Play all audios:
ABSTRACT BACKGROUND Children admitted to hospital with complicated severe malnutrition (CSM) have high mortality despite compliance with standard WHO management guidelines. Limited data
suggests a relationship between intestinal dysfunction and poor prognosis in CSM, but this has not been explicitly studied. This study aimed to evaluate the role of intestinal disturbances
in CSM mortality. METHODS A case-control study nested within a randomized control trial was conducted among children hospitalized with CSM in Kenya and Malawi. Children who died (cases, _n_
= 68) were compared with those who were discharged, propensity matched to the cases on age, HIV and nutritional status (controls, _n_ = 68) on fecal metabolomics that targeted about 70
commonly measured metabolites, and enteropathy markers: fecal myeloperoxidase (MPO), fecal calprotectin, and circulating intestinal fatty acid binding protein (I-FABP). RESULTS The fecal
metabolomes of cases show specific reductions in amino acids, monosaccharides, and microbial fermentation products, when compared to controls. SCFA levels did not differ between groups. The
overall fecal metabolomics signature moderately differentiates cases from controls (AUC = 0.72). Enteropathy markers do not differ between groups overall, although serum I-FABP is elevated
in cases in a sensitivity analysis among non-edematous children. Integrative analysis with systemic data suggests an indirect role of intestinal inflammation in the causal path of mortality.
CONCLUSIONS Intestinal disturbances appear to have an indirect association with acute mortality. Findings of the study improve our understanding of pathophysiological pathways underlying
mortality of children with CSM. PLAIN LANGUAGE SUMMARY Malnourished children are at a high risk of dying when exposed to an acute illness. They often have symptoms like diarrhea that
indicate their gut is not working properly. It is unclear whether these gut problems contribute to their deaths. Feces contain numerous small molecules processed by the gut that reflect gut
health. We compare these fecal molecules between malnourished children who died during hospitalization to those who survived, and relate them to signs of inflammation in the body. We show
that the fecal molecules are different between children who died and those who survived. These differences reveal that poor gut health could increase risk of death, potentially by perturbing
the body’s defensive response to an acute illness. These findings underscore that treatment for ill severely malnourished children should focus on improving gut health. SIMILAR CONTENT
BEING VIEWED BY OTHERS CLINICAL EVIDENCE OF THE ROLE OF _METHANOBREVIBACTER SMITHII_ IN SEVERE ACUTE MALNUTRITION Article Open access 08 March 2021 FECAL AMINE METABOLITE ANALYSIS BEFORE
ONSET OF SEVERE NECROTIZING ENTEROCOLITIS IN PRETERM INFANTS: A PROSPECTIVE CASE–CONTROL STUDY Article Open access 19 July 2022 MALNUTRITION ENTEROPATHY IN ZAMBIAN AND ZIMBABWEAN CHILDREN
WITH SEVERE ACUTE MALNUTRITION: A MULTI-ARM RANDOMIZED PHASE II TRIAL Article Open access 17 April 2024 INTRODUCTION In-hospital mortality among children with severe malnutrition remains
high despite strict adherence to therapeutic and refeeding protocols recommended by the World Health Organization (WHO). This population is commonly referred to as severe malnutrition (SAM),
and more recently as severe malnutrition (SM) to encompass both the chronic and acute aspects of the condition1,2. When severely malnourished children have concurrent serious illnesses
(e.g., acute infections or other complications), they are at higher risks of dying and require hospital-based management compared to those without serious illnesses. Hence, children
hospitalized with severe malnutrition are ‘complicated’ cases, and are referred to as complicated severe malnutrition (CSM)3. Some evidence suggests that diarrhea, reflecting intestinal
dysfunction, is associated with an increased risk of death among these children4,5,6, suggesting a link between intestinal microenvironment disturbances and mortality. Pathophysiological
changes in the intestine, characterized by mucosal atrophy, barrier dysfunction, intestinal inflammation, and nutrition malabsorption, are commonly seen in malnourished children living in
low- and middle-income countries7,8. These subclinical intestinal changes have been linked to increased bacterial translocation across the intestinal lumen leading to systemic inflammation9.
The intestinal microbiota plays a pivotal role in modulating intestinal homeostasis and host metabolism10. This is mediated by an active microbial metabolic repertoire that produces
numerous biologically active substances, such as short-chain fatty acids (SCFAs), vitamins, secondary bile acids, phenolics, aromatic acids, lipids, and neurotransmitters11. Indeed, the
fecal metabolome has been considered as a functional readout of microbial activity of the intestine. The microbiota of severely malnourished children has been reported to have reduced
diversity and persistent immaturity compared to well-nourished children12,13,14. In mouse models of malnutrition, compositional changes of the microbiota were accompanied by shifts in the
profile of fecal metabolites, including those with potentially important health implications such as SCFAs and amino acids15,16. A partial causal relationship between gut microbial
disturbances and the development of enteropathy and malnutrition phenotypes was demonstrated in these preclinical studies15,16,17. The enteropathy observed in children with malnutrition
conceivably puts them at a greater risk of life-threatening infections and complications. In critically ill well-nourished children, an association between intestinal disturbances and
mortality has been shown18,19,20,21. Severely malnourished children are vulnerable to diarrhea-related dehydration, electrolyte imbalances, and gram-negative bacteremia22,23,24. These common
complications point towards an intestinal origin, suggesting that intestinal dysfunction may cause mortality through infectious diarrhea or enteric pathogen-induced bacteremia. Yet, scarce
data are available on the role of intestinal disturbances in the mortality of CSM. A previous case-control study from our group showed that although diarrhea, increased intestinal
inflammation, and reduced fecal SCFAs did not have a direct effect on mortality, they were associated with increased systemic inflammation, which was directly associated with mortality25.
However, this was a relatively small study that examined a few fecal analytes, which may not provide a comprehensive representation of the intestinal microenvironment, nor the complexity of
enteropathy seen in these children. It remains unclear whether and how intestinal disturbances may contribute to inpatient mortality of CSM. The interplay between intestinal enteropathy,
microbiota activity, systemic factors and mortality needs to be investigated to a greater depth. The current study examined the association between intestinal disturbances and mortality in
CSM by comparing the fecal metabolome and a set of enteropathy markers at hospital admission of children who subsequently died to those survived. Differences are seen in the fecal metabolome
of children who die compared with those who are discharged, and intestinal inflammation appears to have an indirect association with mortality. METHODS STUDY DESIGN AND PATIENTS This was a
nested case-control study among children with CSM enrolled to a multicenter randomized controlled trial (NCT02246296) as described in detail by Bandsma et al.26. The current study addresses
some of the pre-specified secondary outcomes of the parent trial. Children with CSM were defined as those with MUAC < 11.5 cm, or WHZ < −3 (age 6 to 59 months, BMI-for-age Z-score <
−3 (age ≥ 60 months), or oedematous malnutrition (at any age), and having medical complications or failed an appetite test according to the WHO guidelines3. This case-control study used
fecal samples collected from participants on admission (before randomization and treatment initiation). Cases (nonsurvivors: NS) were children who died during hospitalization, while controls
(survivors: S) were children discharged alive within 14 days of hospitalization and matched to the cases by propensity score for mortality based on age, MUAC, and HIV status27. The sample
size was limited by sample availability. All available samples collected from NS (_n_ = 68) were included, which represents 54% of all death cases in the parent trial. S were matched with NS
at a 1:1 ratio. Sampling procedures are shown in Supplementary Fig. 1. Baseline patient characteristics were summarized as medians with interquartile ranges (IQRs), mean ± the standard
deviations for continuous variables, or percentages for categorical variables. Patient and public involvement was not part of this study. FECAL METABOLOMIC PROFILING AND WATER CONTENT
QUANTIFICATION Fecal samples were divided into homogeneous aliquots shortly after collection and stored at −80 °C for subsequent analyses. One of the aliquots was further aliquoted into two
parts – one for fecal metabolomic analysis and another for water content quantification. Fecal metabolomic profiling was performed using nuclear magnetic resonance (1H-NMR) spectroscopy,
which targets 68 commonly measured water-soluble fecal metabolites (Supplementary Data 1; TMIC, Edmonton, Canada), as detailed in Supplementary Methods. Briefly, approximately 100 mg of
fecal sample was used to extract 200 μl fecal water, which was mixed with 50 μl NMR buffer for spectral analysis. Lyophilization was performed to quantify fecal water content. Aliquoted
samples were weighted after transferred to pre-weighted Eppendorf tubes. Samples were then frozen before putting into the lyophilizer overnight. After lyophilization, the weight of the
Eppendorf tube was recorded, and the weight of dry sample was calculated. ENTEROPATHY MARKER ASSESSMENT A separate fecal aliquot was used to measure fecal enteropathy markers,
myeloperoxidase (MPO), calprotectin and alpha-1-antitrypsin (AAT), as detailed in Supplementary Methods. Briefly, The Easy Stool Extraction Device was used to extract fecal content from 15
mg stool per the manufacturer’s instructions (ALPCO, Salem, NH). MPO and AAT were quantified by commercially available ELISA kits (ALPCO, Salem, NH), and calprotectin was quantified using
the diagnostic chemiluminescence ELISA kit per the kit insert (ALPCO, Salem, NH). Serum samples were used for quantifying I-FABP using a commercial ELISA kit (Hycult Biotech, Uden,
Netherlands) according to package instructions. Dry weight concentrations were derived based on water content measured by lyophilization. To compare marker ranges with the literature, wet
weight concentrations were also analyzed. Results were related to clinical cut-off concentrations used to diagnose or monitor disease activity in inflammatory bowel disease or celiac
disease: calprotectin: 200 μg/g, MPO: 2000 ng/ml, AAT: 270 ug/g28, and I-FABP: 450 pg/ml29,30. STATISTICAL ANALYSES Metabolites that had coefficient of variation <30% in quality control
samples and were detected in at least 80% of samples in either group were retained for subsequent analysis. Data preprocessing and imputation for missingness were conducted as detailed in
Supplementary Methods. Both univariate and multivariate approaches were used to reveal features and differences in patterns of analytes associated with mortality. First, to identify
individual analytes associated with mortality, univariate conditional logistic regression accounting for the matched design was performed31. Potentially significant analytes were determined
based on _P_ < 0.05, which were obtained from two-sided Wald tests and were adjusted for multiple testing to control for false discovery rate (FDR) according to the Benjamini and
Hochberg. Then, multivariable analysis by elastic net penalized logistic regression was implemented using the “glmnet” R package32, as detailed in Supplementary Methods. Analytes that with
_P_ < 0.05 in univariate analysis or influential (>80% bootstrap confidence interval (CI) of coefficient not crossing zero) in multivariable analysis were considered as differential
analytes. The ‘mixOmics’ R package was used to analyze the differential analytes. To visualize the univariate association between levels of differential metabolites and the probability of
being a case in the study sample, the histSpikeg function from the ‘Hmisc’ R package was used. For enteropathy markers, univariate conditional logistic regression was used for group
comparisons, except for I-FABP. Due to serum sample availability constraints, among the case-control pairs, several pairs had only the case or the control samples measured for I-FABP. To
study I-FABP in an adequate sample size (_n_case = 57, _n_control = 61) without dropping the unpaired samples, I-FABP was analyzed by logistic regression adjusted for the matching variables,
age, MUAC and HIV. Sensitivity analyses were performed to account for potential confounders (edema, recruitment site, and breastfeeding status), as described in Supplementary Methods. To
understand the correlations between intestinal disturbances and systemic inflammation, we integrated the intestinal data in the precent study and systemic data from a previous study. In the
previous study, we quantified systemic inflammatory marker and SCFA levels among children from the same cohort, using the Luminex assay (EMD Millipore, Burlington, USA) and the
LC-MS/MS-based TMIC PRIME® assay (TMIC, Edmonton, Canada), respectively, as detailed in33,34. Cross-correlation analysis was performed amongst the analytes based on pairwise Pearson’s
correlations, with significance level at PFDR < 0.05. PLS path modeling was used to study the causal relationships among intestinal factors, systemic factors, and mortality, using the
“plspm” R package35. Patients with complete data were included to this analysis. All manifested variables were log10-transformed and standardized before modelling. Path coefficients and
their 95%CI were validated using bootstrap resampling. ETHICAL APPROVAL Ethical approval for this study was obtained from the College of Medicine Research and Ethics Committee of the
University of Malawi, the KEMRI Scientific Ethical Review Committee, Kenya, the Oxford Tropical Research Ethics Committee, and the Hospital for Sick Children, Toronto, Canada. The trial
sponsor was the University of Oxford. Informed consent was obtained from parents or caregivers prior to the enrollment of all study participants. REPORTING SUMMARY Further information on
research design is available in the Nature Portfolio Reporting Summary linked to this article. RESULTS CHARACTERISTICS OF STUDY PATIENTS Table 1 is the characteristics of study participants
at admission. Children included in this matched case-control study have a median age of 15 months (IQR: 10–26) and 47% are female. There is a higher proportion of children with nutritional
edema among NS (43% vs. 24%, _p_ = 0.02). The proportion of children with diarrhea does not differ between NS and S, similar to that in the parent trial (_p_ > 0.05). Other
characteristics are similar between NS and S. The median time to death for NS is 6 days (IQR: 4–10) and the time to discharge for S is 8 days (IQR: 7–11) since hospital admission. FECAL
SCFAS ARE NOT ASSOCIATED WITH MORTALITY IN CHILDREN WITH CSM Of 68 metabolites targeted, 61 fulfill the criteria for measurement quality and are retained for further analysis (Supplementary
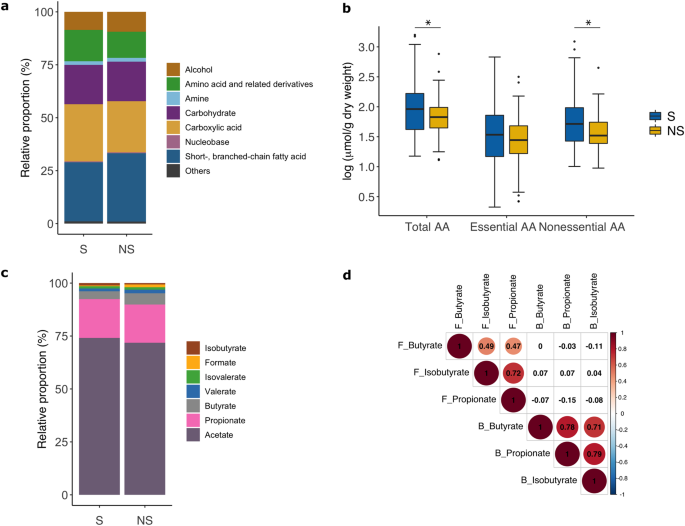
Data 1). These metabolites are grouped into seven classes with SCFA (30%), and carboxylic acids (26%), followed by carbohydrates (19%), amino acids and derivatives (13%), and alcohols (9.0%)
being the dominant metabolite classes constituting the fecal metabolome of the study population (Fig. 1a). Between NS and S, total metabolite abundance, relative proportion, or mean
absolute concentration of individual metabolite classes do not differ, except for the class of amino acids. At admission, mean concentration of total amino acids and derivatives are lower in
NS than S (95.8 ± 107.5 vs. 191.8 ± 314 μmol/g dry weight, OR [95%CI] = 0.39 [0.16, 0.96], _P_raw = 0.04, _P_edema adjusted = 0.01, Fig. 1b). This difference is driven by reductions in
non-essential amino acids (alanine, aspartate, glutamate, glutamine, glycine, proline, serine, and tyrosine) among NS. SCFAs are the main fermentation products of the gut microbiota playing
an important role in intestinal homeostasis. Acetate followed by propionate and butyrate are the most abundant species among the SCFAs, consistent with their relative proportion in
well-nourished populations36. Between NS and S, total SCFAs and individual SCFA species do not differ in their relative proportions nor absolute concentrations (Fig. 1c). Using serum data of
propionate, butyrate and isobutyrate, we examine if levels of these SCFA species in the feces are correlated with systemic levels. We found that although correlations within the same
biofluid are strong, fecal SCFA levels do not correlate with circulating levels (Fig. 1d). FECAL METABOLIC SIGNATURES ARE ASSOCIATED WITH MORTALITY Comparing the fecal metabolome of NS to S,
6 metabolites are univariately lower in NS (_P_raw < 0.05), including 2 amino acids (alanine and glycine), 2 carboxylic acids (fumarate and 3-phenylpropionate) and 2 carbohydrates
(galactose and fucose) (Fig. 2a, b). However, these metabolites do not reach significance after multiple testing correction (_P_FDR > 0.05). It can also be seen from the unsymmetrical
volcano plot that the majority of the fecal metabolites are lower in NS compared to S. The association between individual metabolite and mortality is shown in Supplementary Fig. 2 and
Supplementary Data 2. For example, for an increase from 25th to 75th percentile in alanine concentration (from 7.5 to 23 μmol/g), the associated odds of death decreases by a factor of 0.52
(95% CI: 0.33–0.82). In multivariable analysis, 13 metabolites are identified as influential features in distinguishing NS from S (Fig. 2c), including alanine, fumarate, 3-phenylpropionate,
and galactose that are also identified as univariately differential, and 9 additional influential metabolites (xanthine, β-alanine, isocaproate, isoleucine, creatinine, isobutyrate,
isopropanol, butyrate, methylamalonate). Supplementary Fig. 3a is a Venn diagram showing the overlap between univariate and multivariable analyses. For each matched case-control pair, the
combined profile of the univariate and multivariable differential features (total 15 metabolites) only moderately discriminate NS from S (AUC = 0.72). As illustrated in Fig. 2d, differential
features most strongly driving the separation between NS and S cluster (i.e. arrows perpendicular to the decision line) are reductions of galactose, fucose, and fumarate in NS. As the
concentration of these features increase, the observed probability of death within the case-control sample set decreases steadily (Supplementary Fig. 3b). Conversely, features that are
parallel to the decision line, particularly butyrate, isobutyrate, isocaproate and xanthine, show a more complex relationship with the observed probability of death (Supplementary Fig. 3c).
Further adjusting the data by edema, site, or breastfeeding status do not fundamentally change the results (Supplementary Fig. 4). ENTEROPATHY IS PRESENT IN CSM BUT IS NOT ASSOCIATED WITH
MORTALITY To evaluate associations between enteropathy and mortality, admission levels of one serum and three fecal markers for intestinal function are compared between NS and S. We found
that levels of calprotectin, MPO, or AAT do not differ between NS and S, with or without adjusting for edema or site (_P_raw > 0.05, _P_adjusted > 0.05) (Fig. 3a). The serum marker
I-FABP do not differ between NS and S. Upon sensitivity analysis examining effects of nutritional edema, weak evidence for an interaction between I-FABP and edema status is revealed
(_P_interaction<0.1). Specifically, among children without edema, I-FABP levels are elevated (OR [95%CI] = 1.0003 [1.0000, 1.0006], _P_ = 0.029) in NS, while among children with edema,
I-FABP levels do not differ between groups (Fig. 3b). Notably, I-FABP has a weak association, while the fecal markers have no or inconsistent associations with diarrhea at admission and
within 48 h postadmission (Supplementary Fig. 5). Fecal marker levels and their clinical reference ranges are often expressed as wet weight concentrations in the literature. To compare fecal
marker levels of our study population to these reference ranges, wet weight concentrations are also examined (Fig. 3c). A considerable proportion of patients has elevated levels of
calprotectin, MPO and AAT based on reported references ranges (calprotectin: > 200 μg/g wet feces, MPO: > 2000 ng/ml wet feces, AAT: > 270 ug/g wet feces)28, but the proportion of
patients with elevated levels do not significantly differ between NS and S (calprotectin: 43% vs 40% elevated; MPO: 81% vs 84% elevated; AAT: 18% vs 31%). Similarly, a considerable
proportion of patients have elevated levels of serum I-FABP based on a cut-off used for detecting celiac disease in well-nourished populations (I-FABP: > 450 pg/ml29,30), but the
proportions do not differ between NS and S (79% vs 85%). These data suggest that signs of enteropathy are present in children with CSM upon admission, but the degree of enteropathy is not
associated with mortality. INTERRELATIONSHIPS BETWEEN INTESTINAL DISTURBANCES, SYSTEMIC INFLAMMATION, AND MORTALITY Cross-correlation analysis between enteropathy markers, fecal SCFAs, and
systemic markers analytes is conducted. The two fecal markers for intestinal inflammation, calprotectin and MPO, are strongly correlated with each other (rcalprotectin~MPO = 0.8, _P_FDR <
0.0001). They are moderately correlated with AAT (rMPO~AAT = 0.35, rcalprotectin~AAT = 0.3, _P_FDR < 0.001). Interestingly, calprotectin is also positively correlated with circulating
SCFA levels (rcalprotectin~SCFA = 0.3, PFDR = 0.001) but not with fecal SCFAs. This indicates a link between intestinal inflammation and systemic levels of SCFAs, which are metabolic
products originated from the gut microbiota. To further decipher the interrelationships among intestinal disturbances, systemic inflammation, systemic microbial product levels, and
mortality, PLS path modeling is conducted. Specifically, 4 latent variables are constructed from manifested indicators: 1) Luminal metabolism (indicators: 15 differential fecal metabolites),
2) Intestinal inflammation (indicators: calprotectin and MPO), 3) Systemic inflammation (indicators: proinflammatory mediators IL7, IL8, IL15, TNFa, GCSF, MCP1), and 4) Circulating
microbial products (indicators: serum propionate, butyrate, and isobutyrate), as illustrated in Fig. 4. Communality within each latent variable is high, suggesting that the latent variables
summarize the variability of the indicators well, satisfying model specification requirements. Strengths and directions of the interrelationships are denoted by arrows in Fig. 4. Consistent
with results in the above sections, intestinal inflammation, marked by calprotectin and MPO, do not directly correlate with mortality (direct effect = −0.05 (95%CI: −0.2, −0.08)). However,
the model shows significant indirect effects on mortality via a positive association with circulating microbial products leading to systemic inflammation, as well as a negative association
with luminal metabolic profiles (total indirect effect = 0.23 (95%CI: 0.12–0.33)). Although intestinal inflammation is not directly linked to mortality, intestinal inflammation contributed
to mortality indirectly via reducing luminal metabolism and increasing the systemic load of microbial products, thus indirectly contributing to mortality. We also examined the role of
diarrhea in these causal pathways. A history of diarrhea at admission is not associated with any path nor improves model fit, and thus diarrhea is not included to the final model. DISCUSSION
This case-control study, nested within a clinical trial, compares the fecal metabolome and a set of enteropathy markers between survivors and nonsurvivors of hospitalized children with
severe malnutrition. We assess the association between intestinal disturbances and mortality among severely malnourished children by examining multiple enteropathy markers and the fecal
metabolome. At admission, the fecal metabolome of nonsurvivors is characterized by reductions in certain amino acids, monosaccharides, and other microbial fermentation products. In contrast
to the initial speculation, fecal SCFAs do not differ between groups. A considerable proportion of children in the study has elevated enteropathy marker levels. Overall, enteropathy markers
are not directly associated with mortality, but an indirect association between intestinal inflammation and mortality is observed. The presence of diarrhea at admission is not associated
with mortality and has limited or no association with enteropathy markers. These findings suggest a limited contributory role of enteropathy in CSM mortality. The differences in fecal
metabolome between nonsurvivors and survivors are driven by reductions of galactose, fucose, alanine, glycine, fumarate and 3-hydroxyphenylpropionate. Galactose is a monosaccharide produced
from lactose in milk by digestive enzymes or microbial fermentation. Lower fecal galactose in nonsurvivors compared to survivors may reflect reduced recent dietary intake of carbohydrates.
Fucose can be liberated from fucosylated glycans by certain bacteria, such as _Bifidobacterium_, _Bacteroides_ and mucin degraders; and it can be utilized as energy substrates by bacteria37.
Reduced fecal levels of fucose among nonsurvivors may be attributed to reduced intestinal abundance of fucosylated glycans, reduced fucose producers or increased fucose utilizers in the gut
microbiota. Notably, fucosylation of the intestinal epithelium are necessary for establishing the host-commensal symbiotic relationships and colonization resistance against pathogens.
Deficient of functional expression of the fucosylation enzyme in both mice and humans are associated with increased susceptibility to various intestinal infections and inflammatory diseases,
including Crohn’s disease38. Fumarate is a fermentation intermediate and 3-hydroxyphenylpropionate is a major fermentation end product of aromatic amino acids11. Total amino acids is lower
in nonsurvivors than survivors. In general, reductions in these fecal metabolites may reflect compositional and functional differences of the gut microbiota. It is plausible that, instead of
contributing to subsequent mortality, the reduction in fecal metabolites is attributed to illness-induced anorexia or microbiome perturbations due to preadmission antibiotic exposures. This
may indicate that these children were sicker at admission. However, without microbiota, intestinal absorption, and pre-admission medication data, this remains speculative. SCFAs do not
differ between nonsurvivors and survivors. This contrasts with previous data that showed reductions in fecal butyrate and propionate at admission among children who died in hospital25.
However, the reported significant associations may be biased by the small sample size (_n_recovery = 52, _n_death = 9) of the previous study, considering the known high variability of human
fecal SCFAs39,40. The lack of association with mortality revealed in the present study suggests that the abundances of SCFA-producing bacteria in the gut microbiota may be similar between
survivors and nonsurvivors. Despite that SCFAs, particularly butyrate, are known to have anti-inflammatory and barrier-enhancing effects to the intestine41,42, their roles in mortality are
unclear. A recent study showed that although fecal SCFA levels were lower in patients with sepsis, they are not associated with mortality43. Clearly, fecal SCFA levels are influenced by many
factors, such as intake, absorption, utilization by enterocytes, transit time and antibiotic use, which are not assessed in our study. As a matter of fact, we found no correlation between
fecal and circulating SCFA levels. A set of markers are evaluated covering different domains of enteropathy, including intestinal inflammation, permeability and damage. We found that a
substantial proportion of children has elevated marker levels when compared to reference ranges used for nourished population. This is consistent with the high prevalence of enteropathy seen
in low-resource settings. For example, a birth cohort of 700 infants living in urban slum in Bangladesh reported that over 80% of infants had elevated levels of calprotectin, MPO and AAT,
when applying clinically used references. It’s worth noting that there are no clear clinical cut-offs of enteropathy markers established for malnourished children44. Levels of fecal
enteropathy markers are not statistically different between nonsurvivors and survivors, but a trend towards increased calprotectin was seen in nonsurvivors (_p_ < 0.1). Calprotectin and
MPO are proteins released by neutrophils and their fecal levels indicate mucosal neutrophil activity and inflammation. Aligned with their common roles, calprotectin and MPO levels are
strongly correlated. AAT is a circulating protein synthesized by the liver and increased fecal excretion of AAT is an indicator of gut leakiness. AAT production is affected by hepatic
synthetic activity, which is impaired in malnutrition45. A more comprehensive test, such as AAT clearance calculated from 24-hour blood and stool collection, may be required for reliable use
of AAT to assess of gut permeability46. I-FABP is uniquely expressed at the tips of villi of differentiated enterocytes, and it is only released into systemic circulation upon enterocyte
deaths during inflammation or injury47. Elevated circulating I-FABP is an indicator of villus atrophy and epithelial damages. I-FABP is the only enteropathy marker in our study that has a
weak association with the presence of diarrhea at admission and 48-hour postadmission, which may reflect its physiological relevance with diarrhea compared to the other markers. In
non-edematous patients, admission I-FABP levels are significantly higher in nonsurvivors than survivors, but no difference is found for edematous patients. Further investigation is warranted
to understand if the accumulation of fluid within the interstitial spaces in edema would affect serum I-FABP levels or if physiological differences between malnutrition phenotypes48 would
have impacts on I-FABP expression in the intestine. To reconcile how and to what extent these intestinal factors contribute to mortality, admission intestinal, systemic and clinical data are
integratively analyzed using the path analysis. We found that intestinal inflammation is not directly associated with systemic inflammation nor mortality, but indirectly via increasing
systemic microbial products as marked by circulating SCFAs. In a large multi-country community cohort, using similar causal path modeling methods, Kosek et al. found conflicting
relationships between intestinal inflammation markers (MPO, neopterin) and systemic inflammation, although intestinal inflammation and systemic inflammation are both positively associated
with enteric pathogen exposure49. We also found that increased intestinal inflammation negatively influenced luminal metabolite abundances. It has been reported in patients with inflammatory
bowel disease that fecal metabolites and metabolite classes are frequently depleted50. The study has several limitations. First, fecal material is a complex matrix constituting an array of
inputs from host, microbiota, and diet. It is not unusual to see high variability in fecal data as a result of many confounding factors such as diet, age, bowel activity, and water
content51. Human feces are known to contain about 60–85% water and such a wide range of variation can have a significant impact on statistical inference51. To control for some inherent
variability, we account for sample water content in the quantification of fecal analytes, which is currently not commonly done in majority of the fecal studies. Nonetheless, this study only
examines factors associated with mortality at a single time point on admission and there could have confounding factors that are not measured or accounted for. For example, without
pre-admission data, it is plausible that the findings on reduced fecal metabolite abundances in nonsurvivors are confounded by antibiotic use prior to admission. Similarly, factors
post-admission, such as new onset of infections during hospitalization, could also influence the intestinal environment and mortality risk. Future work should consider integrating
microbiome, pre-admission medication and dietary data with serial fecal metabolomics to better disentangle the interrelationships. Second, despite a relatively large and balanced sample size
compared to the previous study on enteropathy and mortality in CSM25, it may still be insufficient to overcome the inherent variability of fecal analytes, which can reduce statistical power
to detect differences. For this reason, a relaxed statistical significance criterion of _p_ < 0.05 is used in fecal metabolomic analysis with p-values corrected for multiple comparisons
found in Supplementary Data 2. While the chosen cutoff may be considered liberal, a more stringent cutoff could restrict our ability to identify important metabolites with real effects,
particularly given the known high variability of fecal analytes. Therefore, the cutoff is chosen to balance the trade-off between false positives and false negatives. Similarly, when
determining influential features from bootstrapped samples, 80% is chosen as a cutoff instead of a higher probability (i.e., 95%) in order to reduce the risk of missing true effects. It is
worth noting that performing the bootstrap validation step on elastic-net regression, instead of taking results directly from one elastic-net regression, is a procedure implemented to
protect against false positives. Nonetheless, we acknowledge that the significant associations reported in our study may be still influenced by the sample size, considering the high
variability inherent to human fecal analytes. Consequently, our findings should be interpreted with caution and warrant further validation through larger-scale studies to robustly establish
the relationship between intestinal disturbances and mortality in CSM. Third, as SCFAs are not specific markers for microbial translocation, quantifying other indicators of microbial
translocation, such as LPS binding protein, endotoxin core antibody or sCD1452, could be helpful in supporting the associations that we observed. Notably, there is currently no consensus on
a sensitive and reliable marker for microbial translocation. Lastly, the matched case-control design provides efficiency but also inevitably entails limitations. Our study aims to access the
associations between intestinal features at admission and mortality among children with similar underlying mortality risks by controlling for potential confounding effects of age, wasting,
and HIV. It is plausible that intestinal disturbances may have differential effects between different levels of the matched variables; for example, increased intestinal permeability may have
a greater impact on HIV-positive than negative patients. However, effects of the matched variables cannot be evaluated within a matched the design. Therefore, our findings should be
interpreted in the context of the design and population, and may not be generalizable to other conditions. The case-control design does not take into account the temporal relationship
between exposure and outcome. A case-cohort design could be considered for future work aiming to study associations between intestinal features and time-to-death. In conclusion, in children
with CSM, signs of enteropathy appeared indirectly related to mortality via systemic inflammation, suggesting that future interventions should aim at both reducing systemic inflammation and
intestinal inflammation, potentially by modulating the gut microbiome and barrier function, in order to improve clinical outcomes in this vulnerable patient population. DATA AVAILABILITY All
data supporting the findings of the study are in the main text, Supplementary Information, and public data repository. Supplementary Data 3 contains source data for the main figures in this
manuscript. The fecal metabolomics and enteropathy marker data were deposited into the KEMRI-Wellcome data repository on the Harvard Dataverse under https://doi.org/10.7910/DVN/I4EYDR53.
The clinical data of the parent trial and the systemic data are accessible from the same repository under https://doi.org/10.7910/DVN/N4RISX54 and https://doi.org/10.7910/DVN/GI8YL934. All
other data are available from the corresponding author on reasonable request. REFERENCES * Bhutta, Z. A. et al. Severe childhood malnutrition. _Nat. Rev. Dis. Primers._ 3, 17067 (2017).
PubMed PubMed Central Google Scholar * Kerac, M. et al. ‘Severe malnutrition’: Thinking deeplys, communicating simply. _BMJ Glob Health._ 5, e003023 (2020). PubMed PubMed Central Google
Scholar * Guideline: Updates on the management of severe acute malnutrition in infants and children. _World Health Organization, Geneva_ https://www.ncbi.nlm.nih.gov/books/NBK190328
(2013). * Grenov, B. et al. Diarrhea, dehydration, and the associated mortality in children with complicated severe acute malnutrition: A prospective cohort study in uganda. _J. Pediatr._
210, 26–33 (2019). PubMed Google Scholar * Irena, A. H., Mwambazi, M. & Mulenga, V. Diarrhea is a major killer of children with severe acute malnutrition admitted to inpatient set-up
in lusaka, zambia. _Nutrit. J._ 10, 110 (2011). Google Scholar * Talbert, A. et al. Diarrhoea complicating severe acute malnutrition in kenyan children: A prospective descriptive study of
risk factors and outcome. _Plos One._ 7, e38321 (2012). CAS PubMed PubMed Central Google Scholar * Korpe, P. S. & Petri, W. A. Jr. Environmental enteropathy: Critical implications of
a poorly understood condition. _Trends Mol Med._ 18, 328–336 (2012). PubMed PubMed Central Google Scholar * Attia, S., Feenstra, M., Swain, N., Cuesta, M. & Bandsma, R. H. J. Starved
guts: Morphologic and functional intestinal changes in malnutrition. _J Pediatr Gastroenterol Nutr._ 65, 491–495 (2017). PubMed Google Scholar * Oria, R. B. et al. Early-life enteric
infections: Relation between chronic systemic inflammation and poor cognition in children. _Nutrit. Rev._ 74, 374–386 (2016). PubMed PubMed Central Google Scholar * Gill, S. R. et al.
Metagenomic analysis of the human distal gut microbiome. _Science._ 312, 1355–1359 (2006). CAS PubMed PubMed Central Google Scholar * Rowland, I. et al. Gut microbiota functions:
Metabolism of nutrients and other food components. _Eur. J. Nutrit._ 57, 1–24 (2018). CAS Google Scholar * Subramanian, S. et al. Persistent gut microbiota immaturity in malnourished
bangladeshi children. _Nature._ 510, 417 (2014). CAS PubMed PubMed Central Google Scholar * Million, M., Diallo, A. & Raoult, D. Gut microbiota and malnutrition. _Microb Pathog._
106, 127–138 (2017). PubMed Google Scholar * Monira, S. et al. Gut microbiota of healthy and malnourished children in bangladesh. _Front Microbiol._ 2, 228 (2011). PubMed PubMed Central
Google Scholar * Smith, M. I. et al. Gut microbiomes of malawian twin pairs discordant for kwashiorkor. _Science._ 339, 548–554 (2013). CAS PubMed PubMed Central Google Scholar * Brown,
E. M. et al. Diet and specific microbial exposure trigger features of environmental enteropathy in a novel murine model. 6, 7806 (2015). * Mayneris-Perxachs, J. et al. Protein- and
zinc-deficient diets modulate the murine microbiome and metabolic phenotype. 104, 1253-1262 (2016). * MacFie, J. et al. Gut origin of sepsis: A prospective study investigating associations
between bacterial translocation, gastric microflora, and septic morbidity. _Gut._ 45, 223–228 (1999). CAS PubMed PubMed Central Google Scholar * Adelman, M. W. et al. The gut
microbiome’s role in the development, maintenance, and outcomes of sepsis. _Critical Care._ 24, 278 (2020). PubMed PubMed Central Google Scholar * Derikx, J. P. et al. Gut mucosal cell
damage in meningococcal sepsis in children: Relation with clinical outcome. _Crit Care Med._ 38, 133–137 (2010). PubMed Google Scholar * Schuijt, T. J. et al. The gut microbiota plays a
protective role in the host defence against pneumococcal pneumonia. _Gut._ 65, 575–583 (2016). CAS PubMed Google Scholar * Pocket book of hospital care for children: Second edition.
Guidelines for the management of common childhood illnesses. _World Health Organization_ https://www.who.int/maternal_child_adolescent/documents/child_hospital_care/en/ (2013). * Berkley, J.
A. et al. Bacteremia among children admitted to a rural hospital in kenya. _N Engl. J. Med._ 352, 39–47 (2005). CAS PubMed Google Scholar * Chisti, M. J. et al. A prospective study of
the prevalence of tuberculosis and bacteraemia in bangladeshi children with severe malnutrition and pneumonia including an evaluation of xpert mtb/rif assay. _PLoS One._ 9, e93776 (2014).
PubMed PubMed Central Google Scholar * Attia, S. et al. Mortality in children with complicated severe acute malnutrition is related to intestinal and systemic inflammation: An
observational cohort study. _Am J Clin Nutr._ 104, 1441–1449 (2016). CAS PubMed PubMed Central Google Scholar * Bandsma, R. H. J. et al. A reduced-carbohydrate and lactose-free
formulation for stabilization among hospitalized children with severe acute malnutrition: A double-blind, randomized controlled trial. _Plos Med._ 16, e1002747 (2019). CAS PubMed PubMed
Central Google Scholar * Austin, P. C. An introduction to propensity score methods for reducing the effects of confounding in observational studies. _Multivariate Behav. Res._ 46, 399–424
(2011). PubMed PubMed Central Google Scholar * Naylor, C. et al. Environmental enteropathy, oral vaccine failure and growth faltering in infants in bangladesh. _Ebiomedicine._ 2,
1759–1766 (2015). PubMed PubMed Central Google Scholar * Adriaanse, M. P. et al. Serum i-fabp as marker for enterocyte damage in coeliac disease and its relation to villous atrophy and
circulating autoantibodies. _Aliment. Pharmacol. Therap._ 37, 482–490 (2013). CAS Google Scholar * Vreugdenhil, A. C. et al. Additional value of serum i-fabp levels for evaluating celiac
disease activity in children. _Scand J. Gastroenterol._ 46, 1435–1441 (2011). CAS PubMed Google Scholar * Pearce, N. Analysis of matched case-control studies. _Bmj-British Med. J._ 352,
i969 (2016). Google Scholar * Friedman, J. H., Hastie, T. & Tibshirani, R. Regularization paths for generalized linear models via coordinate descent. 2010. 33, 22 (2010). * Wen, B. et
al. Systemic inflammation and metabolic disturbances underlie inpatient mortality among ill children with severe malnutrition. _Sci. Adv._ 8, eabj6779–eabj6779 (2022). CAS PubMed PubMed
Central Google Scholar * Wen, B. et al. Replication data for: Systemic inflammation and metabolic disturbances underlie inpatient mortality among ill children with severe malnutrition.
_Harvard Dataverse_ https://doi.org/10.7910/DVN/GI8YL9 (2021). * Sanchez, G., Trinchera, L. & Russolillo, G. Tools for partial least squares path modeling (pls-pm) r package plspm.
_Comprehensive R Archive Network_ (2017). * Cummings, J. H., Pomare, E. W., Branch, W. J., Naylor, C. P. E. & Macfarlane, G. T. Short chain fatty-acids in human large-intestine, portal,
hepatic and venous-blood. _Gut._ 28, 1221–1227 (1987). CAS PubMed PubMed Central Google Scholar * Tailford, L. E., Crost, E. H., Kavanaugh, D. & Juge, N. Mucin glycan foraging in the
human gut microbiome. _Fronti.Genet._ 6, 81 (2015). Google Scholar * Goto, Y., Uematsu, S. & Kiyono, H. Epithelial glycosylation in gut homeostasis and inflammation. _Nat. Immunol._
17, 1244–1251 (2016). CAS PubMed Google Scholar * Verbeke, K. A. et al. Towards microbial fermentation metabolites as markers for health benefits of prebiotics. _Nutrit. Res Reviews._ 28,
42–66 (2015). CAS Google Scholar * Tsukuda, N. & Yahagi, K. Key bacterial taxa and metabolic pathways affecting gut short-chain fatty acid profiles in early life. _ISME J._ 15,
2574–2590 (2021). CAS PubMed PubMed Central Google Scholar * Zhang, T. H. et al. Sodium butyrate reduces colitogenic immunoglobulin a-coated bacteria and modifies the composition of
microbiota in il-10 deficient mice. _Nutrients._ 8, 728 (2016). PubMed PubMed Central Google Scholar * Sun, M. M., Wu, W., Liu, Z. J. & Cong, Y. Z. Microbiota metabolite short chain
fatty acids, gpcr, and inflammatory bowel diseases. _J. Gastroenterol._ 52, 1–8 (2017). CAS PubMed Google Scholar * Valdes-Duque, B. E. et al. Stool short-chain fatty acids in critically
ill patients with sepsis. _J. Am. College Nutrit._ 39, 706–712 (2020). Google Scholar * Colston, J. M. et al. A methodologic framework for modeling and assessing biomarkers of environmental
enteropathy as predictors of growth in infants: An example from a peruvian birth cohort. _Am. J. Clin. Nutrit._ 106, 245–255 (2017). CAS PubMed Google Scholar * Jahoor, F., Badaloo, A.,
Reid, M. & Forrester, T. Protein metabolism in severe childhood malnutrition. _Ann. Trop. Paediat._ 28, 87–101 (2008). Google Scholar * Florent, C., L’Hirondel, C., Desmazures, C.,
Aymes, C. & Bernier, J. J. Intestinal clearance of alpha 1-antitrypsin. A sensitive method for the detection of protein-losing enteropathy. _Gastroenterology._ 81, 777–780 (1981). CAS
PubMed Google Scholar * Wells, J. M. et al. Homeostasis of the gut barrier and potential biomarkers. _Am. J. Physiol. Gastrointest. Liver Physiol._ 312, G171–G193 (2017). PubMed Google
Scholar * Brunser, O., Araya, M. & Espinoza, J. Gastrointestinal-tract changes in the malnourished child. _Malnourished Child._ 19, 261–276 (1990). Google Scholar * Kosek, M. N. Causal
pathways from enteropathogens to environmental enteropathy: Findings from the mal-ed birth cohort study. _EBioMedicine._ 18, 109–117 (2017). PubMed PubMed Central Google Scholar *
Franzosa, E. A. et al. Gut microbiome structure and metabolic activity in inflammatory bowel disease. _Nat. Microbiol._ 4, 293–305 (2019). CAS PubMed Google Scholar * Karu, N. et al. A
review on human fecal metabolomics: Methods, applications and the human fecal metabolome database. _Anal. Chim. Acta._ 1030, 1–24 (2018). CAS PubMed Google Scholar * Harper, K. M.,
Mutasa, M., Prendergast, A. J., Humphrey, J. & Manges, A. R. Environmental enteric dysfunction pathways and child stunting: A systematic review. _Plos Negl. Trop. Dis._ 12, e0006205
(2018). PubMed PubMed Central Google Scholar * Wen, B. et al. Replication data for: Intestinal disturbances associated with mortality of children with complicated severe malnutrition.
_Harvard Dataverse_ https://doi.org/10.7910/DVN/I4EYDR (2023). * Berkley, J. A., Bandsma, R. H. J. & Ngari, M. M. Modified f75 formula for stabilisation among hospitalised children with
severe acute malnutrition: Double blind, randomised controlled trial. _Harvard Dataverse_ https://doi.org/10.7910/DVN/N4RISX (2019). Download references ACKNOWLEDGEMENTS This work was
supported, in whole or in part, by the Bill & Melinda Gates Foundation. Under the grant conditions of the Foundation, a Creative Commons Attribution 4.0 Generic License has already been
assigned to the Author Accepted Manuscript version that might arise from this submission. The parent clinical trial was funded by the Thrasher Research Fund to R.H.J.B and J.A.B. at the
University of Oxford (9403). Support for C.B., W.V., J.T., M.N., and J.A.B. were provided by the Bill & Melinda Gates Foundation to J.A.B. (Grant Number OPP1131320). J.A.B. and L.M. are
supported by the MRC/DfID/Wellcome Trust Global Health Trials Scheme (Grant Number MR/M007367/1). This work was in part supported by the Canadian Institutes of Health Research (156307). B.W.
is supported by the Research Training Competition (RESTRACOMP) Graduate Scholarship at the Hospital for Sick Children and the Ontario Graduate Scholarship (OGS) at the University of
Toronto. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS *
Department of Nutritional Sciences, Faculty of Medicine, University of Toronto, Toronto, Canada Bijun Wen & Robert H. J. Bandsma * Department of Translational medicine, Hospital for Sick
Children, Toronto, Canada Bijun Wen, Amber Farooqui, Celine Bourdon, Nawar Tarafdar & Robert H. J. Bandsma * The Childhood Acute Illness & Nutrition Network, Nairobi, Kenya Celine
Bourdon, Moses Ngari, Emmanuel Chimwezi, Johnstone Thitiri, Judd L. Walson, Wieger Voskuijl, James A. Berkley & Robert H. J. Bandsma * KEMRI/Wellcome Trust Research Programme, Kilifi,
Kenya Moses Ngari, Johnstone Thitiri, Laura Mwalekwa & James A. Berkley * Department of Paediatrics, Coast General Hospital, Mombasa, Kenya Laura Mwalekwa * Departments of Global Health,
Medicine, Pediatrics and Epidemiology, University of Washington, Seattle, WA, USA Judd L. Walson * Amsterdam Institute for Global Health and Development, Department of Global Health,
Amsterdam University Medical Centres, Amsterdam, The Netherlands Wieger Voskuijl * Department of Paediatrics and Child Health, Kamuzu University of Health Sciences (formerly College of
Medicine), Blantyre, Malawi Wieger Voskuijl * Centre for Tropical Medicine & Global Health, Nuffield Department of Medicine, University of Oxford, Oxford, United Kingdom James A. Berkley
* Department of Biomedical Sciences, Kamuzu University of Health Sciences (formerly College of Medicine), Blantyre, Malawi Robert H. J. Bandsma Authors * Bijun Wen View author publications
You can also search for this author inPubMed Google Scholar * Amber Farooqui View author publications You can also search for this author inPubMed Google Scholar * Celine Bourdon View author
publications You can also search for this author inPubMed Google Scholar * Nawar Tarafdar View author publications You can also search for this author inPubMed Google Scholar * Moses Ngari
View author publications You can also search for this author inPubMed Google Scholar * Emmanuel Chimwezi View author publications You can also search for this author inPubMed Google Scholar
* Johnstone Thitiri View author publications You can also search for this author inPubMed Google Scholar * Laura Mwalekwa View author publications You can also search for this author
inPubMed Google Scholar * Judd L. Walson View author publications You can also search for this author inPubMed Google Scholar * Wieger Voskuijl View author publications You can also search
for this author inPubMed Google Scholar * James A. Berkley View author publications You can also search for this author inPubMed Google Scholar * Robert H. J. Bandsma View author
publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS B.W. contributed to conceptualization, investigation and methodology, statistical analysis, data
curation, data visualization, originally drafting, review and editing the manuscript. A.F. contributed to investigation and methodology, review and editing the manuscript. C.B. contributed
to statistical analysis, data visualization, review and editing the manuscript. N.T. contributed to investigation. M.N. contributed to data curation, review and editing the manuscript. E.C.,
J.T., and L.M. contributed to resources and material, review and editing the manuscript. J.L.W., W.V. and J.A.B. contributed to funding, supervision, review and editing the manuscript.
R.H.J.B. contributed to conceptualization, funding, supervision, review and editing the manuscript. CORRESPONDING AUTHOR Correspondence to Robert H. J. Bandsma. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Communications Medicine_ thanks George Fuchs, Said El Bouhaddani and the other, anonymous,
reviewer(s) for their contribution to the peer review of this work. A peer review file is available. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION DESCRIPTION OF SUPPLEMENTARY MATERIALS SUPPLEMENTARY DATA 1
SUPPLEMENTARY DATA 2 SUPPLEMENTARY DATA 3 REPORTING SUMMARY RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which
permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to
the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless
indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or
exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints
and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Wen, B., Farooqui, A., Bourdon, C. _et al._ Intestinal disturbances associated with mortality of children with complicated severe
malnutrition. _Commun Med_ 3, 128 (2023). https://doi.org/10.1038/s43856-023-00355-0 Download citation * Received: 23 November 2022 * Accepted: 11 September 2023 * Published: 29 September
2023 * DOI: https://doi.org/10.1038/s43856-023-00355-0 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable
link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative