Play all audios:
ABSTRACT Boron, discovered as an element in 1808 and produced in pure form in 1909, has still remained the last elemental material, having stable natural isotopes, with the ground state
crystal phase to be unknown. It has been a subject of long-standing controversy, if α-B or β-B is the thermodynamically stable phase at ambient pressure and temperature. In the present work
this enigma has been resolved based on the α-B-to- β-B phase boundary line which we experimentally established in the pressure interval of ∼4 GPa to 8 GPa and linearly extrapolated down to
ambient pressure. In a series of high pressure high temperature experiments we synthesised single crystals of the three boron phases (α-B, β-B and γ-B) and provided evidence of higher
thermodynamic stability of α-B. Our work opens a way for reproducible synthesis of α-boron, an optically transparent direct band gap semiconductor with very high hardness, thermal and
chemical stability. SIMILAR CONTENT BEING VIEWED BY OTHERS EXPERIMENTAL AND THEORETICAL CONFIRMATION OF AN ORTHORHOMBIC PHASE TRANSITION IN NIOBIUM AT HIGH PRESSURE AND TEMPERATURE Article
Open access 13 August 2020 AB INITIO STUDIES OF MAGNETISM AND TOPOLOGY IN SOLID PD-RICH \({\VARVEC{A}}\)-PDSI ALLOYS Article Open access 17 March 2022 STRUCTURAL, THERMAL, AND RADIATION
SHIELDING PROPERTIES OF ANTIMONY-DOPED ZINC BORATE GLASSES Article Open access 09 May 2025 INTRODUCTION Boron does not exist in nature as a pure elemental phase because of its extreme
chemical activity but, being utilised in compounds it plays an important role in human activities since antiquity1. Boron compounds are widely used as engineering materials (dielectrics,
B-doped semiconductors), superhard materials (cBN, boron carbide), reinforcing chemical additives, for example, for obtaining special glass or corrosion- or heat- resistant alloys)2 and
superconducting materials (ex., MgB2)3 . Surprisingly, despite centuries of application and decades of intensive studies of boron compounds, elemental boron still remains in focus of wide
scientific interest due to its enigmatic properties (largely unknown phase diagram4,5,6,7, pressure induced metallization and superconductivity8, formation of unusual chemical bonds9 and
potential technological applications (exceptional chemical stability combined with very high hardness and interesting semiconducting and optical properties5,10. Among elemental boron
polymorphs, only α-rhombohedral (α-B), β-rhombohedral (β-B) and γ-orthorhombic boron (γ-B) have been currently established as pure phases4. They can be synthesised as single crystals at high
pressures and high temperatures and preserved at ambient conditions4,11,12,13,14. Building blocks of all these polymorphs are quasi-molecular B12 icosahedra arranged in the structures of
different complexity. Among them β-B has the most complex structure, whose details should yet be clarified by further studies. The presence of not fully occupied positions and probably
interstitial atoms allow characterising the structure of β-B as a defect one7,15,16. The γ-B consists of covalently bonded B12 icosahedra in a distorted cubic closest packing with B2
dumbbells placed at the octahedral sites5,9. α-B has the simplest structure with only 12 atoms per a unit cell, where B12 icosahedra are arranged in a distorted cubic closest packing17.
Relative stability of α-B and β-B at ambient conditions remains a puzzle. The β-B crystallizes from melt at ambient pressure and can be also produced by different chemical methods including
vapour deposition18,19. The α-B was crystallized from a variety of metallic solvents in the middle of 1960s20, but later the technology of producing the pure crystalline phase was lost4 and
only recently high-pressure synthesis of α-B single crystals was reported14. On heating at ambient pressure at temperatures above ∼1500 K α-B slowly transforms to β-B and it means that a
stable high-temperature form of boron is the β-phase. The fact that β-B can not be transformed to α-B at ambient pressure may indicate that α-form is metastable21. In this respect, although
α-B is completely ordered, its relative structural simplicity does not make it self-evident that α-B is more stable compared to β-B at ambient conditions. Slow kinetics of transformations
(i.e. large kinetic barriers) and/or high melting temperature of boron have possibly prevented accurate measurements by unambiguous techniques, such as calorimetry22. Theoreticians do not
have consensus on the problem of relative stability of α-B and β-B polymorphs. Using density-functional (DFT) calculations Masaga et al. and Shirai et al.6,23 studied ground-state and
thermodynamic properties (including the effect of atomic disorder and phonons) of α- and β-B and found that at zero temperature α-B is more stable than β-B. That agrees with the conclusion
of Shang et al.24, who considered defect free α- and β-B using first-principle quasi-harmonic phonon calculations. By considering the phonon contribution as the major source of the
temperature dependence of the free energy, Masaga et al.6 obtained 970 K as the transition temperature of α-to-β boron. This is at odds with conclusions of van Setten et al.7, who introduced
the quantum mechanical zero-point vibrational energy as a mechanism to stabilize β-B at absolute zero temperature and made β-B in their DFT calculations the ground state of elemental boron.
Moreover, investigations indicate that it is possible to find an arrangement of partially occupied states in β-boron that also increase its stability with respect to the α-phase7,15,22,25.
Ogitsu et al.22,25, using lattice model Monte Carlo techniques combined with _ab initio_ calculations, found that boron could be a frustrated system and a series of β-boron structures,
nearly degenerate in energy, may be stabilized by a macroscopic amount of intrinsic defects. According to Ogitsu et al.22,25, defects are responsible not only for entropic effects but also
for a reduction in internal energy making β-B more stable than α-B at zero temperature. Thus, if the β-B phase happens to be the ground state, the presence of geometrical frustration will
lead to an exotic thermodynamic property in the vicinity of zero temperature that would be very unusual for a pure elemental material. In the present work we report the results of systematic
experimental exploration of the pressure-temperature (PT) phase diagram of boron at pressures of 3 GPa to 14 GPa and temperatures of 1073 to 2423 K aimed at establishing phase boundaries
and resolving the long-standing problem regarding relative stability of the α- and β-B phases. RESULTS BORON PHASES In order to experimentally constrain relations between α-, β- and γ-boron
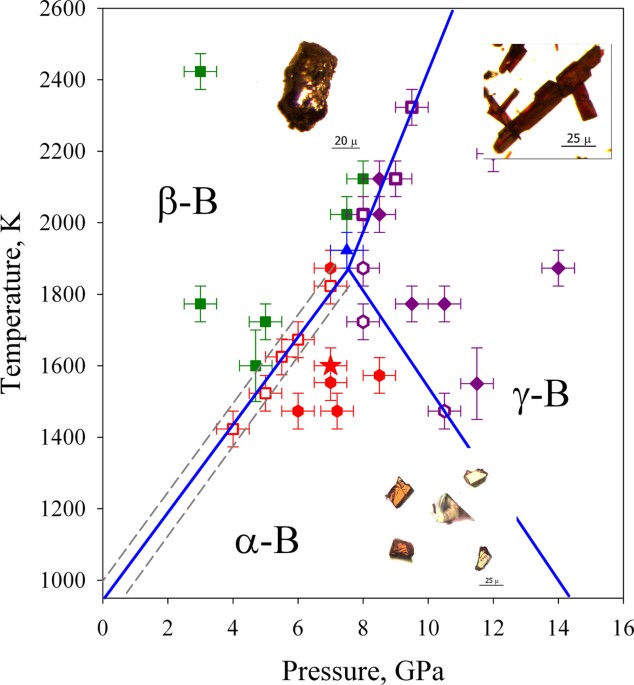
phases we performed more than 30 experiments in a multi-anvil apparatus (Fig. 1, Table 1, see also Methods below). In all experiments a boron source (commercially available polycrystalline
high purity (99.9995%) β-B, see Methods Summary) was enclosed into a metallic (Au or Pt) capsule with or without addition of a Pt powder and treated at various high-pressure high-temperature
(HPHT) conditions. Every trial aimed at establishing the phases that can be crystallised from melt or by solid-solid phase transformation of the precursor. Recovered samples were analysed
by scanning electron microscopy and electron microprobe for chemical purity, X-ray diffraction and Raman spectroscopy for phase composition and some samples were studied by TEM for
characterising their microstructure (Methods Summary). An image of a cross-section of a typical sample chamber recovered after experiment at 7 GPa and 1573 K is shown in Fig. 2. As seen,
single crystals of the boron phase are embedded into the matrix of solidified melt of platinum and platinum borides that form in all experiments at temperatures above eutectic. Dependent on
the pressure-temperature conditions, the experiments resulted in formation of the following pure boron phases: (1) _Re-crystallised β-B_, which is different from the precursor
polycrystalline β-B. It forms black or slightly reddish in thin sections single crystals of an irregular or sometimes hexagonal shape (Fig. 1), gives a typical for single crystals
diffraction pattern consisting of spots (Fig. 3) (space group R_3(-)m_, _a_ = 10.965(2) Å, _c_ = 23.859(4) Å). Its Raman spectrum is distinctly different from that of the precursor and
characterised by much sharper peaks compared to the latter (Fig. 4); (2) _γ-B_, which appears as purple elongated prismatic crystals, gives the characteristic strong Raman spectra (Figs. 1,
4) and the X-ray diffraction pattern (space group P_nnm_, _a_ = 5.0576(4) Å, _b_ = 5.6245(8) Å, _c_ = 6.9884(10) Å). This material is identical to that described in our previous works5,9,26.
(3) _α-B_. It forms single crystals of semi-transparent orange-red colour and relatively isometric shape (Fig. 1,2). Like other boron phases, α-B is easily identified by the Raman
spectrum14,27 (Fig. 4) and X-ray diffraction (space group R_3(-)m_, 4.9065(4) Å, 12.5658(5) Å). The SEM (EDX), microprobe (WDX) and EELS data have shown that boron phases obtained from
crystalline β-boron powders are not contaminated independently on the type of the capsule material or pressure-temperature conditions (Fig. 5). SEM images of the sample surfaces in
backscattered electrons demonstrate homogeneity of the synthesized at HPHT boron phases. High resolution transmission electron microscopy (HRTEM) images of α-B, for example, reveal almost
dislocation free regular packing of spheres (Fig. 5) with a diameter of 3.3-3.4 Å, comparable with that of a circumscribed circle around the B12 icosahedron (3.34 Å)28. BORON PHASE DIAGRAM
Proven chemical and phase purity of boron crystals obtained at different pressure-temperature condition creates a basis for construction of the experimental phase diagram. Different runs
resulted in crystallization of one, two or even all three boron phases simultaneously (Fig. 1, Table 1) that allows defining stability fields of the α−Β, β−Β and γ− Β phases. The phase
boundary separating the β–B and γ-B phase stability fields agrees well with the phase relations experimentally found in our previous work5. The other two phase boundaries (α−/β−Β and α−/γ−
Β) have not been reported so far based on experimental data. We argue that the α−Β has the thermodynamic stability field, because its crystallization is controlled only by pressure and
temperature conditions of the experiments independently on the type of metallic solvent (Au or Pt, Table 1). In the experiment at 5.5 GPa and 1600 K (S5155 MA, see Table 1) the sample was
kept at high temperature for one hour to check if the prolonged heating can affect the result. Like in short-duration experiments at similar P-T conditions we observed two phases,
recrystallized beta-boron and α-boron crystals. The α-B crystals reached up to 0.2 mm in width and up to 0.5 mm in length that is much bigger compared with those (only tens of microns in
length) obtained in other experiments with a short annealing time. Growth of the α-B crystals confirms that it is a stable phase at conditions of the experiment. Observation of simultaneous
crystallization of chemically pure α- and β-B (at 5 GPa and 1520 K, for example) or α- and γ-B (at 8 GPa and 1570 GPa, for example) demonstrates the existence of monovariant points in the
pressure-temperature phase diagram. The invariant (triple) point in the phase diagram could be determined by intersections of α-/β-B, α-/γ-B and β-/γ-B boundaries. The all three lines cross
at 7.6(5) GPa and 1880(50) K (Fig. 1). Indeed, at 7.5 GPa and 1920 K we observed simultaneous crystallisation of all α-, β- and γ-boron phases (Table 1, Fig. 1). The transition of α-to-β
boron upon heating at ambient pressure was already reported in literature20,21. In a diamond anvil cell (DAC) experiment (see Methods Summary) we loaded two pre-synthesized α-B crystals into
the sample chamber along with sodium chloride, NaCl, acting as a pressure transmitting medium and thermal insulator. One of the crystals was laser-heated at 4.7(3) GPa and 1600(100) K and
another one at 11.5(5) GPa and 1550(100) K. In the first case we observed formation of β-B, while at higher pressure α-B transformed directly into the γ-phase (Fig. 6). One more MA
experiment was conducted at 7 GPa and 1623 K in the Au capsule which does not dissolve boron. As in other experiments β-B was used as starting material, but in the recovered sample we found
polycrystalline α-B. Direct solid-solid phase transformation of β-to-α phase proves that α-boron is a thermodynamically stable phase at certain PT conditions (Fig. 1). DISCUSSION
Extrapolation of the α-/β-B boundary to ambient pressure (Fig. 1) suggests that α-boron is the thermodynamically stable low-temperature boron phase below ∼933(50) K. Indeed, in 1960s and
1970s arguments were raised20,29,30,31,32,33 that crystallization of small crystals of α-B from different metallic solvents (Pt, Au, Ag, Cu, Cu-Ni, etc.) at temperature around 1100–1200 K
may indicate stability of the α-polymorph at temperatures below these values. However, inability to grow larger crystals of α-B, its crystallization simultaneously with the β-form and
failure to transform β-B into the α-phase or to synthesize α-B from an amorphous boron precursor supported arguments that α-B may be just a metastable, or even monotropic, form of boron. In
our experiments at appropriate pressure-temperature conditions (Fig. 1) α-B crystals grow at the expense of β-B and in some runs (Table 1) all starting β-boron transforms into the α-phase.
Moreover, we observed direct transformation of β-B into α-B. All mentioned observations prove that α-boron is a thermodynamically stable phase. Previously reported difficulties and even
failure to synthesize α-B at ambient pressure could be explained based on the phase diagram we have experimentally constructed (Fig. 1): α-B is stable below about 1000 K and strictly
speaking, should not crystallize from metallic fluxes with the eutectic point at temperature above ∼1100 K. However, according to the Ostwald step rule at conditions not far from equilibrium
not the most stable but the least stable polymorph that crystallizes first34, so that α-B may appear if a boron-rich metallic flux solidified at relatively low temperature20. Transformation
of β-B or amorphous boron into the α-phase requires very significant rearrangement and/or rupture of B12 icosahedra. It is impossible to activate such a rearrangement at relatively low
temperatures (below 1000 K) in the field of stability of α-B. With a pressure increase the temperature stability field of α-boron increases and, as we demonstrated in an MA experiment, it
becomes possible to realize the direct β-to-α-B transition. Theoretical works7,15,22,25 suggesting that β-B is the ground state of boron are not supported by our experimental results. The
phase diagram drawn by Oganov et al.35 is schematic and based on only a few experimental points related to the HPHT synthesis conditions of β-B. The authors35 sketched the α-/β-B phase
boundary in accordance with the theoretical data of van Setten et al.7 and consequently suggested that β-B is stable down to 0 K at ambient pressure at odds with our conclusions. Combining
_ab initio_ pseudopotential calculations and some experimental data (Grüneisen parameters, particularly), Masaga et al.6 and Shirai et al.23 estimated the phase boundary between α- and β-B
phases and apparently found that α-B is more stable below about 1000 K, in good agreement with our experimental results. However, these authors6,23 calculated total energy of β-B using an
ideal, defects free structural model which contradicts available experimental crystallographic data. Such a simplification of the structure of β-B in calculations could result in
“underestimating” β-boron stability compared to other calculations7; i.e. the agreement with the experimental results could be reached just by chance, because indeed, according to Refs. 7,
15, 22 and 25 structural defects in β-B play key role in stabilization of the phase. Thus, our results call for further detailed theoretical investigations related to stability of boron
polymorphs. Boron has been for a long time known as prospective material4,5 for numerous applications. α-boron demonstrates a truly spectacular combination of properties – it is a direct
band gap semiconductor (with the reported band gap of 2.0 eV (Ref. 36), 2.4 eV (Ref. 37), or 2.15(2) eV as derived by us from EELS data), has a very high hardness (we measured the Vickers
hardness of 38(2) GPa on polycrystalline aggregates), thermally and chemically highly resistive and quite light (the density of α-B is 2.46 g/cm3 vs 4.89 g/cm3 of CdS or 6.11 g/cm3 of GaN
having comparable band gaps). Such properties may make α-B material of choice in many industrial semiconductors applications, and, especially, as a working element of solar cells with high
efficiency of sun light conversion into electrical power. So far research and development on potential applications of α-boron were hindered by concerns of its thermodynamic instability and
the absence of a reliable way of synthesis of single crystals. A phase diagram, as a projection of the fundamental property diagram, allows materials scientist indirect use of
thermodynamics38. It can be utilized to understand materials behaviour and propose optimal ways of their synthesis. The phase diagram of boron (Fig. 1) shows that α-B is not only
thermodynamically stable phase in a large pressure-temperature range, but it also can be reproducibly synthesized14 at conditions readily accessible by modern industry for large-scale
production (like synthetic diamonds, for example). Summarising, our serial exploration of the pressure-temperature field using the large volume press synthesis technique resulted in
establishing the phase diagram of boron in the pressure interval of 3 GPa to 14 GPa at temperatures between 1073 K and 2423 K. Based on our experimental data and linear extrapolation of the
α/β phase boundary down to ambient pressure we could resolve a long-standing controversy on the ground state of boron in favour of the α−Β phase. METHODS Polycrystalline β-boron (purity of
99.9995 at. %, grain size of <1000 microns), purchased from _Chempur Inc._, was used as a boron source material. HIGH-PRESSURE TECHNIQUES Experiments in multianvil apparatuses were
conducted in installed at BGI 1000-ton (Hymag) and 1200-ton (Sumitomo) hydraulic presses39. The Kawai-type multi-anvil system employs six tool-steel outer-anvils and eight tungsten carbide
cubic inner-anvils to focus an applied load on an octahedral high-pressure chamber formed as a result of corner truncations on the inner-anvils. By varying the corner truncation size of the
inner-anvils, various sample-pressure ranges can be attained. An octahedron made of magnesium oxide that matches the pressure chamber was used as a pressure medium. In our experiments 18/11
(the edge-length of an octahedron /anvil truncation edge-length, in millimeters) assemblies for pressures of 7–11 GPa and 25/15 assemblies for pressures of 5–8 GPa were used. Although an
indubitable advantage of using large assemblies is the increase of the amount of synthesized material, reaching highest temperatures in big assemblies is more difficult. Temperature in our
experiments was increased stepwise with a speed of about 80 K/min. Duration of heating was 5 or 3 minutes. Then the samples were either gradually cooled with a speed ∼10 K/min, or quenched.
“Pressure in chamber” vs “hydraulic oil pressure” in experiments was calibrated by observations of phase transitions in standard materials and temperature determined using W3Re/W25Re
thermocouple. Uncertainties estimated in pressure 0.5 GPa and in temperature 50 K. Experiments at pressures below 4 GPa were conducted using an end-loaded piston-cylinder type apparatus40.
The sample material was loaded into 6 mm diameter, 13 mm long Pt capsules (sample area 3 mm diameter, 6 mm long) which were placed into ½ inch talc-pyrex sample assemblies. These sample
assemblies contained an internal, tapered, graphite resistance furnace to ensure minimal temperature gradients along the length of the capsule. Temperature gradients are estimated to be less
than 25°C for the experimental conditions used. Pressure was calibrated against the quartz-coesite and kyanite-sillimanite transitions, as well as the melting point of diopside and
pressures are considered accurate to within less than ± 5% of the stated value. Temperatures were measured with a Pt-Pt10%Rh thermocouple. Run pressures and temperatures were continually
monitored and maintained for the duration of the runs. Experiments were quenched isobarically by turning off power to the heating circuit. Diamond anvil cell experiments we conducted using
diamond anvils with the culet diameter of 300 μm . Pre-synthesized α-B and NaCl (used as a pressure medium and thermal insulating material) were loaded into the pressure chamber in the Re
gasket preindented to about 45 μm thickness with the hole of 125 μm in diameter. Several ruby chips were placed into the sample chamber for pressure measurements. For double-side laser
heating we employed two UniHead systems installed at BGI41. The size of the laser beam was of about 30 µm in diameter with a temperature variation of ±50 K within the beam. The heating
duration was about 5 minutes. Temperature was measured by means of multiwavelength spectroradiometry. ANALYTICAL TECHNIQUES For the phase identification, selection of single crystals and
preliminary structural analysis a high-brilliance Rigaku diffractometer (Mo Kα radiation) equipped with Osmic focusing X-ray optics and Bruker Apex CCD detector was used. The diffraction
patterns were processed using Fit2D software. A LabRam spectrometer (with a resolution of 2 cm−1), a He–Ne laser (632.8 nm) with a power of 15 mW for excitation and a 50× objective were used
for the Raman scattering experiments. The morphology and chemical composition of the synthesized samples of single crystals were studied by means of the scanning electron microscopy (SEM)
(LEO-1530). Chemical purity of the samples was confirmed using WDX microprobe analysis (JEOL JXA-8200; focused beam; 20 keV, 20 nA). Electron transparent foils were prepared by focused ion
beam (FIB) techniques. FIB allows preparation of site-specific TEM foils with typical dimensions of 15–20 µm wide, by approximately 10 µm high and approx. 0.150 µm thick42. TEM
investigations were performed with a TECNAI F20 XTWIN transmission electron microscope operating at 200 kV with a field emission gun electron source. The TEM is equipped with a Gatan
Tridiem™filter, an EDAX Genesis™ X-ray analyzer with ultra thin window and a Fishione high angle annular dark field detector (HAADF). The Tridiem filter was used for the acquisition of
energy-filtered images applying a 20 eV window to the zero loss peak. EEL spectra were acquired with a dispersion of 0.1 eV/channel and an entrance aperture of 2mm. The resolution of the
filter was 0.9 eV at half width, at full maximum of the zero loss peak. Acquisition time was 1 second. Spectra of the different K-edges (B, C, N, O) were acquired in diffraction mode with a
camera length of 770 mm. Spectra processing (background subtraction, removal of plural scattering, quantification) was performed using the DigitalMicrograph software package. EDX spectra
were usually acquired in the scanning transmission mode (STEM) using the TIA™ software package of the TEM. Significant mass loss during analysis was avoided by scanning the beam in a
pre-selected window (20 × 20 nm or larger). Spot size was approx. 1 nm and acquisition time 60 seconds at an average count rate of 60 – 80 counts/second. This resulted in a counting error of
about 4 –5% at a 3σ level. REFERENCES * Garrett, D. E. Borates: handbook of deposits, processing, properties and use. Academic Press (1998). * Perkins, G. L. Boron: Compounds, Production
and Application. Nova Science Pub Inc. (2011). * Braccini, V., Nardelli, D., Penco, R., Grasso, G. Development of ex situ processed MgB2 wires and their applications to magnets. Physica C:
Superconductivity 456, 209–217 (2007). Article CAS ADS Google Scholar * Albert, B., Hillebrecht, H. Boron: elementary challenge for experimenters and theoreticians. Angew. Chem. Int. Ed.
48, 8640–8668 (2009). Article CAS Google Scholar * Zarechnaya, E.Yu., et al. Superhard semiconducting optically transparent high pressure phase of boron. Phys. Rev. Lett. 102, 185501
(2009). Article ADS Google Scholar * Masago, A., Shirai, K., Katayama-Yoshida, H. Crystal stability of α- and β-boron. Phys. Rev. B 73, 104102 (2006). Article ADS Google Scholar * Van
Setten, M. J., Uijttewaal, M. A., de Wijs, G. A. & de Groot, R. A. Thermodynamic stability of boron: The role of defects and zero point motion. J. Am. Chem. Soc. 129, 2458–2465 (2007).
Article CAS Google Scholar * Eremets, M. I., Struzhkin, V. V., Mao, H., Hemley, R. J. Superconductivity in boron. Science 293, 272–274 (2001). Article CAS ADS Google Scholar * Mondal,
S., et al. Electron deficient and multicenter bonds in the high-pressure γ-B28 phase of boron. Phys. Rev. Lett. 106, 215502 (2011). Article ADS Google Scholar * Zhou, W., Sun, H., Chen,
C. Soft bond-deformaton paths in superhard gamma-boron. Phys. Rev. Lett. 105, 215503 (2010). Article ADS Google Scholar * Brazhkin, V. V., Taniguichi, T., Akaishi, M., Popova, S. V.
Fabrication of β-boron by chemical-reaction and melt-quenching methods at high pressures. J. Mat. Res. 19, 1643–1648 (2004). Article CAS ADS Google Scholar * Zarechnaya, E.Yu.,
Dubrovinsky, L., Dubrovinskaia, N., Filinchuk, Y., Chernyshov, D., Dmitriev, V. Growth of singlecrystals of B28 at high pressures and high temperatures. J. Cryst. Growth 312, 3388–3394
(2010). Article CAS ADS Google Scholar * Zarechnaya, E.Yu., et al. L. Pressure-induced isostructural phase transformation in γ-B28. Phys. Rev. B 82, 184111 (2010). Article ADS Google
Scholar * Parakhonskiy, G., Dubrovinskaia, N., Dubrovinsky, L., Mondal, S., van Smaalen, S. High pressure synthesis of single crystals of α-boron. J. Cryst. Growth 321, 162–166 (2011).
Article CAS ADS Google Scholar * Widom, M., Mihalcovic, M. Symmetry-broken crystal structure of elemental boron at low temperature. Phys. Rev. B 77, 064113 (2008). Article ADS Google
Scholar * Slack, G. A., Hejna, C. I., Garbauskas, M. F., Kasper, J. S., J. Sol. Stat. Chem. 76, 52–63 (1988). Article CAS ADS Google Scholar * Decker, B. F., Kasper, J. S. The crystal
structure of simple rhombohedral form boron. Acta Crys. 12, 503–506 (1959). Article CAS Google Scholar * Cueilleron, J., Viala, J. C. The Chemical and Pyrometallurgical Purification of
β-Rhombohedral Boron. J. Less-Com. Met., 67, 333–337 (1979). Article CAS Google Scholar * Greenwood, N. N., in Bailar, J. C. (ed.). Comprehensive Inorganic Chemistry. Pergamon Press,
Oxford-New York 1, 680–689 (1973). Google Scholar * Wald, F. On the stability of the red α-rhombohedral boron modification., Elec. Tech. 3, 103–108 (1970). CAS Google Scholar *
Shalamberidze, S. O., Kalandadze, G. I., Khueldze, D. E., Tsurtsumia, B. D. Production of α-rhombohedral boron by amorphous boron crystallization. J. Sol. St. Chem. 154, 199–203 (2000).
Article CAS ADS Google Scholar * Ogitsu, T., Gygi, F., Reed, J., Motome, Y., Schwergler, E., Galli, G. Imperfect crystal and unusual semiconductor element. J. Am. Chem. Soc. 131,
1903–1909 (2009). Article CAS Google Scholar * Shirai, K., Masago, A., Katayama-Yoshida, H. High-pressure properties and phase diagram of boron. Phys. Stat. Sol. (b) 244, 303–308 (2007).
Article CAS ADS Google Scholar * Shang, S., Wang, Y., Arroyave, R., Liu, Z.-K. Phase stability in alfa and beta-rhombohderal boron. Phys. Rev. B. 75, 092101 (2007). Article ADS Google
Scholar * Ogitsu, T., et al. Geometrical frustration in an elemental solid: An Ising model to explain the defect structure of β-rhombohedral boron. Phys. Rev. B 81, 020102R (2010). Article
ADS Google Scholar * Zarechnaya, E., Dubrovinskaia, N., Dubrovinsky, L. Polarized Raman spectroscopy of high pressure orthorhombic boron phase. J. High Pressure Research 29, 530–535
(2009). Article CAS ADS Google Scholar * Polian, A., Ovsyannikov, S., Gauthier, M., Munsch, P., Chervin, J. C., Lemarchand, G. High pressure crystallography: from fundamental phenomena
to technological applications, in: E.Boldyreva, P. Dera (Eds.). NATO Science for Peace and Security SeriesB: Physics and Biophysics XIV, Springer, Dordrecht, Netherlands, 241–250 (2010). *
Zarechnaya, E. Yu.,. et al. Synthesis of an orthorhombic high pressure boron phase. Sci. Technol. Adv. Mater. 9, 044209 (2008). Article Google Scholar * Hoard, F. L., Hughes, R. E. The
chemistry of boron and its compounds. John Wiley and Sons Inc. 25–98 (1967). * Horn, F. H. “Boron” synthesis, structure and properties. Plenum Press Inc. 110–115 (1960). * Horn, F. H., Taft,
E. A., Oliver, D. W. Boron, 2, preparation, properties and applications. Plenum Press Inc. 231–234 (1965). * Niemyski, T., Zawadzki, W. Some properties of pure polycrystalline boron. Phys.
Let. 2, 30 (1962). Article CAS ADS Google Scholar * Wald, F., Rosenberg, J. A., Constitutional investigations in the boron-platinum system. Trans. Met. Soc. AIME, 233, 796 (1965). CAS
Google Scholar * Van Santen, R. A. The Ostwald step rule., J. Phys. Chem., 88, 5768–5769 (1984) Article CAS Google Scholar * Oganov, A. R., et al. Ionic high-pressure form of elemental
boron. Nature letters 457, 863–867 (2009); 460, 292 (2009). Article CAS ADS Google Scholar * Horn, F. Some electrical and optical properties of simple rhombohedral boron. J. Appl. Phys.
30, 1611–1613 (1959). Article CAS ADS Google Scholar * Terauchi, M., Kawamata, Y., Tanaka, M., Takeda, M., Kimura, K. Electron energy-loss spectroscopy study of the electronic structure
of α-rhombohedral boron. J. Sol. State Chem. 133, 156–159 (1997). Article CAS ADS Google Scholar * Hillert, M. Phase equillibria, phase diagrams and phase transformations: their
thermodynamic basis. Cambridge Univ. Press (2007). * Dubrovinskaia, N., Dubrovinsky, L., Miyajima, N., Langenhorst, F., Crichton, W. A., Dubrovinskaia, N., Dubrovinsky, L., Miyajima, N.,
Langenhorst, F., Crichton, W. A., Braun, H. F. High-pressure high-temperature synthesis and characterization of boron-doped diamond. Zeitschrift für Naturforschung 61b, 1561–1565 (2006).
Article Google Scholar * Dubrovinskaia, N., Dubrovinsky, L., McCammon, C. Iron-magnesium alloying at high pressures and temperatures. J. of Physics: Condensed Matter, 16, S1143–S1150
(2004). CAS ADS Google Scholar * Dubrovinsky, L., et al. Portable laser-heating system for diamond anvil cells. J. Synchrotron Rad., 16, 737–741 (2009). Article CAS Google Scholar *
Wirth, R. Focused Ion Beam (FIB): A novel technology for advanced application of micro- and nanoanalysis in geosciences and applied mineralogy. Eur. Journal Mineralogy, 16, 863–877 (2004).
Article CAS ADS Google Scholar Download references ACKNOWLEDGEMENTS The work was supported by the German Research Foundation (DFG) through the DFG Priority Program 1236. N.D. thanks DFG
for the financial support through the Heisenberg Program. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Bayerisches Geoinstitut, Universität Bayreuth, D-95440, Bayreuth, Germany Gleb
Parakhonskiy, Elena Bykova & Leonid Dubrovinsky * Materialphysik und Technologie, Lehrstuhl für Kristallographie, Physikalisches Institut, Universität Bayreuth, D-95440, Bayreuth,
Germany Gleb Parakhonskiy, Natalia Dubrovinskaia & Elena Bykova * GeoForschungsZentrum Potsdam, Experimental Geochemistry and Mineral Physics, 14473, Potsdam, Germany Richard Wirth
Authors * Gleb Parakhonskiy View author publications You can also search for this author inPubMed Google Scholar * Natalia Dubrovinskaia View author publications You can also search for this
author inPubMed Google Scholar * Elena Bykova View author publications You can also search for this author inPubMed Google Scholar * Richard Wirth View author publications You can also
search for this author inPubMed Google Scholar * Leonid Dubrovinsky View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS L.D. and N.D. designed
research; G.P., N.D., L.D., E.B. and R.W. performed research and analyzed data; G.P., L.D. and N.D. wrote the paper. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing
financial interests. RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license,
visit http://creativecommons.org/licenses/by-nc-nd/3.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Parakhonskiy, G., Dubrovinskaia, N., Bykova, E. _et al._ Experimental
pressure-temperature phase diagram of boron: resolving the long-standing enigma. _Sci Rep_ 1, 96 (2011). https://doi.org/10.1038/srep00096 Download citation * Received: 21 June 2011 *
Accepted: 05 September 2011 * Published: 19 September 2011 * DOI: https://doi.org/10.1038/srep00096 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative