Play all audios:
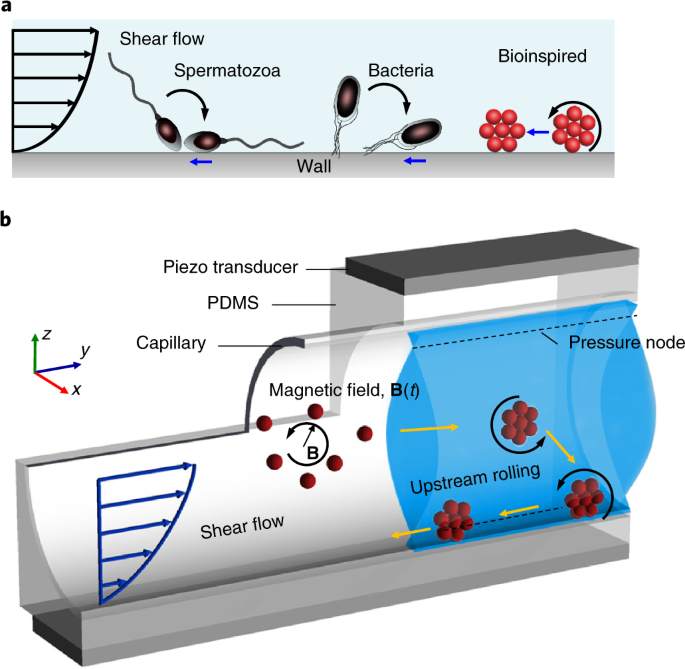
ABSTRACT The ability to propel against flows, that is, to perform positive rheotaxis, can provide exciting opportunities for applications in targeted therapeutics and non-invasive surgery.
So far no biocompatible technologies exist for navigating microparticles upstream when they are in a background fluid flow. Inspired by many naturally occurring microswimmers—such as
bacteria, spermatozoa and plankton—that utilize the no-slip boundary conditions of the wall to exhibit upstream propulsion, here we report on the design and characterization of
self-assembled microswarms that can execute upstream motility in a combination of external acoustic and magnetic fields. Both acoustic and magnetic fields are safe to humans, non-invasive,
can penetrate deeply into the human body and are well-developed in clinical settings. The combination of both fields can overcome the limitations encountered by single actuation methods. The
design criteria of the acoustically induced reaction force of the microswarms, which is needed to perform rolling-type motion, are discussed. We show quantitative agreement between
experimental data and our model that captures the rolling behaviour. The upstream capability provides a design strategy for delivering small drug molecules to hard-to-reach sites and
represents a fundamental step towards the realization of micro- and nanosystem navigation against the blood flow. Access through your institution Buy or subscribe This is a preview of
subscription content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value
online-access subscription $32.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 digital issues and online access to articles $119.00 per year only $9.92 per issue
Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL
ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS PROPULSION OF MAGNETICALLY ACTUATED
ACHIRAL PLANAR MICROSWIMMERS IN NEWTONIAN AND NON-NEWTONIAN FLUIDS Article Open access 27 October 2021 ROLLING MICROSWARMS ALONG ACOUSTIC VIRTUAL WALLS Article Open access 29 November 2022
SURFACE MOTION DYNAMICS AND SWIMMING CONTROL OF PLANAR MAGNETIC MICROSWIMMERS Article Open access 20 March 2025 DATA AVAILABILITY The authors declare that data supporting the findings of
this study are available within the paper and its Supplementary Information. Source data are provided with this paper. REFERENCES * Ashkin, A., Dziedzic, J. M., Bjorkholm, J. E. & Chu,
S. Observation of a single-beam gradient force optical trap for dielectric particles. _Opt. Lett._ 11, 288–290 (1986). Article Google Scholar * Ma, F., Wang, S., Wu, D. T. & Wu, N.
Electric-field induced assembly and propulsion of chiral colloidal clusters. _Proc. Natl Acad. Sci. USA_ 112, 6307–6312 (2015). Article Google Scholar * Snezhko, A. & Aranson, I. S.
Magnetic manipulation of self-assembled colloidal asters. _Nat. Mater._ 10, 698–703 (2011). Article Google Scholar * Lipfert, J., JKerssemakers, J. W. J. & Jager, T. Magnetic torque
tweezers: measuring torsional stiffness in DNA and RecA-DNA filaments. _Nat. Methods_ 7, 977–980 (2010). Article Google Scholar * Sukhov, A. & Berakdar, J. Local control of ultrafast
dynamics of magnetic nanoparticles. _Phys. Rev. Lett_. 102, 057204 (2009). * Miyashita, S., Guitron, S., Li, S. & Rus, D. Robotic metamorphosis by origami exoskeletons. _Sci. Robot_. 2,
eaao4369 (2017). * Grifantini, R. et al. Magnetically driven drug delivery systems improving targeted immunotherapy for colon-rectal cancer. _J. Control. Release_ 280, 76–86 (2018). Article
Google Scholar * Yang, M. & Ripoll, M. Soft matter hydrodynamic simulations of self-phoretic microswimmers. _Soft Matter._ 10, 6208–6218 (2014). Article Google Scholar * Shi, J. et
al. Acoustic tweezers: patterning cells and microparticles using standing surface acoustic waves (SSAW). _Lab Chip_ 9, 2890–2895 (2009). Article Google Scholar * Ahmed, D. et al.
Rotational manipulation of single cells and organisms using acoustic waves. _Nat. Commun._ 7, 11085 (2016). Article Google Scholar * Ding, X. et al. On-chip manipulation of single
microparticles, cells, and organisms using surface acoustic waves. _Proc. Natl Acad. Sci_. _USA_ 109, 11105–11109 (2012). * Friend, J. & Yeo, L. Y. Microscale acoustofluidics:
microfluidics driven via acoustics and ultrasonics. _Rev. Mod. Phys._ 83, 647–704 (2011). Article Google Scholar * Purcell, E. M. Life at low Reynolds number. _Am. J. Phys._ 45, 3–11
(1977). Article Google Scholar * Williams, B. J., Anand, S. V., Rajagopalan, J. & Saif, M. T. A. A self-propelled biohybrid swimmer at low Reynolds number. _Nat. Commun._ 5, 3081
(2014). Article Google Scholar * Medina-Sánchez, M. & Schmidt, O. G. Medical microbots need better imaging and control. _Nature_ 545, 406–408 (2017). Article Google Scholar * Lauga,
E., Diluzio, W. R., Whitesides, G. M. & Stone, H. A. Swimming in circles: motion of bacteria near solid boundaries. _Biophys. J._ 90, 400–412 (2006). Article Google Scholar * Kaya, T.
& Koser, H. Direct upstream motility in _Escherichia coli_ bacteria preparation. _Biophys. J._ 102, 1514–1523 (2012). Article Google Scholar * Mattick, J. S. Type IV pili and twitching
motility. _Annu. Rev. Microbiol._ 56, 289–314 (2002). Article Google Scholar * Kantsler V., Dunkel J., Blayney M. & Goldstein R. E. Rheotaxis facilitates upstream navigation of
mammalian sperm cells. _eLife_ 3, e02403 (2014). * Miki, K. & Clapham, D. E. Article rheotaxis guides mammalian sperm. _Curr. Biol._ 23, 443–452 (2013). Article Google Scholar *
Elgeti, J., Winkler, R. G. & Gompper, G. Physics of microswimmers—single particle motion and collective behavior. _Rep. Prog. Phys._ 78, 056601 (2014). Article MathSciNet Google
Scholar * Durham, W. M. & Stocker, R. Thin phytoplankton layers: characteristics, mechanisms, and consequences. _Annu. Rev. Mar. Sci._ 4, 177–207 (2012). Article Google Scholar *
Miki, K. & Clapham, D. E. Rheotaxis guides mammalian sperm. _Curr. Biol._ 23, 443–452 (2013). Article Google Scholar * Kaupp, U. B. & Strünker, T. Signaling in sperm: more dfferent
than similar. _Trends Cell Biol._ 27, 101–109 (2017). Article Google Scholar * Simmchen, J. et al. Topographical pathways guide chemical microswimmers. _Nat. Commun_. 7, 10598 (2016). *
Das, S. et al. Boundaries can steer active Janus spheres. _Nat. Commun_. 6, 8999 (2015). * Liu, C., Zhou, C., Wang, W. & Zhang, H. P. Bimetallic microswimmers speed up in confining
channels. _Phys. Rev. Lett_. 117, 198001 (2016). * Katuri, J., Uspal, W. E., Simmchen, J., Miguel-lópez, A. & Sánchez, S. Cross-stream migration of active particles. _Sci. Adv_. 4,
eaao1755 (2018). * Palacci, J.et al. Artificial rheotaxis. _Sci. Adv_. 1, –e1400214 (2015). * Palacci, J., Sacanna, S., Steinberg, A. P., Pine, D. J. & Chaikin, P. M. Living crystals of
light-activated colloidal surfers. _Science_ 339, 936–941 (2013). Article Google Scholar * Ren, L. et al. Rheotaxis of bimetallic micromotors driven by driven by chemical–acoustic hybrid
power. _ACS Nano._ 11, 10591–10598 (2017). Article Google Scholar * De Ávila, B. E. et al. Micromotor-enabled active drug delivery for in vivo treatment of stomach infection Berta. _Nat.
Commun_. 8, 272 (2017). * Tierno, P., Golestanian, R., Pagonabarraga, I. & Sague, F. Controlled swimming in confined fluids of magnetically actuated colloidal rotors. _Phys. Rev. Lett_.
101, 218304 (2008). * Martinez-pedrero, F., Ortiz-ambriz, A., Pagonabarraga, I. & Tierno, P. Colloidal microworms propelling via a cooperative hydrodynamic conveyor belt. _Phys. Rev.
Lett._ 115, 138301 (2015). Article Google Scholar * Ahmed, D. et al. Neutrophil-inspired propulsion in a combined acoustic and magnetic field. _Nat. Commun_. 8, 770 (2017). * Shi, J.,
Ahmed, D., Mao, X., Lin, S. S. & Huang, T. J. Acoustic tweezers: patterning cells and microparticles using standing surface acoustic waves (SSAW). _Lab Chip_ 9, 2890–2895 (2009). *
Bruus, H. Acoustofluidics 7: the acoustic radiation force on small particles. _Lab Chip_ 12, 1014–1021 (2012). Article Google Scholar * Bruus, H. Acoustofluidics 10: scaling laws in
acoustophoresis. _Lab Chip_ 12, 1578–1586 (2012). Google Scholar * Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. _Nat. Methods_ 9,
671–675 (2012). Article Google Scholar * Rune Barnkob, H. B., Iranmanesh, I., Wiklund, M. & Bruus, H. Measuring acoustic energy density in microchannel acoustophoresis using a simple
and rapid light-intensity method. _Lab Chip_ 12, 2337–2344 (2012). Article Google Scholar * Wei, W., Thiessen, D. B. & Marston, P. L. Acoustic radiation force on a compressible
cylinder in a standing wave. _J. Acoust. Soc. Am._ 116, 201–208 (2004). Article Google Scholar * Garbin, A., Leibacher, I., Hahn, P. & Le Ferrand, H. Acoustophoresis of disk-shaped
microparticles: a numerical and experimental study of acoustic radiation forces and torques. _J. Acoust. Soc. Am._ 138, 2759 (2015). Article Google Scholar * Klingenberg, D. J., Ulicny, J.
C. & Golden, M. A. Mason numbers for magnetorheology. _J. Rheol_. 51, 883 (2017). * Sherman, S. G., Becnel, A. C. & Wereley, N. M. Relating Mason number to Bingham number in
magnetorheological fluids. _J. Magn. Magn. Mater._ 380, 98–104 (2015). Article Google Scholar * Krishnamurthy, H. et al. Dynamics of rotating paramagnetic particle chains simulated by
particle dynamics, Stokesian dynamics and lattice Boltzmann methods. _Microfluid. Nanofluid._ 5, 33–41 (2008). Article Google Scholar * Sherwood, J. D. Stokes drag on a disc with a Navier
slip condition near a plane wall. _Fluid Dyn. Res._ 45, 055505 (2013). Article MathSciNet Google Scholar * Goldmans, A. J., Cox, R. G. & Brenner, H. Slow viscous motion of a sphere
parallel to a plane wall—I motion through a quiescent fluid. _Chem. Eng. Sci._ 22, 637–651 (1967). Article Google Scholar * Krishnan, G. P. & Leighton, D. T. Inertial lift on a moving
sphere in contact with a plane wall in a shear flow. _Phys. Fluids_. 7, 2538 (1995). * Wu, Y. H. Stokes drag on a disc with a Navier slip condition near a plane wall Stokes drag on a disc
with a Navier slip condition near a plane wall. _Fluid Dyn. Res_. https://doi.org/10.1088/0169-5983/45/5/055505 (2013). * Filho, M. M. C. & Machado, J. C. The ultrasonic attenuation
coefficient for human blood plasma in the frequency range of 7–90 MHz. _Proc. IEEE Ultrason. Symp._ 3, 2073–2077 (2004). Google Scholar * Coey, J. M. D. _Magnetism and Magnetic Materials_
(Cambridge Univ. Press, 2010). * Medina-Sánchez, M. & Schmidt, O. G. Medical microbots need better imaging and control. _Nature_ 545, 406–408 (2017). Article Google Scholar * Wu, Z. et
al. A microrobotic system guided by photoacoustic computed tomography for targeted navigation in intestines in vivo. _Sci. Robotics_ 4, eaax0613 (2019). Article Google Scholar * Awatani,
J. Studies on acoustic radiation pressure. I. (General considerations). _J. Acoust. Soc. Am._ 27, 278–281 (1955). Article MathSciNet Google Scholar Download references ACKNOWLEDGEMENTS
This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme grant agreement no. 853309 (SONOBOTS) and
grant agreement no. 743217 (SOMBOT). In addition, the work has been supported by ETH Zurich Career Seed Grant (grant no. 14 17-2) and DFG Priority Programme SPP 1726, Microswimmers—“From
single particle motion to collective behaviour.” AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Institute of Robotics and Intelligent Systems, ETH Zurich, Zurich, Switzerland Daniel Ahmed,
David Hauri, Dubon Rodrigue, Gian Maranta & Bradley J. Nelson * Helmholtz Institute Erlangen–Nürnberg for Renewable Energy (IEK-11), Forschungszentrum Jülich, Nuremberg, Germany
Alexander Sukhov & Jens Harting * Department of Chemical and Biological Engineering, Friedrich-Alexander-Universität Erlangen-Nürnberg, Nürnberg, Germany Jens Harting * Department of
Physics, Friedrich-Alexander-Universität Erlangen-Nürnberg, Nürnberg, Germany Jens Harting Authors * Daniel Ahmed View author publications You can also search for this author inPubMed Google
Scholar * Alexander Sukhov View author publications You can also search for this author inPubMed Google Scholar * David Hauri View author publications You can also search for this author
inPubMed Google Scholar * Dubon Rodrigue View author publications You can also search for this author inPubMed Google Scholar * Gian Maranta View author publications You can also search for
this author inPubMed Google Scholar * Jens Harting View author publications You can also search for this author inPubMed Google Scholar * Bradley J. Nelson View author publications You can
also search for this author inPubMed Google Scholar CONTRIBUTIONS D.A. initiated, designed, and supervised the project. D.A contributed to the experimental design and scientific
presentation. D.H., M.G., A.S. and D.A. performed all of the experiments and data analysis. A.S., D.R., D.A. and J.H. developed the theoretical studies. D.A. wrote the manuscript with
contribution from all authors. All authors contributed to the scientific discussion. CORRESPONDING AUTHORS Correspondence to Daniel Ahmed or Bradley J. Nelson. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. EXTENDED DATA EXTENDED DATA FIG. 1 Fabrication of the acoustofluidic device. EXTENDED DATA FIG. 2 ULTRASOUND MANIPULATION SYSTEM. (A) Schematic demonstrates the
ultrasound particle manipulation setup. (B) A micrograph shows trapped microparticles in pressure nodes arrays when exposed to an ultrasound at ~2.1 MHz and 12 V, respectively. EXTENDED DATA
FIG. 3 Experimental setup of microparticle manipulation in acoustic and magnetic field. EXTENDED DATA FIG. 4 An electromagnetic setup purchased from MagnebotiX AG (Zurich, Switzerland) with
eight independently controlled coils was integrated with the inverted microscope to generate a rotating magnetic field. EXTENDED DATA FIG. 5 Swarm stability against thermal fluctuation A
plot demonstrates the ratio of the thermal to magnetic forces versus the radius of superparamagnetic particles. Magnetic forces dominate over the thermal effects for particles with radii 3
μm. EXTENDED DATA FIG. 6 Poiseuille flow profile Experimental characterization of the Poiseuille flow profile within the circular channel. EXTENDED DATA FIG. 7 Upstream motion Upstream
migration of swarm of microparticles in a combined acoustic and magnetic field where the pressure node lies outside the capillary, see also Supplementary Movie 6. SUPPLEMENTARY INFORMATION
SUPPLEMENTARY INFORMATION Supplementary Notes 1–5, Extended Data Figs. 1–7, Tables 1 and 2, legends for Supplementary Videos 1–9 and references. SUPPLEMENTARY VIDEO 1 Fluorescent
microparticles getting trapped in the acoustic pressure nodes. SUPPLEMENTARY VIDEO 2 Acoustic switching of microparticles from the centre to the sidewall of the channel. Recorded at 15 fps
and played at 60 fps. SUPPLEMENTARY VIDEO 3 An array of trapped microparticles was acoustically shifted. Recorded at 15 fps and played at 30 fps. SUPPLEMENTARY VIDEO 4 Formation of
microswarms in an acoustic and magnetic field. The top panel shows microswarm configuration sandwiched between two glass slides and in the absence of an acoustic field. The middle panel
shows microswarm structures in the acoustic pressure node. The bottom panel demonstrates microswarms formation in the pressure node when imposed upon an external flow field. SUPPLEMENTARY
VIDEO 5 Recruitment of microswarm near a wall in the presence of an acoustic field. SUPPLEMENTARY VIDEO 6 Upstream migration of microparticles in a combined acoustic and magnetic field with
the pressure node outside the capillary. Recorded at 15 fps and played at 60 fps. SUPPLEMENTARY VIDEO 7 Upstream migration of microparticles with the pressure node lies inside the capillary.
SUPPLEMENTARY VIDEO 8 Deformation of the microswarm when the acoustic field is turned on and off. SUPPLEMENTARY VIDEO 9 Ultrasound manipulation of polystyrene particles (of 5.5 µm) under
pulsatile flow. An external flow of 150 µl min–1 (corresponds to 200 mm s–1) with a periodic flow of 1 Hz is developed along a 1.55 mm outer diameter capillary. A pair of piezoelectric
transducers were bonded across the channel and were actuated at 5.1 MHz, 20 VPP. The video is recorded at 7 fps and played at 15 fps. SUPPLEMENTARY SOFTWARE 1 SUPPLEMENTARY SOFTWARE 2
SUPPLEMENTARY SOFTWARE 3 SOURCE DATA SOURCE DATA FIG. 2 Source data for Fig. 2b,c. SOURCE DATA FIG. 5 Source data for Fig. 5b,c. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS
ARTICLE CITE THIS ARTICLE Ahmed, D., Sukhov, A., Hauri, D. _et al._ Bioinspired acousto-magnetic microswarm robots with upstream motility. _Nat Mach Intell_ 3, 116–124 (2021).
https://doi.org/10.1038/s42256-020-00275-x Download citation * Received: 19 February 2020 * Accepted: 10 November 2020 * Published: 11 January 2021 * Issue Date: February 2021 * DOI:
https://doi.org/10.1038/s42256-020-00275-x SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative