the National
Institute of Chemistry, Slovenia for performing the temperature-programmed desorption experiments. Financing from the Slovenian Research Agency program (P1-0021) is acknowledged. AUTHOR
INFORMATION AUTHORS AND AFFILIATIONS * National Institute of Chemistry, Hajdrihova 19, 1000, Ljubljana, Slovenia Tia K. Tajnšek, Uroš Javornik, Goran Dražić, Nataša Zabukovec Logar &
Matjaž Mazaj * Faculty of Inorganic Chemistry and Technology, University of Ljubljana, Večna pot 113, 1000, Ljubljana, Slovenia Tia K. Tajnšek * Stockholm University, Frescativägen 8, 106
91, Stockholm, Sweden Erik Svensson Grape & Tom Willhammar * Ruđer Bošković Institute, Bijenička cesta 54, 1000, Zagreb, Croatia Tatjana Antonić Jelić * University of Nova Gorica,
Vipavska 13, 5000, Nova Gorica, Slovenia Nataša Zabukovec Logar Authors * Tia K. Tajnšek View author publications You can also search for this author inPubMed Google Scholar * Erik Svensson
Grape View author publications You can also search for this author inPubMed Google Scholar * Tom Willhammar View author publications You can also search for this author inPubMed Google
Scholar * Tatjana Antonić Jelić View author publications You can also search for this author inPubMed Google Scholar * Uroš Javornik View author publications You can also search for this
author inPubMed Google Scholar * Goran Dražić View author publications You can also search for this author inPubMed Google Scholar * Nataša Zabukovec Logar View author publications You can
also search for this author inPubMed Google Scholar * Matjaž Mazaj View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS T.K.T. and M.M.
performed the majority of the synthesis and experimental work, N.Z.L. contributed in manuscript writing, E.S.G. and T.W. performed the structure analysis by ED, T.A.J. performed AAS
analysis, U.J. covered NMR measurements, G.D. performed STEM-EDS elemental mapping. All authors have given approval to the final version of the manuscript. CORRESPONDING AUTHOR
Correspondence to Matjaž Mazaj. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Communications Chemistry_ thanks
Tiantian Kong and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available. ADDITIONAL INFORMATION PUBLISHER’S NOTE
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION PEER REVIEW FILE
RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and
reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes
were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If
material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain
permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS
ARTICLE Tajnšek, T.K., Svensson Grape, E., Willhammar, T. _et al._ Design and degradation of permanently porous vitamin C and zinc-based metal-organic framework. _Commun Chem_ 5, 24 (2022).
https://doi.org/10.1038/s42004-022-00639-x Download citation * Received: 27 October 2021 * Accepted: 04 February 2022 * Published: 25 February 2022 * DOI:
https://doi.org/10.1038/s42004-022-00639-x SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative