Play all audios:
ABSTRACT Seawater is the largest uranium reserve in the world, and the efficient extraction of uranium from seawater could facilitate the sustainable development of the nuclear industry for
thousands of years. However, conventional extraction processes must suffer the dissociation of CO32− ions from [UO2(CO3)3]4− anions to bind the uranyl core, which has a high energy barrier,
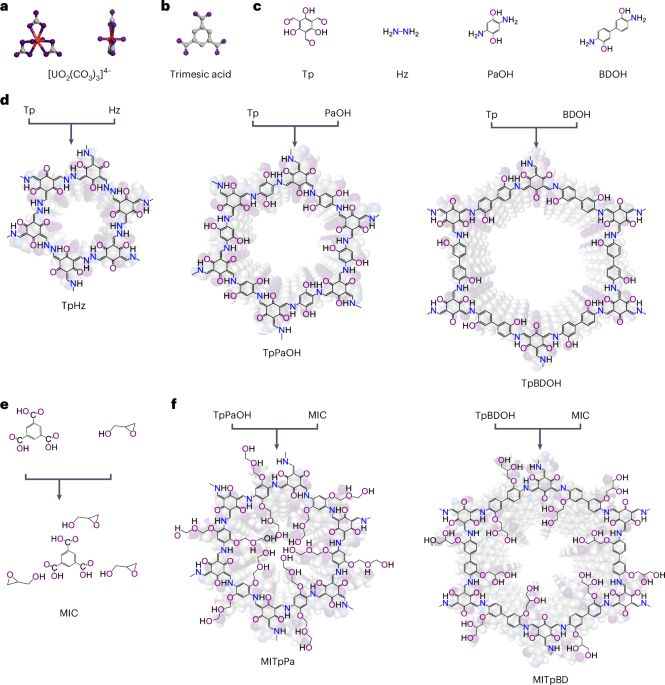
resulting in poor selectivity and long working times. Here we combine a molecular templating strategy to synthesize several hydroxy-rich covalent organic frameworks with tunable nanopore
sizes. In the 1.2-nm-sized covalent organic framework cavity, hydroxyl groups coupled with the hydrogen-bonded NH4+ cations selectively bind uranyl tricarbonate ions via synergistic
electrostatic and hydrogen-bonding interactions. This framework exhibits high uranium extraction capability with a removal ratio of > 99.99% in 400 min (initial concentration of 5 ppm at
298 K, pH = 8–9). Notably, a record uranium adsorption uptake is achieved with a capacity of 23.66 mg g−1 in seven days from natural seawater, surpassing that of classical amidoxime-based
adsorbents by a factor of 350%. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your
institution Subscribe to this journal Receive 12 digital issues and online access to articles $119.00 per year only $9.92 per issue Learn more Buy this article * Purchase on SpringerLink *
Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional
subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS ULTRA-SELECTIVE URANIUM SEPARATION BY IN-SITU FORMATION OF _Π_-_F_ CONJUGATED 2D
URANIUM-ORGANIC FRAMEWORK Article Open access 11 January 2024 DNA NANO-POCKET FOR ULTRA-SELECTIVE URANYL EXTRACTION FROM SEAWATER Article Open access 11 November 2020
PHOTOISOMERIZATION-MEDIATED TUNABLE PORE SIZE IN METAL ORGANIC FRAMEWORKS FOR U(VI)/V(V) SELECTIVE SEPARATION Article Open access 10 March 2025 DATA AVAILABILITY The main data supporting the
findings of this study are available within the article and its Supplementary Information files. Extra data are available from the corresponding author upon request. REFERENCES * _Uranium
2020_ (NEA & IAEA, 2021). * Yang, H. et al. Tuning local charge distribution in multicomponent covalent organic frameworks for dramatically enhanced photocatalytic uranium extraction.
_Angew. Chem. Int. Ed._ 62, e202303129 (2023). Article CAS Google Scholar * Xie, Y. et al. Uranium extraction from seawater: material design, emerging technologies and marine engineering.
_Chem. Soc. Rev._ 52, 97–162 (2023). Article CAS PubMed Google Scholar * Yang, W. et al. Functionalized iron–nitrogen–carbon electrocatalyst provides a reversible electron transfer
platform for efficient uranium extraction from seawater. _Adv. Mater._ 33, 2106621 (2021). Article CAS Google Scholar * Sun, Q. et al. Spatial engineering direct cooperativity between
binding sites for uranium sequestration. _Adv. Sci._ 8, 2001573 (2021). Article CAS Google Scholar * Rhodes, R. More nuclear power can speed CO2 cuts. _Nature_ 548, 281 (2017). Article
CAS PubMed Google Scholar * Wu, Y. et al. Functional nanomaterials for selective uranium recovery from seawater: material design, extraction properties and mechanisms. _Coord. Chem. Rev._
483, 215097 (2023). Article CAS Google Scholar * Lindner, H. & Schneider, E. Review of cost estimates for uranium recovery from seawater. _Energ. Econ._ 49, 9–22 (2015). Article
Google Scholar * Xiong, X. et al. Ammoniating covalent organic framework (COF) for high-performance and selective extraction of toxic and radioactive uranium ions. _Adv. Sci._ 6, 1900547
(2019). Article Google Scholar * Lashley, M. A. et al. Highly preorganized ligand 1,10-phenanthroline-2,9-dicarboxylic acid for the selective recovery of uranium from seawater in the
presence of competing vanadium species. _Inorg. Chem._ 55, 10818–10829 (2016). Article CAS PubMed Google Scholar * Liu, Z. et al. Anti-biofouling and water-stable balanced charged metal
organic framework-based polyelectrolyte hydrogels for extracting uranium from seawater. _ACS Appl. Mater. Interfaces_ 12, 18012–18022 (2020). Article PubMed Google Scholar * Liu, C. et
al. A half-wave rectified alternating current electrochemical method for uranium extraction from seawater. _Nat. Energy_ 2, 17007 (2017). Article CAS Google Scholar * Cui, W. et al. Low
band gap benzoxazole‐linked covalent organic frameworks for photo‐enhanced targeted uranium recovery. _Small_ 17, 2006882 (2021). Article CAS Google Scholar * Wang, Z. et al. Constructing
an ion pathway for uranium extraction from seawater. _Chem_ 6, 1683–1691 (2020). Article CAS Google Scholar * Sun, Q. et al. Bio-inspired nano-traps for uranium extraction from seawater
and recovery from nuclear waste. _Nat. Commun._ 9, 1644 (2018). Article PubMed PubMed Central Google Scholar * Mason, C. F. V. et al. Carbonate leaching of uranium from contaminated
soils. _Environ. Sci. Technol._ 31, 2707–2711 (1997). Article CAS Google Scholar * Santos, E. A. & Ladeira, A. C. Q. Recovery of uranium from mine waste by leaching with
carbonate-based reagents. _Environ. Sci. Technol._ 8, 3591–3597 (2011). Article Google Scholar * Kaushik, A. et al. Large-area self-standing thin film of porous hydrogen-bonded organic
framework for efficient uranium extraction from seawater. _Chem_ 8, 2749–2765 (2022). Article CAS Google Scholar * Wang, Y. et al. Adsorption of U(VI) from aqueous solution by the
carboxyl-mesoporous carbon. _Chem. Eng. J._ 198, 246–253 (2012). Article Google Scholar * Chen, D. et al. Bio-inspired functionalization of electrospun nanofibers with anti-biofouling
property for efficient uranium extraction from seawater. _Chem. Eng. J._ 465, 142844 (2023). Article CAS Google Scholar * Song, Y. et al. Nanospace decoration with uranyl-specific ‘hooks’
for selective uranium extraction from seawater with ultrahigh enrichment index. _ACS Cent. Sci._ 7, 1650–1656 (2021). Article CAS PubMed PubMed Central Google Scholar * Feng, L. et al.
In situ synthesis of uranyl‐imprinted nanocage for selective uranium recovery from seawater. _Angew. Chem. Int. Ed._ 61, e202101015 (2022). Article CAS Google Scholar * Guo, X. et al.
DFT investigations of uranium complexation with amidoxime-, carboxyl-and mixed amidoxime/carboxyl-based host architectures for sequestering uranium from seawater. _Inorg. Chim. Acta_ 441,
117–125 (2016). Article CAS Google Scholar * Gan, J. et al. Phosphorylation improved the competitive U/V adsorption on chitosan-based adsorbent containing amidoxime for rapid uranium
extraction from seawater. _Int. J. Biol. Macromol._ 238, 124074 (2023). Article CAS PubMed Google Scholar * Wang, Z. et al. Constructing uranyl-specific nanofluidic channels for unipolar
ionic transport to realize ultrafast uranium extraction. _J. Am. Chem. Soc._ 143, 14523–14529 (2021). Article CAS PubMed Google Scholar * Wang, D. et al. Significantly enhanced uranium
extraction from seawater with mass produced fully amidoximated nanofiber adsorbent. _Adv. Energy Mater._ 8, 1802607 (2018). Article Google Scholar * Yuan, Y. et al. Rational design of
porous nanofiber adsorbent by blow‐spinning with ultrahigh uranium recovery capacity from seawater. _Adv. Funct. Mater._ 29, 1805380 (2019). Article Google Scholar * Chandra, S. et al.
Molecular level control of the capacitance of two-dimensional covalent organic frameworks, role of hydrogen bonding in energy storage materials. _Chem. Mater._ 29, 2074–2080 (2017). Article
CAS Google Scholar * Liang, Y. et al. Guanidinium-based ionic covalent organic frameworks for capture of uranyl tricarbonate. _Adv. Compos. Hybrid Mater._ 5, 184–194 (2022). Article CAS
Google Scholar * Cao, Q. et al. A study of the potential application of nano-Mg(OH)2 in adsorbing low concentrations of uranyl tricarbonate from water. _Nanoscale_ 4, 2423–2430 (2012).
Article CAS PubMed Google Scholar * Haarmann, N. et al. Modeling of interfacial tensions of long-chain molecules and related mixtures using perturbed chain-statistical associating fluid
theory and the density gradient theory. _J. Chem. Eng. Data_ 65, 1005–1018 (2019). Article Google Scholar * Li, J. et al. Does polysaccharide is an idea template selection for glycosyl
imprinting. _Biosens. Bioelectron._ 99, 438–442 (2018). Article CAS PubMed Google Scholar * Huang, Y. et al. Strategy of choosing templates in molecular imprinting to expand the
recognition width for family-selectivity. _Anal. Chem._ 95, 11070–11077 (2023). Article CAS PubMed Google Scholar * Maia, R. A. et al. Crystal engineering of covalent organic frameworks
based on hydrazine and hydroxy-1,3,5-triformylbenzenes. _Cryst. Growth Des._ 18, 5682–5689 (2018). Article Google Scholar * Yi, S. et al. Ionic liquid modified covalent organic frameworks
for efficient detection and adsorption of ReO4–/TcO4–. _J. Environ. Chem. Eng._ 10, 107666 (2022). Article CAS Google Scholar * Jiang, Y. et al. Stainless-steel-net-supported
superhydrophobic COF coating for oil/water separation. _J. Membr. Sci._ 587, 117177 (2019). Article CAS Google Scholar * Mu, Z. et al. Zwitterionic covalent organic frameworks as
catalysts for hierarchical reduction of CO2 with amine and hydrosilane. _ACS Appl. Mater. Interfaces_ 10, 41350–41358 (2018). Article CAS PubMed Google Scholar * Yuan, Y. et al.
Molecularly imprinted porous aromatic frameworks and their composite components for selective extraction of uranium ions. _Adv. Mater._ 30, 1706507 (2018). Article Google Scholar *
Zeppuhar, A. N. et al. Linkage transformations in a three-dimensional covalent organic framework for high-capacity adsorption of perfluoroalkyl substances. _ACS Appl. Mater. Interfaces_ 15,
28476–28490 (2023). Google Scholar * Humphrey, W. et al. VMD: visual molecular dynamics. _J. Mol. Graph._ 14, 33–38 (1996). Article CAS PubMed Google Scholar * Liu, T. et al. Multiwfn:
a multifunctional wavefunction analyzer. _J. Comput. Chem._ 33, 580–592 (2012). Article CAS Google Scholar * Liu, T. et al. A comprehensive electron wavefunction analysis toolbox for
chemists, Multiwfn. _J. Chem. Phys._ 161, 082503 (2024). Article Google Scholar * Halder, A. et al. Ultrastable imine-based covalent organic frameworks for sulfuric acid recovery, an
effect of interlayer hydrogen bonding. _Angew. Chem. Int. Ed._ 57, 5797–5802 (2018). Article CAS Google Scholar * Zhang, D. et al. Highly efficient extraction of uranium from seawater by
polyamide and amidoxime co-functionalized MXene. _Environ. Pollut._ 317, 120826 (2023). Article CAS PubMed Google Scholar * Sunder, S. & Miller, N. H. XPS and XRD studies of (Th,U)O2
fuel corrosion in water. _J. Nucl. Mater._ 279, 118–126 (2000). Article CAS Google Scholar * Zhong, X. et al. The role of functional-group-tuning in adsorption-photoreduction of U(VI)
onto β-ketoenamine covalent organic frameworks photosystem. _Chem. Eng. J._ 467, 143415 (2023). Article CAS Google Scholar * Qiu, J. et al. Porous covalent organic framework based
hydrogen‐bond nanotrap for the precise recognition and separation of gold. _Angew. Chem. Int. Ed._ 135, e202300459 (2023). Article Google Scholar * Shi, Y. et al. Anchoring LiCl in the
nanopores of metal-organic frameworks for ultra‐high uptake and selective separation of ammonia. _Angew. Chem. Int. Ed._ 61, 202212032 (2022). Article Google Scholar * Lohse, M. S. et al.
Sequential pore wall modification in a covalent organic framework for application in lactic acid adsorption. _Chem. Mater._ 28, 626–631 (2016). Article CAS Google Scholar * Zhou, L. et
al. Suppressing hydrogen evolution in aqueous lithium-ion batteries with double-site hydrogen bonding. _ACS Energy Lett._ 8, 40–47 (2022). Article Google Scholar * Xu, X. et al. Ultrahigh
and economical uranium extraction from seawater via interconnected open-pore architecture poly(amidoxime) fiber. _J. Mater. Chem. A_ 8, 22032–22044 (2020). Article CAS Google Scholar *
Yuan, Y. et al. Ultrafast and highly selective uranium extraction from seawater by hydrogel-like spidroin-based protein fiber. _Angew. Chem. Int. Ed._ 131, 11911–11916 (2019). Article
Google Scholar * Yuan, Y. et al. A bio‐inspired nano‐pocket spatial structure for targeting uranyl capture. _Angew. Chem. Int. Ed._ 59, 4262–4268 (2020). Article CAS Google Scholar *
Wang, N. et al. Accelerated chemical thermodynamics of uranium extraction from seawater by plant‐mimetic transpiration. _Adv. Sci._ 8, 2102250 (2021). Article CAS Google Scholar * Zhang,
C.-R. et al. Alkynyl-based sp2 carbon-conjugated covalent organic frameworks with enhanced uranium extraction from seawater by photoinduced multiple effects. _CCS Chem._ 3, 168–179 (2021).
Article CAS Google Scholar * Feng, X. et al. Highly efficient extraction of uranium from seawater by natural marine crab carapace. _Chem. Eng. J._ 430, 133038 (2022). Article CAS Google
Scholar * Yuan, Y. et al. High-capacity uranium extraction from seawater through constructing synergistic multiple dynamic bonds. _figshare_ https://doi.org/10.6084/m9.figshare.25358452
(2024). Download references ACKNOWLEDGEMENTS G.Z. was supported by the National Key R&D Program of China (grant number 2022YFB3805902), the National Natural Science Foundation of China
(grant numbers 22131004 and U21A20330) and the ‘111’ project (grant number B18012). Y. Yuan was supported by the National Natural Science Foundation of China (grant numbers 21975039 and
22322501), the Fundamental Research Funds for the Central Universities (grant numbers 2412020ZD008 and GFPY202309) and the CNNC Key Laboratory on Uranium Extraction from Seawater
(KLUES202202). Y. Yang was supported by the National Natural Science Foundation of China (grant number 52204389). AUTHOR INFORMATION Author notes * These authors contributed equally: Ye
Yuan, Doudou Cao. AUTHORS AND AFFILIATIONS * Key Laboratory of Polyoxometalate and Reticular Material Chemistry of Ministry of Education, Faculty of Chemistry, Northeast Normal University,
Changchun, China Ye Yuan, Doudou Cao, Fengchao Cui, Cheng Zhang, Yingbo Song, Yue Zheng, Jiarui Cao & Guangshan Zhu * Key Laboratory of Automobile Materials of Ministry of Education and
School of Materials Science and Engineering, Jilin University, Changchun, China Yajie Yang * Beijing Research Institute of Chemical Engineering and Metallurgy, CNNC Key Laboratory on Uranium
Extraction from Seawater, Beijing, China Shusen Chen, Yan Song & Fengju Wang Authors * Ye Yuan View author publications You can also search for this author inPubMed Google Scholar *
Doudou Cao View author publications You can also search for this author inPubMed Google Scholar * Fengchao Cui View author publications You can also search for this author inPubMed Google
Scholar * Yajie Yang View author publications You can also search for this author inPubMed Google Scholar * Cheng Zhang View author publications You can also search for this author inPubMed
Google Scholar * Yingbo Song View author publications You can also search for this author inPubMed Google Scholar * Yue Zheng View author publications You can also search for this author
inPubMed Google Scholar * Jiarui Cao View author publications You can also search for this author inPubMed Google Scholar * Shusen Chen View author publications You can also search for this
author inPubMed Google Scholar * Yan Song View author publications You can also search for this author inPubMed Google Scholar * Fengju Wang View author publications You can also search for
this author inPubMed Google Scholar * Guangshan Zhu View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Y. Yuan and D.C. helped design the
experiments and wrote portions of the paper. D.C. carried out the experiments and performed the data interpretation. F.C., C.Z. and J.C. conducted the theoretical calculations. Y. Yang
helped design the experiments. Yingbo Song, Y.Z., S.C., Yan Song and F.W. performed the characterizations for uranium adsorption. Y. Yuan and G.Z. developed the concept, supervised the
experiments and drafted the paper. CORRESPONDING AUTHOR Correspondence to Guangshan Zhu. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. PEER REVIEW PEER
REVIEW INFORMATION _Nature Water_ thanks Shengqian Ma and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. ADDITIONAL INFORMATION PUBLISHER’S NOTE
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Figs.
1–57 and Tables 1–16. RIGHTS AND PERMISSIONS Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the
author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Yuan, Y., Cao, D., Cui, F. _et al._ High-capacity uranium extraction from seawater through constructing synergistic multiple
dynamic bonds. _Nat Water_ 3, 89–98 (2025). https://doi.org/10.1038/s44221-024-00346-y Download citation * Received: 08 March 2024 * Accepted: 30 October 2024 * Published: 02 January 2025 *
Issue Date: January 2025 * DOI: https://doi.org/10.1038/s44221-024-00346-y SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link
Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative