Play all audios:
ABSTRACT Patients with cancer are presumed to be at increased risk of severe COVID-19 outcomes due to underlying malignancy and treatment-induced immunosuppression. Of the first 178 patients
managed for COVID-19 at the Gustave Roussy Cancer Centre, 125 (70.2%) were hospitalized, 47 (26.4%) developed clinical worsening and 31 (17.4%) died. An age of over 70 years, smoking
status, metastatic disease, cytotoxic chemotherapy and an Eastern Cooperative Oncology Group score of ≥2 at the last visit were the strongest determinants of increased risk of death. In
multivariable analysis, the Eastern Cooperative Oncology Group score remained the only predictor of death. In contrast, immunotherapy, hormone therapy and targeted therapy did not increase
clinical worsening or death risk. Biomarker studies found that C-reactive protein and lactate dehydrogenase levels were significantly associated with an increased risk of clinical worsening,
while C-reactive protein and D-dimer levels were associated with an increased risk of death. COVID-19 management impacted the oncological treatment strategy, inducing a median 20 d delay in
41% of patients and adaptation of the therapeutic strategy in 30% of patients. SIMILAR CONTENT BEING VIEWED BY OTHERS COVID-19 AND CANCER REGISTRIES: LEARNING FROM THE FIRST PEAK OF THE
SARS-COV-2 PANDEMIC Article Open access 25 March 2021 SARS-COV-2 INFECTION IN THE ITALIAN VENETO REGION: ADVERSE OUTCOMES IN PATIENTS WITH CANCER Article 31 July 2020 EFFECTIVENESS OF
REGEN-COV ANTIBODY COMBINATION IN PREVENTING SEVERE COVID-19 OUTCOMES Article Open access 02 August 2022 MAIN By early March 2020, the spread of the coronavirus disease 2019 (COVID-19)
outbreak had reached the Paris area, France. Since then, all medical resources have been reorganized to handle the pandemic. As a tertiary cancer center, Gustave Roussy has followed two
objectives: define processes to safely sustain cancer care in a secured environment and reorganize internally to adapt its capacities to hospitalize patients with cancer and COVID-19
illness. Patients with cancer have been considered at increased risk of COVID-19, on the rationale of the increased systemic immunosuppressive state caused by the underlying malignancy and
anticancer treatments. The first report from a retrospective cohort in China suggested that patients with cancer were observed to have a higher risk of severe events (for example, a
composite endpoint of intensive care unit (ICU) admission, invasive ventilation or death) compared with patients without cancer (seven (39%) of 18 patients versus 124 (8%) of 1,572 patients;
_P_ = 0.0003) and that patients with cancer deteriorated more rapidly than those without cancer1. While general determinants of COVID-19 severity have emerged from large cohorts from China
and Italy2,3, limited data are available on the specificity of patients with cancer to help the oncology community to identify patients at risk of severe COVID-19. Furthermore, the impact of
COVID-19 infection on ongoing cancer care is unexplored. This study investigated the determinants of clinical worsening and death, as well as the impact on cancer care, for the first
patients sequentially managed for COVID-19 and cancer in an academic tertiary cancer center. RESULTS PATIENT POPULATION From 24 March 2020 until 29 April 2020, severe acute respiratory
syndrome coronavirus 2 (SARS-CoV-2) was detected in 196 (12%) of 1,633 tests performed internally at the Gustave Roussy Cancer Centre. Overall, 209 patients were identified (including a few
identified by PCR with reverse transcription (RT-PCR) performed at another facility and some diagnosed by computed tomography scan alone) and the final study population included 178 adult
patients. The following were reasons for exclusion: pediatric population (six patients); non-cancer patients (19 patients); and COVID-19 ultimately ruled out (six patients). Baseline
demographics, comorbidities and underlying cancer characteristics for the population are shown in Table 1. The study population included 102 (57.3%) female patients with a median age of 61
years old and a median body mass index (BMI) of 25. Among 156 patients with a history of solid tumor, the most frequent were breast cancer (20.5%), gynecological cancer (14.7%), head and
neck tumors (14.1%), gastrointestinal cancer (13.5%) and genitourinary malignancies (12.8%). The most common hematological malignancy was mature B cell neoplasm, in 15 patients. The disease
status at the last oncological or hematological follow-up was remission or localized tumor with ongoing curative treatment in 70 patients (39.3%) and locally advanced or metastatic disease
in 108 patients (60.7%). The Eastern Cooperative Oncology Group (ECOG) performance status at the last follow-up was 0–1 in 73% of patients. Systemic anticancer treatment had been
administered in the past 3 months in 117 (66.9%) patients. DIAGNOSIS The vast majority of patients (_n_ = 138; 79.8%) presented with COVID-19 symptoms before any test or imaging. COVID-19
was suspected following symptoms prompting RT-PCR testing in 134 patients (75.7%) and following incidental findings on a computed tomography scan in 16 patients (9%), and was related to
systematic screening (before surgery or another treatment modality) in 27 patients (15.3%). The most common symptoms reported are presented in Table 2. COVID-19 diagnosis was established by
SARS-CoV-2-positive nasal RT-PCR in 166 patients (93.8%) and by computed tomography scan alone (confirmed by observation of the typical appearance of COVID-19 as defined by the American
College of Radiology criteria for patients with a negative RT-PCR test4) in 11 patients (6.2%). Overall, 125 (70.2%) patients were hospitalized for COVID-19 illness. The median time between
the first COVID-19 symptoms and admission was 4 d (Q1–Q3 = 2–8). The median duration of hospital stay was 10 d (range = 1–40 d). COVID-19 SYSTEMIC TREATMENT Based on the available data,
systemic treatment for COVID-19 included a combination of hydroxychloroquine and azithromycin in 45 patients (25.4%), a combination of lopinavir and ritonavir in five patients (2.9%), an
immunomodulatory interleukin-6 inhibitor (tocilizumab) in ten patients (5.6%) and steroids in 21 patients (11.9%), mostly administered intravenously with either dexamethasone (20 mg on days
1–3 and 10 mg on days 4–6) or >1 mg kg−1 equivalent prednisone (Supplementary Table 1). Overall, 91 patients (51.4%) received anticoagulation. Among these 91 patients, anticoagulation was
administered with thromboprophylaxis intent (low or intermediate risk: 62%; high risk: 13%) or with curative intent (25%). Among hospitalized patients, 11 patients (8.8%) experienced a
documented bacterial infection, ten (8.1%) presented neurological or psychiatric symptoms and three (2.4%) developed a thromboembolic event. OUTCOME At data cutoff on 6 May 2020, the median
follow-up of the study population from COVID-19 diagnosis was 23 d (Q1–Q3 = 13–33 d). Among the 178 patients, 47 developed clinical worsening or died (Table 3), corresponding to a clinical
worsening-free survival rate of 73.7% (95% confidence interval (CI) = 66.2–79.9%) 21 d after COVID-19 diagnosis. Sixteen patients (9%) had been admitted to the ICU. The median time from the
first COVID-19 symptoms to clinical worsening was 7 d (minimum = 1 d; maximum = 19 d). Overall, 31 patients died, corresponding to an overall survival rate of 82% (95% CI = 74.9–87.4%) 21 d
after COVID-19 diagnosis. Among them, the primary cause of death was related to COVID-19 in 20 patients (64.5%) and to cancer in 11 patients (35.5%). CANCER-RELATED DETERMINANTS OF CLINICAL
WORSENING-FREE AND OVERALL SURVIVAL IN PATIENTS WITH CANCER AND COVID-19 Univariable and multivariable analyses of both clinical worsening-free and overall survival are presented in Table 4.
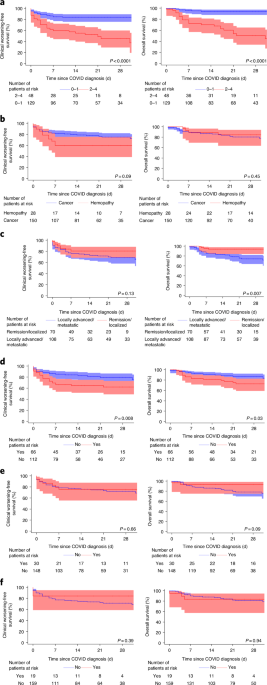
In univariable analysis, an ECOG score of ≥2 at the last follow-up visit, smoking status (current and former) and the use of cytotoxic chemotherapy in the past 3 months were significantly
associated with clinical worsening-free survival. A similar trend was observed according to the type of cancer (hematological malignancy). Determinants of increased risk of death, in
univariable analysis, were age ≥70 years, smoking status (current and former), an ECOG score of ≥2 at the last follow-up, onco-hematological status (metastatic disease) and the use of
cytotoxic chemotherapy in the past 3 months (Figs. 1 and 2). Among patients treated with cytotoxic chemotherapy in the past 3 months and presenting clinical worsening (_n_ = 26), four out of
five (80%) patients in remission or with localized disease were admitted to the ICU versus five out of 21 patients (24%) with locally advanced or metastatic disease (_P_ = 0.035).
Conversely, hormone therapy, targeted therapies and immune checkpoint inhibitors had no impact on the COVID-19 outcomes. Only 11 patients (6%) were receiving radiation therapy at the time of
COVID diagnosis. Three patients presented with clinical deterioration (two with a gynecological cancer and one with a renal cell carcinoma metastatic to the bone). Among them, one patient
eventually died from COVID-19. In multivariable analysis, an ECOG score of ≥2 at the last follow-up was the strongest determinant associated with both clinical worsening and death. BASELINE
BIOLOGICAL DETERMINANTS OF CLINICAL WORSENING-FREE AND OVERALL SURVIVAL IN PATIENTS WITH CANCER AND COVID-19 Several biological factors, markers of infection, inflammatory status and
underlying conditions have been investigated for their association with either the risk of clinical worsening or the risk of death and are presented in Table 5. Among these, C-reactive
protein (CRP) >50 mg ml−1, procalcitonin >0.5 µg l−1, lymphopenia ≤500 cells per µl, monocytopenia ≤200 cells per µl, ferritin >1,000 ng ml−1, lactate dehydrogenase (LDH) >250 IU
l−1, albumin ≤30 g l−1 and troponin >upper limit of normal (ULN) were associated with a significantly increased risk of both clinical worsening and death in univariable analysis. In
multivariable analysis, CRP and LDH were significantly associated with an increased risk of clinical worsening, and CRP and D-dimer >3 µg l−1 were associated with an increased risk of
death. IMPACT OF COVID-19 ON ONCOLOGICAL STRATEGY The results of a post-COVID-19 first-month assessment were available for 146 patients: 75% reported persistent symptoms of asthenia, 42%
reported exertion dyspnea and 30% reported a cough. Among 141 patients with available information, 87 (62%) had cancer-specific treatment already restarted or planned. The overall impact of
COVID-19 on cancer treatment was assessed in the entire cohort (Table 3), leading to no change in oncological care management in 51 patients (29%). These included 28 patients who were under
surveillance before COVID-19 and remained under surveillance, as well as 22 patients with ongoing oncological or hematological treatment for whom COVID-19 did not impact the ongoing care. In
73 patients (41%), COVID-19 diagnosis induced a delay but no change in the strategy, with a median delay of 20 d (interquartile range (IQR): 12–30 d) for systemic therapy and 28 d (IQR:
22–44 d) for surgery or ablative techniques. A change in oncological care management (such as surgery anticipation or switch to radiotherapy) was reported in 4% of patients, and a change in
systemic therapy was reported in 4% of patients. The COVID-19 illness led to a discontinuation of cancer treatment in 4% of patients for the surveillance-only strategy. Among patients with
any impact (delay, change or end of treatment), 78% of these changes were related to COVID-19, 18% were related to underlying cancer progression and 4% were related to cancer treatment
toxicity. Among 11 patients who were diagnosed with COVID-19 while undergoing ongoing radiation therapy, four had an interruption in the radiation plan, and among eight patients with planned
radiation therapy at the time of COVID-19 diagnosis, three had the start of radiation treatment delayed by >7 d. DISCUSSION The outcomes of COVID-19 illness have been analyzed in
previous datasets2,3,5,6 but data on predictors of disease severity specifically in patients with cancer are limited and the impact on cancer treatment is unknown. In this study, we report
on COVID-19 management at a tertiary cancer center and investigate determinants of clinical worsening and death, as well as the impact on cancer care. This study identified that 12% of the
tested population were positive for COVID-19. COVID-19 led to a death rate of 17.4%, similar to the concomitant mortality of admitted patients with COVID-19 in the Paris area
(https://www.gouvernement.fr/info-coronavirus/carte-et-donnees). Conflicting results have been reported recently on a potential increased risk of severe COVID-19 outcome in patients with
cancer. A cohort of 5,688 patients, including 334 patients with cancer, did not identify any increased risk of death in patients with cancer (HR = 1.15 (95% CI = 0.84–1.57))7, while an
analysis of 105 patients with cancer and 536 age-matched patients without cancer suggested that patients with cancer have higher risks for all severe outcomes related to COVID-19 and an
excess odds ratio of 2.17 (_P_ = 0.06) for death8. More recently, the OpenSAFELY study suggested there is increased COVID-19 mortality in patients with a recent diagnosis of solid tumors6.
Recently, two large retrospective international cohorts have been reported on, including all patients with cancer in the COVID-19 and Cancer Consortium (CCC19) study9 and all patients with
thoracic cancer in the Thoracic Cancers International COVID-19 Collaboration (TERAVOLT) study10. Using a similar design, these two studies investigated factors associated with COVID-19
mortality. The design and results of these two studies, along with the current study and two others, are presented in Table 6, which summarizes the specificity of each cohort. In our cohort,
we observed a trend towards severe COVID-19 in patients with hematological malignancies (crude hazard ratio (HR) = 1.80 (95% CI = 0.91–3.55); _P_ = 0.09), as reported in other datasets6,11.
However, in our experience, this factor was not associated with a significant increased risk of death, potentially related to both a more intensive care strategy and the limited number of
hematological patients in our cohort. The granularity of our dataset has enabled the investigation of several determinants including the impact of cancer status (remission or localized
versus metastatic or advanced), the impact of the systemic anticancer treatment used and the ECOG performance status at the last oncological follow-up before COVID-19 diagnosis. Our analysis
suggests that cancer status may not impact the risk of clinical worsening but seems to be associated with an increased risk of death (univariable analysis). With regard to systemic
anticancer therapy, we did not identify a detrimental effect of the use of immune checkpoint inhibitors, hormone therapy or targeted therapy on the risk of clinical worsening or death. While
some initial reports suggested a potential increased severity of COVID-19 with immune checkpoint inhibitors8, a more recent dataset including two cohorts of patients with lung cancer
similarly concluded that programmed cell death protein 1 blockade exposure is not associated with an increased risk of severity of COVID-19 (refs. 10,12). Conversely, the use of cytotoxic
chemotherapy was associated with an increased risk of clinical worsening and death in univariable analysis and showed a trend for a higher risk of death after adjustment of ECOG performance
status and cancer status in multivariable analysis. This may be partially explained by more intensive care solicitation in patients receiving cytotoxic chemotherapy in the setting of
remission or localized disease. Similarly, the UK Coronavirus Cancer Monitoring Project did not identify evidence that patients with cancer who are on cytotoxic chemotherapy or another
anticancer treatment are at an increased risk of mortality from COVID-19 disease compared with those not on active treatment13. In our experience, an ECOG score of ≥2 at the last oncological
follow-up remains in multivariable analysis the strongest determinant of both increased risk of clinical worsening and increased risk of death. Patient characteristics and prognostic models
in our population highlight the fact that patients with cancer may not harbor the specific patterns of comorbidities reported in large COVID-19 series2,3,5. Our dataset identified a trend
towards worse outcomes in patient with a BMI >30, but the lack of a significant impact of BMI may be driven by underlying oncological disease and nutritional status in advanced
oncological states, as captured in patients with a BMI <18.5 or albumin <30 g l−1 in our cohort. Several datasets have reported on the association of current smoking with favorable
outcomes of COVID-19 (refs. 2,14). Conversely, we report a detrimental effect of current or former smoking status on outcomes that may be driven by the underlying smoking-related
malignancies in our population. Furthermore, we investigated the role of several biological markers in disease outcome in patients with cancer. While levels of CRP and other inflammatory
factors have been reported previously, we identified lymphopenia and monocytopenia as relevant markers associated with clinical worsening. Emerging data on a cohort of 142 patients in which
blood markers (CRP, procalcitonin, interleukin-6, lymphocyte count and viral load (ORF1ab Ct)) were explored as predictors of survival in patients with COVID-19 have recently shown that
lymphopenia is the strongest predictor for severity disease in patients with COVID-19 (ref. 15). Of note, lymphopenia has long been established as a prognostic factor for overall survival in
patients with cancer16. We report on the potential role of monocytopenia as a predictor of clinical worsening. This finding is in line with recent immune cell profiling of patients with
COVID-19 identifying that monocyte levels increased in patients in the early recovery stage of COVID-19 (ref. 17). Additionally, recent evidence suggests that pathological macrophages mostly
derive from circulating monocytes that massively infiltrate the lungs18. Monocytes are considered to have the potential to differentiate into pro-inflammatory macrophages via activation of
Janus kinase–signal transducer and activator of transcription (JAK–STAT) pathways and to contribute to hyper-inflammation associated with COVID-19 (ref. 19). Our dataset explores the impact
of COVID-19 on cancer management. Beyond the emerging data raising the concern of fewer cancer diagnoses during the COVID-19 pandemic20, the impact on ongoing cancer care, including delays
of treatment and halting of clinical trials, has been identified21. Our dataset identified a high incidence of delays, but the median time to anticancer systemic therapy or rescheduled
surgery was <1 month. To ensure that patients continue to receive essential care while minimizing exposure to SARS-CoV-2 infection, cancer centers have organized their healthcare systems
at an unprecedented scale and pace22. As such, we have amended our CAPRI telemedicine program, which was initially set up to monitor patients with cancer undergoing oral therapy, to face the
ongoing COVID-19 crisis. The remote telemedicine monitoring strategy has been adapted to monitor outpatients positive for COVID-19 after they have provided informed consent23. Our study did
not address the management of our pediatric patient population. At the institution level, it was decided that all pediatric patients should be tested routinely, and among 122 patients
<18 years of age tested internally, 5% were found to be positive (minimum = 22 months; maximum = 13 years). Dedicated analysis is planned for the management of this specific population.
The retrospective nature of this work from a single institution and the heterogeneity of our cancer center population are inherent limitations to our study. Due to the small sample size and
relatively low number of events, multivariable models were only adjusted on the main prognostic factors. Some analyses may have failed to identify other determinants of clinical
deterioration and overall survival by lack of power. As a result of the testing strategy (mainly in symptomatic patients who were likely to have more severe infection), our results may not
apply to asymptomatic and paucisymptomatic patients with cancer. However, this highlights the challenges that cancer centers have faced when handling the COVID-19 pandemic: the need for
daily fine-tuned patient management and the need to inform the community on strategies to ensure our patients with cancer have access to essential care in an adjusted environment. In
summary, cancer centers had to face the COVID-19 outbreak with the concomitant objective to secure patients’ care while protecting them from the infection. Globally, these objectives have
been reached, with COVID-19 outcomes comparable to those of the general population and cancer care minimally delayed and already safely restarted. METHODS STATISTICS AND REPRODUCIBILITY
STUDY DESIGN We performed a retrospective observational study to describe the management of adult patients with cancer (solid tumors or hematological malignancies) managed at the Gustave
Roussy Cancer Centre after a diagnosis of SARS-CoV-2 infection (COVID-19) between 14 March 2020 and 29 April 2020. The modalities of COVID-19 diagnosis, clinical presentation, treatments
administered for COVID-19 and patient outcomes, including impacts on cancer management, are reported. The aim of the study was to identify clinical and biological prognostic factors of
clinical worsening and/or death. No statistical method was used to predetermine sample size. A total of 31 observations were excluded from the final analysis: six pediatric patients; 19
patients who did not have cancer; and six patients in whom COVID-19 was ultimately ruled out. Due to the retrospective nature of this study, there was neither randomization nor blinding.
MEASURES Prognostic factors included demography (age and gender), comorbidities, solid tumor or hematological malignancies (tumor site and type, disease status and treatment received) and
biological factors from laboratory tests at COVID-19 diagnosis. All of the measurements were performed independently in each patient (no repeated measurements were done). Biological factors
were categorized using pre-defined threshold values based on normal value cutoffs or recently published cutoffs for the study of COVID-19. Chest computed tomography imaging characteristics,
including the extent of lung involvement at diagnosis, were recorded. OUTCOMES The outcomes studied were clinical worsening-free survival and overall survival. Clinical worsening-free
survival was defined as the time from COVID-19 diagnosis to clinical worsening (oxygen needs ≥6 l min−1 or admission to the ICU) or death. Overall survival was defined as the time from
COVID-19 diagnosis to death from any cause. These outcomes were chosen because they are objective, reliably recorded and reflect the increasing severity of COVID-19, as recommended by the
World Health Organization for the assessment of patients in clinical studies24. STATISTICAL ANALYSIS Descriptive statistics (numbers, percentages, medians, IQRs and ranges) were used to
describe population characteristics. The _χ_2 test (or Fisher’s test) and Student’s _t_-test (or Wilcoxon test) were performed for intergroup comparisons, as appropriate. Time-to-event
endpoints (clinical worsening-free survival and overall survival) were reported using the Kaplan–Meier method with Rothman’s CIs. For the study of clinical and biological prognostic factors,
Cox’s proportional hazard models were used to provide _P_ values and HRs with associated 95% CIs in both univariable and multivariable analyses. The choice of variables to include in the
multivariable analyses was driven by the number of events available (three to four variables were included for 47 clinical worsenings/deaths and 31 deaths), the strength of the association
in the univariable analyses and the absence of collinearity between variables included in the model (assessed by _χ_2 test and Fisher’s test for qualitative variables or Spearman’s
correlation coefficient for quantitative variables). The assumption of proportional hazards was checked by testing the existence of an interaction between each variable and log[time] in each
model. Due to the exploratory nature of the analyses, no formal adjustment for multiplicity was done. All tests were two sided and significance was accepted at the 5% level. The analyses
were performed using SAS 9.4 software (SAS institute). DATABASE AND ETHICAL APPROVAL Study data were collected and managed using REDCap 9.8.4 tools hosted at the Gustave Roussy Cancer
Centre25,26. In accordance with the French regulations, there was no requirement for ethical approval to be sought for this observational study, based on medical files. Conforming to the
General Data Protection Regulation and French law about clinical retrospective studies, the patients included in our study all received an information notice (non-opposal information)
introducing the study, following information included in Article 14 of the General Data Protection Regulation, and describing their rights in relation to their data. This study was also
declared to the Gustave Roussy Cancer Centre’s data protection officer and registered on the website of the French Healthcare Data Institute (declaration number: MR4911200520). COVID-19
SCREENING STRATEGY Due to testing resources, the screening strategy for SARS-CoV-2 infection evolved over the course of the reported study. Initially, PCR testing was performed for
symptomatic patients. Subsequently, there was systematic screening of non-symptomatic patients scheduled for surgery and/or radiation therapy, as well as in the pediatric population.
Ultimately, testing was offered to any patients with a solid tumor or hematological malignancies as part of the ongoing ONCOVID clinical trial (Epidemiology of SARS-CoV-2 and Mortality to
COVID-19 Disease in French Cancer Patients; NCT04341207). COVID-19 PCR TESTING SARS-CoV-2 diagnostic testing of clinical samples by RT-PCR was conducted from 14 March to 23 March at an
outside facility (263 patients were tested, of whom 35 were found to be positive) using the Charité protocol27. From 23 March, testing was performed internally at the Gustave Roussy Cancer
Centre. Nasopharyngeal swab samples were collected using flocked swabs (Sigma Virocult) and placed in viral transport media. SARS-CoV-2 RNA was detected using a multiplex real-time RT-PCR
diagnostic kit (the Applied Biosystems TaqPath COVID-19 CE-IVD RT-PCR Kit) targeting three regions (ORF1ab, nucleocapsid and spike genes) with the following modifications. Nucleic acids were
extracted from specimens using automated Maxwell instruments following the manufacturer’s instructions (Maxwell RSC simplyRNA Blood Kit; AS1380; Promega). Real-time RT-PCR was performed on
the QuantiStudio 5 Dx Real-Time PCR System (Thermo Fisher Scientific) in a final reaction volume of 20 μl, including 5 μl of extracted nucleic acids. Samples were reported as positive if at
least two targets were detected. COVID-19 AND ONCOLOGICAL TREATMENT STRATEGIES COVID-19 therapeutic management has been defined through institutional guidelines (Extended Data Fig. 1). These
institutional guidelines were adjusted over time, depending on emerging data from the pandemic28,29, clinical experience30,31,32,33 and onsite activation of clinical trials (NCT04331808,
NCT04341207 and NCT04333914). The oncological treatment strategy was adapted based on international and national guidelines, as previously summarized22. REPORTING SUMMARY Further information
on research design is available in the Nature Research Reporting Summary linked to this article. DATA AVAILABILITY The data underlying the findings of this study cannot be made freely
available because of ethical and legal restrictions. This is because the present study includes an important number of variables that, together, could be used to re-identify the patients
based on a few key characteristics. However, data from this study can be requested by filling out the data request form for Gustave Roussy clinical studies at
https://redcap.gustaveroussy.fr/redcap/surveys/?s=DYDTLPE4AM. The process is similar for every study sponsored by Gustave Roussy. The study steering committee and the sponsor will review the
requests on a case-by-case basis. In case of approval, a specific agreement between the sponsor and the researcher may be required for data transfer. CODE AVAILABILITY SAS software version
9.4 was used for the analysis without customization. Statistical codes (SAS software) will be made available with the data if requested. REFERENCES * Liang, W. et al. Cancer patients in
SARS-CoV-2 infection: a nationwide analysis in China. _Lancet Oncol._ 21, 335–337 (2020). Article CAS Google Scholar * Guan, W.-J. et al. Clinical characteristics of coronavirus disease
2019 in China. _N. Engl. J. Med._ 382, 1708–1720 (2020). Article CAS Google Scholar * Grasselli, G. et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2
admitted to ICUs of the Lombardy region, Italy. _J. Am. Med. Assoc._ https://doi.org/10.1001/jama.2020.5394 (2020). * Simpson, S. et al. Radiological society of North America expert
consensus statement on reporting chest CT Findings related to COVID-19. Endorsed by the society of thoracic radiology, the American College of Radiology, and RSNA. _J. Thorac. Imag._
https://doi.org/10.1097/RTI.0000000000000524 (2020). * Richardson, S. et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New
York City area. _J. Am. Med. Assoc._ https://doi.org/10.1001/jama.2020.6775 (2020). * Williamson, E. J. et al. Factors associated with COVID-19-related death using OpenSAFELY. _Nature_
https://doi.org/10.1038/s41586-020-2521-4 (2020). * Miyashita, H. et al. Do patients with cancer have a poorer prognosis of COVID-19? An experience in New York City. _Ann. Oncol. Off. J.
Eur. Soc. Med. Oncol_. https://doi.org/10.1016/j.annonc.2020.04.006 (2020). * Dai, M. et al. Patients with cancer appear more vulnerable to SARS-COV-2: a multicenter study during the
COVID-19 outbreak. _Cancer Discov_. https://doi.org/10.1158/2159-8290.CD-20-0422 (2020). * Kuderer, N. M. et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study.
_Lancet_ 395, 1907–1918 (2020). Article CAS Google Scholar * Garassino, M. C. et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international,
registry-based, cohort study. _Lancet Oncol._ 21, 914–922 (2020). Article CAS Google Scholar * Mehta, V. et al. Case fatality rate of cancer patients with COVID-19 in a New York hospital
system. _Cancer Discov_. https://doi.org/10.1158/2159-8290.CD-20-0516 (2020). * Luo, J. et al. Impact of PD-1 blockade on severity of COVID-19 in patients with lung cancers. _Cancer Discov_.
https://doi.org/10.1158/2159-8290.CD-20-0596 (2020). * Lee, L. Y. W. et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort
study. _Lancet_ 395, 1919–1926 (2020). Article CAS Google Scholar * Zhang, J.-J. et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. _Allergy_
https://doi.org/10.1111/all.14238 (2020). * Tan, L. et al. Validation of predictors of disease severity and outcomes in COVID-19 patients: a descriptive and retrospective study. _Med._
https://doi.org/10.1016/j.medj.2020.05.002 (2020). * Ray-Coquard, I. et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. _Cancer
Res._ 69, 5383–5391 (2009). Article CAS Google Scholar * Wen, W. et al. Immune cell profiling of COVID-19 patients in the recovery stage by single-cell sequencing. _Cell Discov._ 6, 31
(2020). Article CAS Google Scholar * Zhou, Y. et al. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. _Natl Sci. Rev_.
https://doi.org/10.1093/nsr/nwaa041 (2020). * Merad, M. & Martin, J. C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. _Nat. Rev.
Immunol_. https://doi.org/10.1038/s41577-020-0331-4 (2020). * Dinmohamed, A. G. et al. Fewer cancer diagnoses during the COVID-19 epidemic in the Netherlands. _Lancet Oncol_.
https://doi.org/10.1016/S1470-2045(20)30265-5 (2020). * Richards, M., Anderson, M., Carter, P., Ebert, B. L. & Mossialos, E. The impact of the COVID-19 pandemic on cancer care. _Nat.
Cancer_ https://doi.org/10.1038/s43018-020-0074-y (2020). * Van de Haar, J. et al. Caring for patients with cancer in the COVID-19 era. _Nat. Med._ 26, 665–671 (2020). Article CAS Google
Scholar * Scotté, F. et al. A patient reported outcome platform, a useful tool to improve monitoring and effective management of COVID-19-positive patients with cancer. _Eur. J. Cancer Oxf.
Engl. 1990_ 132, 1–4 (2020). Google Scholar * _COVID-19 Therapeutic Trial Synopsis_ (World Health Organization, 2020);
https://www.who.int/publications-detail/covid-19-therapeutic-trial-synopsis * Harris, P. A. et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow
process for providing translational research informatics support. _J. Biomed. Inform._ 42, 377–381 (2009). Article Google Scholar * Harris, P. A. et al. The REDCap consortium: building an
international community of software platform partners. _J. Biomed. Inform._ 95, 103208 (2019). Article Google Scholar * Corman, V. M. et al. Detection of 2019 novel coronavirus (2019-nCoV)
by real-time RT-PCR. _Euro Surveill._ 25, 2000045 (2020). Google Scholar * Cao, B. et al. A trial of lopinavir–ritonavir in adults hospitalized with severe COVID-19. _N. Engl. J. Med._
382, 1787–1799 (2020). Article Google Scholar * Wang, Y. et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. _Lancet_ 395,
1569–1578 (2020). Article CAS Google Scholar * Michot, J.-M. et al. Tocilizumab, an anti-IL-6 receptor antibody, to treat Covid-19-related respiratory failure: a case report. _Ann.
Oncol._ https://doi.org/10.1016/j.annonc.2020.03.300 (2020). * Gautret, P. et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized
clinical trial. _Int. J. Antimicrob. Agents_ https://doi.org/10.1016/j.ijantimicag.2020.105949 (2020). * Million, M. et al. Early treatment of COVID-19 patients with hydroxychloroquine and
azithromycin: A retrospective analysis of 1061 cases in Marseille, France. _Travel Med. Infect. Dis_. https://doi.org/10.1016/j.tmaid.2020.101738 (2020). * Russell, C. D., Millar, J. E.
& Baillie, J. K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. _Lancet_ 395, 473–475 (2020). Article CAS Google Scholar * Vuagnat, P. et al.
COVID-19 in breast cancer patients: a cohort at the Institut Curie hospitals in the Paris area. _Breast Cancer Res._ 22, 55 (2020). Article CAS Google Scholar Download references
ACKNOWLEDGEMENTS We thank the patients, their families and all of the investigators and caregivers involved in the COVID-19 pandemic management at the Gustave Roussy Cancer Centre. We also
thank E. Gallois, T. Nihouarn, M. Wartelle, F. Lion, C. Mohamed-Djalim, G. Bescher, A. Pinto, H. Emerit, I. Sakraoui, L. Antoun, T. Grinda, C. Alves-Costa-Silva, L. Cerbone, J. C.
Benitez-Montanez, S. Maillard, H. Bompais-Vincent and the Gustave Roussy Clinical Research Department, as well as the ONCOVID clinical trial investigators and researchers. Part of this work
was presented during the virtual Clinical Plenary Session at the AACR 2020 Annual Meeting on 28 April 2020. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Cancer Medicine Department, Gustave
Roussy, Paris-Saclay University, Paris, France Laurence Albiges, Arnaud Bayle, Fanny Pommeret, Emeline Colomba, Giulia Baciarello, Audrey Perret, Antoine Hollebecque, Mathilde Hauchecorne,
Fabrice André & Benjamin Besse * Biostatistics Department, Gustave Roussy, Paris-Saclay University, Paris, France Stéphanie Foulon * Interdisciplinary Cancer Course Department, Gustave
Roussy, Paris-Saclay University, Paris, France Bertrand Gachot, Fanny Pommeret, Annabelle Stoclin, Mansouria Merad & Florian Scotte * Haematology Department, Gustave Roussy, Paris-Saclay
University, Paris, France Christophe Willekens, Thomas Hueso, Nathalie Chaput & Jean-Baptiste Micol * Biopathology Department, Gustave Roussy, Paris-Saclay University, Paris, France
Frank Griscelli, Ludovic Lacroix & Veronique Saada * Pharmacy Department, Gustave Roussy, Paris-Saclay University, Paris, France Florence Netzer * Imaging Department, Gustave Roussy,
Paris-Saclay University, Paris, France Corinne Balleyguier, Samy Ammari & Julien Hadoux * Early Drug Development Department, Gustave Roussy, Paris-Saclay University, Paris, France
Antoine Hollebecque & Jean-Marie Michot * Radiation Oncology Department, Gustave Roussy, Paris-Saclay University, Paris, France Roger Sun * Paediatric Oncology Department, Gustave
Roussy, Paris-Saclay University, Paris, France Dominique Valteau-Couanet * Gustave Roussy, Paris-Saclay University, Paris, France Jean-Charles Soria & Fabrice Barlesi * Aix Marseille
University, CNRS, INSERM, CRCM, Marseille, France Fabrice Barlesi Authors * Laurence Albiges View author publications You can also search for this author inPubMed Google Scholar * Stéphanie
Foulon View author publications You can also search for this author inPubMed Google Scholar * Arnaud Bayle View author publications You can also search for this author inPubMed Google
Scholar * Bertrand Gachot View author publications You can also search for this author inPubMed Google Scholar * Fanny Pommeret View author publications You can also search for this author
inPubMed Google Scholar * Christophe Willekens View author publications You can also search for this author inPubMed Google Scholar * Annabelle Stoclin View author publications You can also
search for this author inPubMed Google Scholar * Mansouria Merad View author publications You can also search for this author inPubMed Google Scholar * Frank Griscelli View author
publications You can also search for this author inPubMed Google Scholar * Ludovic Lacroix View author publications You can also search for this author inPubMed Google Scholar * Florence
Netzer View author publications You can also search for this author inPubMed Google Scholar * Thomas Hueso View author publications You can also search for this author inPubMed Google
Scholar * Corinne Balleyguier View author publications You can also search for this author inPubMed Google Scholar * Samy Ammari View author publications You can also search for this author
inPubMed Google Scholar * Emeline Colomba View author publications You can also search for this author inPubMed Google Scholar * Giulia Baciarello View author publications You can also
search for this author inPubMed Google Scholar * Audrey Perret View author publications You can also search for this author inPubMed Google Scholar * Antoine Hollebecque View author
publications You can also search for this author inPubMed Google Scholar * Julien Hadoux View author publications You can also search for this author inPubMed Google Scholar * Jean-Marie
Michot View author publications You can also search for this author inPubMed Google Scholar * Nathalie Chaput View author publications You can also search for this author inPubMed Google
Scholar * Veronique Saada View author publications You can also search for this author inPubMed Google Scholar * Mathilde Hauchecorne View author publications You can also search for this
author inPubMed Google Scholar * Jean-Baptiste Micol View author publications You can also search for this author inPubMed Google Scholar * Roger Sun View author publications You can also
search for this author inPubMed Google Scholar * Dominique Valteau-Couanet View author publications You can also search for this author inPubMed Google Scholar * Fabrice André View author
publications You can also search for this author inPubMed Google Scholar * Florian Scotte View author publications You can also search for this author inPubMed Google Scholar * Benjamin
Besse View author publications You can also search for this author inPubMed Google Scholar * Jean-Charles Soria View author publications You can also search for this author inPubMed Google
Scholar * Fabrice Barlesi View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS L.A., S.F., A.B. and F.B. conceived of and designed the study.
L.A., S.F., A.B., B.G., F.P., C.W., A.S., M.M., F.G., L.L., F.N., T.H., C.B., S.A., E.C., G.B., A.P., A.H., J.H., J.-M.M., N.C., V.S., M.H., J.-B.M., R.S., D.V.-C., F.A., F.S., B.B., J.-C.S.
and F.B. collected and assembled the data and provided the patients. L.A., S.F., A.B. and F.B. analyzed and interpreted the data. L.A., S.F., A.B., B.G., F.P., C.W., A.S., M.M., F.G., L.L.,
F.N., T.H., C.B., S.A., E.C., G.B., A.P., A.H., J.H., J.-M.M., N.C., V.S., M.H., J.-B.M., R.S., D.V.-C., F.A., F.S., B.B., J.-C.S. and F.B. wrote the manuscript. L.A., S.F., A.B., B.G.,
F.P., C.W., A.S., M.M., F.G., L.L., F.N., T.H., C.B., S.A., E.C., G.B., A.P., A.H., J.H., J.-M.M., N.C., V.S., M.H., J.-B.M., R.S., D.V.-C., F.A., F.S., B.B., J.-C.S. and F.B. gave final
approval of the manuscript. L.A., S.F., A.B., B.G., F.P., C.W., A.S., M.M., F.G., L.L., F.N., T.H., C.B., S.A., E.C., G.B., A.P., A.H., J.H., J.-M.M., N.C., V.S., M.H., J.-B.M., R.S.,
D.V.-C., F.A., F.S., B.B., J.-C.S. and F.B. were accountable for all aspects of the work. CORRESPONDING AUTHOR Correspondence to Fabrice Barlesi. ETHICS DECLARATIONS COMPETING INTERESTS L.A.
reports receiving consulting fees from Pfizer, Novartis, Bristol Myers Squibb, Ipsen, Roche, MSD, AstraZeneca, Merck, Amgen, Astellas, Exelixis, Corvus Pharmaceuticals and Peloton
Therapeutics outside the submitted work. C.B. reports sponsorship for research from GE Healthcare and personal fees from Bracco. A.H. reports sponsorship for research at the Gustave Roussy
Cancer Centre from AbbVie, Agios, Amgen, Astex, AstraZeneca, Bayer, BeiGene, Blueprint Medicines, Bristol Myers Squibb, Boehringer Ingelheim, Celgene, Chugai, Forma, Genentech,
GlaxoSmithKline, H3 Biomedicine, Incyte, Innate Pharma, Janssen, Lilly, Loxo, MedImmune, MSD, Novartis, Oncopeptides, Roche, Sanofi, Taiho and Xencor outside the submitted work. J.-M.M.
reports sponsorship for research at the Gustave Roussy Cancer Centre from AbbVie, Agios, Amgen, Astex, AstraZeneca, Bayer, BeiGene, Blueprint Medicines, Bristol Myers Squibb, Boehringer
Ingelheim, Celgene, Chugai, Forma, Genentech, GlaxoSmithKline, H3 Biomedicine, Incyte, Innate Pharma, Janssen, Lilly, Loxo, MedImmune, MSD, Novartis, Oncopeptides, Roche, Sanofi, Taiho and
Xencor outside the submitted work, as well as personal fees, travel grants or advisory board fees from Astex, iQone, Mundipharma and Bristol Myers Squibb outside the submitted work. J.-B.M.
reports sponsorship for research at the Gustave Roussy Cancer Centre from H3 Biomedicine and personal fees, travel grants or advisory board fees from AbbVie, Novartis, Astellas and Jazz
Pharmaceuticals outside the submitted work. R.S. reports grants from the ARC Foundation and Paris-Saclay University outside the submitted work. N.C. reports sponsorship for research at the
Gustave Roussy Cancer Centre from the Bristol Myers Squibb Foundation, Sanofi, GlaxoSmithKline and Roche outside the submitted work, as well as personal fees, travel grants or advisory board
fees from AstraZeneca, Bayer and Boehringer Ingelheim outside the submitted work. D.V.-C. reports receiving consulting fees from EUSA Pharma and sponsorship for research at the Gustave
Roussy Cancer Centre from Orphelia outside the submitted work. F.A. reports receiving grants from Novartis, AstraZeneca, Pfizer, Lilly and Roche outside the submitted work. F.S. reports
receiving personal fees from Helsinn, MSD, Roche, Amgen, Pierre Fabre Oncology, Pfizer, Mundipharma, Mylan and Leo Pharma outside the submitted work. B.B. reports sponsorship for research at
the Gustave Roussy Cancer Centre from AbbVie, Amgen, AstraZeneca, BeiGene, Blueprint Medicines, Bristol Myers Squibb, Boehringer Ingelheim, Celgene, Cristal Therapeutics, Daiichi Sankyo,
Eli Lilly, GlaxoSmithKline, Ignyta, IPSEN, Inivata, Janssen, Merck, MSD, Nektar, Onxeo, OSE Immunotherapeutics, Pfizer, Pharma Mar, Roche–Genentech, Sanofi, Servier, Spectrum
Pharmaceuticals, Takeda, Tiziana Pharma and Tolero Pharmaceuticals outside the submitted work. J.-C.S. reports receiving consultancy fees from AstraZeneca, Astex, Clovis, GlaxoSmithKline,
GamaMabs, Lilly, MSD, Mission Therapeutics, Merus, Pfizer, Pharma Mar, Pierre Fabre, Roche–Genentech, Sanofi, Servier, Symphogen and Takeda; was a full-time employee for AstraZeneca between
September 2017 and December 2019; and reports receiving other fees from the shareholder Gritstone during the conduct of the study. F.B. reports receiving personal fees from AstraZeneca,
Bayer, Bristol Myers Squibb, Boehringer Ingelheim, Eli Lilly Oncology, F. Hoffmann-La Roche, Novartis, Merck, MSD, Pierre Fabre, Pfizer and Takeda outside the submitted work. S.F., A.B.,
B.G., F.P., C.W., A.S., M.M., F.G., L.L., F.N., T.H., S.A., E.C., G.B., A.P., J.H., V.S. and M.H. declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature
remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. EXTENDED DATA EXTENDED DATA FIG. 1 SUMMARY OF COVID-19 TREATMENTS AND SYMPTOM
PRESENTATION. SOC, standard of care; ICU, intensive care unit HCQ, hydroxychloroquine; AZI, azithromycin; DEXA, dexamethasone; TOCI, tocilizumab; LOPI, Lopinavir; RITO, Ritonavir; CI,
contraindication. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Table 1. REPORTING SUMMARY RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS
ARTICLE Albiges, L., Foulon, S., Bayle, A. _et al._ Determinants of the outcomes of patients with cancer infected with SARS-CoV-2: results from the Gustave Roussy cohort. _Nat Cancer_ 1,
965–975 (2020). https://doi.org/10.1038/s43018-020-00120-5 Download citation * Received: 27 May 2020 * Accepted: 24 August 2020 * Published: 22 September 2020 * Issue Date: October 2020 *
DOI: https://doi.org/10.1038/s43018-020-00120-5 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is
not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative