Play all audios:
ABSTRACT Organoids bridge the gap between 2D cell lines and in vivo studies. With their 3D organization and cellular heterogeneity, adult stem cell-derived organoids closely resemble their
tissue of origin. The development of CRISPR-mediated genome engineering and the recent additions of base and prime editing to the CRISPR toolbox have greatly simplified the generation of
exact, isogenic models for Mendelian diseases. Here, we review recent developments in CRISPR-mediated genome engineering and its application in human adult-stem-cell-derived organoids in the
construction of isogenic disease models. These models allow accurate qualification of the impact of allelic disease variants observed in patients. Furthermore, we discuss the use of
organoids as models for safety and efficacy of CRISPR for gene repair. Although transplantation of repaired tissue remains challenging, benchmarking CRISPR tools in organoids can bring
genome engineering one step closer to patients. KEY POINTS * CRISPR–Cas9-mediated genome engineering acts by introducing double-stranded DNA breaks into the genome. The damage repair process
can be used for gene knockout or precise targeted introduction of exogenous DNA. * Next-generation CRISPR tools, including base and prime editing, allow for induction of precise base
changes and small insertions and deletions, bypassing potentially deleterious double-stranded DNA breaks. * Owing to their 3D organization, adult-stem-cell-derived organoids closely resemble
the tissue of origin and are therefore a good model system to study human health and disease. * CRISPR–Cas9-mediated genome engineering can be used to create isogenic models to investigate
the onset, cause and treatment of human diseases. * CRISPR tools can be benchmarked for efficiency and safety by studying gene repair ex vivo in adult-stem-cell-derived organoids,
facilitating CRISPR–Cas9 clinical translation. * Ex vivo repaired adult-stem-cell-derived organoids can potentially be transplanted into patients to relieve disease phenotypes. Access
through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal
Receive 12 digital issues and online access to articles $119.00 per year only $9.92 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy
now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer
support SIMILAR CONTENT BEING VIEWED BY OTHERS CRISPR SOMATIC GENOME ENGINEERING AND CANCER MODELING IN THE MOUSE PANCREAS AND LIVER Article 14 March 2022 PRIME EDITING FOR FUNCTIONAL
REPAIR IN PATIENT-DERIVED DISEASE MODELS Article Open access 23 October 2020 THE NIH SOMATIC CELL GENOME EDITING PROGRAM Article Open access 07 April 2021 REFERENCES * Visscher, P. M. et al.
10 years of GWAS discovery: biology, function, and translation. _Am. J. Hum. Genet._ 101, 5–22 (2017). Article Google Scholar * Xuan, J., Yu, Y., Qing, T., Guo, L. & Shi, L.
Next-generation sequencing in the clinic: promises and challenges. _Cancer Lett._ 340, 284–295 (2013). Article Google Scholar * Jinek, M. et al. A programmable dual-RNA-guided DNA
endonuclease in adaptive bacterial immunity. _Science_ 337, 816–821 (2012). THIS ARTICLE CONTAINS THE FIRST DESCRIPTION OF THE CRISPR–CAS9 SYSTEM AS A POTENTIAL TOOL FOR RNA-PROGRAMMABLE
GENOME ENGINEERING. Article Google Scholar * Mali, P. et al. RNA-guided human genome engineering via Cas9. _Science_ 339, 823–826 (2013). Article Google Scholar * Cong, L. et al.
Multiplex genome engineering using CRISPR/Cas systems. _Science_ 339, 819–823 (2013). Article Google Scholar * Kapałczyńska, M. et al. 2D and 3D cell cultures — a comparison of different
types of cancer cell cultures. _Arch. Med. Sci._ 14, 910–919 (2018). Google Scholar * Clevers, H. Modeling development and disease with organoids. _Cell_ 165, 1586–1597 (2016). Article
Google Scholar * Kim, J., Koo, B. K. & Knoblich, J. A. Human organoids: model systems for human biology and medicine. _Nat. Rev. Mol. Cell Biol._ 21, 571–584 (2020). Article Google
Scholar * Sato, T. et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. _Gastroenterology_ 141, 1762–1772 (2011). Article
Google Scholar * Barker, N. et al. Lgr5+ve stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. _Cell Stem Cell_ 6, 25–36 (2010). Article Google
Scholar * Schutgens, F. et al. Tubuloids derived from human adult kidney and urine for personalized disease modeling. _Nat. Biotechnol._ 37, 303–313 (2019). Article Google Scholar * Huch,
M. et al. Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis. _EMBO J._ 32, 2708–2721 (2013). Article Google Scholar * Linnemann, J. R.
et al. Quantification of regenerative potential in primary human mammary epithelial cells. _Development_ 142, 3239–3251 (2015). Google Scholar * Boretto, M. et al. Development of organoids
from mouse and human endometrium showing endometrial epithelium physiology and long-term expandability. _Development_ 144, 1775–1786 (2017). Google Scholar * Lõhmussaar, K. et al.
Patient-derived organoids model cervical tissue dynamics and viral oncogenesis in cervical cancer. _Cell Stem Cell_ 28, 1380–1396.e6 (2021). Article Google Scholar * Huch, M. et al. In
vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. _Nature_ 494, 247–250 (2013). Article Google Scholar * Hu, H. et al. Long-term expansion of functional
mouse and human hepatocytes as 3D organoids. _Cell_ 175, 1591–1606.e19 (2018). Article Google Scholar * Sachs, N. et al. Long‐term expanding human airway organoids for disease modeling.
_EMBO J._ 38, e100300 (2019). Article Google Scholar * Nikolić, M. Z. et al. Human embryonic lung epithelial tips are multipotent progenitors that can be expanded in vitro as long-term
self-renewing organoids. _eLife_ 6, e26575 (2017). Article Google Scholar * Ren, W. et al. Single Lgr5- or Lgr6-expressing taste stem/progenitor cells generate taste bud cells ex vivo.
_Proc. Natl Acad. Sci. USA_ 111, 16401–16406 (2014). Article Google Scholar * Bannier-Hélaouët, M. et al. Exploring the human lacrimal gland using organoids and single-cell sequencing.
_Cell Stem Cell_ 28, 1221–1232.e7 (2021). Article Google Scholar * Mullenders, J. et al. Mouse and human urothelial cancer organoids: a tool for bladder cancer research. _Proc. Natl Acad.
Sci. USA_ 116, 4567–4574 (2019). Article Google Scholar * Karthaus, W. R. et al. Identification of multipotent luminal progenitor cells in human prostate organoid cultures. _Cell_ 159,
163–175 (2014). Article Google Scholar * van der Vaart, J. et al. Adult mouse and human organoids derived from thyroid follicular cells and modeling of Graves’ hyperthyroidism. _Proc. Natl
Acad. Sci. USA_ 118, e2117017118 (2021). Article Google Scholar * Ogundipe, V. M. L. et al. Generation and differentiation of adult tissue-derived human thyroid organoids. _Stem Cell
Rep._ 16, 913–925 (2021). Article Google Scholar * Sato, T. et al. Single Lgr5 stem cells build crypt–villus structures in vitro without a mesenchymal niche. _Nature_ 459, 262–265 (2009).
THIS ARTICLE DESCRIBES THE FIRST ADULT-STEM-CELL-DERIVED ORGANOID CULTURES DERIVED FROM THE MOUSE INTESTINE. Article Google Scholar * Wright, A. V., Nuñez, J. K. & Doudna, J. A.
Biology and applications of CRISPR systems: harnessing nature’s toolbox for genome engineering. _Cell_ 164, 29–44 (2016). Article Google Scholar * Sternberg, S. H., Redding, S., Jinek, M.,
Greene, E. C. & Doudna, J. A. DNA interrogation by the CRISPR RNA-guided endonuclease Cas9. _Nature_ 507, 62–67 (2014). Article Google Scholar * Ghezraoui, H. et al. Chromosomal
translocations in human cells are generated by canonical nonhomologous end-joining. _Mol. Cell_ 55, 829–842 (2014). Article Google Scholar * Zhang, X. H., Tee, L. Y., Wang, X. G., Huang,
Q. S. & Yang, S. H. Off-target effects in CRISPR/Cas9-mediated genome engineering. _Mol. Ther. Nucleic Acids_ 4, e264 (2015). Article Google Scholar * Kleinstiver, B. P. et al.
High-fidelity CRISPR-Cas9 nucleases with no detectable genome-wide off-target effects. _Nature_ 529, 490–495 (2016). Article Google Scholar * Vakulskas, C. A. et al. A high-fidelity Cas9
mutant delivered as a ribonucleoprotein complex enables efficient gene editing in human hematopoietic stem and progenitor cells. _Nat. Med._ 24, 1216–1224 (2018). Article Google Scholar *
Tsai, S. Q. et al. GUIDE-seq enables genome-wide profiling of off-target cleavage by CRISPR-Cas nucleases. _Nat. Biotechnol._ 33, 187–197 (2015). Article Google Scholar * Tsai, S. Q. et
al. CIRCLE-seq: a highly sensitive in vitro screen for genome-wide CRISPR–Cas9 nuclease off-targets. _Nat. Methods_ 14, 607–614 (2017). Article Google Scholar * Kosicki, M., Tomberg, K.
& Bradley, A. Repair of double-strand breaks induced by CRISPR–Cas9 leads to large deletions and complex rearrangements. _Nat. Biotechnol._ 36, 765–771 (2018). Article Google Scholar *
Leibowitz, M. L. et al. Chromothripsis as an on-target consequence of CRISPR–Cas9 genome editing. _Nat. Genet._ 53, 895–905 (2021). Article Google Scholar * Branzei, D. & Foiani, M.
Regulation of DNA repair throughout the cell cycle. _Nat. Rev. Mol. Cell Biol._ 9, 297–308 (2008). Article Google Scholar * Maruyama, T. et al. Increasing the efficiency of precise genome
editing with CRISPR-Cas9 by inhibition of nonhomologous end joining. _Nat. Biotechnol._ 33, 538–542 (2015). Article Google Scholar * Lin, S., Staahl, B. T., Alla, R. K. & Doudna, J. A.
Enhanced homology-directed human genome engineering by controlled timing of CRISPR/Cas9 delivery. _eLife_ 3, e04766 (2014). Article Google Scholar * Rees, H. A. & Liu, D. R. Base
editing: precision chemistry on the genome and transcriptome of living cells. _Nat. Rev. Genet._ 19, 770–788 (2018). Article Google Scholar * Qi, L. S. et al. Repurposing CRISPR as an
RNA-guided platform for sequence-specific control of gene expression. _Cell_ 152, 1173–1183 (2013). Article Google Scholar * Chavez, A. et al. Highly-efficient Cas9-mediated
transcriptional programming. _Nat. Methods_ 12, 326–328 (2015). Article Google Scholar * Vojta, A. et al. Repurposing the CRISPR-Cas9 system for targeted DNA methylation. _Nucleic Acids
Res._ 44, 5615–5628 (2016). Article Google Scholar * Komor, A. C., Kim, Y. B., Packer, M. S., Zuris, J. A. & Liu, D. R. Programmable editing of a target base in genomic DNA without
double-stranded DNA cleavage. _Nature_ 533, 420–424 (2016). THIS ARTICLE REPORTS THE FIRST BASE-EDITING SYSTEM BY FUSING CYTIDINE DEAMINASE APOBEC TO NICKASE- AND NUCLEASE-INACTIVE CAS9
ALLOWING FOR C-TO-T BASE EDITING. Article Google Scholar * Cascalho, M. Advantages and disadvantages of cytidine deamination. _J. Immunol._ 172, 6513–6518 (2004). Article Google Scholar
* Komor, A. C. et al. Improved base excision repair inhibition and bacteriophage Mu Gam protein yields C:G-to-T:A base editors with higher efficiency and product purity. _Sci. Adv._ 3,
eaao4774 (2017). Article Google Scholar * Zafra, M. P. et al. Optimized base editors enable efficient editing in cells, organoids and mice. _Nat. Biotechnol._ 36, 888–896 (2018). Article
Google Scholar * Koblan, L. W. et al. Improving cytidine and adenine base editors by expression optimization and ancestral reconstruction. _Nat. Biotechnol._ 36, 843–848 (2018). Article
Google Scholar * Levy, J. M. et al. Cytosine and adenine base editing of the brain, liver, retina, heart and skeletal muscle of mice via adeno-associated viruses. _Nat. Biomed. Eng._ 4,
97–110 (2020). Article Google Scholar * Nishida, K. et al. Targeted nucleotide editing using hybrid prokaryotic and vertebrate adaptive immune systems. _Science_ 353, aaf8729 (2016).
Article Google Scholar * Gaudelli, N. M. et al. Programmable base editing of A•T to G•C in genomic DNA without DNA cleavage. _Nature_ 551, 464–471 (2017). THIS ARTICLE DESCRIBES THE FIRST
ADENINE BASE EDITOR THAT ALLOWS FOR A-TO-G BASE EDITING WITHOUT THE NEED FOR DSBS. Article Google Scholar * Esvelt, K. M., Carlson, J. C. & Liu, D. R. A system for the continuous
directed evolution of biomolecules. _Nature_ 472, 499–503 (2011). Article Google Scholar * Gaudelli, N. M. et al. Directed evolution of adenine base editors with increased activity and
therapeutic application. _Nat. Biotechnol._ 38, 892–900 (2020). Article Google Scholar * Richter, M. F. et al. Phage-assisted evolution of an adenine base editor with improved Cas domain
compatibility and activity. _Nat. Biotechnol._ 38, 883–891 (2020). Article Google Scholar * Hu, J. H. et al. Evolved Cas9 variants with broad PAM compatibility and high DNA specificity.
_Nature_ 556, 57–63 (2018). Article Google Scholar * Nishimasu, H. et al. Engineered CRISPR-Cas9 nuclease with expanded targeting space. _Science._ 9, 1259–1262 (2018). Article Google
Scholar * Walton, R. T., Christie, K. A., Whittaker, M. N. & Kleinstiver, B. P. Unconstrained genome targeting with near-PAMless engineered CRISPR–Cas9 variants. _Science._ 368, 290–296
(2020). Article Google Scholar * Yu, S.-Y. et al. Increasing the targeting scope of CRISPR base editing system beyond NGG. _CRISPR J._ 5, 187–202 (2022). Article Google Scholar *
Pavlov, Y. I. et al. Cytosine, but not adenine, base editors induce genome-wide off-target mutations in rice. _Science_ 8, 647–656 (2019). Google Scholar * Zuo, E. et al. Cytosine base
editor generates substantial off-target single-nucleotide variants in mouse embryos. _Science_ 292, eaav9973 (2019). Google Scholar * Yu, Y. et al. Cytosine base editors with minimized
unguided DNA and RNA off-target events and high on-target activity. _Nat. Commun._ 11, 2052 (2020). Article Google Scholar * Kurt, I. C. et al. CRISPR C-to-G base editors for inducing
targeted DNA transversions in human cells. _Nat. Biotechnol._ 39, 41–46 (2021). Article Google Scholar * Koblan, L. W. et al. Efficient C•G-to-G•C base editors developed using CRISPRi
screens, target-library analysis, and machine learning. _Nat. Biotechnol._ 39, 1414–1425 (2021). Article Google Scholar * Anzalone, A. V. et al. Search-and-replace genome editing without
double-strand breaks or donor DNA. _Nature_ 576, 149–157 (2019). THIS ARTICLE PRESENTS PRIME EDITING AS A TOOL THAT CAN POTENTIALLY REPAIR 89% OF ALL DISEASE-CAUSING MUTATIONS OBSERVED IN
HUMANS WITHOUT THE NEED FOR DSBS. Article Google Scholar * Alexandrov, L. B. et al. Signatures of mutational processes in human cancer. _Nature_ 500, 415–421 (2013). Article Google
Scholar * Anzalone, A. V. et al. Programmable deletion, replacement, integration and inversion of large DNA sequences with twin prime editing. _Nat. Biotechnol._ 40, 731–740 (2021). Article
Google Scholar * Lin, Q. et al. High-efficiency prime editing with optimized, paired pegRNAs in plants. _Nat. Biotechnol._ 39, 923–927 (2021). Article Google Scholar * Choi, J. et al.
Precise genomic deletions using paired prime editing. _Nat. Biotechnol._ 40, 218–226 (2022). Article Google Scholar * Nelson, J. W. et al. Engineered pegRNAs improve prime editing
efficiency. _Nat. Biotechnol._ https://doi.org/10.1038/s41587-021-01039-7 (2021). Article Google Scholar * Chen, P. J. et al. Enhanced prime editing systems by manipulating cellular
determinants of editing outcomes. _Cell_ 184, 5635–5652.e29 (2021). Article Google Scholar * Fearon, E. F. & Vogelstein, B. A genetic model for colorectal tumorigenesis. _Cell_ 61,
759–767 (1990). Article Google Scholar * Drost, J. et al. Sequential cancer mutations in cultured human intestinal stem cells. _Nature_ 521, 43–47 (2015). Article Google Scholar *
Matano, M. et al. Modeling colorectal cancer using CRISPR-Cas9–mediated engineering of human intestinal organoids. _Nat. Med._ 21, 256–262 (2015). Article Google Scholar * Dekkers, J. F.
et al. Modeling breast cancer using CRISPR-Cas9-mediated engineering of human breast organoids. _J. Natl. Cancer Inst._ 112, 540–544 (2020). Article Google Scholar * Artegiani, B. et al.
Probing the tumor suppressor function of BAP1 in CRISPR-engineered human liver organoids. _Cell Stem Cell_ 24, 927–943.e6 (2019). Article Google Scholar * Seino, T. et al. Human pancreatic
tumor organoids reveal loss of stem cell niche factor dependence during disease progression. _Cell Stem Cell_ 22, 454–467.e6 (2018). Article Google Scholar * Lee, J. et al. Reconstituting
development of pancreatic intraepithelial neoplasia from primary human pancreas duct cells. _Nat. Commun._ 8, 14686 (2017). Article Google Scholar * Drost, J. et al. Use of
CRISPR-modified human stem cell organoids to study the origin of mutational signatures in cancer. _Science_ 358, 234–238 (2017). Article Google Scholar * Jager, M. et al. Deficiency of
nucleotide excision repair is associated with mutational signature observed in cancer. _Genome Res._ 29, 1067–1077 (2019). Article Google Scholar * Kawasaki, K. et al. Chromosome
engineering of human colon-derived organoids to develop a model of traditional serrated adenoma. _Gastroenterology_ 158, 638–651.e8 (2020). Article Google Scholar * Artegiani, B. et al.
Fast and efficient generation of knock-in human organoids using homology-independent CRISPR–Cas9 precision genome editing. _Nat. Cell Biol._ 22, 321–331 (2020). Article Google Scholar *
Lo, Y. H. et al. A CRISPR/Cas9-engineered _ARID1A_-deficient human gastric cancer organoid model reveals essential and nonessential modes of oncogenic transformation. _Cancer Discov._ 11,
1562–1581 (2021). Article Google Scholar * Kawasaki, K. et al. An organoid biobank of neuroendocrine neoplasms enables genotype–phenotype mapping. _Cell_ 183, 1420–1435.e21 (2020). Article
Google Scholar * Yan, H. H. N. et al. Organoid cultures of early-onset colorectal cancers reveal distinct and rare genetic profiles. _Gut_ 69, 2165–2179 (2020). Article Google Scholar *
Post, J. B. et al. CRISPR-induced RASGAP deficiencies in colorectal cancer organoids reveal that only loss of NF1 promotes resistance to EGFR inhibition. _Oncotarget_ 10, 1440–1457 (2019).
Article Google Scholar * Bock, C. et al. High-content CRISPR screening. _Nat. Rev. Methods Prim._ 2, 8 (2022). Article Google Scholar * Wang, T. et al. Identification and
characterization of essential genes in the human genome. _Science_ 350, 1096–1101 (2015). Article Google Scholar * Shalem, O. et al. Genome-scale CRISPR–Cas9 knockout screening in human
cells. _Science_ 343, 84–88 (2014). Article Google Scholar * Michels, B. E. et al. Pooled in vitro and in vivo CRISPR–Cas9 screening identifies tumor suppressors in human colon organoids.
_Cell Stem Cell_ 26, 782–792.e7 (2020). Article Google Scholar * Ringel, T. et al. Genome-scale CRISPR screening in human intestinal organoids identifies drivers of TGF-β resistance. _Cell
Stem Cell_ 26, 431–440.e8 (2020). Article Google Scholar * Boettcher, S. et al. A dominant-negative effect drives selection of TP53 missense mutations in myeloid malignancies. _Science_
365, 599–604 (2019). Article Google Scholar * Stolze, B., Reinhart, S., Bulllinger, L., Fröhling, S. & Scholl, C. Comparative analysis of KRAS codon 12, 13, 18, 61, and 117 mutations
using human MCF10A isogenic cell lines. _Sci. Rep._ 5, 8535 (2014). Article Google Scholar * Geurts, M. H. et al. Evaluating CRISPR-based prime editing for cancer modeling and CFTR repair
in organoids. _Life Sci. Alliance_ 4, 1–12 (2021). Article Google Scholar * Schene, I. F. et al. Prime editing for functional repair in patient-derived disease models. _Nat. Commun._ 11,
5352 (2020). Article Google Scholar * van Rijn, J. M. et al. Intestinal failure and aberrant lipid metabolism in patients with DGAT1 deficiency. _Gastroenterology_ 155, 130–143.e15 (2018).
Article Google Scholar * Nanki, K. et al. Somatic inflammatory gene mutations in human ulcerative colitis epithelium. _Nature_ 577, 254–259 (2020). Article Google Scholar * Lamers, M.
M. et al. SARS-CoV-2 productively infects human gut enterocytes. _Science_ 369, 50–54 (2020). Article Google Scholar * Zhou, J. et al. Infection of bat and human intestinal organoids by
SARS-CoV-2. _Nat. Med._ 26, 1077–1083 (2020). Article Google Scholar * Geurts, M. H., van der Vaart, J., Beumer, J. & Clevers, H. The organoid platform: promises and challenges as
tools in the fight against COVID-19. _Stem Cell Rep._ 16, 412–418 (2021). Article Google Scholar * Beumer, J. et al. A CRISPR/Cas9 genetically engineered organoid biobank reveals essential
host factors for coronaviruses. _Nat. Commun._ 12, 5498 (2021). Article Google Scholar * Veres, A. et al. Low incidence of off-target mutations in individual CRISPR-Cas9 and TALEN
targeted human stem cell clones detected by whole-genome sequencing. _Cell Stem Cell_ 15, 27–30 (2014). Article Google Scholar * Wu, X. et al. Genome-wide binding of the CRISPR
endonuclease Cas9 in mammalian cells. _Nat. Biotechnol._ 32, 670–676 (2014). Article Google Scholar * Lombaert, I. M. A. et al. Rescue of salivary gland function after stem cell
transplantation in irradiated glands. _PLoS One_ 3, e2063 (2008). Article Google Scholar * Schwank, G. et al. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of
cystic fibrosis patients. _Cell Stem Cell_ 13, 653–658 (2013). THIS ARTICLE REPORTS THE FIRST PROOF OF THE POTENTIAL CLINICAL APPLICATION OF CRISPR BY REPAIRING THE MOST COMMON MUTATION THAT
CAUSES CYSTIC FIBROSIS IN PATIENT-DERIVED INTESTINAL ORGANOIDS. Article Google Scholar * Sosnay, P. R. et al. Defining the disease liability of variants in the cystic fibrosis
transmembrane conductance regulator gene. _Nat. Genet._ 45, 1160–1167 (2013). Article Google Scholar * Dekkers, J. F. et al. A functional CFTR assay using primary cystic fibrosis
intestinal organoids. _Nat. Med._ 19, 939–945 (2013). Article Google Scholar * Dekkers, J. F. et al. Characterizing responses to CFTR-modulating drugs using rectal organoids derived from
subjects with cystic fibrosis. _Sci. Transl. Med._ 8, 344ra84–344ra84 (2016). Article Google Scholar * Berkers, G. et al. Rectal organoids enable personalized treatment of cystic fibrosis.
_Cell Rep._ 26, 1701–1708.e3 (2019). Article Google Scholar * Zetsche, B. et al. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. _Cell_ 163, 759–771 (2015).
Article Google Scholar * Maule, G. et al. Allele specific repair of splicing mutations in cystic fibrosis through AsCas12a genome editing. _Nat. Commun._ 10, 3556 (2019). Article Google
Scholar * Geurts, M. H. et al. CRISPR-based adenine editors correct nonsense mutations in a cystic fibrosis organoid biobank. _Cell Stem Cell_ 26, 503–510.e7 (2020). THIS ARTICLE REPORTS
THE FIRST PROOF OF DSB-FREE GENE REPAIR IN ADULT-STEM-CELL-DERIVED ORGANOIDS BY REPAIRING MUTATIONS THAT CAUSE CYSTIC FIBROSIS IN PATIENT-DERIVED ORGANOIDS WITHOUT GENOME-WIDE OFF-TARGET
EFFECTS. Article Google Scholar * Schene, I. F. et al. Mutation-specific reporter for optimization and enrichment of prime editing. _Nat. Commun._ 13, 1028 (2022). Article Google Scholar
* van der Vaart, J. et al. Modelling of primary ciliary dyskinesia using patient‐derived airway organoids. _EMBO Rep._ 22, e52058 (2021). Google Scholar * Kuscu, C. et al. CRISPR-STOP:
gene silencing through base-editing-induced nonsense mutations. _Nat. Methods_ 14, 710–712 (2017). Article Google Scholar * Wang, X. et al. Efficient gene silencing by adenine base
editor-mediated start codon mutation. _Mol. Ther._ 28, 431–440 (2020). Article Google Scholar * Kluesner, M. G. et al. CRISPR–Cas9 cytidine and adenosine base editing of splice-sites
mediates highly-efficient disruption of proteins in primary and immortalized cells. _Nat. Commun._ 12, 2437 (2021). Article Google Scholar * Conant, D. et al. Inference of CRISPR edits
from sanger trace data. _CRISPR J._ 5, 123–130 (2022). Article Google Scholar * Arbab, M. et al. Determinants of base editing outcomes from target library analysis and machine learning.
_Cell_ 182, 463–480.e30 (2020). Article Google Scholar * Andersson-Rolf, A. et al. One-step generation of conditional and reversible gene knockouts. _Nat. Methods_ 14, 287–289 (2017).
Article Google Scholar * Sun, D. et al. A functional genetic toolbox for human tissue-derived organoids. _eLife_ 10, e67886 (2021). Article Google Scholar * Yarnall, M. T. N. et al.
Drag-and-drop genome insertion of large sequences without DNA cleavage using CRISPR-directed integrases. _Nat. Biotechnol._ https://doi.org/10.1038/s41587-022-01527-4 (2022). * Price, S. et
al. A suspension technique for efficient large-scale cancer organoid culturing and perturbation screens. _Sci. Rep._ 12, 5571 (2022). Article Google Scholar * Hanna, R. E. et al. Massively
parallel assessment of human variants with base editor screens. _Cell_ 184, 1064–1080.e20 (2021). Article Google Scholar * Drost, J. & Clevers, H. Translational applications of adult
stem cell-derived organoids. _Development_ 144, 968–975 (2017). Article Google Scholar * Yui, S. et al. Functional engraftment of colon epithelium expanded in vitro from a single adult
Lgr5+ stem cell. _Nat. Med._ 18, 618–623 (2012). Article Google Scholar * Pringle, S. et al. Human salivary gland stem cells functionally restore radiation damaged salivary glands. _Stem
Cell_ 34, 640–652 (2016). Article Google Scholar * Sampaziotis, F. et al. Cholangiocyte organoids can repair bile ducts after transplantation in the human liver. _Science_ 371, 839–846
(2021). Article Google Scholar * Gillmore, J. D. et al. CRISPR–Cas9 in vivo gene editing for transthyretin amyloidosis. _N. Engl. J. Med._ 385, 493–502 (2021). THIS ARTICLE DESCRIBES A
LANDMARK CLINICAL TRIAL IN WHICH PATIENTS ARE INJECTED WITH NUCLEASE-ACTIVE CAS9 AND A SGRNA TARGETING THE TRANSTHYRETIN GENE THAT CAUSES AMYLOID PLAQUES IN THE LIVER. Article Google
Scholar * Doman, J. L., Raguram, A., Newby, G. A. & Liu, D. R. Evaluation and minimization of Cas9-independent off-target DNA editing by cytosine base editors. _Nat. Biotechnol._ 38,
620–628 (2020). Article Google Scholar * Aida, T. et al. Prime editing primarily induces undesired outcomes in mice. Preprint at _bioRxiv_
https://www.biorxiv.org/content/10.1101/2020.08.06.239723v1 (2020). * Shen, B. et al. Efficient genome modification by CRISPR-Cas9 nickase with minimal off-target effects. _Nat. Methods_ 11,
399–402 (2014). Article Google Scholar * Muller, H. J. Artificial transmutation of the gene. _Science_ 66, 84–87 (1927). Article Google Scholar * Brenner, S. The genetics of
_Ceanorhabditis elegans_. _Genetics_ 77, 71–94 (1974). Article Google Scholar * Nüsslein-volhard, C. & Wieschaus, E. Mutations affecting segment number and polarity in _Drosophila_.
_Nature_ 287, 795–801 (1980). Article Google Scholar * Doudna, J. A. & Charpentier, E. The new frontier of genome engineering with CRISPR-Cas9. _Science_ 346, 1258096–1258096 (2014).
Article Google Scholar * Scherer, S. & Davis, R. W. Replacement of chromosome segments with altered DNA sequences constructed in vitro. _Proc. Natl Acad. Sci. USA_ 76, 4951–4955
(1979). Article Google Scholar * Smithies, O., Gregg, R. G., Boggst, S. S., Koralewski, M. A. & Kucherlapati, R. S. Insertion of DNA sequences into the human chromosomal β-globin locus
by homologous recombination. _Nature_ 317, 230–236 (1985). Article Google Scholar * Rudin, N., Sugarman, E. & Haber, J. E. Genetic and physical analysis of double-strand break repair
and recombination in _Saccharomyces cerevisiae_. _Genetics_ 122, 519–534 (1989). Article Google Scholar * Rouet, P., Smih, F. & Jasin, M. Introduction of double-strand breaks into the
genome of mouse cells by expression of a rare-cutting endonuclease. _Mol. Cell. Biol._ 14, 8096–8106 (1994). Google Scholar * Epinat, J. C. et al. A novel engineered meganuclease induces
homologous recombination in yeast and mammalian cells. _Nucleic Acids Res._ 31, 2952–2962 (2003). Article Google Scholar * Wood, A. J. et al. Targeted genome editing across species using
ZFNs and TALENs. _Science_ 333, 307 (2011). Article Google Scholar * Hu, J. H., Davis, K. M. & Liu, D. R. Chemical biology approaches to genome editing: understanding, controlling, and
delivering programmable nucleases. _Cell Chem. Biol._ 23, 57–73 (2016). Article Google Scholar * Barrangou, R. et al. CRISPR provides acquired resistance against viruses in prokaryotes.
_Science_ 315, 1709–1712 (2007). Article Google Scholar * Brouns, S. J. J. et al. Small CRISPR RNAs guide antiviral defense in prokaryotes. _Science_ 321, 960–965 (2008). Article Google
Scholar * Akcakaya, P. et al. In vivo CRISPR editing with no detectable genome-wide off-target mutations. _Nature_ 561, 416–419 (2018). Article Google Scholar * Shirley, J. L., de Jong,
Y. P., Terhorst, C. & Herzog, R. W. Immune responses to viral gene therapy vectors. _Mol. Ther._ 28, 709–722 (2020). Article Google Scholar * Wu, Z., Asokan, A. & Samulski, R. J.
Adeno-associated virus serotypes: vector toolkit for human gene therapy. _Mol. Ther._ 14, 316–327 (2006). Article Google Scholar * Nieuwenhuis, B. et al. Optimization of adeno-associated
viral vector-mediated transduction of the corticospinal tract: comparison of four promoters. _Gene Ther._ 28, 56–74 (2021). Article Google Scholar * Burger, C. et al. Recombinant AAV viral
vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. _Mol.
Ther._ 10, 302–317 (2004). Article Google Scholar * Naso, M. F., Tomkowicz, B., Perry, W. L. & Strohl, W. R. Adeno-associated virus (AAV) as a vector for gene therapy. _BioDrugs_ 31,
317–334 (2017). Article Google Scholar * Liu, P. et al. Improved prime editors enable pathogenic allele correction and cancer modelling in adult mice. _Nat. Commun._ 12, 2121 (2021).
Article Google Scholar * Böck, D. et al. In vivo prime editing of a metabolic liver disease in mice. _Sci. Transl. Med._ 14, eabl9238 (2022). Article Google Scholar * Segel M. et al.
Mammalian retrovirus-like protein PEG10 packages its own mRNA and can be pseudotyped for mRNA delivery. _Science_ 185, 882–889 (2021). Article Google Scholar * June, C. H., O’Connor, R.
S., Kawalekar, O. U., Ghassemi, S. & Milone, M. C. CAR T cell immunotherapy for human cancer. _Science._ 359, 1361–1365 (2018). Article Google Scholar * Frangoul, H. et al. CRISPR–Cas9
gene editing for sickle cell disease and β-thalassemia. _N. Engl. J. Med._ 384, 252–260 (2021). Article Google Scholar * Watanabe, S. et al. Transplantation of intestinal organoids into a
mouse model of colitis. _Nat. Protoc._ 17, 649–671 (2022). Article Google Scholar * Sugimoto, S. et al. An organoid-based organ-repurposing approach to treat short bowel syndrome.
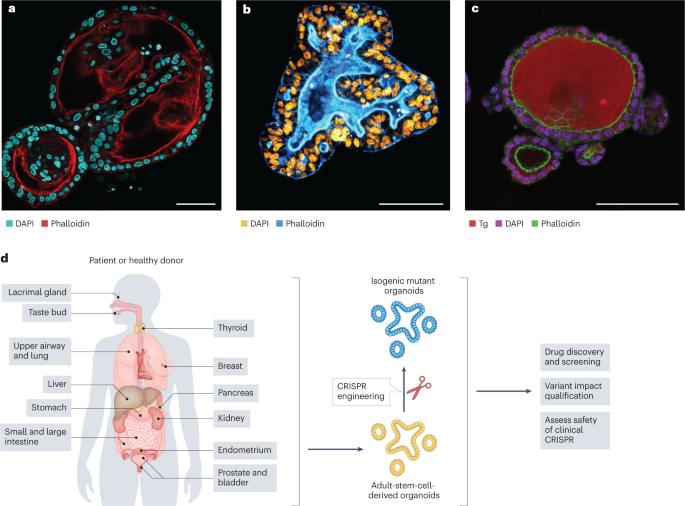
_Nature_ 592, 99–104 (2021). Google Scholar Download references ACKNOWLEDGEMENTS The authors thank J. Beumer for providing confocal images of human intestinal organoids, S. Gandhi for
providing confocal images of human fetal hepatocyte organoids and J. van der Vaart for providing confocal images of murine thyroid organoids. AUTHOR INFORMATION Author notes * Maarten H.
Geurts Present address: Xilis BV, Utrecht, The Netherlands * Hans Clevers Present address: Pharma Research Early Development, Roche, Basel, Switzerland AUTHORS AND AFFILIATIONS * Hubrecht
Institute, Royal Netherlands Academy of Arts and Sciences (KNAW) and University Medical Center Utrecht, Utrecht, The Netherlands Maarten H. Geurts & Hans Clevers * Oncode Institute,
Hubrecht Institute, Utrecht, The Netherlands Maarten H. Geurts & Hans Clevers Authors * Maarten H. Geurts View author publications You can also search for this author inPubMed Google
Scholar * Hans Clevers View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Hans Clevers. ETHICS DECLARATIONS COMPETING
INTERESTS H.C. is inventor on several patents related to organoid technology; his full disclosure is given at https://www.uu.nl/staff/JCClevers/. H.C. is currently head of pharma Research
Early Development (pRED) at Roche. H.C. holds several patents on organoid technology. Their application numbers, followed by their publication numbers (if applicable), are as follows:
PCT/NL2008/050543, WO2009/022907; PCT/NL2010/000017, WO2010/090513; PCT/IB2011/002167, WO2012/014076; PCT/IB2012/052950, WO2012/168930; PCT/EP2015/060815, WO2015/173425; PCT/EP2015/077990,
WO2016/083613; PCT/EP2015/077988, WO2016/083612; PCT/EP2017/054797, WO2017/149025; PCT/EP2017/065101, WO2017/220586; PCT/EP2018/086716, n/a; and GB1819224.5, n/a. M.H.G. is currently a
scientist at Xilis BV. PEER REVIEW PEER REVIEW INFORMATION _Nature Reviews Bioengineering_ thanks Nicholas Zachos and the other, anonymous, reviewer(s) for their contribution to the peer
review of this work. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND
PERMISSIONS Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other
rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law. Reprints and
permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Geurts, M.H., Clevers, H. CRISPR engineering in organoids for gene repair and disease modelling. _Nat Rev Bioeng_ 1, 32–45 (2023).
https://doi.org/10.1038/s44222-022-00013-5 Download citation * Accepted: 17 November 2022 * Published: 19 January 2023 * Issue Date: January 2023 * DOI:
https://doi.org/10.1038/s44222-022-00013-5 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative