Play all audios:
ABSTRACT The clinical translation of regenerative-medicine products, including cell therapies, therapeutic tissue engineering products, and human cell and tissue products, remains limited
because of the so-called ‘valley of death’, that is, the lack of resources necessary to move a product from early preclinical to clinical development. To advance more regenerative-medicine
products into the clinic, academic researchers may benefit greatly from insights into the commercialization process, in particular, through academic startups. In this Review, we discuss key
commercialization aspects, that is, protecting intellectual property, navigating regulatory pathways and obtaining funding, and highlight case studies of academic startups that have
successfully developed US Food and Drug Administration-approved regenerative-medicine products and companies that have received Regenerative Medicine Advanced Therapy designations for their
regenerative-medicine products in development. KEY POINTS * Academic researchers interested in translating regenerative-medicine therapies to approved products may benefit from knowing about
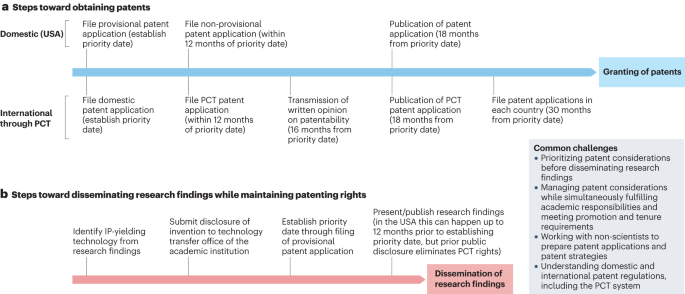
commercialization aspects early in research and development. * Understanding the steps toward establishing intellectual property (IP) within academic institutions is key to conducting and
disseminating IP-yielding research. * Regenerative Medicine Advanced Therapy (RMAT) designations provide a pathway that can expedite the approval process of regenerative-medicine products. *
Grants and non-dilutive mechanisms can support preclinical studies, and dilutive mechanisms of funding and initial public offerings are the major sources of financial capital for clinical
development phases. * Academic institutions and funding agencies should implement policy changes to motivate and drive academic researchers toward the commercialization of
regenerative-medicine technologies. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your
institution Subscribe to this journal Receive 12 digital issues and online access to articles $119.00 per year only $9.92 per issue Learn more Buy this article * Purchase on SpringerLink *
Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional
subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS REGENERATIVE MEDICINE MEETS MATHEMATICAL MODELLING: DEVELOPING SYMBIOTIC RELATIONSHIPS Article
Open access 12 April 2021 ENDURING QUESTIONS IN REGENERATIVE BIOLOGY AND THE SEARCH FOR ANSWERS Article Open access 09 November 2023 ENGINEERING THE NEXT GENERATION OF CELL-BASED
THERAPEUTICS Article 30 May 2022 REFERENCES * Oberweis, C. V., Marchal, J. A., López-Ruiz, E. & Gálvez-Martín, P. A worldwide overview of regulatory frameworks for tissue-based products.
_Tissue Eng. B_ 26, 181–196 (2020). Article Google Scholar * Jacques, E. & Suuronen, E. J. The progression of regenerative medicine and its impact on therapy translation. _Clin.
Transl. Sci._ 13, 440–450 (2020). Article Google Scholar * Goula, A. et al. Advanced therapy medicinal products challenges and perspectives in regenerative medicine. _J. Clin. Med. Res._
12, 780–786 (2020). Article Google Scholar * Sipp, D. & Okano, H. Japan strengthens regenerative medicine oversight. _Cell Stem Cell_ 22, 153–156 (2018). Article Google Scholar *
Kim, D.-S. & Bae, S. Impact and challenges of enactment for advanced regenerative medicine in South Korea. _Front. Bioeng. Biotechnol._ 10, 972865 (2022). Article Google Scholar * Qiu,
T., Hanna, E., Dabbous, M., Borislav, B. & Toumi, M. Regenerative medicine regulatory policies: a systematic review and international comparison. _Health Policy_ 124, 701–713 (2020).
Article Google Scholar * Hogle, L. F. & Das, A. The social production of evidence: regenerative medicine and the 21st Century Cures Act. _Regen. Med._ 12, 581–586 (2017). Article
Google Scholar * Brown, D. G. & Wobst, H. J. A decade of FDA-approved drugs (2010–2019): trends and future directions. _J. Med. Chem._ 64, 2312–2338 (2021). Article Google Scholar *
Butler, D. Translational research: crossing the valley of death. _Nature_ 453, 840–842 (2008). Article Google Scholar * Frederickson, R. M. Escaping the valley of death. _Mol. Ther._ 20,
476–478 (2012). Article Google Scholar * Hinsenkamp, A., Benyó, Z. & Hornyák, I. Overview of tissue engineering patent strategies and patents from 2010 to 2020, including outcomes.
_Tissue Eng. B_ 28, 626–632 (2022). Article Google Scholar * Allen-Hoffmann, L., Comer, A., Conrad, P. B., Hoffmann, M. & Ivarie, C. A.-R. Improved skin substitutes and uses thereof.
Australian patent application 214639 B2 (2010). * Asano, S., Nakanishi, Y. & Sugiyama, D. Intellectual property in the field of regenerative medicine in Japan. _Clin. Ther._ 40,
1823–1827 (2018). Article Google Scholar * Pirnstill, J. & Allen-Hoffmann, B. L. Cold storage of organotypically cultured skin equivalents for clinical applications. US patent 10743533
B2 (2022). * Allen-Hoffmann, B. L., Pirnstill, J. C., Gratz, K. R. & Comer, A. R. Cryopreservation of viable human skin substitutes. Australian patent 232795 B2 (2019). * Koepsel, J.
& Gratz, K. Tissue container systems. US patent application 0330542 A1 (2022). * Smiell, J., Comer, A., Lokuta, M. & Allen-Hoffmann, B. L. Methods for treating acute wounds and
improving outcomes. Australian patent application 353695 A1 (2020). * Wang, S.-J. Patent portfolios for biotech inventions. _Nat. Biotechnol._ 31, 501–503 (2013). Article Google Scholar *
Rachinsky, T. First-to-invent versus first-to-file: impact of the AIA. _Pharm. Pat. Anal._ 3, 353–359 (2014). Article Google Scholar * Begley, C. G. & Ioannidis, J. P. A.
Reproducibility in science. _Circ. Res._ 116, 116–126 (2015). Article Google Scholar * Ozyhar, T., Barnabei, L. & Myrick, D. When speed matters: a discussion on the benefits of a grace
period in patent law to accelerate pharmaceutical innovation in times of pandemic. _J. Law Biosci._ 9, lsac004 (2022). Article Google Scholar * Sherkow, J. S. Preprint servers and patent
prior art. _EMBO Rep._ 23, e54439 (2022). Article Google Scholar * Armstrong, J. P. K. et al. A blueprint for translational regenerative medicine. _Sci. Transl. Med._ 12, eaaz2253 (2020).
Article Google Scholar * Crama, P., Reyck, B. D. & Degraeve, Z. Milestone payments or royalties? Contract design for R&D licensing. _Oper. Res._ 56, 1539–1552 (2008). Article
MathSciNet MATH Google Scholar * Nordberg, R. C., Otarola, G. A., Wang, D., Hu, J. C. & Athanasiou, K. A. Navigating regulatory pathways for translation of biologic cartilage repair
products. _Sci. Transl. Med._ 14, eabp8163 (2022). Article Google Scholar * Zscharnack, M. et al. Preclinical good laboratory practice-compliant safety study to evaluate biodistribution
and tumorigenicity of a cartilage advanced therapy medicinal product (ATMP). _J. Transl. Med._ 13, 160 (2015). Article Google Scholar * Cox, E. M., Edmund, A. V., Kratz, E., Lockwood, S.
H. & Shankar, A. Regulatory affairs 101: introduction to expedited regulatory pathways. _Clin. Transl. Sci._ 13, 451–461 (2020). Article Google Scholar * Kulkarni, T. N. &
Kulkarni, N. G. Authoring a periodic adverse drug experience report…here’s what you need to know! _Perspect. Clin. Res._ 10, 95–99 (2019). Article Google Scholar * Alapati, D., Egan, P.
& Holcombe, J. Understanding conflict of interest for academic entrepreneurs. In _Academic Entrepreneurship for Medical and Health Scientists._ PubPub Edition (2021). * Collins, L. R.
& Shepard, K. A. CIRM tools and technologies: breaking bottlenecks to the development of stem cell therapies. _Stem Cell Transl. Med._ 9, 1129–1136 (2020). Article Google Scholar *
Yuh, D. D. Value analysis committees: not just another committee to get out of. _J. Thorac. Cardiovasc. Surg._ 155, 686–687 (2018). Article Google Scholar * Dang, A. & Kaur, K.
Comparative effectiveness research and its utility in in-clinic practice. _Perspect. Clin. Res._ 7, 9–14 (2016). Article Google Scholar * Westrich, K. D., Wilhelm, J. A. & Schur, C. L.
Comparative effectiveness research in the USA: when will there be an impact on healthcare decision-making? _J. Comp. Eff. Res._ 5, 207–216 (2016). Article Google Scholar * Carter, R. G.
et al. Innovation, entrepreneurship, promotion, and tenure. _Science_ 373, 1312–1314 (2021). Article Google Scholar * Caulfield, T., Harmon, S. H. & Joly, Y. Open science versus
commercialization: a modern research conflict? _Genome Med._ 4, 17 (2012). Article Google Scholar * Tompkins, B. A. et al. IMPACT: Preclinical studies of cell therapy for human disease.
_Circ. Res._ 122, 1006–1020 (2018). Article Google Scholar * Ginty, P., Singh, P., Smith, D., Hourd, P. & Williams, D. Achieving reimbursement for regenerative medicine products in the
USA. _Regen. Med._ 5, 463–469 (2010). Article Google Scholar * Bubela, T. et al. Bringing regenerative medicines to the clinic: the future for regulation and reimbursement. _Regen. Med._
10, 897–911 (2015). Article Google Scholar * Marks, L. V. Collaboration — a competitor’s tool: the story of Centocor, an entrepreneurial biotechnology company. _Bus. History_ 51, 529–546
(2009). Article Google Scholar * Cheever, M. A. & Higano, C. S. PROVENGE (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine. _Clin. Cancer Res._ 17,
3520–3526 (2011). Article Google Scholar * Schmidt, C. Gintuit cell therapy approval signals shift at US regulator. _Nat. Biotechnol._ 30, 479–479 (2012). Article Google Scholar *
Schmidt, C. FDA approves first cell therapy for wrinkle-free visage. _Nat. Biotechnol._ 29, 674–675 (2011). Article Google Scholar * Boss, W. K. et al. Autologous cultured fibroblasts as
cellular therapy in plastic surgery. _Clin. Plastic Surg._ 27, 613–626 (2000). Article Google Scholar * Morrison, C. Boom: 2018’s biotech IPOs. _Nat. Rev. Drug Discov._ 18, 3–6 (2019).
Article Google Scholar * Markert, M. L. et al. Successful formation of a chimeric human thymus allograft following transplantation of cultured postnatal human thymus. _J. Immunol._ 158,
998–1005 (1997). Article Google Scholar * Markert, M. L. et al. Transplantation of thymus tissue in complete DiGeorge syndrome. _N. Engl. J. Med._ 341, 1180–1189 (1999). Article Google
Scholar * Markert, M. L. et al. Thymus transplantation in complete DiGeorge syndrome: immunologic and safety evaluations in 12 patients. _Blood_ 102, 1121–1130 (2003). Article Google
Scholar * Markert, M. Parathyroid and thymus transplantation in DiGeorge syndrome subjects. US patent 0041854 A1 (2009). * Markert, M. L. Parathyroid and thymus transplantation in DiGeorge
syndrome subjects. World patent application WO 107601 A3 (2006). * Markert, M. L. Cultured thymus tissue transplantation promotes donor-specific tolerance to allogeneic solid organ
transplants. World patent application WO 165197 A1 (2019). * Markert, M. L. et al. Methods of determining the suitability of cultured thymus tissue for implantation into humans and
associated methods of use. Canadian patent application 3150732 A1 (2021). * Allen-Hoffmann, B. L. et al. Normal growth and differentiation in a spontaneously immortalized near-diploid human
keratinocyte cell line, NIKS. _J. Investig. Dermatol._ 114, 444–455 (2000). Article Google Scholar * Schurr, M. J. et al. Phase I/II clinical evaluation of stratagraft: a consistent,
pathogen-free human skin substitute. _J. Trauma. Acute Care Surg._ 66, 866 (2009). Article Google Scholar * US National Library of Medicine. StrataGraft™ skin tissue (human donor skin) in
the surgical management of complex skin defects. _ClinicalTrials.gov_ https://clinicaltrials.gov/ct2/show/NCT00618839 (2019). * Centanni, J. M. et al. StrataGraft Skin substitute is
well-tolerated and is not acutely immunogenic in patients with traumatic wounds: results from a prospective, randomized, controlled dose escalation trial. _Ann. Surg._ 253, 672 (2011).
Article Google Scholar * US National Library of Medicine. StrataGraft® skin tissue as an alternative to autografting deep partial-thickness burns. _ClinicalTrials.gov_
https://clinicaltrials.gov/ct2/show/NCT01437852 (2019). * US National Library of Medicine. StrataGraft® skin tissue as an alternative to autografting full-thickness complex skin defects.
_ClinicalTrials.gov_ https://clinicaltrials.gov/ct2/show/NCT03005054 (2016). * Gibson, A. L. F. et al. A phase 3, open-label, controlled, randomized, multicenter trial evaluating the
efficacy and safety of StrataGraft® construct in patients with deep partial-thickness thermal burns. _Burns_ 47, 1024–1037 (2021). Article Google Scholar * US National Library of Medicine.
StrataGraft skin tissue expanded access at specific study sites (StrataCAT). _ClinicalTrials.gov_ https://clinicaltrials.gov/ct2/show/NCT04123548 (2021). * US National Library of Medicine.
Scarring in stratagraft-treated vs. autograft-treated burn wounds: a clinical and histological investigation. _ClinicalTrials.gov_ https://clinicaltrials.gov/ct2/show/NCT04896346 (2022). *
US National Library of Medicine. StrataGraft safety, tolerability and efficacy in pediatric subjects (StrataSTEPS). _ClinicalTrials.gov_ https://clinicaltrials.gov/ct2/show/NCT05517902
(2023). * US National Library of Medicine. StrataGraft overlay of meshed autograft in full-thickness thermal Burns (StrataSOMA). _ClinicalTrials.gov_
https://clinicaltrials.gov/ct2/show/NCT04765202 (2023). * Russell, S. et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal
dystrophy: a randomised, controlled, open-label, phase 3 trial. _Lancet_ 390, 849–860 (2017). Article Google Scholar * Walsh, C. E. & Batt, K. M. Hemophilia clinical gene therapy:
brief review. _Transl. Res._ 161, 307–312 (2013). Article Google Scholar * US National Library of Medicine. Gene transfer clinical trial for spinal muscular atrophy type 1.
_ClinicalTrials.gov_ https://clinicaltrials.gov/ct2/show/NCT02122952 (2022). * Mendell, J. R. et al. Single-dose gene-replacement therapy for spinal muscular atrophy. _N. Engl. J. Med._ 377,
1713–1722 (2017). Article Google Scholar * US National Library of Medicine. Study evaluating the safety and pharmacokinetics of JCAR017 in B-cell non-Hodgkin lymphoma (TRANSCEND-NHL-001).
_ClinicalTrials.gov_ https://www.clinicaltrials.gov/ct2/show/NCT02631044 (2023). * Anderson, D. E., Gridley, A. & Crawford, D. C. Next generation cartilage repair and the
pre-arthroplasty patient. _Oper. Tech. Sports Med._ 30, 150956 (2022). Article Google Scholar Download references ACKNOWLEDGEMENTS The authors thank S. Heimann for assistance with database
searches of regenerative-medicine companies. The authors acknowledge funding support from the NIH through grants R01DE015038, R01AR071457 and R01AR078389. AUTHOR INFORMATION AUTHORS AND
AFFILIATIONS * Department of Biomedical Engineering, University of California Irvine, Irvine, CA, USA Takumi Takahashi, Ryan P. Donahue, Rachel C. Nordberg, Jerry C. Hu & Kyriacos A.
Athanasiou * School of Information Systems and Management, Muma College of Business, University of South Florida, Tampa, FL, USA Steven C. Currall * John A. Paulson School of Engineering and
Applied Sciences, Harvard University, Cambridge, MA, USA Steven C. Currall Authors * Takumi Takahashi View author publications You can also search for this author inPubMed Google Scholar *
Ryan P. Donahue View author publications You can also search for this author inPubMed Google Scholar * Rachel C. Nordberg View author publications You can also search for this author
inPubMed Google Scholar * Jerry C. Hu View author publications You can also search for this author inPubMed Google Scholar * Steven C. Currall View author publications You can also search
for this author inPubMed Google Scholar * Kyriacos A. Athanasiou View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS T.T. conceived the idea
for this Review and contributed to researching the data and literature, discussion, writing and editing. R.P.D. and R.C.N. contributed to researching the data and literature, discussion,
writing and editing of this manuscript. J.C.H. contributed to the discussion, reviewing and editing of the manuscript. S.C.C. and K.A.A. conceived the idea for this Review and contributed to
the discussion, reviewing and editing of the manuscript. CORRESPONDING AUTHOR Correspondence to Kyriacos A. Athanasiou. ETHICS DECLARATIONS COMPETING INTERESTS R.P.D., J.C.H. and K.A.A. are
cofounders of and hold equity in Cartilage Inc. The remaining authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Reviews Bioengineering_ thanks István
Hornyák, Helen Yu and John Blaho for their contribution to the peer review of this work. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional
claims in published maps and institutional affiliations. RELATED LINKS 3/20/2017 (HUMACYL):
https://www.businesswire.com/news/home/20170320005777/en/Humacyte-Receives-FDA-Regenerative-Medicine-Advanced-Therapy-RMAT-Expedited-Review-Designation-for-HUMACYL®-in-Vascular-Access-for-Hemodialysis
4/17/2017 (RVT-802: https://enzyvant.com/enzyvant-receives-fda-designations/ 5/2/2017 (JCELL):
https://www.biospace.com/article/jcyte-receives-regenerative-medicine-advanced-therapy-designation-/ 5/10/2017 (IXMYELOCEL-T):
https://investors.vcel.com/news-releases/news-release-details/vericel-receives-fda-regenerative-medicine-advanced-therapy-rmat 7/18/2017 (APPROVED AS STRATAGRAFT):
https://www.prnewswire.com/news-releases/us-fda-designates-mallinckrodts-stratagraft-as-regenerative-medicine-advanced-therapy-300489200.html 2017 (UD):
https://www.globenewswire.com/en/news-release/2017/08/22/1091116/0/en/Mustang-Bio-Commences-Trading-on-the-NASDAQ-Global-Market.html 9/20/2017 (ATIR101):
https://www.businesswire.com/news/home/20170919006698/en/Kiadis-Pharma-Receives-FDA-Regenerative-Medicine-Advanced-Therapy-RMAT-Designation-for-ATIR101™ 10/1/2017 (LENTIGLOBIN APPROVED AS
ZYNTEGLO FOR THE TREATMENT OF BETA THALASSAEMIA): https://www.raps.org/news-and-articles/news-articles/2019/5/regulatory-intelligence-update-on-regenerative-me 10/2/2017 (AST-OPC1):
https://www.globenewswire.com/news-release/2017/10/02/1138619/0/en/Asterias-Announces-Two-Significant-Developments-for-Spinal-Cord-Injury-Program.html 10/5/2017 (MULTISTEM):
https://seekingalpha.com/news/3299585-fda-designates-athersys-multistem-cell-therapy-for-accelerated-approval-shares-ahead-8 11/1/2017 (JCAR017):
https://www.businesswire.com/news/home/20171101006552/en/Juno-Therapeutics-Reports-Third-Quarter-2017-Financial-Results 11/8/2017 (CEVA101):
https://www.fortressbiotech.com/news-media/press-releases/detail/42/fortress-biotech-announces-cellvations-ceva101-granted 12/21/2017 (MPC-150-IM):
https://www.globenewswire.com/news-release/2017/12/21/1268818/0/en/Mesoblast-Receives-FDA-Regenerative-Medicine-Advanced-Therapy-Designation-for-Its-Cell-Therapy-in-Heart-Failure-Patients-With-Left-Ventricular-Assist-Devices.html
1/29/2018 (EB-101): https://investors.abeonatherapeutics.com/press-releases/detail/109/abeona-receives-fda-regenerative-medicine-advanced-therapy 2/5/2018 (CAP-1002):
https://www.globenewswire.com/news-release/2018/02/05/1333106/29467/en/Capricor-Receives-FDA-Regenerative-Medicine-Advanced-Therapy-RMAT-Designation-for-Duchenne-Muscular-Dystrophy-Therapy.html
3/9/2018 (AMINOFIX INJECTABLE): https://mimedx.gcs-web.com/news-releases/news-release-details/amniofixr-injectable-granted-regenerative-medicine-advanced 4/23/2018 (ABO-102):
https://investors.abeonatherapeutics.com/press-releases/detail/123/abeona-announces-fda-grants-rmat-designationto-abo-102 MAY 2018 (VM-202):
https://www.raps.org/news-and-articles/news-articles/2019/5/regulatory-intelligence-update-on-regenerative-me 2000 (US$53 M):
https://www.fool.com/investing/2018/05/17/sangamo-therapeutics-stock-history.aspx 6/14/2018 (NSR-REP1):
https://www.globenewswire.com/en/news-release/2018/06/14/1524448/0/en/Nightstar-Therapeutics-Receives-Regenerative-Medicine-Advanced-Therapy-RMAT-Designation-for-NSR-REP1-in-Choroideremia.html
6/19/2018 (CLBS14):
https://www.globenewswire.com/news-release/2018/06/19/1526396/18623/en/Caladrius-Receives-FDA-Regenerative-Medicine-Advanced-Therapy-Designation-for-CD34-Cell-Therapy-for-Treating-Refractory-Angina.html
6/21/2018 (VY-AADC):
https://www.globenewswire.com/en/news-release/2018/06/21/1527729/36461/en/Voyager-Therapeutics-Announces-FDA-Regenerative-Medicine-Advanced-Therapy-RMAT-Designation-Granted-for-VY-AADC-for-the-Treatment-of-Parkinson-s-Disease.html
7/2/2018 (ROMYELOCEL-L):
https://www.businesswire.com/news/home/20180702005248/en/Cellerant-Therapeutics-Inc.-Announces-FDA-Grants-Regenerative-Medicine-Advanced-Therapy-RMAT-Designation-for-Romyelocel-L-to-Prevent-Infections-During-Neutropenia
8/21/2018 (AT132):
https://www.prnewswire.com/news-releases/audentes-announces-regenerative-medicine-advanced-therapy-rmat-designation-granted-by-the-fda-to-at132-for-the-treatment-of-x-linked-myotubular-myopathy-300699224.html
10/11/2018 (LIFILEUCEL):
https://www.globenewswire.com/news-release/2018/10/11/1620273/0/en/Iovance-Biotherapeutics-Reports-Results-from-FDA-End-of-Phase-2-meeting-and-Provides-Updates-About-the-Company-s-Clinical-Program.html
10/29/2018 (AVANCE NERVE GRAFT): https://ir.axogeninc.com/press-releases/detail/849/avance-nerve-graft-receives-regenerative-medicine 11/5/2018 (P-BCMA-101):
https://investors.poseida.com/news-releases/news-release-details/poseida-therapeutics-receives-regenerative-medicine-advanced/ 11/27/2018 (RP-L102):
https://ir.rocketpharma.com/news-releases/news-release-details/rocket-pharmaceuticals-receives-fda-regenerative-medicine/ 4/18/2019 (FCR-001):
https://talaristx.com/2019/04/talaris-therapeutics-inc-announces-promising-phase-2-data-of-novel-allogeneic-cell-therapy-in-living-donor-kidney-transplant-recipients/ 4/23/2019 (ECT-001):
https://www.globenewswire.com/news-release/2019/04/23/1807869/0/en/ExCellThera-s-lead-technology-ECT-001-receives-FDA-Regenerative-Medicine-Advanced-Therapy-RMAT-designation.html MAY 2019
(ALOFISEL):
https://www.takeda.com/newsroom/newsreleases/2022/alofisel-darvadstrocel-shows-clinical-remission-rate-at-six-months-in-the-real-world-inspire-study-interim-analysis-consistent-with-the-pivotal-clinical-admire-cd-study/
5/29/2019 (FCX-007):
https://www.globenewswire.com/en/news-release/2019/05/29/1856272/33272/en/Fibrocell-Receives-FDA-Regenerative-Medicine-Advanced-Therapy-Designation-for-FCX-007-Gene-Therapy-for-the-Treatment-of-RDEB.html
6/11/2019 (POSOLEUCEL): https://ir.allovir.com/news-releases/news-release-details/allovir-announces-viralym-m-granted-regenerative-medicine/ 6/24/2019 (KB103):
https://ir.krystalbio.com/news-releases/news-release-details/krystal-biotech-announces-positive-results-phase-2-clinical 7/5/2019 (SB-525):
https://investor.sangamo.com/news-releases/news-release-details/sangamo-and-pfizer-announce-updated-phase-12-results-sb-525 7/29/2019 (OTL-103):
https://ir.orchard-tx.com/news-releases/news-release-details/orchard-therapeutics-announces-fda-regenerative-medicine/ 8/22/2019 (MB-107):
https://ir.mustangbio.com/news-events/press-releases/detail/68/mustang-bio-and-st-jude-childrens-research-hospital 9/4/2019 (MGTA-456):
https://investor.magentatx.com/news-releases/news-release-details/magenta-therapeutics-announces-fda-regenerative-medicine/ 9/20/2019 (SB623):
https://www.pharmaceutical-business-review.com/news/sanbio-rmat-sb623-cell-therapy/ 10/28/2019 (CT053):
https://www.prnewswire.com/news-releases/carsgen-announces-investigational-car-t-therapy-ct053-granted-rmat-designation-by-the-us-fda-for-rr-multiple-myeloma-300945966.html 12/3/2019
(ADP-A2M4): https://www.adaptimmune.com/investors-and-media/news-center/press-releases/detail/11/regenerative-medicine-advanced-therapy-designation-granted 2/27/2020 (TT11):
https://www.thepharmaletter.com/article/tessa-picks-up-rmat-designation-for-novel-car-t-cell-candidate 4/16/2020 (TTAX02):
https://www.prnewswire.com/news-releases/tissuetech-receives-regenerative-medicine-advanced-therapy-rmat-designation-from-us-food-and-drug-administration-301042276.html 4/22/2020
(FDA-APPROVED AS KYMRIAH): https://www.novartis.com/news/media-releases/novartis-kymriah-receives-fda-regenerative-medicine-advanced-therapy-designation-follicular-lymphoma 5/6/2020
(ILIXADENCEL):
https://www.globenewswire.com/news-release/2020/05/06/2028129/0/en/Immunicum-AB-publ-Receives-Regenerative-Medicine-Advanced-Therapy-Designation-from-FDA-for-Ilixadencel-in-Kidney-Cancer.html
5/11/2020 (CTX001):
https://crisprtx.com/about-us/press-releases-and-presentations/crispr-therapeutics-and-vertex-pharmaceuticals-announce-fda-regenerative-medicine-advanced-therapy-rmat-designation-granted-to-ctx001-for-the-treatment-of-severe-hemoglobinopathies
9/22/2020 (MDR-101): https://www.medeortx.com/news/pr-2020-09-22.php 9/23/2020 (MULTISTEM):
https://www.athersys.com/investors/press-releases/press-release-details/2020/FDA-Grants-RMAT-Designation-to-MultiStem-Cell-Therapy-for-the-Treatment-of-Acute-Respiratory-Distress-Syndrome/default.aspx
10/14/2020 (ORCA-T): https://www.globenewswire.com/news-release/2020/10/14/2108307/0/en/Orca-Bio-Receives-Regenerative-Medicine-Advanced-Therapy-RMAT-Designation-for-Orca-T.html 11/11/2020
(AB205): https://angiocrinebioscience.com/news-events/news/ 12/17/2020 (AMDC-USR):
https://www.biospace.com/article/fda-grants-rmat-designation-for-cook-myosite-s-investigational-autologous-muscle-derived-cells-for-urinary-sphincter-repair/ 1/11/2021 (RENU):
https://organogenesis.com/news-events/press-release-01112021.html 3/8/2021 (VALOCTOCOGENE ROXAPARVOVEC):
https://investors.biomarin.com/2021-03-08-BioMarin-Announces-FDA-Regenerative-Medicine-Advanced-Therapy-RMAT-Designation-Granted-to-Valoctocogene-Roxaparvovec-Investigational-Gene-Therapy-for-Hemophilia-A
3/9/2021 (RP-L201): https://ir.rocketpharma.com/news-releases/news-release-details/rocket-pharmaceuticals-receives-fda-regenerative-medicine-0/ 4/21/2021 (ALLO-715):
https://ir.allogene.com/news-releases/news-release-details/allogene-therapeutics-announces-fda-regenerative-medicine/ OCT 2021 (REACT):
https://investors.prokidney.com/news-releases/news-release-details/prokidney-corroborates-mechanism-action-reacttm-cell-marker 11/22/2021 (CTX110):
http://ir.crisprtx.com/news-releases/news-release-details/crispr-therapeutics-announces-fda-regenerative-medicine-advanced/ 12/13/2021 (FT516):
https://ir.fatetherapeutics.com/news-releases/news-release-details/fate-therapeutics-highlights-positive-durability-response-data 2022 SPAC MERGER (UD):
https://medcitynews.com/2022/01/regenerative-med-biotech-prokidney-inks-825m-merger-to-back-ckd-cell-therapy/ 1/5/2022 (POSOLEUCEL):
https://ir.allovir.com/news-releases/news-release-details/fda-grants-regenerative-medicine-advanced-therapy-rmat/ 1/10/2022 (CT041):
https://www.prnewswire.com/news-releases/carsgen-announces-ct041-car-t-cell-product-candidate-granted-rmat-designation-by-the-fda-301456859.html 1/12/2022 (C-CAR039):
https://www.cellbiomedgroup.com/newsroom/fda-rmat?lang=en 2/14/2022 (APROART): https://www.ucsf.edu/news/2022/02/422276/ucsf-gene-therapy-deadly-mutation-fast-tracked-fda-review 4/12/2022
(EXOFLO): https://directbiologics.com/fda-grants-direct-biologics-regenerative-medicine-advanced-therapy-rmat-designation-for-the-use-of-exoflo-in-covid-19-related-ards/ 4/20/2022
(POSOLEUCEL): https://ir.allovir.com/news-releases/news-release-details/fda-grants-regenerative-medicine-advanced-therapy-rmat-0/ 4/25/2022 (OBE-CEL):
https://autolus.gcs-web.com/news-releases/news-release-details/fda-grants-regenerative-medicine-advanced-therapy-rmat/ 5/13/2022 (SKINTE):
https://www.prnewswire.com/news-releases/polarityte-announces-fda-regenerative-medicine-advanced-therapy-designation-granted-to-skinte-301546677.html 5/24/2022 (NEOCART):
https://ir.ocugen.com/news-releases/news-release-details/ocugen-announces-new-cell-therapy-program-following-fda/ 6/8/2022 (ALLO-501A):
https://ir.allogene.com/news-releases/news-release-details/allogene-therapeutics-announces-fda-granted-regenerative/ 9/28/2022 (CTX130):
https://crisprtx.com/about-us/press-releases-and-presentations/crispr-therapeutics-announces-fda-regenerative-medicine-advanced-therapy-rmat-designation-granted-to-ctx130-for-the-treatment-of-cutaneous-t-cell-lymphomas-ctcl
11/8/2022 (ADP-A2M4CD8): https://www.adaptimmune.com/investors-and-media/news-center/press-releases/detail/234/adaptimmune-reports-increased-response-rate-and-durability 11/29/2022
(CB-010): https://investor.cariboubio.com/news-releases/news-release-details/caribou-biosciences-announces-fda-granted-regenerative-medicine/ 1/26/2023 (REBONUPUTEMCEL):
https://www.discgenics.com/news-posts/2023/1/26/discgenics-announces-fda-regenerative-medicine-advanced-therapy-rmat-designation-granted-to-idct-for-degenerative-disc-disease 2/7/2023
(RP-A501): https://ir.rocketpharma.com/news-releases/news-release-details/rocket-pharmaceuticals-receives-fda-regenerative-medicine-1/ 2/8/2023 (REXLEMESTROCEL-L):
https://www.globenewswire.com/en/news-release/2023/02/08/2604535/0/en/FDA-Grants-Regenerative-Medicine-Advanced-Therapy-Rmat-Designation-for-Rexlemestrocel-L-in-Chronic-Low-Back-Pain.html
2/11/2023 (CT103A): https://www.iasobio.com/phone/info.php?id=215 3/21/2023 (NTLA-2002):
https://ir.intelliatx.com/news-releases/news-release-details/intellia-therapeutics-announces-fda-regenerative-medicine 5/4/2023 (HAV):
https://www.globenewswire.com/news-release/2023/05/04/2661452/0/en/Humacyte-s-Human-Acellular-Vessel-HAV-Receives-FDA-s-Regenerative-Medicine-Advanced-Therapy-RMAT-Designation-for-Urgent-Arterial-Repair-Following-Vascular-Trauma.html
5/23/2023 (RP-L301): https://ir.rocketpharma.com/news-releases/news-release-details/rocket-pharmaceuticals-receives-fda-regenerative-medicine-2/ 5/24/2023 (RGX-121):
https://www.prnewswire.com/news-releases/regenxbio-receives-fda-regenerative-medicine-advanced-therapy-rmat-designation-for-rgx-121-gene-therapy-for-hunter-syndrome-301831944.html 1997
(US$20 M): https://investors.vcel.com/node/11886/html 1999 (US$58 M): https://www.bioworld.com/articles/392839-biomarin-completes-58m-ipo-as-first-product-nears-market?v=preview 2004 (US$21
M): https://www.intelligentinvestor.com.au/shares/asx-msb/mesoblast-limited/float 2007 (US$56 M): https://www.pehub.com/tigenix-ipo-prices-in-brussels/ 2007 REVERSE MERGER (UD):
https://www.cleveland.com/business/2007/06/athersys_goes_public_in_revers.html 2008 (UD): https://roic.ai/company/IOVA 2008 REVERSE MERGER (UD):
https://www.biospace.com/article/mimedx-announces-post-split-name-change-/ 2011 REVERSE MERGER (UD):
https://www.cfodive.com/news/reverse-merger-helped-recession-hit-axogen-regain-footing/602531/ 2013 (SEK 21 M):
https://www.marketscreener.com/quote/stock/MENDUS-AB-PUBL-17946401/news/Immunicum-AB-has-completed-an-IPO-in-the-amount-of-SEK-21-40-million-39111826/ 2013 (US$116 M):
https://investor.bluebirdbio.com/news-releases/news-release-details/bluebird-bio-announces-closing-initial-public-offering-and 2013 (US$40 M):
https://www.fiercebiotech.com/r-d/fate-ipo-flop-raises-fresh-concerns-about-investors-appetite-for-biotech 2013 REVERSE MERGER (UD):
https://www.biospace.com/article/china-s-b-cellular-biomedicine-group-b-announces-reverse-merger-/ 2013 REVERSE MERGER (UD):
https://www.businesswire.com/news/home/20131121005377/en/Capricor-and-Nile-Therapeutics-Complete-Merger-to-Form-Capricor-Therapeutics-Inc. 2014 (US$265 M):
https://www.fiercebiotech.com/r-d/updated-juno-banks-a-265m-ipo-pushing-next-big-thing-oncology 2015 (US$124 M):
https://www.businesswire.com/news/home/20150408005269/en/SanBio-Announces-Pricing-of-Initial-Public-Offering-on-Tokyo-Stock-Exchange 2015 (US$159 M):
https://www.globenewswire.com/news-release/2015/09/22/770163/10150220/en/REGENXBIO-Announces-Closing-of-Initial-Public-Offering-and-Full-Exercise-of-Underwriters-Option-to-Purchase-Additional-Shares.html
2015 (US$191 M): https://www.nasdaq.com/articles/adaptimmune-therapeutics-prices-upsized-ipo-17-high-end-range-2015-05-05 2015 (US$36 M):
https://www.fiercebiotech.com/r-d/kiadis-banks-36m-a-euro-ipo-to-fund-its-stem-cell-r-d 2015 (US$5.5 M):
https://www.prnewswire.com/news-releases/asterias-biotherapeutics-completes-public-offering-and-private-placement-of-common-stock-300033976.html 2015 (US$81 M):
https://ir.voyagertherapeutics.com/news-releases/news-release-details/voyager-therapeutics-announces-closing-initial-public-offering/ 2016 (US$113 M):
https://ir.intelliatx.com/news-releases/news-release-details/intellia-therapeutics-announces-closing-initial-public-offering 2016 (US$56 M):
https://www.fiercebiotech.com/biotech/crispr-therapeutics-raises-a-56m-ipo-but-patent-battles-potential-stock-drops-loom 2016 (US$75 M):
https://pitchbook.com/newsletter/audentes-raises-75m-in-ipo 2016 ACQUIRED BY MALLINCKRODT (UD):
https://www.fiercebiotech.com/medical-devices/mallinckrodt-acquires-regenerative-medicine-company-stratatech 2017 (US$46 M):
https://www.globenewswire.com/en/news-release/2017/09/22/1131592/0/en/Krystal-Biotech-Announces-Closing-of-Initial-Public-Offering-and-Full-Exercise-of-Underwriters-Option-to-Purchase-Additional-Shares.html
2017 (US$77 M):
https://www.globenewswire.com/en/news-release/2017/10/02/1139020/0/en/Nightstar-Announces-Closing-of-Initial-Public-Offering-and-Full-Exercise-of-Underwriters-Option-to-Purchase-Additional-American-Depositary-Shares.html
2017 REVERSE MERGER (UD):
https://www.globenewswire.com/news-release/2017/04/07/1052850/0/en/PolarityTE-TM-Inc-Announces-Completion-of-Merger-and-Acquires-Important-Intellectual-Property-for-Regenerative-Medicine-Programs.html
2018 (US$100 M): https://www.businesswire.com/news/home/20180625006210/en/Magenta-Therapeutics-Announces-Closing-of-Initial-Public-Offering 2018 (US$160 M):
https://autolus.gcs-web.com/news-releases/news-release-details/autolus-announces-closing-initial-public-offering-and-full/ 2018 (US$225 M):
https://ir.orchard-tx.com/news-releases/news-release-details/orchard-therapeutics-announces-closing-225-million-initial/ 2018 (US$373 M):
https://ir.allogene.com/news-releases/news-release-details/allogene-therapeutics-announces-closing-initial-public-offering/ 2018 ACQUIRED BY CELGENE (US$9,000 M):
https://www.geekwire.com/2018/juno-therapeutics-acquired-celgene-9b-dramatic-deal-rising-biotech-star/ 2018 ACQUIRED BY TAKEDA PHARMACEUTICAL COMPANY (US$608 M):
https://www.pharmaceutical-technology.com/news/takeda-tigenix-acquisition-608m/ 2018 REVERSE MERGER (UD):
https://endpts.com/gene-therapy-startup-rocket-pharma-reverse-merges-with-troubled-inotek-after-25m-raise/ 2018 REVERSE MERGER (UD):
https://organogenesis.com/news-events/press-release-12102018.html 2019 ACQUIRED BY BIOGEN (US$800 M):
https://investors.biogen.com/news-releases/news-release-details/biogen-completes-acquisition-nightstar-therapeutics 2019 ACQUIRED BY BIOTIME (UD):
https://www.spglobal.com/marketintelligence/en/news-insights/trending/dgy96s7WowE7oCnW-Mu7KA2 2019 ACQUIRED BY CASTLE CREEK PHARMACEUTICAL (US$63 M):
https://www.globenewswire.com/en/news-release/2019/09/12/1915187/33272/en/Castle-Creek-Pharmaceutical-Holdings-to-Acquire-Fibrocell.html 2019 ACQUIRED BY SUMITOMO DAINIPPON PHARMA (UD):
https://enzyvant.com/sumitomo-dainippon-pharma-completes-the-formation-of-the-strategic-alliance-with-roivant-sciences/ 2019 REVERSE MERGER (UD):
https://www.nbcphiladelphia.com/news/local/malvern-gene-therapy-company-completes-reverse-merger/219782/ 2020 (US$224 M):
https://investors.poseida.com/news-releases/news-release-details/poseida-therapeutics-announces-pricing-initial-public-offering/ 2020 (US$318 M):
https://www.globenewswire.com/en/news-release/2020/08/03/2072010/0/en/AlloVir-Announces-Closing-of-Upsized-Initial-Public-Offering-and-Full-Exercise-of-the-Underwriters-Option-to-Purchase-Additional-Shares.html
2020 ACQUIRED BY ASTELLAS PHARMA (US$3,000 M): https://www.spglobal.com/marketintelligence/en/news-insights/trending/VpqpVltOd5YKifSvTvBf2A2 2021 (US$150 M):
https://ir.talaristx.com/news-releases/news-release-details/talaris-therapeutics-announces-closing-initial-public-offering/ 2021 (US$304 M):
https://www.businesswire.com/news/home/20210727006010/en/Caribou-Biosciences-Announces-Closing-of-Upsized-Initial-Public-Offering 2021 (US$400 M):
https://www.bioworld.com/articles/508424-car-t-developer-carsgen-debuts-on-hkex-with-400m-ipo-targets-bcma-first?v=preview 2021 ACQUIRED BY CONSORTIUM (US$411 M):
https://www.prnewswire.com/news-releases/cellular-biomedicine-group-inc-announces-completion-of-merger-301231873.html 2021 ACQUIRED BY SANOFI (US$358 M):
https://www.fiercebiotech.com/biotech/sanofi-inks-358m-kiadis-takeover-to-acquire-nk-cell-platform 2021 REVERSE MERGER (UD):
https://www.marketscreener.com/quote/stock/HUMACYTE-INC-126300449/news/Humacyte-Inc-completed-the-acquisition-of-Alpha-Healthcare-Acquisition-Corp-from-a-group-of-share-36263997/ 2022
ACQUIRED BY CANARIA BIO (UD): https://www.koreabiomed.com/news/articleView.html?idxno=20085 2022 MERGER WITH CEND THERAPEUTICS (UD):
https://www.globenewswire.com/en/news-release/2022/09/15/2516989/18623/en/Caladrius-Biosciences-and-Cend-Therapeutics-Announce-Closing-of-Merger-and-the-Emergence-of-Lisata-Therapeutics.html
20 CELLULAR AND GENE THERAPY PRODUCTS: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products 21 CFR 312:
https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRsearch.cfm?CFRPart=312 525 NON-REGENERATIVE-MEDICINE DRUGS:
https://www.fda.gov/drugs/development-approval-process-drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products 7% IN BANK FEES FOR A US$100 MILLION IPO:
https://news.crunchbase.com/public/want-to-take-your-startup-public-heres-what-it-actually-costs-to-ipo/ 86 RMAT DESIGNATIONS:
https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/cumulative-cber-regenerative-medicine-advanced-therapy-rmat-designation-requests-received-fiscal A LIST AND
RANKING OF INCUBATORS AND ACCELERATORS WORLDWIDE: https://www.andeglobal.org/publication/the-ubi-global-world-rankings-of-business-incubators-and-accelerators/ A US$20 MILLION AWARD FROM THE
CALIFORNIA INSTITUTE FOR REGENERATIVE MEDICINE (CIRM): https://www.fiercebiotech.com/biotech/stemcells-inc-awarded-20-million-from-california-institute-for-regenerative-medicine-for ABECMA
(2021): https://www.fda.gov/news-events/press-announcements/fda-approves-first-cell-based-gene-therapy-adult-patients-multiple-myeloma ACCELERATORS:
https://www.feedough.com/accelerator-vs-incubator/ ACQUIRED BY CELGENE: https://www.geekwire.com/2018/juno-therapeutics-acquired-celgene-9b-dramatic-deal-rising-biotech-star/ ACQUIRED BY
MALLINCKRODT PHARMACEUTICALS: https://www.fiercebiotech.com/medical-devices/mallinckrodt-acquires-regenerative-medicine-company-stratatech ACQUIRED BY NOVARTIS:
https://www.novartis.com/news/media-releases/novartis-enters-agreement-acquire-avexis-inc-usd-87-bn-transform-care-sma-and-expand-position-gene-therapy-and-neuroscience-leader ACQUIRED IN A
REVERSE MERGER:
https://www.businesswire.com/news/home/20190930005241/en/Ocugen-Announces-Completion-of-its-Merger-with-Histogenics-to-Create-Nasdaq-Listed-Clinical-Stage-Company-Developing-Novel-Ocular-Gene-Therapies-and-Biotherapeutics
ACQUIRED THROUGH A REVERSE MERGER: https://www.massdevice.com/stemcells-soars-on-reverse-merger-with-israels-microbot-medical/ ADSTILADRIN (2022):
https://www.ferring.com/ferring-receives-approval-from-u-s-fda-for-adstiladrin-for-high-risk-bcg-unresponsive-non-muscle-invasive-bladder-cancer/ AMGEN:
https://sciencebusiness.net/news/74714/UCL-spin-out-sold-to-Amgen-in-%241-billion-deal AMOUNT FOR A SEED ROUND: https://www.paddle.com/resources/guide-to-seed-funding-for-startups ANALYSTS
CONSIDER: https://www.statnews.com/2016/06/01/stem-cell-company-shutters APPROVED AS BREYANZI:
https://news.bms.com/news/details/2021/U.S.-Food-and-Drug-Administration-Approves-Bristol-Myers-Squibbs-Breyanzi-lisocabtagene-maraleucel-a-New-CAR-T-Cell-Therapy-for-Adults-with-Relapsed-or-Refractory-Large-B-cell-Lymphoma/default.aspx
APPROVED AS RETHYMIC): https://endpts.com/after-three-decades-and-a-surprise-rejection-first-treatment-for-babies-born-without-a-thymus-secures-fda-approval/ APPROVED BY THE US FOOD AND
DRUG ADMINISTRATION: https://www.fda.gov/vaccines-blood-biologics/zolgensma APPROVED: https://www.fda.gov/vaccines-blood-biologics/stratagraft APROART:
https://www.cirm.ca.gov/our-progress/awards/ex-vivo-transduction-human-artemis-dclre1c-cdna-lentiviral-vector-aproart-cd34 ATIR101:
https://www.cancer.gov/publications/dictionaries/cancer-drug/def/allodepleted-t-cell-immunotherapeutic-atir101 AVERAGES FOR VC SERIES A, B, AND C:
https://www.evaluate.com/vantage/articles/insights/venture-financing-ipo/venture-financing-holds-steady-device-makers AVEXIS:
https://www.novartis.com/news/media-releases/novartis-enters-agreement-acquire-avexis-inc-usd-87-bn-transform-care-sma-and-expand-position-gene-therapy-and-neuroscience-leader BAYLOR COLLEGE
OF MEDICINE: https://elevate.bio/press-releases/elevatebio-announces-that-allovir-joins-its-portfolio-of-highly-innovative-cell-and-gene-therapy-companies/;
https://www.tessacell.com/about-tessa-therapeutics/ BIOVEX: https://mergr.com/amgen-acquires-biovex-group BLA CLINICAL REVIEW: https://www.fda.gov/vaccines-blood-biologics/stratagraft BLA
PHARMACOLOGY/TOXICOLOGY REVIEW OF STRATAGRAFT: https://www.fda.gov/vaccines-blood-biologics/stratagraft BLUEBIRD BIO (FOUNDED AS GENETIX PHARMACEUTICALS):
https://investor.bluebirdbio.com/news-releases/news-release-details/genetix-pharmaceuticals-renamed-bluebird-bio-announces BREYANZI (2021):
https://news.bms.com/news/details/2021/U.S.-Food-and-Drug-Administration-Approves-Bristol-Myers-Squibbs-Breyanzi-lisocabtagene-maraleucel-a-New-CAR-T-Cell-Therapy-for-Adults-with-Relapsed-or-Refractory-Large-B-cell-Lymphoma/default.aspx
CALIFORNIA INSTITUTE FOR REGENERATIVE MEDICINE (CIRM): https://www.cirm.ca.gov/ CARTILAGE REPAIR PRODUCTS:
https://www.fda.gov/regulatory-information/search-fda-guidance-documents/preparation-ides-and-inds-products-intended-repair-or-replace-knee-cartilage CARVYKTI (2022):
https://www.jnj.com/u-s-fda-approves-carvykti-ciltacabtagene-autoleucel-janssens-first-cell-therapy-a-bcma-directed-car-t-immunotherapy-for-the-treatment-of-patients-with-relapsed-or-refractory-multiple-myeloma
CASE WESTERN RESERVE UNIVERSITY:
https://case.edu/medicine/about/newsroom/our-latest-news/case-western-reserve-center-stem-cell-and-regenerative-medicine-and-athersys-receive-1m-third-frontier-grant-support-research-spinal-cord-injury
CELLULAR IMMUNOTHERAPY PRODUCT PROVENGE: https://stanmed.stanford.edu/pioneering-cancer-drug-provenge-fresh-look CFR TITLE 21:
https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm CGT PRODUCTS:
https://www.fda.gov/regulatory-information/search-fda-guidance-documents/preclinical-assessment-investigational-cellular-and-gene-therapy-products CHILDREN’S HOSPITAL OF PHILADELPHIA
UNIVERSITY OF PENNSYLVANIA: https://www.chop.edu/news/childrens-hospital-philadelphia-celebrates-fda-approval-gene-therapy-inherited-blindness CITY OF HOPE NATIONAL MEDICAL CENTER:
https://www.cityofhope.org/news/phase-1-clinical-trial-of-mb-101 CLINICAL TRIALS:
https://www.fda.gov/patients/clinical-trials-what-patients-need-know/what-are-different-types-clinical-research COMPLETED AN INITIAL PUBLIC OFFERING (IPO):
https://www.globenewswire.com/en/news-release/2016/02/11/809635/0/en/AveXis-Announces-Pricing-of-Initial-Public-Offering.html CONSIDERED SAFETY DATA GENERATED FROM THE PHASE I/IIA AND PHASE
II TRIALS FOR AN ALTERNATE INDICATION: https://www.fda.gov/vaccines-blood-biologics/stratagraft CONVERTIBLE NOTE: https://www.investopedia.com/terms/s/senior-convertible-note.asp COOPERATIVE
PATENT CLASSIFICATION SYSTEM: https://www.cooperativepatentclassification.org/about CURRENT GOOD MANUFACTURING PRACTICE (CGMP) REGULATIONS:
https://www.fda.gov/drugs/pharmaceutical-quality-resources/current-good-manufacturing-practice-cgmp-regulations DILUTION OF EQUITY:
https://www.svb.com/startup-insights/startup-equity/startup-equity-dilution DUKE UNIVERSITY, MASSACHUSETTS INSTITUTE OF TECHNOLOGY:
https://wraltechwire.com/2021/02/18/a-blooming-success-the-rise-of-humacyte-from-humble-beginnings-to-1-1b-valuation/ DUKE UNIVERSITY:
https://endpts.com/after-three-decades-and-a-surprise-rejection-first-treatment-for-babies-born-without-a-thymus-secures-fda-approval/ DUKE UNIVERSITY:
https://endpts.com/after-three-decades-and-a-surprise-rejection-first-treatment-for-babies-born-without-a-thymus-secures-fda-approval/ ‘END OF PHASE’ MEETINGS:
https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/otp-ind-milestone-type-b-meetings ENZYVANT THERAPEUTICS GMBH LICENCED THE TECHNOLOGY FROM DUKE UNIVERSITY:
https://www.prnewswire.com/news-releases/enzyvant-to-develop-novel-biologic-therapy-for-complete-digeorge-syndrome-300386922.html ESTABLISHED IP:
https://patents.justia.com/assignee/histogenics-corporation EXCEEDING US$20 BILLION IN 2021: https://alliancerm.org/sector-report/2021-annual-report/ EXCESS OF THESE AMOUNTS:
https://grants.nih.gov/grants/guide/notice-files/NOT-HL-22-065.html FDA APPROVAL OF JCAR017:
https://news.bms.com/news/details/2021/U.S.-Food-and-Drug-Administration-Approves-Bristol-Myers-Squibbs-Breyanzi-lisocabtagene-maraleucel-a-New-CAR-T-Cell-Therapy-for-Adults-with-Relapsed-or-Refractory-Large-B-cell-Lymphoma/default.aspx
FERRING PHARMACEUTICALS: https://ferringusa.com/?press=ferring-and-blackstone-life-sciences-invest-over-570-million-usd-in-novel-gene-therapy-for-bladder-cancer-patients FINANCIAL EXIT
STRATEGIES: https://www.investopedia.com/terms/b/business-exit-strategy.asp FOUNDED IN 2013:
https://www.prnewswire.com/news-releases/spark-therapeutics-launched-with-50-million-in-financing-to-advance-late-and-mid-stage-gene-therapy-programs-with-clinical-proof-of-concept-228752221.html
FRED HUTCHINSON CANCER CENTER, ST JUDE CHILDREN’S RESEARCH HOSPITAL: https://ir.mustangbio.com/news-events/press-releases/detail/68/mustang-bio-and-st-jude-childrens-research-hospital FRED
HUTCHINSON CANCER RESEARCH CENTER MEMORIAL SLOAN-KETTERING CANCER CENTER SEATTLE CHILDREN’S RESEARCH INSTITUTE:
https://www.forbes.com/sites/matthewherper/2014/08/05/why-this-cancer-fighting-company-has-raised-300-million-in-just-12-months/ FRED HUTCHINSON CANCER RESEARCH CENTER MEMORIAL
SLOAN-KETTERING CANCER CENTER SEATTLE CHILDREN’S RESEARCH INSTITUTE UNIVERSITY OF CALIFORNIA, SAN FRANCISCO ST JUDE CHILDREN’S RESEARCH HOSPITAL:
https://www.businesswire.com/news/home/20171101006552/en/Juno-Therapeutics-Reports-Third-Quarter-2017-Financial-Results FUNDING CAPS: https://www.sbir.gov/about GENESIS SEED FUND:
https://research.rutgers.edu/researcher-support/commercialize-your-innovation/launch-startup/new-ventures/genesis-seed-fund GILEAD SCIENCES:
https://www.gilead.com/news-and-press/company-statements/kite-pharma HARVARD UNIVERSITY, MASSACHUSETTS INSTITUTE OF TECHNOLOGY: https://news.mit.edu/2001/sicklecell HARVARD UNIVERSITY,
WASHINGTON UNIVERSITY IN ST LOUIS, STANFORD UNIVERSITY, SAN RAFFAELE TELETHON INSTITUTE FOR GENE THERAPY, UNIVERSITY OF BASEL:
https://investor.magentatx.com/news-releases/news-release-details/magenta-therapeutics-announces-485m-series-financing-transform/ HARVARD UNIVERSITY:
https://hsci.harvard.edu/company-startups HARVARD UNIVERSITY: https://hsci.harvard.edu/company-startups HEMGENIX (2022):
https://www.fda.gov/news-events/press-announcements/fda-approves-first-gene-therapy-treat-adults-hemophilia-b HIGHLY COMPETITIVE: https://grantengine.com/sbir-sttr-paylines-by-nih-institute/
IMLYGIC (2015): https://www.amgen.com/newsroom/press-releases/2015/10/fda-approves-imlygic-talimogene-laherparepvec-as-first-oncolytic-viral-therapy-in-the-us INITIAL TARGETED ENGAGEMENT
FOR REGULATORY ADVICE ON CBER/CDER PRODUCTS (INTERACT) MEETING: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/otp-interact-meeting INTERPRETATIONS OF SECTION
506(G)(8) BY THE US FOOD AND DRUG ADMINISTRATION (FDA): https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/resources-related-regenerative-medicine-therapies
‘INVENTIVE STEP’: https://www.wipo.int/pct/en/texts/ispe/13_01_02.html INVESTIGATIONAL NEW DRUG APPLICATION: https://www.fda.gov/vaccines-blood-biologics/stratagraft IPO:
https://pitchbook.com/blog/ipo-process-explained JLABS: https://jnjinnovation.com/home JOHNS HOPKINS UNIVERSITY: https://www.sdbj.com/technology/capricor-therapeutics-relocates-la-jolla/
JOHNSON & JOHNSON: https://johnsonandjohnson.gcs-web.com/news-releases/news-release-details/johnson-johnson-announces-completion-merger-centocor-inc/ JUNO THERAPEUTICS (FOUNDED AS FC
THERAPEUTICS): https://pitchbook.com/profiles/company/60580-27#overview JUNO THERAPEUTICS:
https://www.geekwire.com/2018/juno-therapeutics-acquired-celgene-9b-dramatic-deal-rising-biotech-star/ KATHOLIEKE UNIVERSITEIT LEUVEN GHENT UNIVERSITY:
https://www.flandersinvestmentandtrade.com/invest/en/news/tigenix-raise-20-million-euro-product-trials KYMRIAH (2017):
https://www.novartis.com/news/media-releases/novartis-receives-first-ever-fda-approval-car-t-cell-therapy-kymriahtm-ctl019-children-and-young-adults-b-cell-all-refractory-or-has-relapsed-least-twice
KYMRIAH: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/kymriah-tisagenlecleucel LUXTURNA (2017):
https://sparktx.com/press_releases/fda-approves-spark-therapeutics-luxturna-voretigene-neparvovec-rzyl-a-one-time-gene-therapy-for-patients-with-confirmed-biallelic-rpe65-mutation-associated-retinal-dystrophy/
LUXTURNA TO BE APPROVED BY THE US FOOD AND DRUG ADMINISTRATION:
https://sparktx.com/press_releases/fda-approves-spark-therapeutics-luxturna-voretigene-neparvovec-rzyl-a-one-time-gene-therapy-for-patients-with-confirmed-biallelic-rpe65-mutation-associated-retinal-dystrophy/
M&A: https://www.sba.gov/business-guide/grow-your-business/merge-acquire-businesses MACI (2016):
https://investors.vcel.com/news-releases/news-release-details/fda-approves-maci-treatment-symptomatic-cartilage-defects-knee MALLINCKRODT PHARMACEUTICALS:
https://mallinckrodt.gcs-web.com/news-releases/news-release-details/mallinckrodt-announces-us-fda-approval-stratagraftr-allogeneic/ MASSACHUSETTS INSTITUTE OF TECHNOLOGY:
https://organogenesis.com/about-us/ MASSACHUSETTS INSTITUTE OF TECHNOLOGY: https://organogenesis.com/about-us/ MGTA-456:
https://investor.magentatx.com/news-releases/news-release-details/magenta-therapeutics-announces-updated-phase-2-data-mgta-456/ NATIONAL CANCER INSTITUTE:
https://www.stocktitan.net/news/IOVA/iovance-biotherapeutics-announces-first-patient-randomized-in-phase-1fnhlejx69wn.html NATIONWIDE CHILDREN’S HOSPITAL OHIO STATE UNIVERSITY:
https://www.nationwidechildrens.org/newsroom/news-releases/2013/10/avexis-biolife-licenses-spinal-muscular-atrophy-sma-patent-portfolio-from-nationwide-childrens NATIONWIDE CHILDREN’S
HOSPITAL:
https://www.prnewswire.com/news-releases/abeona-therapeutics-and-nationwide-childrens-hospital-receive-prestigious-global-genes-champions-of-hope-award-for-patient-advocacy-227092241.html
NIH DATA MANAGEMENT AND SHARING POLICY: https://oir.nih.gov/sourcebook/intramural-program-oversight/intramural-data-sharing/2023-nih-data-management-sharing-policy NIH’S COMMERCIALIZATION
READINESS PILOT (CRP) PROGRAMME: https://seed.nih.gov/support-for-small-businesses/commercialization-enhancement-programs/crp NO MAJOR SAFETY CONCERNS WERE GENERATED FROM THE PRECLINICAL
STUDIES: https://www.fda.gov/vaccines-blood-biologics/stratagraft NSF-FUNDED INNOVATION-CORPS (I-CORPS): https://beta.nsf.gov/funding/initiatives/i-corps/about-i-corps OBTAINED REGENERATIVE
MEDICINE ADVANCED THERAPY DESIGNATION: https://www.prnewswire.com/news-releases/us-fda-designates-mallinckrodts-stratagraft-as-regenerative-medicine-advanced-therapy-300489200.html ONLY THE
PHASE IB AND PHASE III TRIALS WERE CONSIDERED FOR EFFICACY: https://www.fda.gov/vaccines-blood-biologics/stratagraft ORCA-T:
https://ashpublications.org/blood/article/140/Supplement1/654/487614/Precision-Engineered-Cell-Therapy-Orca-T OTL-103:
https://ir.orchard-tx.com/news-releases/news-release-details/orchard-therapeutics-announces-fda-regenerative-medicine/ OVER 1,000 NEW DEVICES:
https://www.fda.gov/about-fda/center-devices-and-radiological-health/cdrh-reports UNIVERSITY OF OXFORD, UNIVERSITY OF PENNSYLVANIA: https://www.adaptimmune.com/our-company PATENT COOPERATION
TREATY (PCT): https://www.wipo.int/pct/en/faqs/faqs.html PEAK SALES FORECAST: https://www.europeanpharmaceuticalreview.com/news/179320/cd19-car-t-agents-to-boost-blood-cancer-market/ PHASE
0 GRANTS: https://www.sbir.gov/sites/default/files/SBIR-Table_StateMatchingPhase0_Sept2020.pdf PLEDGED A US$50 MILLION COMMITMENT FROM THE CHILDREN’S HOSPITAL OF PHILADELPHIA:
https://www.prnewswire.com/news-releases/spark-therapeutics-launched-with-50-million-in-financing-to-advance-late-and-mid-stage-gene-therapy-programs-with-clinical-proof-of-concept-228752221.html
PRE-IND MEETING: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/otp-pre-ind-meetings PRIVATE EQUITY (PE): https://www.investopedia.com/terms/p/privateequity.asp
PRODUCT REVENUE OF BREYANZI: https://bioprocessintl.com/bioprocess-insider/therapeutic-class/bms-sees-car-t-sales-rocket-in-line-with-increased-capacity/ PROMOTION AND TENURE-INNOVATION AND
ENTREPRENEURSHIP (PTIE) COALITION: https://ptie.org/ PROVENGE (2010): https://stanmed.stanford.edu/pioneering-cancer-drug-provenge-fresh-look/ QUALITY MANAGEMENT SYSTEM:
https://www.nidcr.nih.gov/sites/default/files/2017-12/quality-management-of-clinical-research-brief-overview.doc RAISED A TOTAL OF US$310 MILLION:
https://www.crunchbase.com/organization/juno-therapeutics/company_financials RAISED AN ADDITIONAL US$72.8 MILLION IN SERIES B FUNDING:
https://www.prnewswire.com/news-releases/spark-therapeutics-raises-728-million-in-oversubscribed-financing-260806381.html RAISED US$304 MILLION THROUGH ITS INITIAL PUBLIC OFFERING:
https://www.nasdaq.com/articles/juno-therapeutics-prices-upsized-ipo-24-largest-2014-biotech-ipo-22-billion-market-cap REASON FOR NONCOMPLIANCE:
https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=312&showFR=1 REGENERATIVE MEDICINE ADVANCED THERAPY (RMAT) DESIGNATION:
https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/regenerative-medicine-advanced-therapy-designation REGENERATIVE MEDICINE MINNESOTA: https://www.regenmedmn.org/
RETHYMIC (2021):
https://enzyvant.com/enzyvant-receives-fda-approval-for-rethymic-allogeneic-processed-thymus-tissue-agdc-a-one-time-regenerative-tissue-based-therapy-for-pediatric-congenital-athymia/
REVERSE MERGER: https://www.investopedia.com/terms/r/reversetakeover.asp REXLEMESTROCEL-L: https://go.drugbank.com/drugs/DB17606 RISK EVALUATION AND MITIGATION STRATEGY PROGRAMME:
https://www.fda.gov/drugs/drug-safety-and-availability/risk-evaluation-and-mitigation-strategies-rems ROCHE:
https://technical.ly/startups/spark-therapeutics-acquired-roche-bill-marrazzo-university-city-science-center-gene-therapy/ ROUGHLY 90% OF APPLICATIONS FILED ARE UTILITY PATENTS:
https://www.uspto.gov/web/offices/ac/ido/oeip/taf/data/patdesc.htm SAN RAFFAELE-TELETHON INSTITUTE FOR GENE THERAPY:
https://ir.orchard-tx.com/news-releases/news-release-details/orchard-therapeutics-announces-fda-regenerative-medicine/ SBA LOANS: https://www.sba.gov/funding-programs/loans SBIR AND STTR
PROGRAMMES: https://www.sbir.gov/node/2120519 SECONDARY PUBLIC OFFERINGS: https://www.cbinsights.com/research-what-is-a-secondary-offering SECTION 361:
https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=1271 SECTION 506(G)(8) OF THE UNITED STATES (US) FEDERAL FOOD, DRUG, AND COSMETIC ACT:
https://www.congress.gov/114/plaws/publ255/PLAW-114publ255.htm SEOUL NATIONAL UNIVERSITY: https://www.koreabiomed.com/news/articleView.html?idxno=20085 SETTLED A LEGAL DISPUTE:
https://www.wsj.com/articles/juno-therapeutics-settles-patent-dispute-with-novartis-1428326691 SEVERAL EXPEDITED PROGRAMMES:
https://www.fda.gov/patients/learn-about-drug-and-device-approvals/fast-track-breakthrough-therapy-accelerated-approval-priority-review SKINTE: https://www.woundsource.com/product/skinte
SKYSONA (2022): https://investor.bluebirdbio.com/news-releases/news-release-details/bluebird-bio-receives-fda-accelerated-approval-skysonar-gene SPARK THERAPEUTICS:
https://sparktx.com/press_releases/spark-therapeutics-enters-into-definitive-merger-agreement-with-roche/ STANFORD UNIVERSITY: https://orcabio.com/who-we-are/ STANFORD UNIVERSITY:
https://www.medeortx.com/about-us.php STRATAGRAFT (2021): https://mallinckrodt.gcs-web.com/news-releases/news-release-details/mallinckrodt-announces-us-fda-approval-stratagraftr-allogeneic/
STRATAGRAFT: https://www.stratagraft.com/ STRATATECH: https://www.fiercebiotech.com/medical-devices/mallinckrodt-acquires-regenerative-medicine-company-stratatech SUBSEQUENTLY APPROVED:
https://www.ns-healthcare.com/news/breyanzi-large-b-cell-lymphoma/ SUMITOMO PHARMA:
https://endpts.com/after-three-decades-and-a-surprise-rejection-first-treatment-for-babies-born-without-a-thymus-secures-fda-approval/ TECARTUS (2020):
https://www.gilead.com/news-and-press/press-room/press-releases/2020/7/us-fda-approves-kites-tecartus-the-first-and-only-car-t-treatment-for-relapsed-or-refractory-mantle-cell-lymphoma
TECHNOLOGY FOR BRENYANZI: https://www.forbes.com/sites/matthewherper/2014/08/05/why-this-cancer-fighting-company-has-raised-300-million-in-just-12-months/?sh=449f6d8b50d5 TECHNOLOGY FOR
STRATAGRAFT: https://pathology.wisc.edu/2021/06/21/stratagraft-approved-by-fda/ TECHNOLOGY OF ZOLGENSMA:
https://www.nationwidechildrens.org/newsroom/news-releases/2013/10/avexis-biolife-licenses-spinal-muscular-atrophy-sma-patent-portfolio-from-nationwide-childrens THREE FDA-APPROVED PRODUCTS:
https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/cber-regenerative-medicine-advanced-therapy-rmat-approvals THREE TYPES OF PATENT:
https://www.uspto.gov/patents/basics/general-information-patents “TREAT, MODIFY, REVERSE, OR CURE A SERIOUS OR LIFE-THREATENING CONDITION”:
https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/regenerative-medicine-advanced-therapy-designation TT11: https://www.tessacell.com/pipeline/ UNIQURE (FOUNDED AS
AMSTERDAM MOLECULAR THERAPEUTICS): https://www.biopharmadive.com/news/hemophilia-gene-therapy-fda-approval-hemgenix-csl-uniqure/636999/ UNIVERSITÉ DE MONTRÉAL UNIVERSITY OF TORONTO:
https://www.signalsblog.ca/catching-up-with-excellthera-and-co-founder-guy-sauvageau/ UNIVERSITY COLLEGE LONDON: https://www.uclb.com/portfolio/our-spinouts/orchard-theapeutics-plc/
UNIVERSITY OF CALIFORNIA SAN DIEGO (UCSD): https://innovation.ucsd.edu/commercialize/licensing.html UNIVERSITY OF CALIFORNIA, BERKELEY: https://ipira.berkeley.edu/caribou-biosciences-inc
UNIVERSITY OF CALIFORNIA, BERKELEY: https://ipira.berkeley.edu/intellia UNIVERSITY OF CALIFORNIA, IRVINE:
https://innovation.uci.edu/2016/11/20161129special-feature-stem-cell-strides-asterias-biotherapeutics/ UNIVERSITY OF CALIFORNIA, IRVINE:
https://innovation.uci.edu/2020/06/uci-startup-company-jcyte-partners-with-santen-pharmaceutical/ UNIVERSITY OF CALIFORNIA, LOS ANGELES: https://labusinessjournal.com/news/arie-belldegrun/
UNIVERSITY OF CALIFORNIA, LOS ANGELES: https://www.biopharmadive.com/news/Kite-Pharma-UCLA-tcell-allogeneic/423263/ UNIVERSITY OF CALIFORNIA, SAN FRANCISCO:
https://www.ucsf.edu/news/2022/02/422276/ucsf-gene-therapy-deadly-mutation-fast-tracked-fda-review UNIVERSITY COLLEGE LONDON: https://www.autolus.com/about-us/ UNIVERSITY OF COLORADO
UNIVERSITY OF ILLINOIS AT CHICAGO: https://www.fiercebiotech.com/biotech/eye-specialist-ocugen-looks-east-for-second-round-fundraising UNIVERSITY OF EASTERN FINLAND:
https://www.uef.fi/en/article/university-of-eastern-finland-and-ferring-ventures-to-continue-collaboration-in-gene-and-cell UNIVERSITY OF FLORIDA:
https://ir.axogeninc.com/press-releases/detail/811/axogen-inc-receives-david-j-gury-company-of-the-year UNIVERSITY OF FLORIDA:
https://news.drgator.ufl.edu/2013/09/26/uf-teams-with-company-to-advance-gene-therapy-treatments/ UNIVERSITY OF LOUISVILLE:
https://www.uoflnews.com/post/uofltoday/uofl-born-company-secures-100-million-to-advance-cell-therapy/ UNIVERSITY OF MASSACHUSETTS UNIVERSITY OF CALIFORNIA, SAN FRANCISCO STANFORD
UNIVERSITY: https://www.umassmed.edu/es/news/news-archives/2014/02/voyager-therapeutics-targets-novel-gene-therapies-to-combat-diseases/ UNIVERSITY OF MINNESOTA:
https://www.nytimes.com/2002/12/11/business/technology-politically-correct-stem-cell-is-licensed-to-biotech-concern.html UNIVERSITY OF OXFORD:
https://www.ox.ac.uk/news/2015-11-09-£23-million-boost-oxford-spinout-company UNIVERSITY OF PENNSYLVANIA:
https://pci.upenn.edu/regenxbio-receives-fda-fast-track-designation-for-its-novel-gene-therapy-candidate/ UNIVERSITY OF PENNSYLVANIA:
https://www.novartis.com/news/media-releases/novartis-kymriah-receives-fda-regenerative-medicine-advanced-therapy-designation-follicular-lymphoma UNIVERSITY OF PENNSYLVANIA:
https://www.pennmedicine.org/news/news-releases/2019/september/university-pennsylvania-announces-exclusive-alliance-novartis-development-new-focused-relationship UNIVERSITY OF WASHINGTON,
CHILDREN’S HOSPITAL BOSTON MASS GENERAL HOSPITAL FOR CHILDREN:
https://iscrm.uw.edu/fate-therapeutics-a-company-started-in-seattle-with-intellectual-property-from-iscrm-has-announced-its-first-clinical-trials/ UNIVERSITY OF WISCONSIN:
https://pathology.wisc.edu/2021/06/21/stratagraft-approved-by-fda/ UNIVERSITY OF WISCONSIN — MADISON: https://news.wisc.edu/gov-evers-state-leaders-tour-uw-biotech-spinoff-stratatech/
UPDATED THE US POLICY GUIDANCE: https://www.whitehouse.gov/ostp/news-updates/2022/08/25/ostp-issues-guidance-to-make-federally-funded-research-freely-available-without-delay/ US$10 MILLION
IN SERIES C: https://www.wsj.com/articles/DJFVW00120150109eb19ozrd9 US$13.1 MILLION: https://www.pehub.com/histogenics-adds-131-million/ US$34 MILLION:
https://www.massdevice.com/histogenics-raises-34-million-after-prochon-biotech-merger/ US$49 MILLION:
https://www.fiercebiotech.com/biotech/histogenics-closes-49-million-series-a-fundraising-to-support-commercial-development-of-0 US$5.35 MILLION:
https://www.prweb.com/releases/2008/05/prweb908944.htm/ US$65 MILLION IN SERIES D: https://www.fiercebiotech.com/biotech/avexis-secures-65-million-financing US$65 MILLION THROUGH AN INITIAL
PUBLIC OFFERING: https://www.bizjournals.com/boston/blog/bioflash/2014/12/with-65m-offering-histogenics-becomes-states-17th.html US$9.0 MILLION:
https://www.fiercebiotech.com/biotech/histogenics-secures-9-million-second-round-of-financing VYJUVEK (2023): https://www.fda.gov/vaccines-blood-biologics/vyjuvek WEIZMANN INSTITUTE:
https://www.haaretz.com/israel-news/2017-08-31/ty-article/kite-pharmas-success-is-nothing-compared-to-tevas-failure/0000017f-dbae-df9c-a17f-ffbef7f70000 WEILL CORNELL MEDICINE:
https://angiocrinebioscience.com/about-us/our-story/ WESTERN NEW YORK INCUBATOR NETWORK SBIR/STTR ASSISTANCE PROGRAMME: https://www.wnyincubators.com/sbir-sttr-assistance Y COMBINATOR
MANAGEMENT: https://www.ycombinator.com/ YESCARTA (2017):
https://www.gilead.com/news-and-press/press-room/press-releases/2020/7/us-fda-approves-kites-tecartus-the-first-and-only-car-t-treatment-for-relapsed-or-refractory-mantle-cell-lymphoma
ZOLGENSMA (2019): https://www.novartis.com/news/media-releases/avexis-receives-fda-approval-zolgensma-first-and-only-gene-therapy-pediatric-patients-spinal-muscular-atrophy-sma ZYNTEGLO
(2022): https://investor.bluebirdbio.com/news-releases/news-release-details/bluebird-bio-announces-fda-approval-zynteglor-first-gene-therapy RIGHTS AND PERMISSIONS Springer Nature or its
licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the
accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE
Takahashi, T., Donahue, R.P., Nordberg, R.C. _et al._ Commercialization of regenerative-medicine therapies. _Nat Rev Bioeng_ 1, 906–929 (2023). https://doi.org/10.1038/s44222-023-00095-9
Download citation * Accepted: 13 July 2023 * Published: 04 September 2023 * Issue Date: December 2023 * DOI: https://doi.org/10.1038/s44222-023-00095-9 SHARE THIS ARTICLE Anyone you share
the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative