Play all audios:
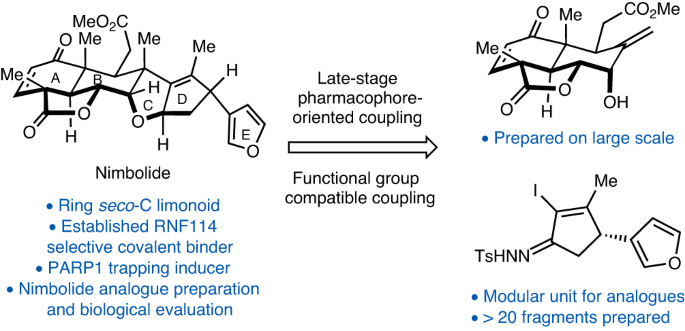
ABSTRACT Nimbolide, a ring seco-C limonoid natural product, was recently found to inhibit the poly(ADP)-ribosylation (PARylation)-dependent ubiquitin E3 ligase RNF114. In doing so, it
induces the ‘supertrapping’ of both PARylated PARP1 and PAR-dependent DNA-repair factors. PARP1 inhibitors have reshaped the treatment of cancer patients with germline _BRCA1_/_2_ mutations
partly through the PARP1 trapping mechanism. To this end, modular access to nimbolide analogues represents an opportunity to develop cancer therapeutics with enhanced PARP1 trapping
capability. Here we report a convergent synthesis of nimbolide through a late-stage coupling strategy. Through a sulfonyl hydrazone-mediated etherification and a radical cyclization, this
strategy uses a pharmacophore-containing building block and diversifiable hydrazone units to enable the modular synthesis of nimbolide and its analogues. The broad generality of our
synthetic strategy allowed access to a variety of analogues with their preliminary cellular cytotoxicity and PARP1 trapping activity reported. Access through your institution Buy or
subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 digital issues and online
access to articles $119.00 per year only $9.92 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local
taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING
VIEWED BY OTHERS POLY(ADP-RIBOSE) POLYMERASE INHIBITION: PAST, PRESENT AND FUTURE Article 03 September 2020 MINIMIZING DNA TRAPPING WHILE MAINTAINING ACTIVITY INHIBITION VIA SELECTIVE PARP1
DEGRADER Article Open access 18 December 2024 MECHANISM OF SUBSTRATE HYDROLYSIS BY THE HUMAN NUCLEOTIDE POOL SANITISER DNPH1 Article Open access 26 October 2023 DATA AVAILABILITY
Experimental data as well as characterization data for all new compounds prepared in the course of these studies and Supplementary figures and schemes are provided in Supplementary
Information of this paper. Crystallographic data for the structures reported in the present article have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition
nos. CCDC 2289527 (10), 2289528 (22), 2289529 (41) and 2289530 (48) (see ‘X-ray crystallographic data’ in Supplementary Information). Copies of the data can be obtained free of charge via
https://www.ccdc.cam.ac.uk/structures. REFERENCES * Huang, A., Garraway, L. A., Ashworth, A. & Weber, B. Synthetic lethality as an engine for cancer drug target discovery. _Nat. Rev.
Drug Discov._ 19, 23–38 (2020). Article CAS PubMed Google Scholar * Bryant, H. E. et al. Specific killing of _BRCA2_-deficient tumours with inhibitors of poly(ADP-ribose) polymerase.
_Nature_ 434, 913–917 (2005). Article CAS PubMed ADS Google Scholar * Farmer, H. et al. Targeting the DNA repair defect in _BRCA_ mutant cells as a therapeutic strategy. _Nature_ 434,
917–921 (2005). Article CAS PubMed ADS Google Scholar * Lord, C. J. & Ashworth, A. PARP inhibitors: synthetic lethality in the clinic. _Science_ 355, 1152–1158 (2017). Article CAS
PubMed PubMed Central ADS Google Scholar * Ashworth, A. & Lord, C. J. Synthetic lethal therapies for cancer: what’s next after PARP inhibitors? _Nat. Rev. Clin. Oncol._ 15, 564–576
(2018). Article CAS PubMed Google Scholar * Chan, C. Y., Tan, K. V. & Cornelissen, B. PARP inhibitors in cancer diagnosis and therapy. _Clin. Cancer Res._ 27, 1585–1594 (2021).
Article CAS PubMed Google Scholar * Chambon, P., Weill, J. D. & Mandel, P. Nicotinamide mononucleotide activation of a new DNA-dependent polyadenylic acid synthesizing nuclear
enzyme. _Biochem. Biophys. Res. Commun._ 11, 39–43 (1963). Article CAS PubMed Google Scholar * Kraus, W. L. PARPs and ADP-ribosylation: 50 years … and counting. _Mol. Cell_ 58, 902–910
(2015). Article CAS PubMed PubMed Central Google Scholar * Pommier, Y., O’Connor, M. J. & De Bono, J. Laying a trap to kill cancer cells: PARP inhibitors and their mechanisms of
action. _Sci. Transl. Med._ 8, 362ps17 (2016). Article PubMed Google Scholar * Murai, J. et al. Trapping of PARP1 and PARP2 by clinical PARP inhibitors. _Cancer Res._ 72, 5588–5599
(2012). Article CAS PubMed PubMed Central Google Scholar * Wang, S. et al. Uncoupling of PARP1 trapping and inhibition using selective PARP1 degradation. _Nat. Chem. Biol._ 15,
1223–1231 (2019). Article CAS PubMed PubMed Central Google Scholar * Kim, C., Wang, X.-D. & Yu, Y. PARP1 inhibitors trigger innate immunity via PARP1 trapping-induced DNA damage
response. _eLife._ 9, e60637 (2020). Article CAS PubMed PubMed Central Google Scholar * Hopkins, T. A. et al. PARP1 trapping by PARP inhibitors drives cytotoxicity in both cancer cells
and healthy bone marrow. _Mol. Cancer Res._ 17, 409–419 (2019). Article CAS PubMed Google Scholar * Rodrigues, T., Reker, D., Schneider, P. & Schneider, G. Counting on natural
products for drug design. _Nat. Chem._ 8, 531–541 (2016). Article CAS PubMed Google Scholar * Li, P. et al. Nimbolide targets RNF114 to induce the trapping of PARP1 and synthetic
lethality in BRCA-mutated cancer. _Sci. Adv._ 9, eadg7752 (2023). Article CAS PubMed PubMed Central ADS Google Scholar * Yu, Y. et al. Nimbolide analogs and methods of use thereof.
Patent WO 2022/150667 (2022). * Spradlin, J. N. et al. Harnessing the anti-cancer natural product nimbolide for targeted protein degradation. _Nat. Chem. Biol._ 15, 747–755 (2019). Article
CAS PubMed PubMed Central Google Scholar * Ekong, D. E. U. Chemistry of the meliacins (limonoids). The structure of nimbolide, a new meliacin from _Azadirachta indica_. _Chem. Commun._
https://doi.org/10.1039/C1967000808A (1967). * Breslin, C. et al. The XRCC1 phosphate-binding pocket binds poly(ADP-ribose) and is required for XRCC1 function. _Nucleic Acids Res._ 43,
6934–6944 (2015). Article CAS PubMed PubMed Central Google Scholar * Dias, D. A., Urban, S. & Roessner, U. A historical overview of natural products in drug discovery. _Metabolites_
2, 303–336 (2012). Article CAS PubMed PubMed Central Google Scholar * Nicolaou, K. C. & Montagnon, T. _Molecules That Changed the World_ (Wiley-VCH, 2008). * Li, Q. et al.
Synthetic group A streptogramin antibiotics that overcome Vat resistance. _Nature_ 586, 145–150 (2020). Article CAS PubMed PubMed Central ADS Google Scholar * Murphy, S. K., Zeng, M.
& Herzon, S. B. A modular and enantioselective synthesis of the pleuromutilin antibiotics. _Science_ 356, 956–959 (2017). Article CAS PubMed PubMed Central ADS Google Scholar * Li,
J., Chen, F. & Renata, H. Concise chemoenzymatic synthesis of gedunin. _J. Am. Chem. Soc._ 144, 19238–19242 (2022). Article CAS PubMed Google Scholar * Renata, H., Zhou, Q. &
Baran, P. S. Strategic redox relay enables a scalable synthesis of ouabagenin, a bioactive cardenolide. _Science_ 339, 59–63 (2013). Article CAS PubMed PubMed Central ADS Google Scholar
* Logan, M. M., Toma, T., Thomas-Tran, R. & Du Bois, J. Asymmetric synthesis of batrachotoxin: enantiomeric toxins show functional divergence against Nav. _Science_ 354, 865–869
(2016). Article CAS PubMed ADS Google Scholar * Jorgensen, L. et al. 14-step synthesis of (+)-ingenol from (+)-3-carene. _Science_ 341, 878–882 (2013). Article CAS PubMed ADS Google
Scholar * Wan, K. K. & Shenvi, R. A. Conjuring a supernatural product—delmarine. _Synlett._ 27, 1145–1164 (2016). Article CAS Google Scholar * Abbasov, M. E. et al. Simplified
immunosuppressive and neuroprotective agents based on gracilin A. _Nat. Chem._ 11, 342–350 (2019). Article CAS PubMed PubMed Central Google Scholar * Veitch, G. E., Boyer, A. & Ley,
S. V. The azadirachtin story. _Angew. Chem. Int. Ed._ 47, 9402–9429 (2008). Article CAS Google Scholar * Veitch, G. E. et al. Synthesis of azadirachtin: a long but successful journey.
_Angew. Chem. Int. Ed._ 46, 7629–7632 (2007). Article CAS Google Scholar * Li, F.-Z. et al. A chiral pool approach for asymmetric syntheses of (−)-antrocin, (+)-asperolide C, and
(−)-_trans_-ozic acid. _Chem. Commun._ 52, 12426–12429 (2016). Article CAS ADS Google Scholar * Hamulić, D. et al. Synthesis and biological studies of (+)-liquiditerpenoic acid A
(abietopinoic acid) and representative analogues: SAR studies. _J. Nat. Prod._ 82, 823–831 (2019). Article PubMed Google Scholar * Michl, G., Rettenbacher, C. & Haslinger, E.
Synthesis of 12-methoxyabietic acid methylester, a feeding deterrent of the larch sawfly _Pristiphora erichsonii_ (Hartig). _Monatshefte Chem. Chem. Mon._ 119, 833–838 (1988). Article CAS
Google Scholar * Pelletier, S. W., Iyer, K. N. & Chang, C. W. J. Oxidative degradation of resin acids. _J. Org. Chem._ 35, 3535–3538 (1970). Article CAS Google Scholar * Larock, R.
C., Hightower, T. R., Kraus, G. A., Hahn, P. & Zheng, D. A simple, effective, new, palladium-catalyzed conversion of enol silanes to enones and enals. _Tetrahedron Lett._ 36, 2423–2426
(1995). Article CAS Google Scholar * Kawamura, S., Chu, H., Felding, J. & Baran, P. S. Nineteen-step total synthesis of (+)-phorbol. _Nature_ 532, 90–93 (2016). Article CAS PubMed
PubMed Central ADS Google Scholar * Curci, R., D’Accolti, L. & Fusco, C. A novel approach to the efficient oxygenation of hydrocarbons under mild conditions. Superior oxo transfer
selectivity using dioxiranes. _Acc. Chem. Res._ 39, 1–9 (2006). Article CAS PubMed Google Scholar * Kawamata, Y. et al. Scalable, electrochemical oxidation of unactivated C–H bonds. _J.
Am. Chem. Soc._ 139, 7448–7451 (2017). Article CAS PubMed PubMed Central Google Scholar * Aranda, G. et al. Practical and efficient 1α-hydroxylation of 4,4-dimethyl-2-ene derivatives in
terpenic series. _Synth. Commun._ 27, 45–60 (1997). Article CAS Google Scholar * Barluenga, J., Barrio, P., Riesgo, L., López, L. A. & Tomás, M. A general and regioselective
synthesis of cyclopentenone derivatives through nickel (0)-mediated [3+ 2] cyclization of alkenyl Fischer carbene complexes and internal alkynes. _J. Am. Chem. Soc._ 129, 14422–14426 (2007).
Article CAS PubMed Google Scholar * Ohashi, M., Taniguchi, T. & Ogoshi, S. Nickel-catalyzed formation of cyclopentenone derivatives via the unique cycloaddition of α,β-unsaturated
phenyl esters with alkynes. _J. Am. Chem. Soc._ 133, 14900–14903 (2011). Article CAS PubMed Google Scholar * Jenkins, A. D., Herath, A., Song, M. & Montgomery, J. Synthesis of
cyclopentenols and cyclopentenones via nickel-catalyzed reductive cycloaddition. _J. Am. Chem. Soc._ 133, 14460–14466 (2011). Article CAS PubMed Google Scholar * Ahlin, J. S. E., Donets,
P. A. & Cramer, N. Nickel (0)‐catalyzed enantioselective annulations of alkynes and arylenoates enabled by a chiral NHC ligand: efficient access to cyclopentenones. _Angew. Chem. Int.
Ed._ 53, 13229–13233 (2014). Article CAS Google Scholar * Shi, X., Gorin, D. J. & Toste, F. D. Synthesis of 2-cyclopentenones by gold(I)-catalyzed Rautenstrauch rearrangement. _J. Am.
Chem. Soc._ 127, 5802–5803 (2005). Article CAS PubMed Google Scholar * Sha, C.-K. & Huang, S.-J. Synthesis of β-substituted α-iodocycloalkenones. _Tetrahedron Lett._ 36, 6927–6928
(1995). Article CAS Google Scholar * Barluenga, J., Tomás‐Gamasa, M., Aznar, F. & Valdés, C. Straightforward synthesis of ethers: metal‐free reductive coupling of tosylhydrazones with
alcohols or phenols. _Angew. Chem. Int. Ed._ 49, 4993–4996 (2010). Article CAS Google Scholar * Chandrasekhar, S., Rajaiah, G., Chandraiah, L. & Swamy, D. N. Direct conversion of
tosylhydrazones to _tert_-butyl ethers under Bamford–Stevens reaction conditions. _Synlett._ 2001, 1779–1780 (2001). Article Google Scholar * Crossley, S. W. M., Obradors, C., Martinez, R.
M. & Shenvi, R. A. Mn-, Fe-, and Co-catalyzed radical hydrofunctionalizations of olefins. _Chem. Rev._ 116, 8912–9000 (2016). Article CAS PubMed PubMed Central Google Scholar *
Nicolaou, K. C. et al. Studies toward the synthesis of azadirachtin. Part 2: construction of fully functionalized ABCD ring frameworks and unusual intramolecular reactions induced by
close-proximity effects. _Angew. Chem. Int. Ed._ 44, 3447–3452 (2005). Article CAS Google Scholar * Hasegawa, E. et al. Tris(trimethylsilyl)silane promoted radical reaction and
electron-transfer reaction in benzotrifluoride. _Tetrahedron_ 64, 7724–7728 (2008). Article CAS Google Scholar * Chatgilialoglu, C., Ferrei, C., Landais, Y. & Timokhin, V. I. Thirty
years of (TMS)3SiH: a milestone in radical-based synthetic chemistry. _Chem. Rev._ 118, 6516–6572 (2008). Article Google Scholar * Overman, L. E., Abelman, M. M., Kucera, D. J., Tran, V.
D. & Ricca, D. J. Palladium-catalyzed, polyene cyclizations. _Pure Appl. Chem._ 64, 1813–1819 (1992). Article CAS Google Scholar * Green, S. A., Huffman, T. R., McCourt, R. O., van
der Puyl, V. & Shenvi, R. A. Hydroalkylation of olefins to form quaternary carbons. _J. Am. Chem. Soc._ 141, 7709–7714 (2019). Article CAS PubMed PubMed Central Google Scholar *
Cui, B. et al. Limonoids from _Azadirachta excelsa_. _Phytochemistry._ 47, 1283–1287 (1998). Article CAS PubMed Google Scholar * Bokel, M., Cramer, R., Gutzeit, H., Reeb, S. & Kraus,
W. Tetranortriterpenoids related to nimbin and nimbolide from _Azadirachta_ _indica_ A. Juss (Meliaceae). _Tetrahedron._ 46, 775–782 (1990). Article CAS Google Scholar * Kraus, W. &
Cramer, R. Pentanortriterpenoide aus _Azadirachta indica_ A. Juss (Meliaceae). _Chem. Ber._ 114, 2375–2381 (1981). Article CAS Google Scholar * Peterson, L. A. Reactive metabolites in the
biotransformation of molecules containing a furan ring. _Chem. Res. Toxicol._ 26, 6–25 (2013). Article CAS PubMed ADS Google Scholar Download references ACKNOWLEDGEMENTS Financial
support for this work was provided by the National Institutes of Health (grant nos. R01GM141088 to T.Q. and 5R35GM134883, 1R01NS122533 and 1R21CA261018 to Y.Y.), the Welch Foundation (grant
nos. I-2155-20230405 and I-2010-20190330 to T.Q. and I-1800 to Y.Y.) and UT Southwestern Eugene McDermott Scholarship (to T.Q.). We thank F. Lin (University of Texas Southwestern (UTSW)) for
assistance with nuclear magnetic resonance (NMR) spectroscopy, H. Baniasadi (UTSW) for the high resolution mass spectrometry (HRMS) and V. Lynch (University of Texas, Austin) for X-ray
crystallographic analysis. We thank U. Tambar (UTSW) for generous access to chiral high-performance liquid chromatography equipment, P. Baran (Scripps Research) and J. Porco (Boston
University) for helpful discussions. AUTHOR INFORMATION Author notes * Chiho Kim, Xudong Wang & Yonghao Yu Present address: Department of Molecular Pharmacology and Therapeutics,
Columbia University Vagelos College of Physicians and Surgeons, New York, NY, USA * These authors contributed equally: Heping Deng, Hejun Deng. AUTHORS AND AFFILIATIONS * Department of
Biochemistry, The University of Texas Southwestern Medical Center, Dallas, TX, USA Heping Deng, Hejun Deng, Chiho Kim, Peng Li, Xudong Wang, Yonghao Yu & Tian Qin Authors * Heping Deng
View author publications You can also search for this author inPubMed Google Scholar * Hejun Deng View author publications You can also search for this author inPubMed Google Scholar * Chiho
Kim View author publications You can also search for this author inPubMed Google Scholar * Peng Li View author publications You can also search for this author inPubMed Google Scholar *
Xudong Wang View author publications You can also search for this author inPubMed Google Scholar * Yonghao Yu View author publications You can also search for this author inPubMed Google
Scholar * Tian Qin View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Heping D. and Hejun D. performed synthetic experiments. C.K., P.L. and
X.W. performed the cellular cytotoxicity, chromatin PAPR1 trapping and microirradiation experiments. Y.Y. and T.Q. designed and supervised the project. All the authors wrote the paper.
CORRESPONDING AUTHORS Correspondence to Yonghao Yu or Tian Qin. ETHICS DECLARATIONS COMPETING INTERESTS Patent WO 2022/150667 ‘Nimbolide analogs and methods of use thereof’ has been filed on
some aspects of the work in this paper, and H.D., H.D., C.K., P.L., Y.Y. and T.Q. are listed as inventors. Y.Y. and T.Q. are co-founders and shareholders of ProteoValent Therapeutics. The
other authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Synthesis_ thanks the anonymous reviewers for their contribution to the peer review of this work.
Primary Handling Editor: Thomas West, in collaboration with the _Nature Synthesis_ team. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional
claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION General experimental. Summary of first-generation synthesis and optimization.
Model studies to construct the central THF ring. Summary of second-generation synthesis and optimization. The overview of final route. Experimental procedures and characterization data.
Stereochemistry assignment for the CBS reduction of linear ketone. C–H oxidation of lactone substrate; radical _ipso_ substitution. General synthetic route and procedure to access analogues.
Natural products NMR comparisons. X-ray crystallography data. Reference. NMR spectra. REPORTING SUMMARY SUPPLEMENTARY DATA 1 Crystallographic data for 10, CCDC 2289527. SUPPLEMENTARY DATA 2
Crystallographic data for 22, CCDC 2289528. SUPPLEMENTARY DATA 3 Crystallographic data for 41, CCDC 2289529. SUPPLEMENTARY DATA 4 Crystallographic data for 48, CCDC 2289530. SUPPLEMENTARY
DATA 5 Source spreadsheet data for Supplementary Fig. 1. SUPPLEMENTARY DATA 6 Source spreadsheet data for Supplementary Fig. 2. SOURCE DATA SOURCE DATA FIG. 6 Source spreadsheet data for
Fig. 6b. SOURCE DATA FIG. 6 Original blots scan for Fig. 6a (also included in Supplementary Information). RIGHTS AND PERMISSIONS Springer Nature or its licensor (e.g. a society or other
partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this
article is solely governed by the terms of such publishing agreement and applicable law. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Deng, H., Deng, H., Kim, C. _et al._
Synthesis of nimbolide and its analogues and their application as poly(ADP-ribose) polymerase-1 trapping inducers. _Nat. Synth_ 3, 378–385 (2024). https://doi.org/10.1038/s44160-023-00437-w
Download citation * Received: 27 December 2022 * Accepted: 11 October 2023 * Published: 27 November 2023 * Issue Date: March 2024 * DOI: https://doi.org/10.1038/s44160-023-00437-w SHARE THIS
ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard
Provided by the Springer Nature SharedIt content-sharing initiative