Play all audios:
ABSTRACT The catalytic conversion of CO2 into valuable chemicals is an effective strategy for reducing its adverse impact on the environment. In this work, the formation of formic acid via
CO2 hydrogenation on bare and ligated Ti6Se8 clusters is investigated with gradient-corrected density functional theory. It is shown that attaching suitable ligands (i.e., PMe3, CO) to a
metal-chalcogenide cluster transforms it into an effective donor/acceptor enabling it to serve as an efficient catalyst. Furthermore, by controlling the ratio of the attached donor/acceptor
ligands, it is possible to predictably alter the barrier heights of the CO2 hydrogenation reaction and, thereby, the rate of CO2 conversion. Our calculation further reveals that by using
this strategy, the barrier heights of CO2 hydrogenation can be reduced to ~0.12 eV or possibly even lower, providing unique opportunities to control the reaction rates by using different
combinations of donor/acceptor ligands. SIMILAR CONTENT BEING VIEWED BY OTHERS UNIFIED MECHANISTIC UNDERSTANDING OF CO2 REDUCTION TO CO ON TRANSITION METAL AND SINGLE ATOM CATALYSTS Article
25 November 2021 ORDERED SINGLE ACTIVE SITES FOR CASCADE HYDROGENATION AND HYDROFORMYLATION REACTIONS Article 26 May 2025 ATOMICALLY PRECISE ULTRASMALL COPPER CLUSTER FOR ROOM-TEMPERATURE
HIGHLY REGIOSELECTIVE DEHYDROGENATIVE COUPLING Article Open access 28 October 2023 INTRODUCTION The rapid increase of the CO2 level in the atmosphere has become a serious concern to mankind
in recent times1,2. One of the direct solutions to the problem is utilizing porous or mesoporous adsorbents as a medium for CO2 capture3. However, the high cost associated with storage and
transport restricts such procedures from large-scale industrial applications4. As an alternative, the conversion of CO2 into useful chemicals represents a relatively cost-effective
strategy4,5,6. Aside from reducing CO2 concentration in the atmosphere, the converted products can also be utilized as a resource for value-added chemicals. Among the assortment of chemicals
that CO2 can be chemically converted into, formic acid (FA) represents a compelling choice for a multitude of reasons7,8,9. As a chemical, formic acid is extensively used as feedstock
material and can also be transformed into value-added products with relative ease. As an energy-dense material, it can also be used as an alternative to fossil fuels, thereby being effective
in reducing the carbon footprint. Additionally, having a high volumetric hydrogen density, it also has immense potential as an effective hydrogen storage vector8. At room temperature,
formic acid is a low-toxic liquid; therefore, the storage and transportation of formic acid are significantly cost-efficient. As a result of these advantages, a significant amount of
worldwide CO2 conversion is now performed in the form of formic acid, and the net conversion quantity is rising at a rapid pace every year4,8. One of the major challenges of converting CO2
to formic acid is the inherent inertness of CO2. Being thermodynamically and kinetically stable10, CO2 hydrogenation without an effective catalyst is a difficult task under normal
conditions. Nowadays, a range of homogeneous and heterogeneous catalysts are available for CO2 hydrogenation, and the reaction can be carried out thermochemically or electrochemically as
required11,12,13,14,15,16,17,18. One of the focuses of this current paper is to evaluate the potential of the metal chalcogenide clusters (e.g., Ti6Se8) toward thermochemical CO2 conversion
to formic acid. In recent times, ligated metal chalcogenide clusters have gained considerable attention due to their high stability and ease of synthesis in a solvent medium19,20,21,22,23.
These stable ligated clusters can also be assembled as ionic solids with complementary units like fullerenes. In the past decades, significant numbers of such clusters and assemblies have
been experimentally synthesized by Roy, Nuckolls, and coworkers20,21,22,23,24,25. In such solids, the ligand-protected cluster cores usually act as charge donors, whereas the fullerene
moieties are charge acceptors, resulting in extended three-dimensional ionic crystals that are similar in structure to CdI2 or NaCl. Incidentally, it was also shown26,27,28,29,30,31,32,33
that within such solids, the ligated clusters maintain their chemical identity, and the attached ligands control the donor/acceptor characteristics of the clusters, providing a unique
opportunity to predictably alter the properties of the whole superstructure altogether. Historically, ligands have always played a pivotal role in cluster chemistry34,35,36,37. Apart from
protecting the sensitive cluster core or preventing a cluster from coalescence or leaching, they can also be utilized to electronically stabilize a metal cluster by filling up the valence
shell to the nearby magic number. In some rare instances, it was observed that ligands could also enhance the reactivity of a cluster via the formation of localized active sites or
donor-acceptor pairs38,39,40,41. In that regard, the effect of ligands on the electronic structure of the metal chalcogenide cluster is observed to be unique. It has been
shown26,27,28,29,30,31,32,33 that by controlling the number and type of the attached ligands, one can transform a metal chalcogenide cluster into a strong electron donor or an acceptor by
shifting the whole electronic spectrum without altering the valence shell configuration. For example, we have shown that strong σ-donor ligands like phosphines induce an upward shift of the
electronic spectrum, whereas a shift in the reverse direction is noticed for strong π-acceptor ligands like CO. The overall effect of the ligands in such cases can be conceptualized as a
Coulombic well that surrounds the cluster core and thereby influencing the discrete energy levels of the cluster. In the present paper, we have considered Ti6Se8 as a model cluster catalyst
and investigated its catalytic potential toward formic acid synthesis via CO2 hydrogenation. Our calculation of the minimum energy pathway reveals that in comparison to other reported
catalysts, the unligated Ti6Se8 cluster is a really good catalyst for CO2 hydrogenation with significantly low barrier heights ranging from ~ 0.3-0.4 eV42,43,44. However, what sets the
Ti6Se8 cluster apart from any conventional catalyst, is not the lower barriers for hydrogenation but rather the dependence of the barrier heights on the attached ligands. Controlling the
rate of a chemical reaction has always remained an elusive goal for chemists and materials scientists. However, apart from a few exceptional examples, reports of such achievements are rare.
Among the notable examples, controlling reactivity by microwave irradiation45 is observed earlier for an electron transfer reaction. Recently, Pan and Liu46 have shown an interesting example
where control of chemical reactivity is achieved by Fermi-coupled vibrational states. In this work, we show that a similar goal can also be achieved by ligated Ti6Se8 clusters simply by
varying the number and type of the attached ligands. It is observed that attaching π-acceptor ligands, e.g., CO, to the cluster results in an increase of hydrogenation barriers compared to
pristine Ti6Se8, whereas σ-donor ligands like PMe3 reduce the barriers for the reaction. Thus, by selectively controlling the ratio of attached acceptor and donor ligands to the cluster, one
can increase or decrease the barrier heights for CO2 hydrogenation in a stepwise manner. Our calculation further indicates that by attaching only 3 PMe3 ligands to the cluster, one of the
CO2 hydrogenation barriers can be reduced to as low as 0.12 eV. Investigation of the electronic structure of the ligated clusters proves that the alteration of the barrier heights is due to
the ligand-induced shift in the electronic levels. The upward shift of the electronic levels by PMe3 facilitates the electron transfer as well as energetically destabilizes the
cluster-hydrogen bond and assists the release of H atom(s) and, thereby, the reaction. An opposite effect hinders the reactivity when one or more CO ligands are introduced. Thus, we have
shown that apart from protecting the cluster core, ligands can also be utilized as a tool for controlling the reactivity of the cluster. In regard to that, our current investigation offers a
simplistic, cost-efficient, and easily achievable approach to the problem. RESULTS CALCULATION OF THE REACTION PATHWAY OF CO2 → HCOOH CONVERSION ON THE TI6SE8 CLUSTER To investigate the
catalytic potential of the metal chalcogenide cluster toward CO2 hydrogenation, we have chosen the Ti6Se8 cluster as our template catalyst. In previous investigations, titanium-doped
nanoparticles and surfaces have shown remarkable promises and performances toward CO2 reduction reactions47,48,49,50. Our choice of Ti6Se8 cluster is influenced by such experimental and
theoretical results. It is noteworthy that although the specific Ti6Se8 cluster reported in this paper is not yet reported experimentally, similar metal chalcogenide clusters with identical
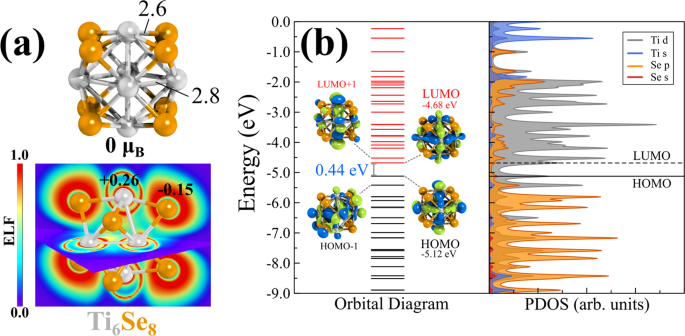
stoichiometric compositions are chemically synthesized20,21,22,23,24,25. The optimized ground state geometry of Ti6Se8 is a distorted face-capped octahedron with 0 μB spin magnetic moment.
Figure 1a shows the ground state optimized geometry of the Ti6Se8 cluster along with some bond lengths (in Å). The structure with 2 μB spin magnetic moment is ~0.14 eV higher in energy
compared to the ground state geometry (see Supplementary Table 1). The HOMO-LUMO gap, adiabatic ionization energy (AIE), and adiabatic electron affinity (AEA) of the ground state (0 μB)
cluster are 0.44, 6.79, and 2.98 eV, respectively. Electron localization function (ELF) calculation (Fig. 1a) further reveals that the electrons are mostly localized on the Se atoms, which
also explains the observed negative Hirshfeld51 charge (−0.15 |e|) on the selenium atoms. In contrast, the Ti atoms are seen to be positively charged (+0.26 | e |). The projected density of
states (PDOS) and the molecular orbital (MO) diagram of the cluster is included in Fig. 1b. The PDOS diagram and the frontier orbital isosurfaces clearly show that both the HOMO and LUMO are
composed of Ti-d orbitals, and Se has minor contributions. As a next step, we proceed to compute the favorable reaction pathway of CO2 hydrogenation on the Ti6Se8 cluster catalyst. Figure
2a shows a general schematic of the CO2 → HCOOH conversion on a cluster surface. For the present study, we have considered that the first step of the reaction is the adsorption of
dissociated H atoms on the cluster surface, which is followed by the adsorption of a CO2 molecule at an adjacent site of the cluster. Our calculation (see Supplementary Fig. 1) reveals that
the H2 dissociation barrier on the Ti6Se8 cluster is 1.08 eV (PBE/TZ2P); however, dissociated H atoms produced by any other experimental methods52,53,54,55 can also be used for the reaction
as alternatives. Since the adsorption sites of CO2 and H atoms are different, the sequence by which they are adsorbed on the cluster surface is irrelevant to the present reaction. Further
discussion regarding this and related arguments, specifically for the Ti6Se8 cluster, is given later on. Following the adsorption steps, we have found that the reaction has the possibility
to proceed via two pathways depending on to which atom of the CO2 molecule (C or O) the first H atom gets transferred. As shown in Fig. 2a, we have designated these two pathways as (A) and
(B) in red and blue color, respectively. In pathway A, the first H gets transferred to the nearest O atom of the CO2 molecule resulting in a COOH intermediate, and subsequent transfer of the
2nd H to the C atom of the intermediate yields the formic acid. In path B, the reaction proceeds via an HCOO intermediate since the first H gets attached to the C atom of CO2. The formic
acid is then produced via the transfer of the second hydrogen from the cluster surface to the nearest O atom of the intermediate. In Fig. 2b, we have shown both the calculated pathways on
Ti6Se8 clusters. As we have shown in the schematics, the first step is considered to be the adsorption of the H atoms on the cluster surface. Although we expected that the H atoms would bind
only to the Ti or Se site, upon adsorption, it was noticed that the dissociated H atoms favorably formed bridging bonds with the Ti and one of the nearby Se atoms with a net binding energy
of 3.47 eV. This is evident by looking into the geometric structure of intermediate 2, as shown in Fig. 2b. This is due to chalcogens like S or Se being able to form a much stronger bond
with H compared to Ti. Wiberg bond indices (WBI) calculated by Natural bond orbital (NBO) analysis56 further revealed that the Se−H bond order is significantly higher (0.82-0.85) compared to
the Ti−H bond order (0.07-0.09), showing that the Se−H interaction is the dominant one. Important to note that after H adsorption, the ground state spin-only magnetic moment is also altered
to 2μB. The bridging orientation of H and stronger Se−H interaction proves two things. First, even after the adsorption of H atoms, the Ti site remains free for CO2 adsorption. So there is
minimal competition between H and CO2 adsorption on the cluster as long as the number of H atoms is reasonably low, and hence their adsorption order (i.e., which one among H or CO2 is
adsorbed first) does not influence the calculated reaction pathway. Second, since the interaction of the dissociated H atoms is much stronger with Se than Ti, the presence of nearby H atoms
does not affect the binding energy of the incoming CO2 at a lower H concentration. Therefore, it is expected that the reaction parameters will remain the same as long as the number of
adsorbed H that are in close vicinity to CO2 remains low. Hence, although here we have considered the whole reaction pathway by coadsorbing both the hydrogen atoms, sequential adsorption,
i.e., one H atom at a time, should not alter the reaction parameters. These observations and conclusions were also confirmed in previous investigations based on the Mo6S8 cluster57,58. It is
noteworthy that the computed reaction pathways presented here are all based on the aforementioned assumptions, i.e., the number of adsorbed H atoms remains low. The influence of a higher
number of adsorbed H on the reaction mechanism demands a separate study and is not the current focus of the paper. Following the H adsorption, the resulting cluster complex, i.e.,
intermediate 2 gets stabilized by 0.34 eV via the adsorption of CO2 on the Ti atom. This leads to structure 3, as shown in Fig. 2b. We have observed that, in contrast to H, the CO2 molecule
prefers to bind with the Ti site via one of the O atoms. Upon adsorption, the molecule adopts an angular orientation with respect to the cluster surface. As shown previously, starting from
3, the reaction can possibly proceed via two different pathways, namely A and B respectively. We have investigated both the pathways for Ti6Se8 clusters, and the barrier heights are included
in Fig. 2b. From the diagram, it is seen that the two barrier heights of the first (4*) and second (6*) H transfer along pathway A (COOH intermediate, shown as red-colored 5 in Fig. 2b) are
0.54 and 0.59 eV, respectively. In contrast, the reaction pathway via the HCOO intermediate (i.e., pathway B) shows significantly lower barrier heights, 0.31 and 0.44 eV, respectively. The
lowering of the second barrier height in pathway B can be attributed to the lowering of the intermediate (5) along with the transition states (6*), as shown in Fig. 2b. Due to the lower
barriers, it is evident that the CO2 → HCOOH conversion on the Ti6Se8 clusters will preferably proceed via pathway B and through the HCOO intermediate (blue-colored 5 in Fig. 2b) despite the
energies of the final products (7 in Fig. 2b) from both pathways are near identical. The sum of energies of the free cluster and formic acid are included in the same figure as 8 for
reference purposes. It is also important to note that the ground state spin moments do not remain constant throughout the reaction. A second spin alteration is noticed after the first
transition state. Thus, although 2μB spin magnetic moment is maintained till 4*, beginning from intermediate 5 in both A and B pathways, the ground state moment has altered to 0μB, which
remained the same till the end of the reaction. The relative energies of all species in the reaction pathway considering the different magnetic moments are provided in the Supplementary
Table 2 and 3. EFFECT OF LIGANDS ON THE REACTION PATHWAY OF CO2 → HCOOH CONVERSION Following the calculation of the most favorable CO2 → HCOOH conversion route on the pristine Ti6Se8
cluster, we proceed to understand how ligands will influence the barrier heights of CO2 hydrogenation. To achieve this, we have chosen two different dative ligands, namely, PMe3 and CO.
Based on the electronic effect, these two ligands differ widely from each other. The PMe3 ligand is a strong σ-donor but a poor π-acceptor. In contrast, the CO ligand is a strong electron
donor as well as a good π-acceptor. Starting from the pristine Ti6Se8, we have sequentially increased the number of attached ligands with the Ti atoms of the cluster. In all cases, we have
kept the reaction site unligated so that the reaction could proceed without any hindrance. In this present work, we have restricted ourselves to a maximum of three ligands of each type. The
major reason behind this is that we have observed that going beyond three ligands (especially for PMe3) results in a major steric crowding in the vicinity of the reaction site. This results
in a weakening of CO2/H binding and subsequent desorption from the cluster surface. Our test calculation on Ti6Se8(CO)3 cluster (see Supplementary Fig. 2) indicated that pathway B remains
energetically favorable even after ligand attachment. Hence, for the ligated systems, only pathway B is computed and included in this study. Figures 3a, b show the reaction pathway of CO2 →
HCOOH conversion on the Ti6Se8(PMe3)3 and Ti6Se8(CO)3 clusters, respectively. The results for the rest of the ligated clusters (i.e., Ti6Se8(PMe3)n and Ti6Se8(CO)n, n = 1-2) are included in
the Supplementary Table 4–6. As shown, the trend of the barrier heights for CO2 hydrogenation on the ligated clusters is observed to be very interesting. As reported, for all the CO-ligated
clusters, including Ti6Se8(CO)3, both the CO2 hydrogenation barriers are shown to be higher compared to the Ti6Se8 cluster. It is interesting to note that the first barriers for all three
Ti6Se8(CO)n clusters are nearly the same ~0.40 eV. However, the second barrier is observed to increase by ~0.03-0.04 eV with the increment of the number of the attached CO ligands one at a
time (see Supplementary Table 6). In contrast, an opposite trend is noticed for Ti6Se8(PMe3)n clusters. All the PMe3 attached clusters show relatively lower barrier heights compared to
Ti6Se8 for CO2 hydrogenation. In the case of PMe3 ligated clusters, both the hydrogenation barriers are observed to decrease with the increment of the number of ligands attached to the
cluster. However, the first hydrogenation barrier falls off more rapidly compared to the second one. In the case of Ti6Se8(PMe3)3 cluster (Fig. 3a), the first hydrogenation barrier was
calculated as 0.12 eV, which is a significant reduction compared to that of Ti6Se8 (i.e., 0.31 eV). The alteration of the second CO2 hydrogenation barrier (0.38 eV) was not as drastic as the
first one, and in this case, a reduction of 0.06 eV was noticed compared to for Ti6Se8 cluster (0.44 eV). As shown in Fig. 3, upon analyzing the relative energies of all the chemical
species, we have observed the overall reduction of the second hydrogenation barrier in the case of Ti6Se8(PMe3)3 cluster compared to Ti6Se8(CO)3 is due to the energetic lowering of the
transition state (6*) as well as due to the energetic destabilization of the respective intermediate (5). Both of these factors simultaneously result in a lower second hydrogenation barrier
for the Ti6Se8(PMe3)3 cluster. At this point, it is important to mention that we have observed that using a hybrid functional (e.g., PBE0) instead of PBE is altering the barrier heights
marginally (see Supplementary Fig. 3). Therefore, it is expected that even with a different level of theory, the relative trends and the conclusions drawn herewith will still remain the
same. So far, we have shown that the barrier heights of CO2 hydrogenation on Ti6Se8 can effectively be decreased/increased by decorating the cluster with σ-donor (e.g., PMe3) or π-acceptor
(e.g., CO) ligands. This provides a unique opportunity to predictably alter the reactivity of a cluster simply by changing the ratio of the attached ligands of both types. To further
elaborate on this possibility, we started from the Ti6Se8(PMe3)3 cluster and sequentially replaced one PMe3 with one CO ligand at each step till we reached Ti6Se8(CO)3 cluster. The reaction
profiles of these two intermediate clusters, i.e., [Ti6Se8(PMe3)3-m(CO)m] (m = 1,2) clusters along with all the optimized geometries are shown in Fig. 4. The relative energies of all species
in the reaction pathway considering the different magnetic moments of [Ti6Se8(PMe3)3-m(CO)m] (m = 1,2) clusters are included the Supplementary Tables 7–8. As expected, both the barrier
heights of these two clusters are observed to be intermediate in magnitude compared to the barrier heights obtained for the Ti6Se8(PMe3)3 and Ti6Se8(CO)3 clusters (Fig. 3). In Fig. 5, we
have combined the barrier heights of both hydrogenation steps for all these four ligated clusters, i.e., [Ti6Se8(PMe3)3-m(CO)m] (m = 0-3), and as shown, it is now evident that by
sequentially changing the ratio of the attached PMe3 and CO ligands, both the hydrogenation barriers can be altered in a controlled and stepwise manner. The range of alteration in the first
and second barriers at each step is observed to be 0.07–0.13 and 0.02–0.10 eV, respectively. It is important to mention that by observing the stepwise reduction of barrier heights, one can
argue that some of the barrier height differences between the adjacent step are not that significant considering the error range of the DFT methodology; however, it is impossible to ignore
the observed systematic trend as the Fig. 5 show a near-smooth decrease of both the hydrogenation barriers as we sequentially increase the number of attached PMe3 ligands. This observation
is crucial since, by using such strategies, it might be possible for experimentalists to predictably alter and control the rate of a particular reaction and hence the reactivity of the
cluster. Moreover, by using a larger cluster with more available sites for ligand attachment and by using a stronger donor, the magnitude of stepwise barrier height reduction can further be
increased. Additionally, it is noteworthy that although the stepwise reduction in barrier height for the present cluster may be low in some cases, the overall reduction of the hydrogenation
barrier heights (0.14-0.28 eV, considering both hydrogenation steps) from Ti6Se8(CO)3 to Ti6Se8(PMe3)3 cluster is reasonably significant. UNDERSTANDING THE LIGAND-INDUCED REACTIVITY
ALTERATION OF THE TI6SE8 CLUSTER TOWARD CO2 CONVERSION We now consider the underlying electronic effects that are responsible for such alteration of the reactivity of the clusters. To
achieve this, we have plotted the molecular orbital (MO) diagram and the projected density of states (PDOS) (Fig. 6) of intermediate 2 for the four ligated clusters. An alternative version
of the same figure, along with the electronic structure of Ti6Se8 cluster, is included in the Supplementary Fig. 4. As shown earlier (Figs. 3 and 4), intermediate 2 is obtained via the
adsorption of the H atoms on the cluster surface, and hence it can be considered one of the crucial species in the reaction pathway. From Fig. 6, we can see that increasing the number of
PMe3 ligands at each step (right to left for 6A and top to bottom for 6B) creates an energetical upward shift of the whole electronic (MO) spectrum of intermediate 2. This shift is observed
for both spin channels of intermediate 2 with minimal alteration in the HOMO-LUMO gaps of the same. It is noteworthy that the upward shift has also not perturbed the order of molecular
orbitals or the occupation number of both spin channels (intermediate 2). In Fig. 6, we have also included the MO diagram and PDOS of a free CO2 molecule for reference purposes. The
ligand-induced upward energetic shift of intermediate 2 results in the alteration of multiple important properties. First, the upward shift of the electronic (MO) spectrum results in the
reduction of the energetic gap between the HOMO of intermediate 2 and LUMO of the free CO2 molecule, thereby favoring the orbital overlap and the charge transfer during the course of the
reaction. As one can see from Fig. 6, the respective gap is considerably lower, i.e., 3.2 eV for [Ti6Se8(PMe3)3H2] cluster compared to 4.3 eV as obtained for [Ti6Se8(CO)3H2] cluster.
Additionally, due to the ligand-induced upward shift of the electronic (MO) spectrum, intermediate 2 also becomes a better electron donor due to the reduction in the ionization energy. In
Fig. 7, we have shown the trends in the ionization energy of intermediate 2 for all four clusters. As depicted, the adiabatic ionization energy (AIE) of the [Ti6Se8(PMe3)3H2] is observed to
be significantly lower (5.08 eV) compared to the AIE of the [Ti6Se8(CO)3H2] cluster (6.34 eV) which further facilitates the charge transfer from the intermediate 2 to CO2. This argument can
also be proven simply by looking at the accumulated charges on the CO2 molecule at the first transition state (i.e., 4* in Figs. 3 and 4). The Hirshfeld51 charge on CO2 at the respective
transition state is more negative (-0.36 | e | ) in the Ti6Se8(PMe3)3 pathway compared to the Ti6Se8(CO)3 pathway (-0.26 | e | ). The rest of the two clusters show intermediate charges on
CO2 and also follow the expected trend (see Supplementary Table 9). Apart from facilitating the charge transfer, it is also observed that the ligand-induced shift alters the binding energy
of H atoms as well. Figure 7 also shows the net H binding energies (i.e., the binding energy of the H atom pair with the ligated cluster) for all four intermediates (2), and as shown,
increasing the number of PMe3 ligands results in a reduction of the same. According to our calculation, the net H binding energy has reduced monotonically from 3.45 eV to 3.26 eV as we move
from [Ti6Se8(CO)3H2] to [Ti6Se8(PMe3)3H2] cluster. One possible explanation for such reduction is that as the electronic spectrum shifts upward, the bonding orbitals of the attached
hydrogens get energetically destabilized, thereby facilitating their release from the cluster surface. In other words, the upward shifting of the electronic (MO) spectrum along with the H
bonding molecular orbitals results in the weakening of the bond and, thereby, the binding energy with the cluster. To summarize, we can conclude that the ligand-induced upward shifting of
the electronic spectrum assists the H release from the cluster surface and also expedites the charge transfer from the cluster to CO2 by improving the orbital overlap and reducing the
ionization energy. Since the overall ligand-induced alteration of the H binding energy is smaller compared to the AIE, we think that the facilitation of the charge transfer due to the donor
ligand attachment plays a dominant role in the observed barrier height reduction. Therefore, controlling the degree of the shift via ligands, as shown in this article, provides a way to
predictably alter the barrier heights and hence the reactivity of the cluster. DISCUSSION The present study provides valuable insight into how the rate of CO2 conversion to formic acid can
be controlled by attaching suitable combinations of donor/acceptor ligands to the Ti6Se8 cluster catalyst. Our investigation reveals that attaching σ-donor (e.g., PMe3)/ π-acceptor (e.g.,
CO) ligands to the cluster induces an upward/downward shift of the electronic spectrum of the cluster without altering the sequence or the occupancy of the electronic levels. We have further
shown that such ligand-induced alteration of the electronic levels also results in a change in the reactivity of the cluster in a predictable manner. This provides a unique opportunity to
alter the barrier heights of the CO2 conversion in a progressive and predictable manner simply by attaching different combinations of donor/acceptor ligands to the cluster. Analysis of our
computational results reveals that the alteration of the barrier is due to two different electronic effects. First, the upward shift of the electronic levels induces by the donor ligands
lowers the ionization energy of the cluster and thereby facilitates the charge transfer toward CO2. Second, the same shift also weakens the binding of H atoms to the cluster and hence
assists the H-transfer process. While the present studies are based on Ti6Se8 clusters, the insight gained into the various mechanisms controlling the barrier should be applicable to other
metal chalcogenide clusters as well. It is noteworthy that the influence of other donor/acceptor ligands and the effect of various solvents on the barrier heights remain as open questions
that demand separate in-depth studies. Despite the fact, as mentioned earlier, using synthetic chemical techniques, it is now possible to synthesize the ligated metal chalcogenide clusters
in solutions. Therefore, we hope the present study will motivate experimental investigation into the facile synthesis of formic acid, thereby reducing the environmental CO2 concentration and
converting it into useful products using metal chalcogenide clusters. METHODS COMPUTATIONAL DETAILS All the reported results in this paper are calculated by using the Amsterdam Density
Functional (ADF) package59. The gradient-corrected Perdew, Burke, and Ernzerhof (GGA-PBE) exchange-correlation functional is used for all computations60. The Slater-type triple ζ basis sets
with two polarization functions (TZ2P) and large frozen electron cores are used for all the elements61,62. The frozen core orbitals are expressed in an auxiliary set of Slater-type basis
functions that is different from the valence set. The scalar relativistic corrections are incorporated via the zero-order regular approximation (ZORA)63,64. The Hessian-based quasi-Newton
method, without any symmetry constraints, is utilized for all optimizations65. To check the validity of the optimized structures, analytical frequency calculations66,67 are performed, and it
is ensured that all the normal modes of vibrations for the minima structures are real and positive, whereas all the transition states are first-order saddle points having only one imaginary
frequency with significant magnitude. To verify that the respective transition states are connected to the reported minima at the left and the right-hand side of the transition state, the
intrinsic reaction coordinate (IRC) calculations68 are also performed. Since the geometry of all the transition states remained similar even after ligand attachment, the IRC calculations are
performed only for the pristine cluster. This is also due to the high computational costs associated with each IRC calculation. The dispersion correction is included via Grimme’s DFT-D3
method with the Becke−Johnson damping69. The natural bond orbital (NBO) calculations are performed with NBO 6.0 program56 as implemented within ADF59 package. It is also confirmed that the
spin contamination errors associated with all the open shell systems are negligible and within a reasonable level. A wide range of spin multiplicities are investigated during all
optimizations, including for all the species along the reaction pathways, and only the ground state structures were selected for all cases. DATA AVAILABILITY The optimized cartesian
coordinates of the transition state structures, relative energies, barrier heights, and Hirshfeld charges are given in the Supplementary Information. Any additional data reported herewith
are available from the corresponding authors upon reasonable request. REFERENCES * Rosa, E. A. & Dietz, T. Human drivers of national greenhouse-gas emissions. _Nat. Clim. Change_ 2,
581–586 (2012). Article CAS Google Scholar * Hardy, J. T. _Climate change: causes, effects, and solutions_. (John Wiley & Sons), (2003). * Singh, G. et al. Emerging trends in porous
materials for CO2 capture and conversion. _Chem. Soc. Rev._ 49, 4360–4404 (2020). Article CAS PubMed Google Scholar * MacDowell, N. et al. An overview of CO2 capture technologies.
_Energy Environ. Sci._ 3, 1645–1669 (2010). Article CAS Google Scholar * Taheri Najafabadi, A. CO2 chemical conversion to useful products: an engineering insight to the latest advances
toward sustainability. _Int. J. Energy Res._ 37, 485–499 (2013). Article CAS Google Scholar * Song, C. Global challenges and strategies for control, conversion and utilization of CO2 for
sustainable development involving energy, catalysis, adsorption and chemical processing. _Catal. Today_ 115, 2–32 (2006). Article CAS Google Scholar * Ma, Z., Legrand, U., Pahija, E.,
Tavares, J. R. & Boffito, D. C. From CO2 to formic acid fuel cells. _Ind. Eng. Chem. Res._ 60, 803–815 (2020). Article Google Scholar * Moret, S., Dyson, P. J. & Laurenczy, G.
Direct synthesis of formic acid from carbon dioxide by hydrogenation in acidic media. _Nat. Commun._ 5, 1–7 (2014). Article Google Scholar * Weilhard, A., Argent, S. P. & Sans, V.
Efficient carbon dioxide hydrogenation to formic acid with buffering ionic liquids. _Nat. Commun._ 12, 1–7 (2021). Article Google Scholar * Reda, T., Plugge, C. M., Abram, N. J. &
Hirst, J. Reversible interconversion of carbon dioxide and formate by an electroactive enzyme. _Proc. Natl Acad. Sci._ 105, 10654–10658 (2008). Article CAS PubMed PubMed Central Google
Scholar * Saeidi, S. et al. Mechanisms and kinetics of CO2 hydrogenation to value-added products: A detailed review on current status and future trends. _Renew. Sustain. Energy Rev._ 80,
1292–1311 (2017). Article CAS Google Scholar * Ye, R.-P. et al. CO2 hydrogenation to high-value products via heterogeneous catalysis. _Nat. Commun._ 10, 1–15 (2019). Article Google
Scholar * Yang, H. et al. A review of the catalytic hydrogenation of carbon dioxide into value-added hydrocarbons. _Catal. Sci. Technol._ 7, 4580–4598 (2017). Article CAS Google Scholar
* Saeidi, S. et al. Recent advances in CO2 hydrogenation to value-added products—Current challenges and future directions. _Prog. Energy Combust. Sci._ 85, 100905 (2021). Article Google
Scholar * Bai, S.-T. et al. Homogeneous and heterogeneous catalysts for hydrogenation of CO2 to methanol under mild conditions. _Chem. Soc. Rev._ 50, 4259–4298 (2021). Article CAS PubMed
Google Scholar * Wiedner, E. S. & Linehan, J. C. Making a splash in homogeneous CO2 hydrogenation: elucidating the impact of solvent on catalytic mechanisms. _Chem. Eur. J._ 24,
16964–16971 (2018). Article CAS PubMed Google Scholar * Nitopi, S. et al. Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. _Chem. Rev._ 119,
7610–7672 (2019). Article CAS PubMed Google Scholar * Li, W. et al. A short review of recent advances in CO2 hydrogenation to hydrocarbons over heterogeneous catalysts. _RSC Adv._ 8,
7651–7669 (2018). Article CAS PubMed PubMed Central Google Scholar * Corrigan, J. F., Fuhr, O. & Fenske, D. Metal chalcogenide clusters on the border between molecules and
materials. _Adv. Mater._ 21, 1867–1871 (2009). Article CAS Google Scholar * Champsaur, A. M. et al. Two-dimensional nanosheets from redox-active superatoms. _ACS Cent. Sci._ 3, 1050–1055
(2017). Article CAS PubMed PubMed Central Google Scholar * Pinkard, A., Champsaur, A. M. & Roy, X. Molecular clusters: nanoscale building blocks for solid-state materials. _Acc.
Chem. Res._ 51, 919–929 (2018). Article CAS PubMed Google Scholar * Roy, X. et al. Nanoscale atoms in solid-state chemistry. _Science_ 341, 157–160 (2013). Article CAS PubMed Google
Scholar * Yang, J. et al. Shape Matching in Superatom Chemistry and Assembly. _J. Am. Chem. Soc._ 142, 11993–11998 (2020). Article CAS PubMed Google Scholar * Gadjieva, N. A.,
Champsaur, A. M., Steigerwald, M. L., Roy, X. & Nuckolls, C. Dimensional Control of Assembling Metal Chalcogenide Clusters. _Eur. J. Inorg. Chem._ 2020, 1245–1254 (2020). Article CAS
Google Scholar * Lee, C. H. et al. Ferromagnetic ordering in superatomic solids. _J. Am. Chem. Soc._ 136, 16926–16931 (2014). Article CAS PubMed Google Scholar * Chauhan, V., Reber, A.
C. & Khanna, S. N. Metal chalcogenide clusters with closed electronic shells and the electronic properties of alkalis and halogens. _J. Am. Chem. Soc._ 139, 1871–1877 (2017). Article
CAS PubMed Google Scholar * Chauhan, V., Reber, A. C. & Khanna, S. N. Transforming Ni9Te6 from electron donor to acceptor via ligand exchange. _J. Phys. Chem. A_ 120, 6644–6649
(2016). Article CAS PubMed Google Scholar * Chauhan, V., Sahoo, S. & Khanna, S. N. Ni9Te6(PEt3)8C60 is a superatomic superalkali superparamagnetic cluster assembled material
(S3-CAM). _J. Am. Chem. Soc._ 138, 1916–1921 (2016). Article CAS PubMed Google Scholar * Reber, A. C., Chauhan, V. & Khanna, S. N. Symmetry and magnetism in Ni9Te6 clusters ligated
by CO or phosphine ligands. _J. Chem. Phys._ 146, 024302 (2017). Article PubMed Google Scholar * Liu, G. et al. Ligand Effect on the Electronic Structure of Cobalt Sulfide Clusters: A
Combined Experimental and Theoretical Study. _J. Phys. Chem. C._ 123, 25121–25127 (2019). Article CAS Google Scholar * Liu, G. et al. Tuning the electronic properties of hexanuclear
cobalt sulfide superatoms via ligand substitution. _Chem. Sci._ 10, 1760–1766 (2019). Article CAS PubMed Google Scholar * Chauhan, V., Reber, A. C. & Khanna, S. N. Strong lowering of
ionization energy of metallic clusters by organic ligands without changing shell filling. _Nat. Commun._ 9, 1–7 (2018). Article CAS Google Scholar * Reber, A. C., Bista, D., Chauhan, V.
& Khanna, S. N. Transforming Redox Properties of Clusters Using Phosphine Ligands. _J. Phys. Chem. C._ 123, 8983–8989 (2019). Article CAS Google Scholar * Jena, P. & Castleman, A.
W. _Nanoclusters: a bridge across disciplines_. (Elsevier), (2010). * Walter, M. et al. A unified view of ligand-protected gold clusters as superatom complexes. _Proc. Natl Acad. Sci._ 105,
9157–9162 (2008). Article CAS PubMed PubMed Central Google Scholar * Pichugina, D. A., Kuz’menko, N. E. & Shestakov, A. F. Ligand-protected gold clusters: the structure, synthesis
and applications. _Russ. Chem. Rev._ 84, 1114 (2015). Article CAS Google Scholar * Xu, W. W., Zeng, X. C. & Gao, Y. Application of electronic counting rules for ligand-protected gold
nanoclusters. _Acc. Chem. Res._ 51, 2739–2747 (2018). Article CAS PubMed Google Scholar * Roach, P. J., Woodward, W. H., Castleman, A. W. Jr, Reber, A. C. & Khanna, S. N.
Complementary active sites cause size-selective reactivity of aluminum cluster anions with water. _Science_ 323, 492–495 (2009). Article CAS PubMed Google Scholar * Reber, A. C. &
Khanna, S. N. Superatoms: electronic and geometric effects on reactivity. _Acc. Chem. Res._ 50, 255–263 (2017). Article CAS PubMed Google Scholar * Abreu, M. B., Powell, C., Reber, A. C.
& Khanna, S. N. Ligand-induced active sites: reactivity of iodine-protected aluminum superatoms with methanol. _J. Am. Chem. Soc._ 134, 20507–20512 (2012). Article CAS PubMed Google
Scholar * Luo, Z. et al. What determines if a ligand activates or passivates a superatom cluster? _Chem. Sci._ 7, 3067–3074 (2016). Article CAS PubMed PubMed Central Google Scholar *
Peng, G., Sibener, S. J., Schatz, G. C., Ceyer, S. T. & Mavrikakis, M. CO2 hydrogenation to formic acid on Ni (111). _J. Phys. Chem. C._ 116, 3001–3006 (2012). Article CAS Google
Scholar * Yan, G., Gao, Z., Zhao, M., Yang, W. & Ding, X. CO2 hydrogenation to formic acid over platinum cluster doped defective graphene: A DFT study. _Appl. Surf. Sci._ 517, 146200
(2020). Article CAS Google Scholar * Sarma, P. J. et al. Tuning the transition barrier of H2 dissociation in the hydrogenation of CO2 to formic acid on Ti-doped Sn2O4 clusters. _Phys.
Chem. Chem. Phys._ 23, 204–210 (2021). Article CAS PubMed Google Scholar * Kishimoto, F. et al. Remote control of electron transfer reaction by microwave irradiation: kinetic
demonstration of reduction of bipyridine derivatives on surface of nickel particle. _J. Phys. Chem. Lett._ 10, 3390–3394 (2019). Article CAS PubMed Google Scholar * Pan, H. & Liu, K.
Fermi-phase-induced interference in the reaction between Cl and vibrationally excited CH3D. _Nat. Chem._ 14, 545–549 (2022). Article CAS PubMed Google Scholar * Aljabour, A. et al.
Active sulfur sites in semimetallic titanium disulfide enable CO2 electroreduction. _ACS Catal._ 10, 66–72 (2019). Article Google Scholar * Hasan, M. R., Abd Hamid, S. B., Basirun, W. J.,
Suhaimy, S. H. M. & Mat, A. N. C. A sol–gel derived, copper-doped, titanium dioxide–reduced graphene oxide nanocomposite electrode for the photoelectrocatalytic reduction of CO2 to
methanol and formic acid. _RSC Adv._ 5, 77803–77813 (2015). Article CAS Google Scholar * Handoko, A. D. et al. Two-dimensional titanium and molybdenum carbide MXenes as electrocatalysts
for CO2 reduction. _Iscience_ 23, 101181 (2020). Article CAS PubMed PubMed Central Google Scholar * Esrafili, M. D. & Dinparast, L. A DFT study on the catalytic hydrogenation of CO2
to formic acid over Ti-doped graphene nanoflake. _Chem. Phys. Lett._ 682, 49–54 (2017). Article CAS Google Scholar * Hirshfeld, F. L. Bonded-Atom Fragments for Describing Molecular
Charge Densities. _Theor. Chim. Acta_ 44, 129–138 (1977). * Kaufmann, S., Schwarzer, D., Reichardt, C., Wodtke, A. M. & Bünermann, O. Generation of ultra-short hydrogen atom pulses by
bunch-compression photolysis. _Nat. Commun._ 5, 1–5 (2014). Article Google Scholar * Comerford, D. W., Smith, J. A., Ashfold, M. N. & Mankelevich, Y. A. On the mechanism of H atom
production in hot filament activated H2 and CH4/H2 gas mixtures. _J. Chem. Phys._ 131, 044326 (2009). Article PubMed Google Scholar * LaVerne, J. A. & Huestis, P. L. H Atom Production
and reaction in the gamma radiolysis of thermally modified boehmite. _J. Phys. Chem. C._ 123, 21005–21010 (2019). Article CAS Google Scholar * Kovács, T., Blitz, M. A. & Seakins, P.
W. H-atom yields from the photolysis of acetylene and from the reaction of C2H with H2, C2H2, and C2H4. _J. Phys. Chem. A_ 114, 4735–4741 (2010). Article PubMed Google Scholar *
Glendening, E. D., Landis, C. R. & Weinhold, F. NBO 6.0: Natural bond orbital analysis program. _J. Comput. Chem._ 34, 1429–1437 (2013). Article CAS PubMed Google Scholar * Liu, P.,
Choi, Y., Yang, Y. & White, M. G. Methanol synthesis from H2 and CO2 on a Mo6S8 cluster: a density functional study. _J. Phys. Chem. A_ 114, 3888–3895 (2010). Article CAS PubMed
Google Scholar * Liu, C. & Liu, P. Mechanistic study of methanol synthesis from CO2 and H2 on a modified model Mo6S8 cluster. _ACS Catal._ 5, 1004–1012 (2015). Article CAS Google
Scholar * te Velde, G. et al. Chemistry with ADF. _J. Comput. Chem._ 22, 931–967 (2001). Article Google Scholar * Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized Gradient
Approximation Made Simple. _Phys. Rev. Lett._ 77, 3865–3868 (1996). Article CAS PubMed Google Scholar * Van Lenthe, E. & Baerends, E. J. Optimized Slater-type basis sets for the
elements 1–118. _J. Comput. Chem._ 24, 1142–1156 (2003). Article PubMed Google Scholar * Chong, D. P., Van Lenthe, E., Van Gisbergen, S. & Baerends, E. J. Even-tempered slater-type
orbitals revisited: From hydrogen to krypton. _J. Comput. Chem._ 25, 1030–1036 (2004). Article CAS PubMed Google Scholar * Lenthe, E., van, Snijders, J. G. & Baerends, E. J. The
zero‐order regular approximation for relativistic effects: The effect of spin–orbit coupling in closed shell molecules. _J. Chem. Phys._ 105, 6505–6516 (1996). Article Google Scholar * Van
Lenthe, E., Ehlers, A. & Baerends, E.-J. Geometry optimizations in the zero order regular approximation for relativistic effects. _J. Chem. Phys._ 110, 8943–8953 (1999). Article Google
Scholar * Fan, L. & Ziegler, T. Optimization of molecular structures by self-consistent and nonlocal density-functional theory. _J. Chem. Phys._ 95, 7401–7408 (1991). Article CAS
Google Scholar * Bérces, A. et al. An implementation of the coupled perturbed Kohn-Sham equations: perturbation due to nuclear displacements. _Comput. Phys. Commun._ 100, 247–262 (1997).
Article Google Scholar * Jacobsen, H., Bérces, A., Swerhone, D. P. & Ziegler, T. Analytic second derivatives of molecular energies: a density functional implementation. _Comput. Phys.
Commun._ 100, 263–276 (1997). Article CAS Google Scholar * Deng, L. & Ziegler, T. The determination of Intrinsic Reaction Coordinates by density functional theory. _Int. J. Quantum
Chem._ 52, 731–765 (1994). Article CAS Google Scholar * Grimme, S., Ehrlich, S. & Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. _J.
Comput. Chem._ 32, 1456–1465 (2011). Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS The authors gratefully acknowledge funding by the US Air Force Office of
Scientific Research (AFOSR), Grant No. FA 9550-18-1-0511. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Physics, Virginia Commonwealth University, Richmond, VA, 23284-2000, USA
Turbasu Sengupta & Shiv N. Khanna Authors * Turbasu Sengupta View author publications You can also search for this author inPubMed Google Scholar * Shiv N. Khanna View author
publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS T.S. has carried out all the theoretical calculations. Both T.S. and S.N.K. have participated in the
scientific discussion and preparation of the manuscript. CORRESPONDING AUTHORS Correspondence to Turbasu Sengupta or Shiv N. Khanna. ETHICS DECLARATIONS COMPETING INTERESTS The authors
declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Communications Chemistry_ thanks the anonymous reviewers for their contribution to the peer review of this work.
ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION
SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation,
distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and
indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to
the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will
need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE
CITE THIS ARTICLE Sengupta, T., Khanna, S.N. Converting CO2 to formic acid by tuning quantum states in metal chalcogenide clusters. _Commun Chem_ 6, 53 (2023).
https://doi.org/10.1038/s42004-023-00851-3 Download citation * Received: 22 November 2022 * Accepted: 07 March 2023 * Published: 21 March 2023 * DOI:
https://doi.org/10.1038/s42004-023-00851-3 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative