Play all audios:
ABSTRACT OBJECTIVES: The study aimed to use functional magnetic resonance imaging to ascertain changes in sensorimotor system function in patients with hereditary spastic paraplegia and to
correlate it with severity of spasticity and paresis. SETTING: Tartu University Hospital, Tartu, Estonia. METHODS: Nine patients with autosomal-dominant pure HSP and 14 age- and sex-matched
healthy controls were investigated with a 1.5T fMRI scanner during flexion/extension of the right-hand fingers and right ankle. Images were analysed with a general linear model and
Statistical Parametrical Mapping software. Highest _Z_-scores were identified from probability maps, and weighted laterality indices were calculated using combined bootstrap/histogram
analysis; these were correlated with clinical severity of spasticity and paresis. RESULTS: During hand movements, clusters located in contralateral primary sensorimotor and premotor areas
activated in both controls and patients. Bilateral activation occurred in the supplementary motor area, parietal operculum and cerebellum (predominantly ipsilateral). During the ankle task,
bilateral activation was noted in the primary sensorimotor area, supplementary motor area and cerebellum. Activation clusters in HSP patients were smaller than those in controls in the
sensorimotor area, especially during the ankle task, and more pronounced ipsilaterally in cerebellum both during hand and ankle motor tasks. Spasticity was significantly associated with
contralateral activation in the sensory area and correlated negatively with the highest _Z_-scores in Brodmann areas 1-2-3 and 4. CONCLUSION: Our results suggest changes in cortical
sensorimotor network function in patients with HSP compared with healthy subjects. Lower activation in patients might reflect damage to the corticospinal tract, be influenced by compensatory
mechanisms, and/or be a reflection of neurorehabilitation. SIMILAR CONTENT BEING VIEWED BY OTHERS SHARED AND DISTINCT VOXEL-BASED LESION-SYMPTOM MAPPINGS FOR SPASTICITY AND IMPAIRED
MOVEMENT IN THE HEMIPARETIC UPPER LIMB Article Open access 17 June 2022 EXPLORATORY STUDY OF HOW COGNITIVE MULTISENSORY REHABILITATION RESTORES PARIETAL OPERCULUM CONNECTIVITY AND IMPROVES
UPPER LIMB MOVEMENTS IN CHRONIC STROKE Article Open access 20 November 2020 FUNCTIONAL CONNECTIVITY AND AMPLITUDE OF LOW-FREQUENCY FLUCTUATIONS CHANGES IN PEOPLE WITH COMPLETE SUBACUTE AND
CHRONIC SPINAL CORD INJURY Article Open access 03 December 2022 INTRODUCTION Hereditary spastic paraplegia or the Strümpell-Lorrain disease is a group of rare chronic neurodegenerative
disorders marked by clinical and genetic heterogeneity. It has been classified into two forms, ‘pure’ and ‘complex’ (cHSP). pHSP presents with spasticity and motor deficit in the legs, brisk
reflexes and Babinski’s signs; deep sensory impairment and sphincter disturbances are also common. For cases of cHSP, other neurological or extra-neurological features can be present.1 The
disease affects substantially health related quality of life of persons with HSP.2 The primary pathology in HSP is axonal degeneration that is maximal at the distal ends of the corticospinal
tracts and at the distal ends of dorsal column fibres.3 Previous studies in HSP patients have revealed cerebral involvement as well, including cognitive decline, white matter abnormalities,
involvement of motor cortex and alterations of regional cerebral blood flow and brain activity.4, 5, 6, 7, 8 However, the pattern of possible cortical functional reorganisation and its
correlates with motor impairments are yet poorly understood. The objectives of this study were to ascertain changes in sensorimotor system function in patients with HSP by using functional
magnetic resonance imaging and to correlate fMRI changes with the severity of spasticity and paresis in legs. MATERIALS AND METHODS SUBJECTS Patient data were acquired from an
epidemiological study database consisting of 59 persons with HSP.9 Patients with autosomal-dominant pHSP and mutations in the _SPAST_ gene were invited to participate in the study as were
their affected family members with the same phenotype. Other Estonian patients with HSP were not included in this study in order to ensure the genetic and clinical homogeneity of the study
group. Nine right-handed patients and 14 age- and sex-matched right-handed healthy controls consented for participation. All nine patients studied had a positive family history for HSP and
belonged to three different pedigrees before the study, but were taken off the medication for 3 days before the time of the study. Spasticity was evaluated using the Modified Ashworth Scale
to assess the antagonist muscles: hamstrings, thigh adductor, gastrocnemius and soleus.11 A 0–5 grading system was applied Scale for Muscle Strength grading it from 0–5 (5, normal strength;
0, no movement).12 FUNCTIONAL MRI SCANNING PARADIGM Before examination, the subjects were informed about the procedure, tasks and length of the examination. A block design with six
alternating task-rest cycles was used, starting with a rest cycle. Two paradigms were used for sensorimotor function assessment: flexion/extension of the right-hand fingers; and
flexion/extension of the right ankle. Brief verbal instructions to move or rest were given. Before the experiment, all subjects were trained to perform movements at a frequency of 1 Hz.
Performance of the task was monitored visually, and the number of movements was registered. The frequency of movements in patients and controls did not differ significantly (Wilcoxon
two-sample test). IMAGE ACQUISITION Images were obtained on a 1.5-T MR scanner (Magnetom Symphony; Siemens Medical Systems, Erlangen, Germany). Before obtaining functional scans, a
high-resolution T1-weighted anatomical image was obtained with the gradient echo, fast low-angle shot sequence (repetition time/echo time (TR/TE)=12/5.68 ms, flip angle 15°, resolution 224 ×
256, voxel size 1 × 1 × 1 mm3, 176 sagittal planes). Functional T2*-weighted images were obtained using the gradient echo, echo-planar imaging sequence (TR/TE=4/50 ms, resolution 64 × 64,
voxel size 3 × 3 × 3 mm3, slice gap 0.75 mm, 36 axial planes, interleaved scan). Altogether, 60 whole-brain functional images were obtained for each patient and control subject while the
subjects were performing the described tasks. IMAGE PROCESSING Image processing was performed using Statistical Parametrical Mapping software functions to process and analyse functional
neuroimaging data.13 First step of spatial pre-processing was realignment of functional images, where movement effects were discounted. Then high-resolution anatomical images were
co-registered with functional images to maximise the mutual information. Pre-processing continued with segmentation of high-resolution anatomical images, where the Montreal Neurological
Institute 452 white matter, grey matter and cerebrospinal fluid probability maps were used to yield a parametric description for normalisation.14 In normalisation processing, images were
also bias corrected. Image pre-processing ended with smoothing by 8 × 8 × 8 mm3 full-width at half maximum isotropic Gaussian kernel. STATISTICAL ANALYSIS Image processing was followed by a
general linear model-based statistical analysis of the functional images. Multiple comparisons problem was corrected by masking images with Brodmann area masks from MRIcro and by doing
region-of-interest analysis using WFU PickAtlas.15, 16, 17 Unmasked t-maps were divided into two groups and were taken onto second level, that is, random effect analysis. One-sample _t_-test
was used to explore group-specific activations. The resulting t-maps for each subject were analysed within BA 1-2-3, BA 4 and BA 6, defined by MRIcro templates were calculated using
combined bootstrap/histogram analysis after Wilke and Schmithorst; these were correlated with clinical severity of spasticity and paresis.18 The wLI<−1 corresponds to right-hemispheric,
wLI–0.2…0.2 to bilateral and wLI >0.2 to left-hemispheric brain activation. Images were thresholded at a given value (_t_), and only voxels at which all images exceed the threshold were
included. Inter- and intrasubject reproducibility of the used methodology has been assessed previously.19 Additional statistical analysis was performed using the statistical package SAS
Version 9.1. Differences between the groups were studied with the nonparametric Mann–Whitney _U_-test. The Kolmogorov–Smirnov criterion was employed for assessment of normality. To examine
the association between the variables, the Spearman’s correlation test was used. All _P_ values were two-sided, and differences were considered statistically significant if _P_ values were
less than 0.05. STATEMENT OF ETHICS We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course
of this research. This study was approved by the Ethics Review Committee on Human Research of the University of Tartu, and informed consent was obtained from all study participants. RESULTS
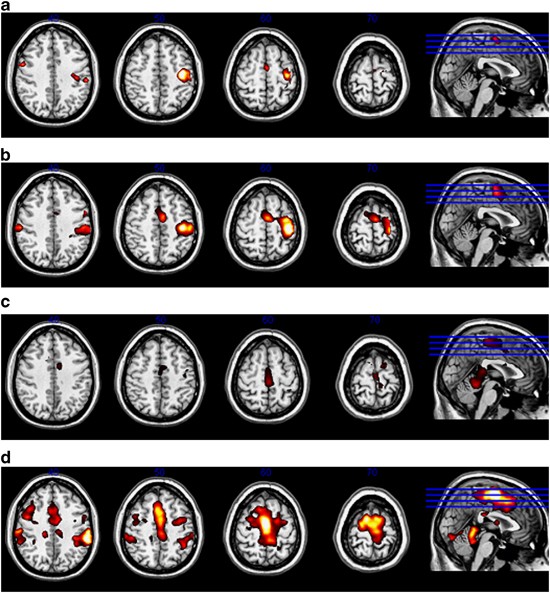
During hand movements, clusters located in the contralateral primary sensorimotor and premotor areas activated in both the control subjects and the patients (Table 2, Figure 2). Bilateral
activation occurred in the supplementary motor area, in the parietal operculum and in the cerebellum (predominantly ipsilateral). During the ankle task, bilateral activation was noted in the
primary sensorimotor area, in supplementary motor area and in the cerebellum. Activation clusters in HSP patients were smaller than those in controls in the sensorimotor area, especially
during the ankle task, and more pronounced ipsilaterally in the cerebellum during both the hand and ankle motor tasks. During the ankle task, less lateralised activation in BA 4 occurred in
patients than in controls both in patients and in controls. In control subjects, lateralisation was significantly more pronounced in BA 4 than in BA 1-2-3 and BA 6 during both the hand and
ankle tasks (_P_<0.001). In patients with HSP, mean maximal _Z_-scores during the hand task, and especially during the ankle task, were lower than those in controls, but a statistically
significant difference was revealed only in BA 1-2-3 during the ankle task (Table 3). Maximal _Z_-scores in BA 1-2-3 and in BA 4 were negatively correlated with the scores for spasticity in
leg muscles (Figure 3). Spasticity significantly correlated with wLI only in BA 1-2-3 (_P_<0.05), indicating that more severe spasticity was associated with contralateral activation in
the sensory area (Figure 4). Paresis did not correlate significantly with wLI and _Z_-scores. Also, no correlation was noted with either disease duration or patients’ age or sex. DISCUSSION
The results of our study suggest changes in cortical sensorimotor network function in patients with HSP compared with healthy subjects. Activation in the sensorimotor cortex was associated
with spasticity, but not with the degree of paresis. The exact mechanism behind these findings is unknown, but it can be related to the clinical peculiarity of HSP that separates it from
other causes of spastic paraparesis: it has been shown, that the spasticity contributes to gait disturbance disproportionally more than the paresis, with a notable discrepancy between the
degrees of spasticity and of muscle weakness.20 It is difficult to compare our results with similar ones because, to our knowledge, there is only one published study on this topic that also
used the same technique.7 It is also hard to find a disease to compare with that could be similar to HSP in terms of both underlying neuropathological process and clinical presentation
(almost isolated pyramidal involvement with clear domination of spasticity over paresis and slow rate of progression). This is because, to date, the extent of cortical involvement in the
pathogenesis of HSP and the pattern of possible cortical functional reorganisation with its correlates with motor impairment are poorly investigated. Nevertheless, despite the main
pathological process of distal axonal degeneration, cortical involvement was studied previously in a few works that used different techniques.4, 5, 6, 7, 8 Erichsen _et al._4 used magnetic
resonance spectroscopy on a small but genetically well-defined group of _SPAST_-related HSP patients. The cholin/creatin ratio in motor cortex of the patients was significantly lower than
that in controls and was significantly associated with age-related verbal learning and memory reduction. Authors concluded that these findings support involvement of motor cortex in HSP.
Duning _et al._8 examined six patients with pHSP because of _SPAST_ mutations by using conventional MRI, diffusion tensor imaging and brain volumetry. The only pathology found was revealed
by diffusion tensor imaging: widespread disturbance of white matter integrity, mainly affecting the corticospinal tract. This finding is consistent with the clinical representation of the
disorder, but probably not specific to HSP. A Danish group published two articles on the same cohort of HSP patients in order to investigate the extent of motor cortical functional
reorganisation in patients with _SPAST_-related HSP, by using positron emission tomography.5, 6 They found that the patients with HSP had significantly decreased regional cerebral blood flow
in the left fronto-temporal cortex at resting state and that patients had a tendency toward more widespread activation in sensorimotor cortical and cerebellar regions upon movements (right
ankle flexion-extension and right shoulder flexion-extension were studied). Although statistically significant differences were found only for the ankle movement response, patients showed
significantly increased regional cerebral blood flow in the right and left primary motor cortices, the supplementary motor areas and the right premotor cortex compared with controls. The
hypothesis of an impaired function was investigated using neurophysiological methods as well. For instance Satrucci _et al._21 used short latency somatosensory evoked potentials in patients
with _SPAST_-related AD-pHSP. Somewhat similarly to the present study authors showed that cortical somatosensory evoked potentials from lower limbs were abnormal and correlated with
spasticity. But there were no abnormalities found in neurophysiological parameters from upper limbs. To our knowledge, only one study has been published, in which, like the present study,
fMRI was used to explore cortical activation: Koritnik _et al._7 found that there was increased activation in bilateral posterior parietal cortex and left primary sensorimotor cortex in
patients with HSP. As the authors used two different levels of difficulty for performing the selected motor movements, they rightfully concluded that these changes probably reflect
reorganisation of the cortical motor system and not just increased activation because of a greater relative motor effort. Unfortunately, there were some methodological concerns in the study.
First, the authors used a heterogeneous group of HSP patients, which affects the interpretation of the results. It is unclear at the present time to what extent having additional
neurological features, as in cHSP, can influence the cortical activation. Second, in their study, only finger movements were selected as a motor task to evaluate cortical activation;
however, patients with HSP have more pronounced motor deficit in legs than in arms, including both spasticity and paresis.22 Hence, it is important to include motor tests evaluating legs as
well. Perhaps because of the latter, the authors also did not mention any correlation between spasticity and cortical activation, although spasticity was measured. Our group included only
patients with AD-pHSP; hence, it represents a homogeneous group of HSP patients. In fact our study group is genetically and clinically the most homogeneous of all similar studies published
so far on this topic. We also temporary discontinued all antispastic medications to exclude the possibility of drugs affecting the results of the study, although the precise possible
mechanism of influence of these medications on cortical activation is unknown. The motor tasks covered both upper and lower extremities. As, to date, it is unclear which of the main two
neurological features influences cortical reorganisation more—spasticity or paresis—these clinical correlates were investigated. As in HSP, spasticity dominates over paresis, it can be
speculated that this might be a reason for the more significant correlations with cortical activation found in our study. Lower activation in patients compared with controls might reflect
damage to the corticospinal tract producing reduced motor output during activation, which is associated with lower afferent feedback and activity in sensory areas. It may also be influenced
by the compensatory mechanism, cortical plasticity.23 It is not excluded that certain patterns of cortical activation can be a reflection of active neurorehabilitation. All these hypotheses
are still to be answered in future studies specifically designed to do so and taking into consideration the fact that all possible mechanisms remain to be fully elucidated. Interestingly, in
the group of patients there was one person with neither complaints, nor paresis or spasticity, but with notable hyperreflexia in legs and bilateral Babinski’s sign. He belongs to a larger
pedigree of patients with the same mutation in the _SPAST_ gene.10 This 32 year-old man probably didn’t reach the age of the first notable deficit to appear clinically. When this person’s
results were analysed separately, a few interesting trends appeared. He had higher _Z_-scores for almost all the investigated movements, except for BA 1-2-3 _Z_-score (4, 14), which was
somewhat smaller than in controls (4, 44), but higher than in patients (3, 65). On finger tasks this person’s cortical activation was more lateralized. His results for the hand’s motor tasks
were resembling controls’ ones. On the contrary, cortical activation during the motor tasks in legs is more suggestive for the pattern seen in the patients group. These findings might
represent individual variations and are not conclusive. But such a trend may suggest that pathological process of cortical reorganisation probably starts before the symptomatic phase of the
disease and initially is represented in legs’ motor areas, correlating with clinical pattern of the initial presentation of HSP. This hypothesis is indirectly supported by the differences
found during the hand task between controls and patients, who actually had no obvious motor deficit in upper extremities (in present study and one by Koritnik _et al._7). This is one of the
most interesting findings of our study and these trends have never been described in literature so far. Hence, further research can also concentrate on asymptomatic patients with known
genetic pathology of HSP. If the results of such research could confirm our findings, this might represent the possibility of using fMRI for the preclinical stage of the disease and can
possibly be used as a prognostic marker for the symptomatic phase of HSP. LIMITATIONS There are some limitations associated with this study. The number of participants in this study is
somewhat low. A larger study would have been more robust for statistical analyses, and therefore, more conclusive. Despite the clear-cut phenotype of AD-pHSP, not all patients had the
pathogenic mutations in the _SPAST_ gene. Hence, the results are not to be implemented strictly to the _SPAST_-related HSP. Somatosensory evoked potentials were not used because of the
unavailability of the method in Estonia. CONCLUSION Our results suggest changes in cortical sensorimotor network function in patients with HSP compared with the healthy subjects. More
studies to increase the conclusiveness of the data and to gain more evidence in the field of this subject are needed. It could represent a necessary preclinical basis for planning
evidence-based neurorehabilitation of persons with HSP. DATA ARCHIVING There were no data to deposit. REFERENCES * McDermott CJ, White K, Bushby K, Shaw P . Hereditary spastic paraparesis: a
review of new developments. _J Neurol Neurosurg Psychiatry_ 2000; 69: 150–160. Article CAS Google Scholar * Braschinsky M, Rannikmäe K, Krikmann Ü, Lüüs S-M, Raidvee A, Gross-Paju K _et
al_ Health-related quality of life in patients with hereditary spastic paraplegia in Estonia. _Spinal Cord_ 2011; 49: 175–181. Article CAS Google Scholar * Harding AE . Hereditary spastic
paraplegias. _Semin Neurol_ 1993; 13: 333–336. Article CAS Google Scholar * Erichsen AK, Server A, Landrø NI, Sandvik L, Tallaksen CM . Proton magnetic resonance spectroscopy and
cognition in patients with spastin mutations. _Brain_ 2009; 132: 1577–1588. Article Google Scholar * Scheuer KH, Nielsen JE, Krabbe K, Simonsen C, Koefoed P, Sørensen SA _et al_ Reduced
regional cerebral blood flow in SPG4-linked hereditary spastic paraplegia. _J Neurol Sci_ 2005; 235: 23–32. Article CAS Google Scholar * Scheuer KH, Nielsen JE, Krabbe K, Paulson OB, Law
I . Motor activation in SPG4-linked hereditary spastic paraplegia. _J Neurol Sci_ 2006; 244: 31–39. Article CAS Google Scholar * Koritnik B, Azam S, Knific J, Zidar J . Functional changes
of the cortical motor system in hereditary spastic paraparesis. _Acta Neurol Scand_ 2009; 120: 182–190. Article CAS Google Scholar * Duning T, Warnecke T, Schirmacher A, Schiffbauer H,
Lohmann H, Mohammadi S _et al_ Specific pattern of early white-matter changes in pure hereditary spastic paraplegia. _Mov Disord_ 2010; 25: 1986–1992. Article Google Scholar * Braschinsky
M, Lüüs S-M, Gross-Paju K, Haldre S . The prevalence of hereditary spastic paraplegia and the occurrence of SPG4 mutations in Estonia. _Neuroepidemiology_ 2009; 32: 89–93. Article Google
Scholar * Braschinsky M, Tamm R, Beetz C, Sachez-Ferrero E, Raukas E, Lüüs SM _et al_ Unique spectrum of SPAST variants in Estonian HSP patients: presence of benign missense changes but
lack of exonic rearrangements. _BMC Neurol_ 2010; 10: 17. Article Google Scholar * Bohannon RW, Smith MB . Interrater reliability of a modified Ashworth scale of muscle spasticity. _Phys
Ther_ 1987; 67: 206–207. Article CAS Google Scholar * Medical Research CouncilAids to the examination of the peripheral nervous system, Memorandum no. 45, Her Majesty’s Stationery Office,
London, 1981. * Ashburner J, Chen CC, Flandin G, Henson R, Kiebel S, Kilner J _et al_ _Statistical Parametric Mapping v.8_. The FIL Methods Group: London,
Ukhttp://www.fil.ion.ucl.ac.uk/spm/ (August, 2010). * International Consortium for Brain MappingICBM 452 T1 Atlas, 2010. http://www.loni.ucla.edu/ICBM/Downloads/Downloads_452T1.shtml
(August, 2010). * Rorden C . _MRIcro v.1.40_. Center for Advanced Brain Imaging: Atlanta, GA, USA. 2005. Google Scholar * Maldjian JA, Laurienti PJ, Kraft RA, Burdette JB . An automated
method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. _NeuroImage_ 2003; 19: 1233–1239. Article Google Scholar * Maldjian JA, Laurienti PJ, Burdette
JH . Precentral gyrus discrepancy in electronic versions of the Talairach Atlas. _NeuroImage_ 2004; 21: 450–455. Article Google Scholar * Wilke M, Schmithorst VJ . A combined
bootstrap/histogram analysis approach for computing a lateralization index from neuroimaging data. _Neuroimage_ 2006; 33: 522–530. Article Google Scholar * Kepler J, Kepler K, Tomberg T,
Ilves P . Uncertainty in functional magnetic resonance imaging methods for cortex motor and language area examinations. In: Medical Physics in the Baltic States: 8th International Conference
on Medical Physics; Kaunas, Lithuania; 14-16 October 2010.. _Kaunas Technol_ 2010; 2: 95–100. Google Scholar * Braschinsky M, Parts K, Maamägi H, Gross-Paju K, Haldre S . Functional
assessment of lower extremities in hereditary spastic paraplegia. _Arch Phys Med Rehabil_ 2009; 90: 1887–1890. Article Google Scholar * Sartucci F, Tovani S, Murri L, Sagliocco L . Motor
and somatosensory evoked potentials in Autosomal Dominant Hereditary Spastic Paraparesis linked to chromosome 2p, SPG4. _Brain Res Bull_ 2007; 74: 243–249. Article CAS Google Scholar *
Tallaksen CM, Dürr A, Brice A . Recent advances in hereditary spastic paraplegia. _Curr Opin in Neurol_ 2001; 14: 457–463. Article CAS Google Scholar * Konrad C . Brain plasticity and
functional reorganization in progressive motor system degeneration. _J Neurol Sci_ 2006; 244: 3–4. Article Google Scholar Download references ACKNOWLEDGEMENTS We would like to thank Pille
Kool for her assistance in statistical analysis. This study was supported by Estonian Science Foundation research Grant no. 7868 and targeted financing from the Estonian Ministry of
Education and Research (Grant no. SF0180064s07). AUTHOR INFORMATION Author notes * T Tomberg and M Braschinsky: These authors contributed equally to this work. AUTHORS AND AFFILIATIONS *
Department of Neurology and Neurosurgery, University of Tartu, Tartu, Estonia T Tomberg * Department of Radiology, Tartu University Hospital, Tartu, Estonia T Tomberg * Department of
Neurology and Neurosurgery, University of Tartu, Tartu, Estonia M Braschinsky, K Rannikmäe, J Kõrv, Ü Linnamägi & T Asser * Diagnostics Service, Pärnu Hospital, Pärnu, Estonia J Kepler *
Institute of Physics, University of Tartu, Tartu, Estonia K Kepler Authors * T Tomberg View author publications You can also search for this author inPubMed Google Scholar * M Braschinsky
View author publications You can also search for this author inPubMed Google Scholar * K Rannikmäe View author publications You can also search for this author inPubMed Google Scholar * J
Kepler View author publications You can also search for this author inPubMed Google Scholar * K Kepler View author publications You can also search for this author inPubMed Google Scholar *
J Kõrv View author publications You can also search for this author inPubMed Google Scholar * Ü Linnamägi View author publications You can also search for this author inPubMed Google Scholar
* T Asser View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to M Braschinsky. ETHICS DECLARATIONS COMPETING INTERESTS
The authors declare no conflict of interest. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Tomberg, T., Braschinsky, M., Rannikmäe, K. _et al._
Functional MRI of the cortical sensorimotor system in patients with hereditary spastic paraplegia. _Spinal Cord_ 50, 885–890 (2012). https://doi.org/10.1038/sc.2012.70 Download citation *
Received: 20 February 2012 * Revised: 20 April 2012 * Accepted: 11 May 2012 * Published: 03 July 2012 * Issue Date: December 2012 * DOI: https://doi.org/10.1038/sc.2012.70 SHARE THIS ARTICLE
Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard
Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * cortical reorganisation * functional magnetic resonance imaging * hereditary spastic paraplegia *
Strümpell–Lorrain disease