Play all audios:
ABSTRACT Accessible drug modalities have continued to increase in number in recent years. Peptides play a central role as pharmaceuticals and biomaterials in these new drug modalities.
Although traditional peptide synthesis using chain-elongation from C- to N-terminus is reliable, it produces large quantities of chemical waste derived from protecting groups and
condensation reagents, which place a heavy burden on the environment. Here we report an alternative N-to-C elongation strategy utilizing catalytic peptide thioacid formation and oxidative
peptide bond formation with main chain-unprotected amino acids under aerobic conditions. This method is applicable to both iterative peptide couplings and convergent fragment couplings
without requiring elaborate condensation reagents and protecting group manipulations. A recyclable N-hydroxy pyridone additive effectively suppresses epimerization at the elongating chain.
We demonstrate the practicality of this method by showcasing a straightforward synthesis of the nonapeptide DSIP. This method further opens the door to clean and atom-efficient peptide
synthesis. SIMILAR CONTENT BEING VIEWED BY OTHERS PHOTOCHEMICALLY-ENABLED, POST-TRANSLATIONAL PRODUCTION OF C-TERMINAL AMIDES Article Open access 30 November 2024 THIOCARBAZATE BUILDING
BLOCKS ENABLE THE CONSTRUCTION OF AZAPEPTIDES FOR RAPID DEVELOPMENT OF THERAPEUTIC CANDIDATES Article Open access 28 November 2022 EXTENDABLE STAPLING OF UNPROTECTED PEPTIDES BY CROSSLINKING
TWO AMINES WITH _O_-PHTHALALDEHYDE Article Open access 14 January 2022 INTRODUCTION Amide bonds are a recurring structural motif found in both naturally occurring and man-made molecules, as
ubiquitously observed in the backbone of proteins/peptides, drugs, and functional materials. Amide bond formation is the most frequently used chemical reaction in medicinal chemistry;
approximately 50% of drug discovery papers contain amide bond formation, twice as many as 30 years prior1,2. In recent years, efficient amide bond formations are especially important due to
the emergence of medium-sized peptide drugs, which frequently exhibit unique characteristics and advantages over small molecule drugs and antibodies3,4. The traditional peptide synthesis
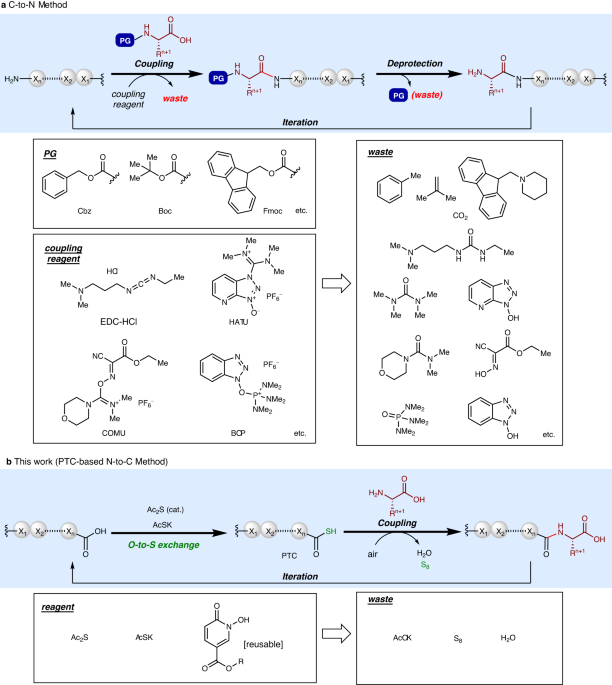
iteratively elongates the chain from the C-terminus to N-terminus (C-to-N) using excess N-carbamate-protected amino acids and condensation reagents to minimize epimerization (Fig. 1a)5.
Combined with solid-phase synthesis, the C-to-N elongation method has enabled facile construction of peptides of up to ca. 50 amino acid residues, and the introduction of revolutionary
automated flow systems promises to increase this number6. Further, combined with native chemical ligation methods, synthesis of proteins with even 400 or more amino acid residues is also
possible7. The maximum size of peptides/proteins that can be produced by chemical synthesis is rapidly increasing. Despite high fidelity and reliability, every C-to-N peptide bond formation
requires multiple protecting-group manipulations and non-recoverable condensation reagents that produce waste. For example, the average molecular weight of an amino acid is ca. 110, but the
molecular weights of commonly used protecting groups (Cbz: 135, Boc: 101, Fmoc: 223) or condensation reagents (EDC-HCl: 191, HATU: 380, COMU: 428, BOP: 442) are comparable to or much greater
than the substrate. Furthermore, C-to-N elongation of anything longer than dipeptides often suffers from diketopiperadine formation, when a simple C-terminus ester protecting group is
used8. Therefore, traditional peptide synthesis is of low atom efficiency and high environmental impact9,10,11,12. In an era more conscious of environmental preservation and sustainability,
greener peptide synthesis is in high demand. Pursuing this, nonclassical amide bond formations13,14 have been extensively studied, and some of them have been applied to C-to-N oligopeptide
synthesis15,16,17,18,19,20,21,22,23. However, many protocols still require harsh conditions (high temperatures for azeotropic removal of water), super-stoichiometric reagents of sometimes
poor accessibility, and protecting groups. N-to-C peptide elongation (Fig. 1b) is less explored than C-to-N elongation due to difficulty in suppressing epimerization of the C-terminus amino
acid residue’s stereocenter24,25,26,27,28,29,30,31,32. Because the two amino groups, one in the elongating peptide strand and the other in the amino acid to be introduced, are already
differentiated as amide and amine groups, respectively, this strategy is potentially advantageous in improving both atom and step efficiency by minimizing protecting group manipulations.
Here we report an iterative and practical N-to-C peptide synthesis in liquid phase, in which epimerization is minimal. This method enables the use of unprotected amino acids and does not
require elaborate condensation reagents, thus markedly improving the atom and step efficiency of liquid-phase peptide synthesis. Moreover, this method is applicable to convergent fragment
coupling, as demonstrated in the short and scalable synthesis of a bioactive nonapeptide. RESULTS AND DISCUSSION OPTIMIZATION OF CONDITIONS To realize N-to-C peptide synthesis, we employed
the peptide thiocarboxylic acid (PTC) platform (Fig. 1b)33. Amide bond formation using PTC under various conditions has been reported34,35,36,37,38,39,40,41,42,43, but PTCs have never been
used in iterative N-to-C peptide synthesis. Based on our previous development of a general, one-step PTC synthesis from peptides using a catalytic diacetyl sulfide (Ac2S) and potassium
thioacetate (AcSK)44, we envisioned the scheme shown in Fig. 1b. Converting the C-terminus carboxylic acid to PTC differentiates the elongating peptide strand from the amino acid to be
introduced without protecting groups. After peptide bond formation, the new C-terminus carboxylic acid can be directly used for the PTC formation to start the next elongation cycle.
Elemental sulfur and water are the only waste produced in this peptide bond formation step. We started optimizing the PTC-based N-to-C elongation using 1A to produce tripeptide 2AA (Table
1). Although PTC is inert to amide formation by itself, oxidatively dimerized diacyl disulfide is the active acylating species45. We first searched for aerobic conditions to convert 1A to
diacyl disulfides in situ, which would be captured by alanine calcium salt (Ca(Ala)2)46. Using an iron(II) phthalocyanine (FePc) catalyst in open-air DMF, tripeptide 2AA was obtained in 22%
yield (entry 1). Next, N-hydroxy amine/amide/imide additives were screened to improve the reactivity while maintaining the low epimerization level (entries 2–6)37. Among the additives
tested, 3-hydroxy-1,2,3-benzotriazin-4(3H)-one (HOObt) afforded acceptable results, producing 2AA in 33% yield and <1% epimerization (entry 6). When the concentration was increased to 100
mM, yield improved to 68%, while epimerization remained suppressed (<1% epi. level, entry 7). Then, the same conditions as in entry 7 were applied to the more sterically hindered
dipeptide 1B. Product tripeptide 2BA, however, was produced only in a low yield (35%) with an increased epimerization level (6.1% epi. level, entry 8). HPLC analysis revealed that FcPc
degraded the diacyl disulfide intermediate derived from 1B prior to condensation. Therefore, we eliminated FePc, resulting in improved yield (64%, entry 9), although the reaction was
sluggish (22 h) and the epimerization level was still high (4.9% epi. level). When alanine (H-Ala-OH), instead of Ca(Ala)2, was used to mitigate basicity of the reaction system, the
epimerization level was reduced to 1.3% (entry 10). Using DMSO as a solvent to promote diacyl disulfide formation47, yield improved (70%) but the epimerization level increased (4.5% epi.
level, entry 11). The epimerization level was decreased when the reaction was performed in a less-polar DMSO/toluene mixed solvent system (1.8% epi. level, entry 12; Table S1). Further
investigation for N-hydroxy amide additives led us to identify that N-hydroxy-2-pyridinone methyl ester (HOPOMe)48,49 improved yield while reducing the epimerization level to <1% (entry
13). Increasing the amounts of alanine (2.0 equiv) and HOPOMe (2.0 equiv) enhanced the reactivity to give 86% yield of 2BA after 6 hours (entry 14). However, separation of the crystalline
HOPOMe from the tripeptide product was difficult. Further structural tuning afforded the optimal additive, HOPOPhy (entry 15), which could be easily recovered by a simple hexane washing and
reused (Table S2). SCOPE AND LIMITATIONS After developing a practical isolation protocol of the products (recrystallization or flash chromatography, see the Method section), the optimized
conditions were used to survey substrate generality (Fig. 2). First, we investigated the generality of amino acids to be introduced using 1B as a peptide substrate (Fig. 2a). The reaction
proceeded in high yield (78–99%) with <1−1.8% epimerization for amino acids bearing hydrophobic (Val: 2BB, Phe: 2BD, Ile: 2BE, Met: 2BF, Trp: 2BG, _t_Leu: 2BP) or protected (Cys: 2BH,
Lys: 2BN, Arg: 2BO) side chains, due to their acceptable solubility in the optimized solvent. Specifically, the sterically hindered amino acid, _t_Leu, was introduced to 1B to produce
tripeptide 2BP containing a highly congested sequence (Phe-Val-_t_Leu) in high yield (93%) with <1% epimerization. Ala and Gly were barely soluble in the solvent and produced slightly
higher epimerization levels (ca. 2.1% for: 2BA and 1.0% for 2BC). For relatively less soluble amino acids bearing polar side chains (Thr, Tyr, Asp, Asn), however, the reaction under the
above optimized conditions resulted in low yield (9–48%), likely due to insufficient concentration of the amino acids. In these cases, PTC hydrolysis preceded the desired peptide coupling.
Further modifying the reaction conditions, we found that by using DMSO solvent without added toluene, the HOPOMe additive which is more active than HOPOPhy, a desiccant (MgSO4), and/or
microwave irradiation (40 °C, see section 1-1 in Supplementary Methods for detailed parameters), the desired tripeptides were obtained in high yield (67–94%) with <1% epimerization. Side
chain protection was not necessary for amino acids containing functional groups of moderate nucleophilicity (Trp: 2BG, Ser: 2BI, Thr: 2BJ, Tyr: 2BK, Asn: 2BL, and Asp: 2BM). Regarding the
N-terminus protecting group on the PTC, Fmoc and Boc groups were also compatible with the current protocol (Fig. 2b: 2CB and 2DB). As for the C-terminus amino acids of the elongating
peptides, the reaction proceeded without any detectable epimerization at Phe (2AB) and Pro (2GB) residues (Fig. 2c). Furthermore, this method can be expanded to the convergent couplings of
two peptide fragments. After liberation of the N-terminus amine from trifluoroacetic acid (TFA) salt of the peptide to be introduced with N,N-diisopropylethylamine (_i_Pr2NEt), the fragment
coupling between dipeptides proceeded affording tetrapeptide 2BR in 99% yield without epimerization (Fig. 2d, see Fig. 3b for more examples of fragment coupling). Due to the higher
solubility of peptide fragments compared to unprotected monoamino acids, only a slight excess (1.2 equiv) of C-terminus fragments was necessary. A preliminary application of this method to
solid-phase peptide synthesis (SPPS), however, resulted in only low-yield product formation (Table S3). SCALABLE, CONVERGENT LIQUID-PHASE SYNTHESIS OF BIOACTIVE PEPTIDE We applied our method
to the synthesis of a bioactive nonapeptide, delta-sleep-inducing peptide (DSIP) (Fig. 3). DSIP was retrosynthesized to three tripeptides, FRAGMENTS 1–3. As the starting amino acids for
each fragment, we selected Boc-Trp for FRAGMENT 1 aiming at global deprotection under acidic conditions at the final step, and Fmoc-Gly and Fmoc-Ser for FRAGMENT 2 and FRAGMENT 3,
respectively, for selective deprotection prior to two fragment couplings. After converting a C-terminus carboxylic acid to PTC, an unprotected amino acid was coupled under the conditions
described above (Fig. 3a). The crude product obtained after extraction with ethyl acetate (AcOEt) and evaporation of the solvent, was washed with hexane to extract HOPOPhy (75–95% recovery).
The recovered and purified HOPOPhy was reusable without any loss of its activity. Then, the residue containing the product peptide was dissolved in AcOEt or MeOH, and the solution was
treated with activated carbon. This process efficiently eliminated residual sulfur compounds, which were often problematic for the next peptide coupling and purification. The subsequent
simple purification by silica gel column chromatography afforded the desired di- and tripeptides in good yield with sufficient purity for the next iteration or fragment coupling. For the
removal of Fmoc group, we used triethylamine as a base. After evaporation, the crude mixture was dissolved in a biphasic solvent comprised of water and ether, which contained the product
peptides and Fmoc-derived side products, respectively. The water phase was separated and freeze dried. Consequently, FRAGMENTS 1–3 were synthesized in pure forms in a scalable manner
(>300 mg prepared for each). Then, fragment couplings were performed (Fig. 3b). After converting FRAGMENT 1 to PTC, the reaction with FRAGMENT 2 yielded hexapeptide 3 in 66% yield (2
steps) without column chromatography. Hexapeptide 3 was further converted to PTC and coupled with FRAGMENT 3 to yield protected DSIP 4 in 56% yield (2 steps) without column chromatography.
Finally, global deprotection and purification by reverse-phase column chromatography afforded 130 mg of DSIP in 62% yield (Fig. 3c, d), showcasing that the current protocol is practical in
supplying middle-sized bioactive peptides. MECHANISTIC STUDIES To gain insight into the mechanism of the peptide coupling, the reaction was monitored over time by HPLC. When PTC 1B was
stirred under air without a coupling partner, 1B was oxidatively dimerized to 5 within 1 h (Fig. 4a). Under the indicated conditions in the presence of HOPOPhy, PTC 1B was consumed in 1 h,
producing tripeptide 2BA in 81% yield. The formation of elemental sulfur (S8) was confirmed by HPLC during the reaction. Dimer 5 was observed at the initial stage of the reaction (t < 30
min) but was consumed within 3 h and converted to active ester 6. Then, 6 gradually reacted with alanine and was fully converted to the tripeptide after 4.5 h (Fig. 4b, c). From these
reaction profiles, the rate-limiting step is likely the peptide bond-forming step between active ester 6 and the amino acid (Figs. S1‒S3). Based on the above observations, a plausible
reaction mechanism is proposed as shown in Fig. 5. First, PTC 1 dimerizes under aerobic conditions to form diacyl disulfide 7. DMSO solvent accelerates this oxidation step47. Diacyl
disulfide 7 then reacts with the additive HOPOR to produce active ester 8, thus suppressing undesired epimerization through oxazolidine formation. The liberated acyl disulfide 9 reacts with
1 to generate 7 and H2S, or with HOPOR to generate active ester 8 and H2S2. Active ester 8 gradually reacts with an unprotected amino acid or peptide fragment to produce elongated peptide 2.
H2S and H2S2 undergo oxidation to release stable S8 and water as the only byproducts. CONCLUSION In this study, we developed an iterative, liquid-phase N-to-C peptide synthesis relying on
the PTC platform, which enabled the use of unprotected amino acids as starting materials50. Only a one atom difference (O _vs_. S) distinguished the C-termini of elongating peptides and the
unprotected amino acids being introduced. Therefore, protecting group manipulations and the number of synthetic steps were minimal compared with conventional C-to-N synthesis. A reusable
additive (HOPOPhy) bearing a long-alkyl chain allowed for coupling of amino acids or peptide fragments in high yield with minimal epimerization (<1% in most cases). The only waste
byproducts were water and elemental sulfur, making the current process highly atom efficient. A straightforward workup (extraction, hexane washing, activated carbon treatment, and/or
chromatography/recrystallization) after the coupling reaction provided peptides with sufficient purity for the next iteration. This method was applicable not only to sequential elongation of
single amino acids but also to convergent fragment couplings, which allowed for the rapid increase in molecular complexity. Taking advantage of the characteristics of this method, a
practical synthesis of a bioactive nonapeptide was demonstrated in a sub-gram scale. Although there are several previous examples of N-to-C peptide elongation, their scopes were limited or
not thoroughly studied. Furthermore, these earlier works require protecting groups or activating groups at the N- or C-terminus, which diminished the potential advantages of N-to-C
elongation regarding atom efficiency. Our achievement will open a green route to practically supplying peptides of ca. 10 residues in length, a common size for synthetic peptide drugs.
Further studies investigating ways to accelerate the reaction rate while reducing epimerization, as well as the applicability to late-stage peptide functionalization, lateral coupling,
cyclic peptide synthesis, and SPPS, are ongoing. METHODS PROCEDURE FOR GRAM-SCALE N-TO-C PEPTIDE COUPLING (REPRESENTED BY THE SYNTHESIS OF FRAGMENT 1) To a solution of BOC-WA (1.18 g, 3.14
mmol) and potassium thioacetate (3.59 g, 31.4 mmol, 10 equiv) in DMF (31.4 mL), diacetyl sulfide (65.7 μL, 0.628 mmol, 0.2 equiv) was added dropwise at 0 °C and the mixture was stirred for
3.5 hours at 0 °C under an argon atmosphere. Ethyl acetate, water, and 1 M HCl aq. were added to the reaction mixture to stop the reaction. The products were extracted with ethyl acetate.
The combined organic layers were washed with water, 1 M HCl aq., and brine, dried over Na2SO4, and filtered. Volatiles were removed under reduced pressure to afford the crude PTC
(BOC-WA-SH). This crude product was used for the peptide coupling reaction without further purification. BOC-WA-SH (estimated as 3.14 mmol) dissolved in DMSO (6.5 mL) was added to a mixture
of glycine (471 mg, 6.18 mmol, 2.0 equiv), HOPOPhy (2.05 g, 4.71 mmol, 1.5 equiv), and toluene (6.5 mL) in a test tube for a microwave apparatus. The mixture was stirred under microwave
irradiation at 40 °C for 3 hours. Ethyl acetate, water, and 1 M HCl aq. were added to the reaction mixture. The mixture was extracted with ethyl acetate. The combined organic layers were
washed with brine, dried over Na2SO4, filtered, and volatiles were removed under reduced pressure. Hexane (150 mL) was added to the residue and the mixture was sonicated to precipitate out
the peptide product. The precipitates were collected by filtration and washed with hexane. Then, the filtrate was evaporated under reduced pressure for recovering HOPOPhy. The resulting
residue from hexane was purified by column chromatography (neutral silica gel, hexane/ethyl acetate = 80:20 → 50:50) to afford HOPOPhy, which was reusable for another peptide coupling
reaction (1.95 g, 95% recovery). Meanwhile, the sticky precipitate on the filter containing tripeptide was once dissolved into a large amount of methanol and ethyl acetate. Then, the solvent
was removed under reduced pressure. Ethyl acetate (50 mL) and activated carbon (400 mg) were added to the mixture. This suspension was stirred at 80 °C for 10 min under an argon atmosphere
and then cooled to room temperature. Activated carbon was filtered over Celite and washed with ethyl acetate. The filtrate was evaporated under reduced pressure to afford the tripeptide
product, FRAGMENT 1. This crude product was purified by column chromatography (silica gel, hexane/ethyl acetate = 70:30 → 0:100 then ethyl acetate/methanol = 90:10 → 80:20) to afford pure
FRAGMENT 1 (931 mg, 69%). PROCEDURE FOR PEPTIDE FRAGMENT COUPLING (REPRESENTED BY THE SYNTHESIS OF 2BR) To a solution containing Cbz-Phe-Val-SH (1B, 50 mg, 0.12 mmol), H-Ala-Pro-OH TFA salt
(43 mg, 0.144 mmol, 1.2 equiv), and HOPOPhy (78.4 mg, 0.18 mmol, 1.5 equiv) in DMSO (600 μL) and toluene (600 μL), _i_Pr2NEt (25 μL, 0.144 mmol, 1.2 equiv) was added. After stirring at 30 °C
for 6 hours, a HPLC sample was prepared (12 μL of the reaction mixture was picked up into 68 μL of 1% TFA/DMSO) for yield determination. HPLC yield was determined as 99% (method B in ESI).
TFA (68 μL) was added to the reaction mixture to quench the reaction. After transferring the reaction mixture into a separatory funnel, ethyl acetate and 1 M HCl aq. were added. Organic
compounds were extracted with ethyl acetate (three times). The combined organic layers were washed with brine and dried over Na2SO4. After filtration, volatiles were removed under reduced
pressure. The crude mixture was purified by column chromatography (silica gel, hexane/ethyl acetate = 80:20, then chloroform/methanol = 100:0 → 80:20). The obtained material was further
purified by reverse-phase preparative HPLC (method D in section 1-4 of Supplementary Methods, tR = 52.0 min). Fractions containing the pure desired product were combined and lyophilized to
afford analytically pure 2BR (43.3 mg, 64% isolated yield). For NMR data of isolated new compounds, see Supplementary Data 1. DATA AVAILABILITY All relevant data are presented in the main
article or the supporting information. Detailed experimental procedures for the syntheses and characterizations of new compounds, mechanistic studies, and HPLC analysis are available in
Electronic Supplementary Information. 1H and 13C NMR charts of isolated new compounds can be found in the Supplementary Data 1. REFERENCES * Brown, D. G. & Boström, J. Analysis of Past
and Present Synthetic Methodologies on Medicinal Chemistry: Where Have All the New Reactions Gone? _J. Med. Chem._ 59, 4443–4458 (2016). Article CAS PubMed Google Scholar * Pattabiraman,
V. R. & Bode, J. W. Rethinking amide bond synthesis. _Nature_ 480, 471–479 (2011). Article CAS PubMed Google Scholar * Henninot, A., Collins, J. C. & Nuss, J. M. The Current
State of Peptide Drug Discovery: Back to the Future? _J. Med. Chem._ 61, 1382–1414 (2018). Article CAS PubMed Google Scholar * Muttenthaler, M., King, G. F., Adams, D. J. & Alewood,
P. F. Trends in peptide drug discovery. _Nat. Rev. Drug Discov._ 20, 309–325 (2021). Article CAS PubMed Google Scholar * Merrifield, R. B. Solid Phase Peptide Synthesis. I. The Synthesis
of a Tetrapeptide. _J. Am. Chem. Soc._ 85, 2149–2154 (1963). Article CAS Google Scholar * Hartrampf, N. et al. Synthesis of proteins by automated flow chemistry. _Science_ 368, 980–987
(2020). Article CAS PubMed Google Scholar * Sun, H. & Brik, A. The Journey for the Total Chemical Synthesis of a 53 kDa Protein. _Acc. Chem. Res._ 52, 3361–3371 (2019). Article CAS
PubMed Google Scholar * Gisin, B. F. & Merrifield, R. B. Carboxyl-catalyzed intramolecular aminolysis. Side reaction in solid-phase peptide synthesis. _J. Am. Chem. Soc._ 94,
3102–3106 (1972). Article CAS PubMed Google Scholar * Dunetz, J. R., Magano, J. & Weisenburger, G. A. Large-scale applications of amide coupling reagents for the synthesis of
pharmaceuticals. _Org. Process Res. Dev._ 20, 140–177 (2016). Article CAS Google Scholar * Lawrenson, S. B., Arav, R. & North, M. The greening of peptide synthesis. _Green. Chem._ 19,
1685–1691 (2017). Article CAS Google Scholar * Isidro-Llobet, A. et al. Sustainability Challenges in Peptide Synthesis and Purification: From R&D to Production. _J. Org. Chem._ 84,
4615–4628 (2019). Article CAS PubMed Google Scholar * Ferrazzano, L. et al. Sustainability in peptide chemistry: current synthesis and purification technologies and future challenges.
_Green. Chem._ 24, 975–1020 (2022). Article CAS Google Scholar * de Figueiredo, R. M., Suppo, J.-S. & Campagne, J.-M. Nonclassical routes for amide bond formation. _Chem. Rev._ 116,
12029–12122 (2016). Article PubMed Google Scholar * Sabatini, M. T., Boulton, L. T., Sneddon, H. F. & Sheppard, T. D. A green chemistry perspective on catalytic amide bond formation.
_Nat. Catal._ 2, 10–17 (2019). Article CAS Google Scholar * Hu, L. et al. Ynamides as Racemization-Free Coupling Reagents for Amide and Peptide Synthesis. _J. Am. Chem. Soc._ 138,
13135–13138 (2016). Article CAS PubMed Google Scholar * Aspin, S. J., Taillemaud, S., Cyr, P. & Charette, A. B. 9-silafluorenyl dichlorides as chemically ligating coupling agents and
their application in peptide synthesis. _Angew. Chem. Int. Ed._ 55, 13833–13837 (2016). Article CAS Google Scholar * Liu, Z., Noda, H., Shibasaki, M. & Kumagai, N. Catalytic
oligopeptide synthesis. _Org. Lett._ 20, 612–615 (2018). Article CAS PubMed Google Scholar * Muramatsu, W., Hattori, T. & Yamamoto, H. Substrate-Directed Lewis-Acid Catalysis for
Peptide Synthesis. _J. Am. Chem. Soc._ 141, 12288–12295 (2019). Article CAS PubMed Google Scholar * Handoko, Satishkumar, S., Panigrahi, N. R. & Arora, P. S. Rational Design of an
Organocatalyst for Peptide Bond Formation. _J. Am. Chem. Soc._ 141, 15977–15985 (2019). Article CAS PubMed Google Scholar * Michigami, K., Sakaguchi, T. & Takemoto, Y. Catalytic
dehydrative peptide synthesis with gem-diboronic acids. _ACS Catal._ 10, 683–688 (2020). Article CAS Google Scholar * Lee, H.-J., Huang, X., Sakaki, S. & Maruoka, K. Metal-free
approach for hindered amide-bond formation with hypervalent iodine(iii) reagents: application to hindered peptide synthesis. _Green. Chem._ 23, 848–855 (2021). Article CAS Google Scholar
* Nagahara, S., Okada, Y., Kitano, Y. & Chiba, K. Biphasic electrochemical peptide synthesis. _Chem. Sci._ 12, 12911–12917 (2021). Article CAS PubMed PubMed Central Google Scholar *
Lee, C., Thomson, B. J. & Sammis, G. M. Rapid and column-free syntheses of acyl fluorides and peptides using ex situ generated thionyl fluoride. _Chem. Sci._ 13, 188–194 (2021). Article
PubMed PubMed Central Google Scholar * Letsinger, R. L. & Kornet, M. J. Popcorn polymer as a support in multistep syntheses. _J. Am. Chem. Soc._ 85, 3045–3046 (1963). Article CAS
Google Scholar * Felix, A. M. & Merrifield, R. B. Azide solid phase peptide synthesis. _J. Am. Chem. Soc._ 92, 1385–1391 (1970). Article CAS PubMed Google Scholar * Matsueda, R.,
Maruyama, H., Kitazawa, E., Takahagi, H. & Mukaiyama, T. Solid phase peptide synthesis by oxidation-reduction condensation. _J. Am. Chem. Soc._ 97, 2573–2575 (1975). Article CAS PubMed
Google Scholar * Henkel, B., Zhang, L. & Bayer, E. Investigations on solid-phase peptide synthesis in N-to-C direction (inverse synthesis). _Liebigs Ann._ 1997, 2161–2168 (1997).
Article Google Scholar * Stamm, S. & Heimgartner, H. The “azirine/oxazolone method” under solid-phase conditions. _Eur. J. Org. Chem._ 2004, 3820–3827 (2004). Article Google Scholar
* Rai, A. & Gutheil, W. G. A Dde resin based strategy for inverse solid-phase synthesis of amino terminated peptides, peptide mimetics and protected peptide intermediates. _J. Pept.
Sci._ 11, 69–73 (2005). Article CAS PubMed Google Scholar * Pourvali, A., Cochrane, J. R. & Hutton, C. A. A new method for peptide synthesis in the N→C direction: amide assembly
through silver-promoted reaction of thioamides. _Chem. Commun._ 50, 15963–15966 (2014). Article CAS Google Scholar * Suppo, J.-S., Subra, G., Bergès, M., Marcia de Figueiredo, R. &
Campagne, J.-M. Inverse peptide synthesis via activated α-aminoesters. _Angew. Chem. Int. Ed._ 53, 5389–5393 (2014). Article CAS Google Scholar * Li, J. et al. An Atom-Economic Inverse
Solid-Phase Peptide Synthesis Using Bn or BcM Esters of Amino Acids. _Org. Lett._ 23, 7571–7574 (2021). Article CAS PubMed Google Scholar * Narendra et al. Thioacids – synthons for amide
bond formation and ligation reactions: assembly of peptides and peptidomimetics. _Org. Biomol. Chem._ 16, 3524–3552 (2018). Article Google Scholar * Blake, J. & Li, C. H. New
Segment-Coupling Method for Peptide Synthesis in Aqueous Solution: Application to Synthesis of Human [Gly17]-β-endorphin. _Proc. Natl. Acad. Sci. USA_ 78, 4055–4058 (1981). Article CAS
PubMed PubMed Central Google Scholar * Crich, D., Sana, K. & Guo, S. Amino acid and peptide synthesis and functionalization by the reaction of thioacids with
2,4-dinitrobenzenesulfonamides. _Org. Lett._ 9, 4423–4426 (2007). Article CAS PubMed Google Scholar * Crich, D. & Sasaki, K. Reaction of thioacids with isocyanates and
isothiocyanates: a convenient amide ligation process. _Org. Lett._ 11, 3514–3517 (2009). Article CAS PubMed PubMed Central Google Scholar * Wang, P. & Danishefsky, S. J. Promising
general solution to the problem of ligating peptides and glycopeptides. _J. Am. Chem. Soc._ 132, 17045–17051 (2010). Article CAS PubMed PubMed Central Google Scholar * Pan, J.,
Devarie-Baez, N. O. & Xian, M. Facile amide formation via _S_-nitrosothioacids. _Org. Lett._ 13, 1092–1094 (2011). Article CAS PubMed PubMed Central Google Scholar * Wu, W., Zhang,
Z. & Liebeskind, L. S. In situ carboxyl activation using a silatropic switch: a new approach to amide and peptide constructions. _J. Am. Chem. Soc._ 133, 14256–14259 (2011). Article CAS
PubMed PubMed Central Google Scholar * Mali, S. M., Jadhav, S. V. & Gopi, H. N. Copper(II) mediated facile and ultrafast peptide synthesis in methanol. _Chem. Commun._ 48, 7085–7087
(2012). Article CAS Google Scholar * Chen, W. et al. A traceless approach to amide and peptide construction from thioacids and dithiocarbamate-terminal amines. _Chem. Sci._ 4, 970–976
(2013). Article CAS Google Scholar * Mali, S. M. & Gopi, H. N. Thioacetic acid/ NaSH-mediated synthesis of N-protected amino thioacids and their utility in peptide synthesis. _J. Org.
Chem._ 79, 2377–2383 (2014). Article CAS PubMed Google Scholar * Nomura, K., Maki, Y., Okamoto, R., Satoh, A. & Kajihara, Y. Glycoprotein Semisynthesis by Chemical Insertion of
Glycosyl Asparagine Using a Bifunctional Thioacid-Mediated Strategy. _J. Am. Chem. Soc._ 143, 10157–10167 (2021). Article CAS PubMed Google Scholar * Matsumoto, T., Sasamoto, K., Hirano,
R., Oisaki, K. & Kanai, M. A catalytic one-step synthesis of peptide thioacids: the synthesis of leuprorelin via iterative peptide-fragment coupling reactions. _Chem. Commun._ 54,
12222–12225 (2018). Article CAS Google Scholar * Liu, R. & Orgel, L. E. Oxidative acylation using thioacids. _Nature_ 389, 52–54 (1997). Article CAS PubMed Google Scholar *
Hashimoto, C., Takeguchi, K. & Kodomari, M. An efficient synthetic method of N-protected dipeptide acids using amino acid calcium carboxylates in an organic solvent. _Synlett_ 2011,
1427–1430 (2011). Article Google Scholar * Tam, J. P., Wu, C. R., Liu, W. & Zhang, J. W. Disulfide bond formation in peptides by dimethyl sulfoxide. Scope and applications. _J. Am.
Chem. Soc._ 113, 6657–6662 (1991). Article CAS Google Scholar * Ho, G.-J. et al. Carbodiimide-Mediated Amide Formation in a Two-Phase System. A High-Yield and Low-Racemization Procedure
for Peptide Synthesis. _J. Org. Chem._ 60, 3569–3570 (1995). Article CAS Google Scholar * Ando, M. et al. Discovery of pyridone-containing imidazolines as potent and selective inhibitors
of neuropeptide Y Y5 receptor. _Bioorg. Med. Chem._ 17, 6106–6122 (2009). Article CAS PubMed Google Scholar * During preparation of this manuscript, Zhao reported another strategy for
N-to-C peptide synthesis using unprotected amino acids. See; Liu, T., Peng, Z., Zhao, J. Peptide Synthesis Using Unprotected Amino Acids. _ChemRxiv_
https://doi.org/10.26434/chemrxiv-2023-5776w Download references ACKNOWLEDGEMENTS This research was supported partly by MEXT/JSPS KAKENHI Grant number JP23H05466 and 23H04909 (M.K.) and
JP21H05077 (K. Oisaki), and Inamori Research Grant (to K. Oisaki). T.T. thanks JSPS Fellowship JP2101365 for financial support. T.M. thanks Graduate Program for Leaders in Life Innovation
(GPLLI). We thank Professor Akira Otaka in Tokushima University for valuable suggestions. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Graduate School of Pharmaceutical Sciences, The
University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo, 113-0033, Japan Toshifumi Tatsumi, Koki Sasamoto, Takuya Matsumoto, Ryo Hirano, Kazuki Oikawa, Kounosuke Oisaki & Motomu Kanai *
Interdisciplinary Research Center for Catalytic Chemistry (IRC3), National Institute of Advanced Industrial Science and Technology (AIST), Tsukuba Central 5-2, 1-1-1 Higashi, Tsukuba,
Ibaraki, 305-8565, Japan Masato Nakano, Masaru Yoshida & Kounosuke Oisaki Authors * Toshifumi Tatsumi View author publications You can also search for this author inPubMed Google Scholar
* Koki Sasamoto View author publications You can also search for this author inPubMed Google Scholar * Takuya Matsumoto View author publications You can also search for this author inPubMed
Google Scholar * Ryo Hirano View author publications You can also search for this author inPubMed Google Scholar * Kazuki Oikawa View author publications You can also search for this author
inPubMed Google Scholar * Masato Nakano View author publications You can also search for this author inPubMed Google Scholar * Masaru Yoshida View author publications You can also search
for this author inPubMed Google Scholar * Kounosuke Oisaki View author publications You can also search for this author inPubMed Google Scholar * Motomu Kanai View author publications You
can also search for this author inPubMed Google Scholar CONTRIBUTIONS T.T., K.S., T.M., R.H., K. Oikawa, and M.N. conducted experimental studies and collected the data. K. Oisaki and M. K.
designed, advised, and directed the project. M.Y., K. Oisaki and M.K. co-wrote the manuscript. All the authors analyzed the data, discussed the results, and edited the manuscript.
CORRESPONDING AUTHORS Correspondence to Kounosuke Oisaki or Motomu Kanai. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. PEER REVIEW PEER REVIEW
INFORMATION : _Communications Chemistry_ thanks Rita Petracca and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION PEER
REVIEW FILE SUPPLEMENTARY INFORMATION DESCRIPTION OF ADDITIONAL SUPPLEMENTARY FILES RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0
International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the
source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative
Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Tatsumi, T., Sasamoto, K., Matsumoto, T. _et al._ Practical N-to-C peptide
synthesis with minimal protecting groups. _Commun Chem_ 6, 231 (2023). https://doi.org/10.1038/s42004-023-01030-0 Download citation * Received: 12 July 2023 * Accepted: 13 October 2023 *
Published: 26 October 2023 * DOI: https://doi.org/10.1038/s42004-023-01030-0 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link
Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative