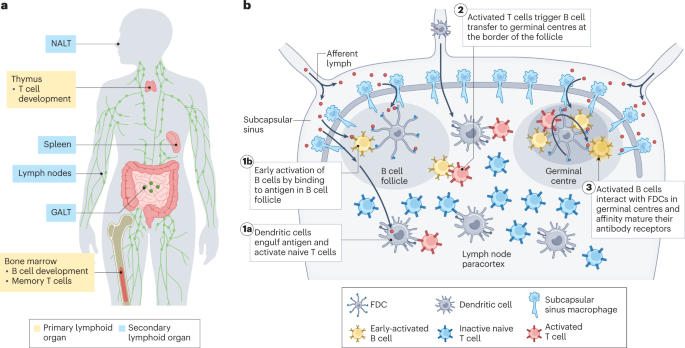
Play all audios:
ABSTRACT Therapies modulating the immune system offer the prospect of treating a wide range of conditions including infectious diseases, cancer and autoimmunity. Biomaterials can promote
specific targeting of immune cell subsets in peripheral or lymphoid tissues and modulate the dosage, timing and location of stimulation, thereby improving the safety and efficacy of vaccines
and immunotherapies. Here, we review recent advances in biomaterials-based strategies, focusing on targeting of lymphoid tissues, circulating leukocytes, tissue-resident immune cells and
immune cells at disease sites. These approaches can improve the potency and efficacy of immunotherapies by promoting immunity or tolerance against different diseases. KEY POINTS * In
immunotherapy, choosing the right target cell, tissue and treatment duration is essential to ensure effective immunomodulation while avoiding toxicity. * Biomaterial-mediated targeting of
immune cells in lymph nodes improves the potency and efficacy of vaccines by promoting immunity or tolerance. * Circulating migratory immune cells can be targeted to perform as living
chaperones to carry therapeutics into tissues. * Systemic administration or intratumoral injection of nanomaterials and therapeutic depots can selectively accumulate and target immune cells
in tumours. * Reducing biomaterial complexity is essential to facilitate clinical translation. Access through your institution Buy or subscribe This is a preview of subscription content,
access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 digital issues and online access to articles $119.00 per year only $9.92 per
issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL
ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS DESIGN OF THERAPEUTIC BIOMATERIALS TO
CONTROL INFLAMMATION Article 28 February 2022 BIOMATERIALS TO ENHANCE ADOPTIVE CELL THERAPY Article 26 January 2024 RESPONSIVE BIOMATERIALS: OPTIMIZING CONTROL OF CANCER IMMUNOTHERAPY
Article 22 December 2023 REFERENCES * Urquhart, L. Top companies and drugs by sales in 2021. _Nat. Rev. Drug Discov._ 21, 251 (2022). Article Google Scholar * Dougan, M., Luoma, A. M.,
Dougan, S. K. & Wucherpfennig, K. W. Understanding and treating the inflammatory adverse events of cancer immunotherapy. _Cell_ 184, 1575–1588 (2021). Article Google Scholar * Seung,
E. et al. A trispecific antibody targeting HER2 and T cells inhibits breast cancer growth via CD4 cells. _Nature_ 603, 328–334 (2022). Article Google Scholar * Muik, A. et al. Preclinical
characterization and phase I trial results of a bispecific antibody targeting PD-L1 and 4-1BB (GEN1046) in patients with advanced refractory solid tumors. _Cancer Discov._ 12, 1248–1265
(2022). Article Google Scholar * Neri, D. Antibody–cytokine fusions: versatile products for the modulation of anticancer immunity. _Cancer Immunol. Res._ 7, 348–354 (2019). Article Google
Scholar * Jones, D. S. II et al. Cell surface-tethered IL-12 repolarizes the tumor immune microenvironment to enhance the efficacy of adoptive T cell therapy. _Sci. Adv_. 8, eabi8075
(2022). * Ren, Z. et al. Selective delivery of low-affinity IL-2 to PD-1+ T cells rejuvenates antitumor immunity with reduced toxicity. _J. Clin. Invest._ 132, e153604 (2022). Article
Google Scholar * Tzeng, A., Kwan, B. H., Opel, C. F., Navaratna, T. & Wittrup, K. D. Antigen specificity can be irrelevant to immunocytokine efficacy and biodistribution. _Proc. Natl
Acad. Sci. USA_ 112, 3320–3325 (2015). Article Google Scholar * Reddy, S. T. et al. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. _Nat. Biotechnol._
25, 1159–1164 (2007). Article Google Scholar * McLennan, D. N., Porter, C. J. H. & Charman, S. A. Subcutaneous drug delivery and the role of the lymphatics. _Drug. Discov. Today
Technol._ 2, 89–96 (2005). Article Google Scholar * Schudel, A., Francis, D. M. & Thomas, S. N. Material design for lymph node drug delivery. _Nat. Rev. Mater._ 4, 415–428 (2019).
Article Google Scholar * Mehta, N. K. et al. Pharmacokinetic tuning of protein–antigen fusions enhances the immunogenicity of T-cell vaccines. _Nat. Biomed. Eng._ 4, 636–648 (2020).
Article Google Scholar * Kourtis, I. C. et al. Peripherally administered nanoparticles target monocytic myeloid cells, secondary lymphoid organs and tumors in mice. _PLoS ONE_ 8, e61646
(2013). Article Google Scholar * Silva, M. et al. A particulate saponin/TLR agonist vaccine adjuvant alters lymph flow and modulates adaptive immunity. _Sci. Immunol._ 6, eabf1152 (2021).
Article Google Scholar * Moon, J. J. et al. Enhancing humoral responses to a malaria antigen with nanoparticle vaccines that expand Tfh cells and promote germinal center induction. _Proc.
Natl Acad. Sci. USA_ 109, 1080–1085 (2012). Article Google Scholar * Boyoglu-Barnum, S. et al. Quadrivalent influenza nanoparticle vaccines induce broad protection. _Nature_ 592, 623–628
(2021). Article Google Scholar * Jardine, J. G. et al. HIV-1 broadly neutralizing antibody precursor B cells revealed by germline-targeting immunogen. _Science_ 351, 1458–1463 (2016).
Article Google Scholar * Walls, A. C. et al. Elicitation of potent neutralizing antibody responses by designed protein nanoparticle vaccines for SARS-CoV-2. _Cell_ 183, 1367–1382 (2020).
Article Google Scholar * Marcandalli, J. et al. Induction of potent neutralizing antibody responses by a designed protein nanoparticle vaccine for respiratory syncytial virus. _Cell_ 176,
1420–1431 (2019). Article Google Scholar * Walls, A. C. et al. Elicitation of broadly protective sarbecovirus immunity by receptor-binding domain nanoparticle vaccines. _Cell_ 184,
5432–5447.e16 (2021). Article Google Scholar * Houser, K. V. et al. Safety and immunogenicity of a ferritin nanoparticle H2 influenza vaccine in healthy adults: a phase 1 trial. _Nat.
Med._ 28, 383–391 (2022). Article Google Scholar * Song, J. Y. et al. Safety and immunogenicity of a SARS-CoV-2 recombinant protein nanoparticle vaccine (GBP510) adjuvanted with AS03: a
randomized, placebo-controlled, observer-blinded phase 1/2 trial. _EClinicalMedicine_ 51, 101569 (2022). Article Google Scholar * Kuai, R., Ochyl, L. J., Bahjat, K. S., Schwendeman, A.
& Moon, J. J. Designer vaccine nanodiscs for personalized cancer immunotherapy. _Nat. Mater._ 16, 489–496 (2016). Article Google Scholar * Karabin, N. B. et al. Sustained micellar
delivery via inducible transitions in nanostructure morphology. _Nat. Commun._ 9, 624 (2018). Article Google Scholar * Heath, P. T. et al. Safety and efficacy of NVX-CoV2373 Covid-19
vaccine. _N. Engl. J. Med._ 385, 1172–1183 (2021). Article Google Scholar * Liu, H. et al. Structure-based programming of lymph-node targeting in molecular vaccines. _Nature_ 507, 519–522
(2014). Article Google Scholar * Moynihan, K. D. et al. Enhancement of peptide vaccine immunogenicity by increasing lymphatic drainage and boosting serum stability. _Cancer Immunol. Res._
6, 1025–1038 (2018). Article Google Scholar * Rakhra, K. et al. Exploiting albumin as a mucosal vaccine chaperone for robust generation of lung-resident memory T cells. _Sci. Immunol._ 6,
eabd8003 (2021). Article Google Scholar * Vrieze, J. D. et al. Potent lymphatic translocation and spatial control over innate immune activation by polymer-lipid amphiphile conjugates of
small-molecule TLR7/8 agonists. _Angew. Chem. Int. Edn_ 58, 15390–15395 (2019). Article Google Scholar * Pant, S. et al. First-in-human phase 1 trial of ELI-002 immunotherapy as treatment
for subjects with Kirsten rat sarcoma (KRAS)-mutated pancreatic ductal adenocarcinoma and other solid tumors. _J. Clin. Oncol._ 40, TPS2701–TPS2701 (2022). Article Google Scholar * Cao, S.
et al. Hybrid nanocarriers incorporating mechanistically distinct drugs for lymphatic CD4+ T cell activation and HIV-1 latency reversal. _Sci. Adv._ 5, eaav6322 (2019). Article Google
Scholar * Capini, C. et al. Antigen-specific suppression of inflammatory arthritis using liposomes. _J. Immunol._ 182, 3556–3565 (2009). Article Google Scholar * Zhou, K. et al. Targeting
peripheral immune organs with self‐assembling prodrug nanoparticles ameliorates allogeneic heart transplant rejection. _Am. J. Transpl._ 21, 3871–3882 (2021). Article Google Scholar *
Kishimoto, T. K. & Maldonado, R. A. Nanoparticles for the induction of antigen-specific immunological tolerance. _Front. Immunol._ 9, 230 (2018). Article Google Scholar * Maldonado, R.
A. et al. Polymeric synthetic nanoparticles for the induction of antigen-specific immunological tolerance. _Proc. Natl Acad. Sci. USA_ 112, E156–E165 (2015). Article Google Scholar * Ali,
O. A., Huebsch, N., Cao, L., Dranoff, G. & Mooney, D. J. Infection-mimicking materials to program dendritic cells in situ. _Nat. Mater._ 8, 151–158 (2009). Article Google Scholar *
Ali, O. A., Emerich, D., Dranoff, G. & Mooney, D. J. In situ regulation of DC subsets and T cells mediates tumor regression in mice. _Sci. Transl Med._ 1, 8ra19 (2009). Article Google
Scholar * Shah, N. J. et al. A biomaterial-based vaccine eliciting durable tumour-specific responses against acute myeloid leukaemia. _Nat. Biomed. Eng._ 4, 40–51 (2020). Article Google
Scholar * Kim, J. et al. Injectable, spontaneously assembling inorganic scaffolds modulate immune cells in vivo and increase vaccine efficacy. _Nat. Biotechnol._ 33, 64–72 (2014). Article
Google Scholar * Super, M. et al. Biomaterial vaccines capturing pathogen-associated molecular patterns protect against bacterial infections and septic shock. _Nat. Biomed. Eng._ 6, 8–18
(2022). Article Google Scholar * Lee, J. A. et al. Recruitment of dendritic cells using ‘find-me’ signaling microparticles for personalized cancer immunotherapy. _Biomaterials_ 282, 121412
(2022). Article Google Scholar * Lewis, J. S. et al. A combination dual-sized microparticle system modulates dendritic cells and prevents type 1 diabetes in prediabetic NOD mice. _Clin.
Immunol._ 160, 90–102 (2015). Article Google Scholar * Allen, R., Chizari, S., Ma, J. A., Raychaudhuri, S. & Lewis, J. S. Combinatorial, microparticle-based delivery of immune
modulators reprograms the dendritic cell phenotype and promotes remission of collagen-induced arthritis in mice. _ACS Appl. Bio Mater._ 2, 2388–2404 (2019). Article Google Scholar * Cho,
J. J. et al. An antigen-specific semi-therapeutic treatment with local delivery of tolerogenic factors through a dual-sized microparticle system blocks experimental autoimmune
encephalomyelitis. _Biomaterials_ 143, 79–92 (2017). Article Google Scholar * Gerner, M. Y., Casey, K. A., Kastenmuller, W. & Germain, R. N. Dendritic cell and antigen dispersal
landscapes regulate T cell immunity. _J. Exp. Med._ 214, 3105–3122 (2017). Article Google Scholar * Eickhoff, S. et al. Robust anti-viral immunity requires multiple distinct T
cell-dendritic cell interactions. _Cell_ 162, 1322–1337 (2015). Article Google Scholar * Junt, T. et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present
them to antiviral B cells. _Nature_ 450, 110–114 (2007). Article Google Scholar * Gretz, J. E., Norbury, C. C., Anderson, A. O., Proudfoot, A. E. I. & Shaw, S. Lymph-borne chemokines
and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node
cortex. _J. Exp. Med._ 192, 1425–1440 (2000). Article Google Scholar * Sixt, M. et al. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in
the T cell area of the lymph node. _Immunity_ 22, 19–29 (2005). Article Google Scholar * Roozendaal, R. et al. Conduits mediate transport of low-molecular-weight antigen to lymph node
follicles. _Immunity_ 30, 264–276 (2009). Article Google Scholar * Reynoso, G. V. et al. Lymph node conduits transport virions for rapid T cell activation. _Nat. Immunol._ 20, 602–612
(2019). Article Google Scholar * Kim, E. H. et al. Squalene emulsion-based vaccine adjuvants stimulate CD8 T cell, but not antibody responses, through a RIPK3-dependent pathway. _eLife_ 9,
e52687 (2020). Article Google Scholar * Detienne, S. et al. Central role of CD169+ lymph node resident macrophages in the adjuvanticity of the QS-21 component of AS01. _Sci. Rep._ 6,
39475 (2016). Article Google Scholar * Zhang, Y.-N., Poon, W., Sefton, E. & Chan, W. C. W. Suppressing subcapsular sinus macrophages enhances transport of nanovaccines to lymph node
follicles for robust humoral immunity. _ACS Nano_ 14, 9478–9490 (2020). Article Google Scholar * Schudel, A. et al. Programmable multistage drug delivery to lymph nodes. _Nat.
Nanotechnol._ 15, 491–499 (2020). Article Google Scholar * Hafiz, M. M. et al. Immunosuppression and procedure-related complications in 26 patients with type 1 diabetes mellitus receiving
allogeneic islet cell transplantation. _Transplantation_ 80, 1718–1728 (2005). Article Google Scholar * Burke, J. A. et al. Subcutaneous nanotherapy repurposes the immunosuppressive
mechanism of rapamycin to enhance allogeneic islet graft viability. _Nat. Nanotechnol._ 17, 319–330 (2022). Article Google Scholar * Kenison, J. E. et al. Tolerogenic nanoparticles
suppress central nervous system inflammation. _Proc. Natl Acad. Sci. USA_ 117, 32017–32028 (2020). Article Google Scholar * Lynn, G. M. et al. Peptide–TLR-7/8a conjugate vaccines
chemically programmed for nanoparticle self-assembly enhance CD8 T-cell immunity to tumor antigens. _Nat. Biotechnol._ 38, 320–332 (2020). Article Google Scholar * Wilson, D. S. et al.
Antigens reversibly conjugated to a polymeric glyco-adjuvant induce protective humoral and cellular immunity. _Nat. Mater._ 18, 175–185 (2019). Article Google Scholar * Shae, D. et al.
Co-delivery of peptide neoantigens and stimulator of interferon genes (STING) agonists enhances response to cancer vaccines. _ACS Nano_ 14, 9904–9916 (2020). Article Google Scholar * Wang,
S. et al. Rational vaccinology with spherical nucleic acids. _Proc. Natl Acad. Sci. USA._ 116, 10473–10481 (2019). Article Google Scholar * Callmann, C. E. et al. Tumor cell lysate-loaded
immunostimulatory spherical nucleic acids as therapeutics for triple-negative breast cancer. _Proc. Natl Acad. Sci. USA_ 117, 17543–17550 (2020). Article Google Scholar * Froimchuk, E.,
Oakes, R. S., Kapnick, S. M., Yanes, A. A. & Jewell, C. M. Biophysical properties of self-assembled immune signals impact signal processing and the nature of regulatory immune function.
_Nano Lett._ 21, 3762–3771 (2021). Article Google Scholar * Hess, K. L. et al. Engineering immunological tolerance using quantum dots to tune the density of self-antigen display. _Adv.
Funct. Mater._ 27, 1700290 (2017). Article MathSciNet Google Scholar * Liang, Y., Zhang, T. & Tang, M. Toxicity of quantum dots on target organs and immune system. _J. Appl. Toxicol._
42, 17–40 (2022). Article Google Scholar * Tokatlian, T. et al. Innate immune recognition of glycans targets HIV nanoparticle immunogens to germinal centers. _Science_ 363, 649–654
(2019). Article Google Scholar * Read, B. J. et al. Mannose-binding lectin and complement mediate follicular localization and enhanced immunogenicity of diverse protein nanoparticle
immunogens. _Cell Rep._ 38, 110217 (2022). Article Google Scholar * Venkatesan, P. Preliminary phase 1 results from an HIV vaccine candidate trial. _Lancet Microbe_ 2, e95 (2021). Article
Google Scholar * Zhang, Y.-N. et al. Nanoparticle size influences antigen retention and presentation in lymph node follicles for humoral immunity. _Nano Lett._ 19, 7226–7235 (2019).
Article Google Scholar * Veneziano, R. et al. Role of nanoscale antigen organization on B-cell activation probed using DNA origami. _Nat. Nanotechnol._ 15, 716–723 (2020). Article Google
Scholar * Xiao, P. et al. Engineering nanoscale artificial antigen-presenting cells by metabolic dendritic cell labeling to potentiate cancer immunotherapy. _Nano Lett._ 21, 2094–2103
(2021). Article Google Scholar * Ma, L. et al. Enhanced CAR–T cell activity against solid tumors by vaccine boosting through the chimeric receptor. _Science_ 365, 162–168 (2019). Article
Google Scholar * Pennock, N. D., Kedl, J. D. & Kedl, R. M. T cell vaccinology: beyond the reflection of infectious responses. _TRENDS Immunol._ 37, 170–180 (2016). Article Google
Scholar * Ols, S. et al. Route of vaccine administration alters antigen trafficking but not innate or adaptive immunity. _Cell Rep._ 30, 3964–3971 (2020). Article Google Scholar *
Golombek, S. K. et al. Tumor targeting via EPR: strategies to enhance patient responses. _Adv. Drug. Deliv. Rev._ 130, 17–38 (2018). Article Google Scholar * Kranz, L. M. et al. Systemic
RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. _Nature_ 534, 396–401 (2016). Article Google Scholar * Beyaert, S., Machiels, J.-P. & Schmitz, S.
Vaccine-based immunotherapy for head and neck cancers. _Cancers_ 13, 6041 (2021). Article Google Scholar * Sahin, U. et al. An RNA vaccine drives immunity in checkpoint-inhibitor-treated
melanoma. _Nature_ 585, 107–112 (2020). Article Google Scholar * Baharom, F. et al. Intravenous nanoparticle vaccination generates stem-like TCF1+ neoantigen-specific CD8+ T cells. _Nat.
Immunol._ 52, 41–52 (2020). Google Scholar * Creemers, J. H. A. et al. Assessing the safety, tolerability and efficacy of PLGA-based immunomodulatory nanoparticles in patients with advanced
NY-ESO-1-positive cancers: a first-in-human phase I open-label dose-escalation study protocol. _BMJ Open_ 11, e050725 (2021). Article Google Scholar * Kishimoto, T. K. et al. Improving
the efficacy and safety of biologic drugs with tolerogenic nanoparticles. _Nat. Nanotechnol._ 11, 890–899 (2016). Article Google Scholar * Sands, E. et al. Tolerogenic nanoparticles
mitigate the formation of anti-drug antibodies against pegylated uricase in patients with hyperuricemia. _Nat. Commun._ 13, 272 (2022). Article Google Scholar * Prasad, S. et al.
Tolerogenic Ag-PLG nanoparticles induce tregs to suppress activated diabetogenic CD4 and CD8 T cells. _J. Autoimmun._ 89, 112–124 (2018). Article Google Scholar * Luo, X., Miller, S. D.
& Shea, L. D. Immune tolerance for autoimmune disease and cell transplantation. _Annu. Rev. Biomed. Eng._ 18, 181–205 (2016). Article Google Scholar * Leon, M. A. et al. Soluble
antigen arrays displaying mimotopes direct the response of diabetogenic T cells. _ACS Chem. Biol._ 14, 1436–1448 (2019). Article Google Scholar * Liu, Q. et al. Use of polymeric
nanoparticle platform targeting the liver to induce Treg-mediated antigen-specific immune tolerance in a pulmonary allergen sensitization model. _ACS Nano_ 13, 4778–4794 (2019). Article
Google Scholar * Ishii, Y. et al. Alpha-galactosylceramide-driven immunotherapy for allergy. _Front. Biosci._ 13, 6214–6228 (2008). Article Google Scholar * Duramad, O., Laysang, A., Li,
J., Ishii, Y. & Namikawa, R. Pharmacologic expansion of donor-derived, naturally occurring CD4+ Foxp3+ regulatory T cells reduces acute graft-versus-host disease lethality without
abrogating the graft-versus-leukemia effect in murine models. _Biol. Blood Marrow Transplant._ 17, 1154–1168 (2011). Article Google Scholar * Chen, Y.-B. et al. Pharmacokinetics and safety
of KRN7000 following intravenous infusion of RGI-2001 in allogeneic transplant patients. _Blood_ 136, 15–16 (2020). Google Scholar * Okhrimenko, A. et al. Human memory T cells from the
bone marrow are resting and maintain long-lasting systemic memory. _Proc. Natl Acad. Sci. USA_ 111, 9229–9234 (2014). Article Google Scholar * Mulder, W. J. M., Ochando, J., Joosten, L. A.
B., Fayad, Z. A. & Netea, M. G. Therapeutic targeting of trained immunity. _Nat. Rev. Drug Discov._ 18, 553–566 (2019). Article Google Scholar * Netea, M. G. et al. Trained immunity:
a program of innate immune memory in health and disease. _Science_ 352, aaf1098 (2016). Article Google Scholar * Netea, M. G. & van der Meer, J. W. M. Trained immunity: an ancient way
of remembering. _Cell Host Microbe_ 21, 297–300 (2017). Article Google Scholar * Priem, B. et al. Trained immunity-promoting nanobiologic therapy suppresses tumor growth and potentiates
checkpoint inhibition. _Cell_ 183, 786–801 (2020). Article Google Scholar * Finck, A. V., Blanchard, T., Roselle, C. P., Golinelli, G. & June, C. H. Engineered cellular immunotherapies
in cancer and beyond. _Nat. Med._ 28, 678–689 (2022). Article Google Scholar * Ribas, A. T cells as the future of cancer therapy. _Cancer Discov._ 11, 798–800 (2021). Article Google
Scholar * Hong, M., Clubb, J. D. & Chen, Y. Y. Engineering CAR-T cells for next-generation cancer therapy. _Cancer Cell_ 38, 473–488 (2020). Article Google Scholar * Barros, L. R. C.
et al. Systematic review of available CAR-T cell trials around the world. _Cancers_ 14, 2667 (2022). Article Google Scholar * National Cancer Institute. CAR T cells: engineering patients’
immune cells to treat their cancers. _NCI_ https://www.cancer.gov/about-cancer/treatment/research/car-t-cells (2022). * Larson, R. C. & Maus, M. V. Recent advances and discoveries in the
mechanisms and functions of CAR T cells. _Nat. Rev. Cancer_ 21, 145–161 (2021). Article Google Scholar * Siriwon, N. et al. CAR–T cells surface-engineered with drug-encapsulated
nanoparticles can ameliorate intratumoral T-cell hypofunction. _Cancer Immunol. Res._ 6, 812–824 (2018). Article Google Scholar * Huang, B. et al. Active targeting of chemotherapy to
disseminated tumors using nanoparticle-carrying T cells. _Sci. Transl Med._ 7, 291ra94–291ra94 (2015). Article Google Scholar * Eskandari, S. K. et al. Regulatory T cells engineered with
TCR signaling–responsive IL-2 nanogels suppress alloimmunity in sites of antigen encounter. _Sci. Transl Med._ 12, eaaw4744 (2020). Article Google Scholar * Stephan, M. T., Moon, J. J.,
Um, S. H., Bershteyn, A. & Irvine, D. J. Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. _Nat. Med._ 16, 1035–1041 (2010). Article Google Scholar * Tang,
L. et al. Enhancing T cell therapy through TCR-signaling-responsive nanoparticle drug delivery. _Nat. Biotechnol._ 36, 707–716 (2018). Article Google Scholar * Romee, R. et al.
First-in-human phase 1 clinical study of the IL-15 superagonist complex ALT-803 to treat relapse after transplantation. _Blood_ 131, 2515–2527 (2018). Article Google Scholar * Hamilton, E.
et al. 801 PRIMETM IL-15 (RPTR-147): preliminary clinical results and biomarker analysis from a first-in-human phase 1 study of IL-15 loaded peripherally-derived autologous T cell therapy
in solid tumor patients. _J. Immunother. Cancer_ 8, A850–A850 (2020). Google Scholar * ShieldsIV, C. W. et al. Cellular backpacks for macrophage immunotherapy. _Sci. Adv._ 6, eaaz6579
(2020). Article Google Scholar * Champion, J. A. & Mitragotri, S. Role of target geometry in phagocytosis. _Proc. Natl Acad. Sci. USA._ 103, 4930–4934 (2006). Article Google Scholar
* Berlin, C. et al. α4β7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. _Cell_ 74, 185–195 (1993). Article Google Scholar * Mora, J. R. et al. Selective
imprinting of gut-homing T cells by Peyer’s patch dendritic cells. _Nature_ 424, 88–93 (2003). Article Google Scholar * Cao, S., Jiang, Y., Zhang, H., Kondza, N. & Woodrow, K. A.
Core–shell nanoparticles for targeted and combination antiretroviral activity in gut-homing T cells. _Nanomed. Nanotechnol. Biol. Med._ 14, 2143–2153 (2018). Article Google Scholar *
Dammes, N. et al. Conformation-sensitive targeting of lipid nanoparticles for RNA therapeutics. _Nat. Nanotechnol._ 16, 1030–1038 (2021). Article Google Scholar * Parayath, N. N., Parikh,
A. & Amiji, M. M. Repolarization of tumor-associated macrophages in a genetically engineered nonsmall cell lung cancer model by intraperitoneal administration of hyaluronic acid-based
nanoparticles encapsulating microRNA-125b. _Nano Lett._ 18, 3571–3579 (2018). Article Google Scholar * Sofias, A. M. et al. Tumor targeting by αvβ3-integrin-specific lipid nanoparticles
occurs via phagocyte hitchhiking. _ACS Nano_ 14, 7832–7846 (2020). Article Google Scholar * Hou, J. et al. Accessing neuroinflammation sites: monocyte/neutrophil-mediated drug delivery for
cerebral ischemia. _Sci. Adv._ 5, eaau8301 (2019). Article Google Scholar * Tsai, S. et al. Reversal of autoimmunity by boosting memory-like autoregulatory T cells. _Immunity_ 32, 568–580
(2010). Article Google Scholar * Singha, S. et al. Peptide–MHC-based nanomedicines for autoimmunity function as T-cell receptor microclustering devices. _Nat. Nanotechnol._ 12, 701–710
(2017). Article Google Scholar * Umeshappa, C. S. et al. Suppression of a broad spectrum of liver autoimmune pathologies by single peptide-MHC-based nanomedicines. _Nat. Commun._ 10, 2150
(2019). Article Google Scholar * Rhodes, K. R. et al. Biodegradable cationic polymer blends for fabrication of enhanced artificial antigen presenting cells to treat melanoma. _ACS Appl.
Mater. Interf._ 13, 7913–7923 (2021). Article Google Scholar * Rhodes, K. R., Meyer, R. A., Wang, J., Tzeng, S. Y. & Green, J. J. Biomimetic tolerogenic artificial antigen presenting
cells for regulatory T cell induction. _Acta Biomater._ 112, 136–148 (2020). Article Google Scholar * Yip, A. & Webster, R. M. The market for chimeric antigen receptor T cell
therapies. _Nat. Rev. Drug Discov._ 17, 161–162 (2018). Article Google Scholar * Agarwal, S., Weidner, T., Thalheimer, F. B. & Buchholz, C. J. In vivo generated human CAR T cells
eradicate tumor cells. _Oncoimmunology_ 8, e1671761 (2019). Article Google Scholar * Parayath, N. N. & Stephan, M. T. In situ programming of CAR T cells. _Annu. Rev. Biomed. Eng._ 23,
385–405 (2021). Article Google Scholar * Nayak, S. & Herzog, R. W. Progress and prospects: immune responses to viral vectors. _Gene Ther._ 17, 295–304 (2010). Article Google Scholar
* Barnes, C., Scheideler, O. & Schaffer, D. Engineering the AAV capsid to evade immune responses. _Curr. Opin. Biotech._ 60, 99–103 (2019). Article Google Scholar * Hinderer, C. et al.
Severe toxicity in nonhuman primates and piglets following high-dose intravenous administration of an adeno-associated virus vector expressing human SMN. _Hum. Gene Ther._ 29, 285–298
(2018). Article Google Scholar * Smith, T. T. et al. In situ programming of leukaemia-specific T cells using synthetic DNA nanocarriers. _Nat. Nanotechnol._ 12, 813–820 (2017). Article
Google Scholar * Rurik, J. G. et al. CAR T cells produced in vivo to treat cardiac injury. _Science_ 375, 91–96 (2022). Article Google Scholar * Li, W. et al. Biomimetic nanoparticles
deliver mRNAs encoding costimulatory receptors and enhance T cell mediated cancer immunotherapy. _Nat. Commun._ 12, 7264 (2021). Article Google Scholar * Billingsley, M. M. et al.
Ionizable lipid nanoparticle-mediated mRNA delivery for human CAR T cell engineering. _Nano Lett._ 20, 1578–1589 (2020). Article Google Scholar * McKinlay, C. J., Benner, N. L., Haabeth,
O. A., Waymouth, R. M. & Wender, P. A. Enhanced mRNA delivery into lymphocytes enabled by lipid-varied libraries of charge-altering releasable transporters. _Proc. Natl Acad. Sci. USA_
115, 201805358 (2018). Article Google Scholar * Agarwalla, P. et al. Bioinstructive implantable scaffolds for rapid in vivo manufacture and release of CAR-T cells. _Nat. Biotechnol._ 40,
1250–1258 (2022). Article Google Scholar * Mora, J. R. et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. _Science_ 314, 1157–1160 (2006). Article Google
Scholar * Allie, S. R. et al. The establishment of resident memory B cells in the lung requires local antigen encounter. _Nat. Immunol._ 20, 97–108 (2018). Article Google Scholar *
Kiyono, H. & Fukuyama, S. NALT- versus PEYER’S-patch-mediated mucosal immunity. _Nat. Rev. Immunol._ 4, 699–710 (2004). Article Google Scholar * Bowen, A., Sweeney, E. E. &
Fernandes, R. Nanoparticle-based immunoengineered approaches for combating HIV. _Front. Immunol._ 11, 789 (2020). Article Google Scholar * Li, M. et al. Mucosal vaccines: strategies and
challenges. _Immunol. Lett._ 217, 116–125 (2019). Article Google Scholar * Iwasaki, A. Exploiting mucosal immunity for antiviral vaccines. _Annu. Rev. Immunol._ 34, 575–608 (2016). Article
Google Scholar * Sockolosky, J. T. & Szoka, F. C. The neonatal Fc receptor, FcRn, as a target for drug delivery and therapy. _Adv. Drug Deliv. Rev._ 91, 109–124 (2015). Article
Google Scholar * Fieux, M. et al. FcRn as a transporter for nasal delivery of biologics: a systematic review. _Int. J. Mol. Sci._ 22, 6475 (2021). Article Google Scholar * Hartwell, B. L.
et al. Intranasal vaccination via lipid-conjugated immunogens promotes antigen persistence and transmucosal uptake to drive mucosal and systemic immunity. _Sci. Transl Med._ 14, eabn1413
(2022). Article Google Scholar * Lee, Y. et al. Hyaluronic acid–bilirubin nanomedicine for targeted modulation of dysregulated intestinal barrier, microbiome and immune responses in
colitis. _Nat. Mater._ 19, 118–126 (2019). Article Google Scholar * Lázaro, Ide & Mooney, D. J. Obstacles and opportunities in a forward vision for cancer nanomedicine. _Nat. Mater._
20, 1469–1479 (2021). Article Google Scholar * Wilhelm, S. et al. Analysis of nanoparticle delivery to tumours. _Nat. Rev. Mater._ 1, 16014 (2016). Article Google Scholar * Barber, G. N.
STING: infection, inflammation and cancer. _Nat. Rev. Immunol._ 15, 760–770 (2015). Article Google Scholar * Dane, E. L. et al. STING agonist delivery by tumour-penetrating PEG-lipid
nanodiscs primes robust anticancer immunity. _Nat. Mater._ 21, 710–720 (2022). Article Google Scholar * Miller, I. C. et al. Enhanced intratumoural activity of CAR T cells engineered to
produce immunomodulators under photothermal control. _Nat. Biomed. Eng._ 5, 1348–1359 (2021). Article Google Scholar * Longmire, M., Choyke, P. L. & Kobayashi, H. Clearance properties
of nano-sized particles and molecules as imaging agents: considerations and caveats. _Nanomedicine_ 3, 703–717 (2008). Article Google Scholar * Schmid, D. et al. T cell-targeting
nanoparticles focus delivery of immunotherapy to improve antitumor immunity. _Nat. Commun._ 8, 1747 (2017). Article Google Scholar * Turk, M. J., Waters, D. J. & Low, P. S.
Folate-conjugated liposomes preferentially target macrophages associated with ovarian carcinoma. _Cancer Lett._ 213, 165–172 (2004). Article Google Scholar * Miller, M. A. et al.
Tumour-associated macrophages act as a slow-release reservoir of nano-therapeutic Pt(IV) pro-drug. _Nat. Commun._ 6, 8692 (2015). Article Google Scholar * Dai, Q. et al. Quantifying the
ligand-coated nanoparticle delivery to cancer cells in solid tumors. _ACS Nano_ 12, 8423–8435 (2018). Article Google Scholar * Rodell, C. B. et al. TLR7/8-agonist-loaded nanoparticles
promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy. _Nat. Biomed. Eng._ 2, 578–588 (2018). Article Google Scholar * Kulkarni, A. et al. A designer
self-assembled supramolecule amplifies macrophage immune responses against aggressive cancer. _Nat. Biomed. Eng._ 2, 589–599 (2018). Article Google Scholar * Zhang, F. et al. Genetic
programming of macrophages to perform anti-tumor functions using targeted mRNA nanocarriers. _Nat. Commun._ 10, 3974 (2019). Article Google Scholar * Gustafson, H. H., Holt-Casper, D.,
Grainger, D. W. & Ghandehari, H. Nanoparticle uptake: the phagocyte problem. _Nano Today_ 10, 487–510 (2015). Article Google Scholar * Chen, D. et al. NIR-II fluorescence imaging
reveals bone marrow retention of small polymer nanoparticles. _Nano Lett._ 21, 798–805 (2021). Article Google Scholar * Sorensen, A. G. et al. A “vascular normalization index” as potential
mechanistic biomarker to predict survival after a single dose of cediranib in recurrent glioblastoma patients. _Cancer Res._ 69, 5296–5300 (2009). Article Google Scholar * Dickson, P. V.
et al. Bevacizumab-induced transient remodeling of the vasculature in neuroblastoma xenografts results in improved delivery and efficacy of systemically administered chemotherapy. _Clin.
Cancer Res._ 13, 3942–3950 (2007). Article Google Scholar * Willett, C. G. et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal
cancer. _Nat. Med._ 10, 145–147 (2004). Article Google Scholar * Inai, T. et al. Inhibition of vascular endothelial growth factor (VEGF) signaling in cancer causes loss of endothelial
fenestrations, regression of tumor vessels, and appearance of basement membrane ghosts. _Am. J. Pathol._ 165, 35–52 (2004). Article Google Scholar * Goel, S., Wong, A. H.-K. & Jain, R.
K. Vascular normalization as a therapeutic strategy for malignant and nonmalignant disease. _Cold Spring Harb. Perspect. Med._ 2, a006486 (2012). Article Google Scholar * Tong, R. T. et
al. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. _Cancer Res._
11, 3731–3736 (2004). Article Google Scholar * Boucher, Y. et al. Bevacizumab improves tumor infiltration of mature dendritic cells and effector T-cells in triple-negative breast cancer
patients. _npj Precis. Oncol._ 5, 62 (2021). Article Google Scholar * Fukumura, D., Kloepper, J., Amoozgar, Z., Duda, D. G. & Jain, R. K. Enhancing cancer immunotherapy using
antiangiogenics: opportunities and challenges. _Nat. Rev. Clin. Oncol._ 15, 325–340 (2018). Article Google Scholar * Shigeta, K. et al. Regorafenib combined with PD1 blockade increases CD8
T-cell infiltration by inducing CXCL10 expression in hepatocellular carcinoma. _J. Immunother. Cancer_ 8, e001435 (2020). Article Google Scholar * Manning, E. A. et al. A vascular
endothelial growth factor receptor-2 inhibitor enhances antitumor immunity through an immune-based mechanism. _Clin. Cancer Res._ 13, 3951–3959 (2007). Article Google Scholar * Yasuda, S.
et al. Simultaneous blockade of programmed death 1 and vascular endothelial growth factor receptor 2 (VEGFR2) induces synergistic anti‐tumour effect in vivo. _Clin. Exp. Immunol._ 172,
500–506 (2013). Article Google Scholar * Li, B. et al. Vascular endothelial growth factor blockade reduces intratumoral regulatory T cells and enhances the efficacy of a GM-CSF-secreting
cancer immunotherapy. _Clin. Cancer Res._ 12, 6808–6816 (2006). Article Google Scholar * Voron, T. et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors.
_J. Exp. Med._ 212, 139–148 (2015). Article Google Scholar * Allen, E. et al. Combined antiangiogenic and anti–PD-L1 therapy stimulates tumor immunity through HEV formation. _Sci. Transl
Med._ 9, eaak9679 (2017). Article Google Scholar * Huang, Y. et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and
enhance immunotherapy. _Proc. Natl Acad. Sci. USA_ 109, 17561–17566 (2012). Article Google Scholar * Yang, R. K. et al. Intratumoral hu14.18-IL-2 (IC) induces local and systemic antitumor
effects that involve both activated T and NK cells as well as enhanced IC retention. _J. Immunol_. https://doi.org/10.4049/jimmunol.1200934 (2012). * Momin, N. et al. Maximizing response to
intratumoral immunotherapy in mice by tuning local retention. _Nat. Commun._ 13, 109 (2022). Article Google Scholar * Momin, N. et al. Anchoring of intratumorally administered cytokines to
collagen safely potentiates systemic cancer immunotherapy. _Sci. Transl Med._ 11, eaaw2614 (2019). Article Google Scholar * Ishihara, J. et al. Matrix-binding checkpoint immunotherapies
enhance antitumor efficacy and reduce adverse events. _Sci. Transl Med._ 9, eaan0401 (2017). Article Google Scholar * Agarwal, Y. et al. Intratumourally injected alum-tethered cytokines
elicit potent and safer local and systemic anticancer immunity. _Nat. Biomed. Eng._ 6, 129–143 (2022). Article Google Scholar * Huang, Z. N., Callmann, C. E., Cole, L. E., Wang, S. &
Mirkin, C. A. Synergistic immunostimulation through the dual activation of toll-like receptor 3/9 with spherical nucleic acids. _ACS Nano_ 15, 13329–13338 (2021). Article Google Scholar *
O’Day, S. et al. 423 Safety and preliminary efficacy of intratumoral cavrotolimod (AST-008), a spherical nucleic acid TLR9 agonist, in combination with pembrolizumab in patients with
advanced solid tumors. _Regul. Young Invest. Award. Abstr_. https://doi.org/10.1136/jitc-2020-sitc2020.0423 (2020). * Exicure provides interim results from ongoing phase 1b/2 clinical trial
of cavrotolimod (AST-008). sec.report https://sec.report/Document/0001698530-21-000079/exhibit992pressrelease0805.htm (2021). * Wang, C., Fiering, S. N. & Steinmetz, N. F. Cowpea mosaic
virus promotes anti‐tumor activity and immune memory in a mouse ovarian tumor model. _Adv. Ther._ 2, 1900003 (2019). Article Google Scholar * Lizotte, P. H. et al. In situ vaccination with
cowpea mosaic virus nanoparticles suppresses metastatic cancer. _Nat. Nanotechnol._ 11, 295–303 (2016). Article Google Scholar * Nguyen, K. G. et al. Localized interleukin-12 for cancer
immunotherapy. _Front. Immunol._ 11, 575597 (2020). Article Google Scholar * Zaharoff, D. A., Hance, K. W., Rogers, C. J., Schlom, J. & Greiner, J. W. Intratumoral immunotherapy of
established solid tumors with chitosan/IL-12. _J. Immunother._ 33, 697–705 (2010). Article Google Scholar * Mills, B. N. et al. Stereotactic body radiation and interleukin-12 combination
therapy eradicates pancreatic tumors by repolarizing the immune microenvironment. _Cell Rep._ 29, 406–421 (2019). Article Google Scholar * Park, C. G. et al. Extended release of
perioperative immunotherapy prevents tumor recurrence and eliminates metastases. _Sci. Transl Med._ 10, eaar1916 (2018). Article Google Scholar * Chen, Q. et al. In situ sprayed
bioresponsive immunotherapeutic gel for post-surgical cancer treatment. _Nat. Nanotechnol._ 14, 89–97 (2018). Article Google Scholar * Stephan, S. B. et al. Biopolymer implants enhance the
efficacy of adoptive T cell therapy. _Nat. Biotechnol._ 33, 97–101 (2014). Article Google Scholar * Hu, Q. et al. Inhibition of post-surgery tumour recurrence via a hydrogel releasing
CAR-T cells and anti-PDL1-conjugated platelets. _Nat. Biomed. Eng._ 5, 1038–1047 (2021). Article Google Scholar * Coon, M. E., Stephan, S. B., Gupta, V., Kealey, C. P. & Stephan, M. T.
Nitinol thin films functionalized with CAR-T cells for the treatment of solid tumours. _Nat. Biomed. Eng._ 4, 195–206 (2020). Article Google Scholar * Shae, D. et al. Endosomolytic
polymersomes increase the activity of cyclic dinucleotide STING agonists to enhance cancer immunotherapy. _Nat. Nanotechnol._ 14, 269–278 (2019). Article Google Scholar * Liu, Y. et al.
Intrapleural nano-immunotherapy promotes innate and adaptive immune responses to enhance anti-PD-L1 therapy for malignant pleural effusion. _Nat. Nanotechnol._ 17, 206–216 (2022). Article
Google Scholar * Hewitt, S. L. et al. Durable anticancer immunity from intratumoral administration of IL-23, IL-36γ, and OX40L mRNAs. _Sci. Transl Med._ 11, eaat9143 (2019). Article Google
Scholar * Hotz, C. et al. Local delivery of mRNA-encoding cytokines promotes antitumor immunity and tumor eradication across multiple preclinical tumor models. _Sci. Transl Med._ 13,
eabc7804 (2021). Article Google Scholar * Tzeng, S. Y. et al. In situ genetic engineering of tumors for long-lasting and systemic immunotherapy. _Proc. Natl Acad. Sci. USA_ 117, 4043–4052
(2020). Article Google Scholar * Patel, M. et al. 539 Phase 1 study of mRNA-2752, a lipid nanoparticle encapsulating mRNAs encoding human OX40L/IL-23/IL-36γ, for intratumoral (ITu)
injection +/− durvalumab in advanced solid tumors and lymphoma. _J Immunother. Cancer_ 9, A569 (2021). Article Google Scholar * Myerson, J. W. et al. Supramolecular arrangement of protein
in nanoparticle structures predicts nanoparticle tropism for neutrophils in acute lung inflammation. _Nat. Nanotechnol._ 17, 86–97 (2022). Article Google Scholar * Robertson, J. D., Ward,
J. R., Avila-Olias, M., Battaglia, G. & Renshaw, S. A. Targeting neutrophilic inflammation using polymersome-mediated cellular delivery. _J. Immunol._ 198, 3596–3604 (2017). Article
Google Scholar * Sofias, A. M. et al. Cyclic arginine–glycine–aspartate‐decorated lipid nanoparticle targeting toward inflammatory lesions involves hitchhiking with phagocytes. _Adv. Sci._
8, 2100370 (2021). Article Google Scholar * Braza, M. S. et al. Inhibiting inflammation with myeloid cell-specific nanobiologics promotes organ transplant acceptance. _Immunity_ 49,
819–828 (2018). Article Google Scholar * Park, J. et al. Intravascular innate immune cells reprogrammed via intravenous nanoparticles to promote functional recovery after spinal cord
injury. _Proc. Natl Acad. Sci. USA_ 116, 14947–14954 (2019). Article Google Scholar * Li, Y. et al. Immunosuppressive PLGA TGF-β1 microparticles induce polyclonal and antigen-specific
regulatory T cells for local immunomodulation of allogeneic islet transplants. _Front. Immunol._ 12, 653088 (2021). Article Google Scholar * Fisher, J. D. et al. Treg-inducing
microparticles promote donor-specific tolerance in experimental vascularized composite allotransplantation. _Proc. Natl Acad. Sci. USA_ 116, 25784–25789 (2019). Article Google Scholar *
Hautz, T. et al. Lymphoid neogenesis in skin of human hand, nonhuman primate, and rat vascularized composite allografts. _Transpl. Int._ 27, 966–976 (2014). Article Google Scholar * Azzi,
J. et al. Targeted delivery of immunomodulators to lymph nodes. _Cell Rep._ 15, 1202–1213 (2016). Article Google Scholar * Bahmani, B. et al. Targeted delivery of immune therapeutics to
lymph nodes prolongs cardiac allograft survival. _J. Clin. Invest._ 128, 4770–4786 (2018). Article Google Scholar * Li, Z. et al. In vivo labeling reveals continuous trafficking of TCF-1+
T cells between tumor and lymphoid tissue. _J. Exp. Med._ 219, e20210749 (2022). Article Google Scholar * Torcellan, T. et al. In vivo photolabeling of tumor-infiltrating cells reveals
highly regulated egress of T-cell subsets from tumors. _Proc. Natl Acad. Sci. USA_ 114, 5677–5682 (2017). Article Google Scholar * Gearty, S. V. et al. An autoimmune stem-like CD8 T cell
population drives type 1 diabetes. _Nature_ 602, 156–161 (2022). Article Google Scholar * Im, S. J. et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy.
_Nature_ 537, 417–421 (2016). Article Google Scholar * Connolly, K. A. et al. A reservoir of stem-like CD8+ T cells in the tumor-draining lymph node preserves the ongoing anti-tumor immune
response. _Sci. Immunol._ 6, eabg7836 (2021). Article Google Scholar * Schenkel, J. M. et al. Conventional type I dendric cells maintain a reservoir of proliferative tumor-antigen
specific TCF-1+ CD8+ T cells in tumor-draining lymph nodes. _Immunity_ 54, 2338–2353 (2021). Article Google Scholar * Siddiqui, I. et al. Intratumoral Tcf1+ PD-1+ D8+ T cells with
stem-like properties promote tumor control in response to vaccination and checkpoint blockade immunotherapy. _Immunity_ 50, 195–211 (2018). Article Google Scholar * Tahtinen, S. et al.
IL-1 and IL-1ra are key regulators of the inflammatory response to RNA vaccines. _Nat. Immunol._ 23, 532–542 (2022). Article Google Scholar * Alameh, M.-G. et al. Lipid nanoparticles
enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. _Immunity_ 54, 2877–2892 (2021). Article Google Scholar * Li,
C. et al. Mechanisms of innate and adaptive immunity to the Pfizer-BioNTech BNT162b2 vaccine. _Nat. Immunol._ 23, 543–555 (2022). Article Google Scholar * Yang, X. et al. Lactate-modulated
immunosuppression of myeloid-derived suppressor cells contributes to the radioresistance of pancreatic cancer. _Cancer Immunol. Res._ 8, 1440–1451 (2020). Article Google Scholar * Certo,
M., Tsai, C.-H., Pucino, V., Ho, P.-C. & Mauro, C. Lactate modulation of immune responses in inflammatory versus tumour microenvironments. _Nat. Rev. Immunol._ 21, 151–161 (2021).
Article Google Scholar * Sangsuwan, R. et al. Lactate exposure promotes immunosuppressive phenotypes in innate immune cells. _Cell Mol. Bioeng._ 13, 541–557 (2020). Article MathSciNet
Google Scholar * Allen, R. P., Bolandparvaz, A., Ma, J. A., Manickam, V. A. & Lewis, J. S. Latent, immunosuppressive nature of poly(lactic-co-glycolic acid) microparticles. _ACS
Biomater. Sci. Eng._ 4, 900–918 (2018). Article Google Scholar * Shimabukuro, T. T., Cole, M. & Su, J. R. Reports of anaphylaxis after receipt of mRNA COVID-19 vaccines in the
US—December 14, 2020-January 18, 2021. _JAMA_ 325, 1101–1102 (2021). Article Google Scholar * Szebeni, J. et al. Applying lessons learned from nanomedicines to understand rare
hypersensitivity reactions to mRNA-based SARS-CoV-2 vaccines. _Nat. Nanotechnol._ 17, 337–346 (2022). Article Google Scholar * McKinlay, C. J. et al. Charge-altering releasable
transporters (CARTs) for the delivery and release of mRNA in living animals. _Proc. Natl Acad. Sci. USA._ 114, E448–E456 (2017). Article Google Scholar * Kowalski, P. S., Rudra, A., Miao,
L. & Anderson, D. G. Delivering the messenger: advances in technologies for therapeutic mRNA delivery. _Mol. Ther._ 27, 710–728 (2019). Article Google Scholar * Kong, N. et al.
Synthetic mRNA nanoparticle-mediated restoration of p53 tumor suppressor sensitizes p53-deficient cancers to mTOR inhibition. _Sci. Transl Med._ 11, eaaw1565 (2019). Article Google Scholar
* Leuschner, F. et al. Therapeutic siRNA silencing in inflammatory monocytes in mice. _Nat. Biotechnol._ 29, 1005–1010 (2011). Article Google Scholar * Cheng, Q. et al. Selective organ
targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR–Cas gene editing. _Nat. Nanotechnol._ 15, 313–320 (2020). Article Google Scholar * Hewitt, S. L. et al.
Intratumoral IL12 mRNA therapy promotes TH1 transformation of the tumor microenvironment. _Clin. Cancer Res._ 26, 6284–6298 (2020). Article Google Scholar * Youngblood, B., Hale, J. S.
& Ahmed, R. T-cell memory differentiation: insights from transcriptional signatures and epigenetics. _Immunology_ 139, 277–284 (2013). Article Google Scholar * Gounari, F. &
Khazaie, K. TCF-1: a maverick in T cell development and function. _Nat. Immunol._ 23, 671–678 (2022). Article Google Scholar * Zhao, X., Shan, Q. & Xue, H.-H. TCF1 in T cell immunity:
a broadened frontier. _Nat. Rev. Immunol._ 22, 147–157 (2022). Article Google Scholar Download references ACKNOWLEDGEMENTS This work was supported in part by the Marble Center for
Nanomedicine, the Ragon Institute of MGH, MIT and Harvard, the NIH (awards CA247632, EB031082, U01-CA265706, AI147845, AI162307, AI161297 and CA235375 to D.J.I.) and the Mark Foundation for
Cancer Research. This material is based upon work supported in part by the US Army Research Office through the Institute for Soldier Nanotechnologies at MIT, under Cooperative Agreement
Number W911NF-18-2-0048. D.J.I. is an investigator of the Howard Hughes Medical Institute. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Koch Institute for Integrative Cancer Research,
Massachusetts Institute of Technology, Cambridge, MA, USA Parisa Yousefpour, Kaiyuan Ni & Darrell J. Irvine * Department of Biological Engineering, Massachusetts Institute of Technology,
Cambridge, MA, USA Darrell J. Irvine * Department of Materials Science and Engineering, Massachusetts Institute of Technology, Cambridge, MA, USA Darrell J. Irvine * Ragon Institute of
Massachusetts General Hospital, Massachusetts Institute of Technology and Harvard University, Cambridge, MA, USA Darrell J. Irvine * Howard Hughes Medical Institute, Chevy Chase, MD, USA
Darrell J. Irvine Authors * Parisa Yousefpour View author publications You can also search for this author inPubMed Google Scholar * Kaiyuan Ni View author publications You can also search
for this author inPubMed Google Scholar * Darrell J. Irvine View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS The manuscript was drafted and
revised by P.Y., K.N. and D.J.I. CORRESPONDING AUTHOR Correspondence to Darrell J. Irvine. ETHICS DECLARATIONS COMPETING INTERESTS D.J.I. is an inventor on patents related to albumin
hitchhiking (discussed under ‘Principles of lymph node targeting’), nanoparticle modification of T cells (discussed under ‘Backpacking’ cells’) and alum-binding cytokines (discussed under
‘Intratumoral delivery of biomaterials for immune cell targeting’). These patents have been licensed to Elicio Therapeutics, Repertoire Immune Medicines and Ankyra Therapeutics,
respectively, and D.J.I. holds equity in these companies. The remaining authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Reviews Bioengineering_ thanks the
anonymous reviewers for their contribution to the peer review of this work. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in
published maps and institutional affiliations. RIGHTS AND PERMISSIONS Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a
publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing
agreement and applicable law. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Yousefpour, P., Ni, K. & Irvine, D.J. Targeted modulation of immune cells and tissues using
engineered biomaterials. _Nat Rev Bioeng_ 1, 107–124 (2023). https://doi.org/10.1038/s44222-022-00016-2 Download citation * Accepted: 28 November 2022 * Published: 30 January 2023 * Issue
Date: February 2023 * DOI: https://doi.org/10.1038/s44222-022-00016-2 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry,
a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative