Play all audios:
ABSTRACT As an integral part of a porous framework and uniformly distributed throughout the internal pore space, the high density of the exposed B–H bond in zeolite-like porous BIF-20 (BIF =
Boron Imidazolate Framework) is shown here to effectively produce nanoparticles within its confined pore space. Small noble-metal nanoparticles (Ag or Au) are directly synthesized into its
pores without the need for any external reducing agent or photochemical reactions and the resulting Ag@BIF-20 (or Au@BIF-20) samples show high catalytic activities for the reduction of
4-nitrophenol. SIMILAR CONTENT BEING VIEWED BY OTHERS SYNTHESIS AND CHARACTERIZATION OF NOVEL NANOMATERIAL ZN-MOF-NH2-ZIF-8@CU BASED ON MOF-ON-MOF ARCHITECTURE AND CU NANOPARTICLE AS A
STABLE NANOCATALYST IN SOLVENT-FREE A3 COUPLING REACTION Article Open access 23 May 2025 SYNTHESIS OF ZEOLITIC IMIDAZOLATE FRAMEWORK-8 AND GOLD NANOPARTICLES IN A SUSTAINED
OUT-OF-EQUILIBRIUM STATE Article Open access 07 January 2022 SYNTHESIS OF NEW DFNS/ZNTIO3 NANOPARTICLES AS A NANOCATALYST FOR THE REACTION OF QUINAZOLINE-2, 4(1H, 3H)-DIONE WITH CO2 Article
Open access 04 April 2025 INTRODUCTION Metal-organic frameworks (MOFs) or porous coordination polymers (PCPs) continue to attract much attention in new emerging areas, particularly those
related to energy use and environmental conservation1,2,3,4,5,6. With large internal surface areas and uniform pore and cavity sizes, MOFs share a number of structural and catalytical
properties of inorganic zeolites7,8,9. Typical zeolite-like MOF examples are zeolitic imidazolate frameworks (ZIFs) and boron imidazolate frameworks (BIFs)10,11,12,13,14,15,16,17. Both ZIFs
an BIFs adopt tunable zeolite-type topologies (e.g., SOD, RHO, LTA etc) and have promising applications for gas storage and separation11,16. Among these, the ZIF-8 framework (Zn(mim)2, mim =
2-methylimidazole) has become popular for use as support of select metal nanoparticles (M-NPs) for heterogeneous catalysis. However, loading of M-NPs into these zeotype MOF materials is
typically achieved by the impregnation of a metal precursor via grinding or diffusion, followed by reduction of the metal precursor to metal(0) atoms via external reducing agents such as
NaBH4 or H2 gas18,19,20. Such multi-step procedure is also commonly used to incorporate metal or metal oxide NPs into other MOFs21,22,23,24. This method has an intrinsic limitation for
preparing M-NP@MOF with uniformly distributed M-NPs throughout the pore space, because the diffusion of external reducing agents through internal pore space of MOFs is complicated and
sometimes impeded by many factors such as pore size and geometry and even the particle size and morphology of the host materials. External reducing agents can also enable the possible
reduction of noble metal precursors on external surfaces of MOF particles, thus diminishing the important role of pore confinement effects and size selectivity of resulting catalysts.
Zeolitic BIFs are the most recent addition to the family of zeolite-like inorganic-organic hybrid materials. They contain tetrahedral cations (e.g., Li+ Cu+ or Zn2+) linked by various
pre-synthesized boron imidazolate complexes12,13,14,15. One distinct advantage of the BIFs system is that, both four-substituted B(mim)4− ligand and three-substituted BH(mim)3− ligand can be
readily synthesized prior to solvothermal assembly. Of particular interest to the goal of this work is the unique BH(mim)3− ligand that has been integrated into two BIFs (BIF-20 and BIF-21)
with interrupted zeolite LTA and ATN topologies, respectively15. For these BH(mim)3−-based BIFs, there exists a high density of B–H bonds on the framework BH(mim)3− ligands. These B–H
groups decorate the internal pore surface and their chemical functionality including the reducing property could clearly introduce novel functionality to these BIFs. Yet, prior to this work,
such framework B-H functionality, with its potential for pore confinement effects and size control, has never been explored. In this work, we report that BIF-20 crystal can directly produce
small noble-metal nanoparticles (Ag or Au) in its pores without the need for any external reducing agent or photochemical reactions that are often employed for reduction of noble metal ions
and the resulting Ag@BIF-20 (or Au@BIF-20) samples exhibit high catalytic activities for the reduction of 4-nitrophenol (4-NP). RESULTS BIF-20 (Zn2(BH(mim)3)2(obb); obb =
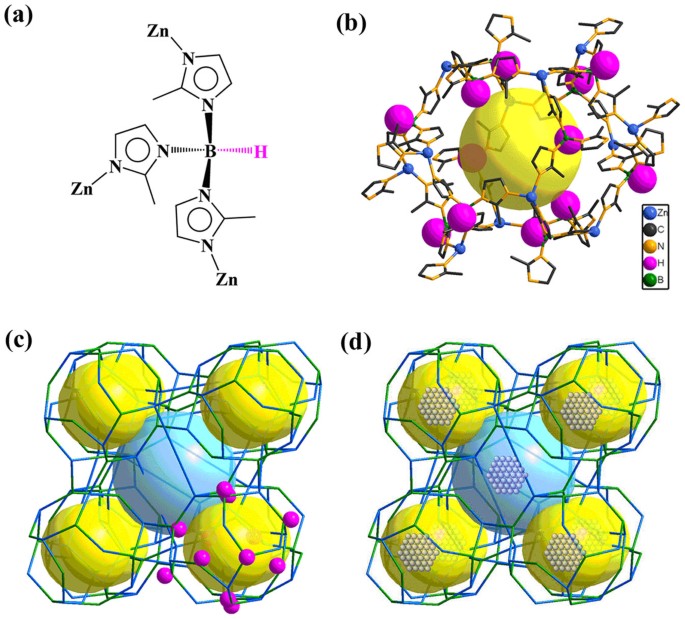
4,4′-oxybis(benzoate)) possesses a neutral interrupted zeolite LTA structure with large cavities and permanent porosity15. Rich naked B–H bonds are present on the surface of porous space
(Figure 1a–b). The as-synthesized BIF-20 crystals were used directly to produce Ag@BIF-20 and Au@BIF-20 materials (Figure 1c–d). Ag@BIF-20 can be quickly prepared by immersing fresh BIF-20
crystals in a methanol or water solution of AgNO3 at room temperature (Figure 2a–b). The amount of deposited Ag NPs and the rate of deposition increase with increasing AgNO3 concentration,
as shown by the color change of the BIF-20 crystals (Figure 2c).The color change of BIF-20 crystals from colorless to brown can be attributed to the surface plasmons of spherical silver
nanoparticles. After the loading, inductively coupled plasma atomic emission spectroscopy (ICP-AES) demonstrated that the weight percentage of Ag NPs in BIF-20 is 3.01%. The field-emission
(FE) TEM image of the resulting dark-brown Ag@BIF-20 crystals prepared from methanol and water solutions, respectively, indicated the formation of ca. 3.0 nm Ag-NPs in the crystals (Figure
3a–b and S2). Moreover, the sizes of these particles are independent on the preparation conditions (e.g., concentration, solvent or time) (Figure S1), which suggests that the porous surface
structure of BIF-20 may provide steric restriction to confine and limit the growth of Ag NPs although the particles are larger than the pore size of BIF-20, which is accordance with the
reported metal NPs/MOF21,22,23,24,25,26,27,28,29. The electron paramagnetic resonance (EPR) spectrum of the resulting solid shows a peak at _g_ = 2.046 for metallic silver (Figure 3c). The
X-ray photoelectron spectra (XPS) as well as the energy-dispersive X-ray spectroscopy (EDS) data indicate that Ag(0) and Zn(II) coexist in the solid (Figure S3). In the XPS, the 3d5/2 and
3d3/2 peaks for Ag(0) appear at 368.6 and 374.6 eV, respectively (Figure 3d)26,30. The 2p3/2 and 2p1/2 peaks for Zn(II) appear at 1022.0 and 1045.0 eV, respectively (Figure S4). The powder
X-ray diffraction (PXRD) patterns further reveal that the host framework of BIF-20 is retained after the loading of Ag NPs (Figure S5). The silver peaks are hardly seen in the PXRD pattern
because the particle size is too small and the amount of silver is too little. Similar procedure was employed to load Au NPs into BIF-20 crystals. However, the process is more complicated
and slower compared to the loading of Ag NPs. As shown in Figure 4a, when BIF-20 crystals were immersed in the methanol solution of NaAuCl4 at room temperature for 50 minutes, the color of
BIF-20 crystals changed from colorless to yellow. The (FE)TEM image of the yellow solid indicated the formation of <2 nm Au NPs (Figure 4d). Once the soaking time increased to 6 hours and
the resulting yellow crystals were further exposed in air for more than 24 hours, Au@BIF-20 crystal containing obvious pink Au-NP “core” was formed (Figure 4b). That means that the Au NPs
are growing in BIF-20, which is also demonstrated by the (FE)TEM image of this pink solid. About 4.0 nm Au NPs were found in pink Au@BIF-20 (Figure 4e). If the concentration of Au(III)
solution and soaking time further increased, the final red Au@BIF-20 crystals were obtained (Figure 4c) and the size of the Au NPs increased to ca. 6.60 nm (Figure 4f). ICP-AES measurement
indicated that the weight content of Au NPs in BIF-20 is 1.63%. The EPR spectrum of the yellow solid shows a peak at _g_ = 2.052 for Au(0) and a peak at _g_ = 2.376 for Au(III), respectively
(Figure S7a). These values are consistent with the results of the XPS and EDS data of pink Au@BIF-20 solids (Figures S3–4 and S7), which indicate that Au(0), Au(III) and Zn(II) coexist in
the solid. In the XPS traces, the 4f7/2 and 4f5/2 peaks of Au(0) appear at 84.35 and 88.00 eV and those of Au(III) appear at 87.05 and 90.65 eV (Figure S7)31,32. The existence of Au(III) may
be attributed to the unreacted AuCl4− that is mixed with the Au(0) nanocomposite. The PXRD patterns of the pink and red Au@BIF-20 solids show that the host framework are still retained and
the gold peaks are also hardly seen in the PXRD patterns (Figure S6). DISCUSSION Based on above (FE)TEM, EPR and XPS data, the color change of the crystals from colorless to yellow may be
attributed to the migration of AuCl4− ions into the pores of BIF-20. At this first stage, the special color of Au NPs is hardly seen because the amount of Au NPs is too little. As for the
pink Au@BIF-20 crystals, the NPs are found predominantly in the core of the crystal surrounded by a clear, NP-free shell (Figure 4b). This result illustrated that the redox reaction to
produce Au NPs might slowly progress from the core towards the outside. Finally, the deposition of Au NPs became uniform throughout the BIF-20 crystal (Figure 4c). Since the reduction of
Au(III) needs multiple electrons, this Au@BIF-20 formation process is thus slow, but its formation mechanism becomes easy to understand. Considering the structural feature of BIF-20, the
presence of potentially active B–H bonds from the coordinated BH(mim)3− ligands may act as the reducing agent and contribute to the direct formation of M-NPs in BIF-20 crystals. To
demonstrate the vital role of these B–H bonds, further experiments have been performed. Another BIF-25 (Co2[B(im)4]4, im = imidazolate) without any B–H bond was employed to run the similar
NPs-loading experiments as BIF-20. However, no M-NPs can be formed in the BIF-25 samples (Figure S9). It is notable that the BH(mim)3− ligand itself can reduce Ag(I) or Au(III) to metal(0).
When the KBH(mim)3 salt was dissolved in the methanol solution of NaAuCl4 or the water solution of AgNO3, respectively, Au or Ag particles were produced. Specifically, two oxidation products
in the mother liquid, B(mim)3(OCH3)− (_Mw_ = 285.14) and B(mim)3(OH)− (_Mw_ = 271.11) were also identified by the mass spectrum, respectively (Figure S13). These results reveal that the B–H
bonds in BIF-20 do play a dominant role on producing M-NPs. It is also widely recognized that the B–H bonds are traditional reducing groups. However, as part of a porous framework
structure, such a reducing function of the B–H bond, to the best of our knowledge, is first realized in BIF-20 material. The reduction of 4-nitrophenol (4-NP) by KBH4 was chosen as a model
reaction for studying the catalytic performance of Ag@BIF-20 and Au@BIF-20, respectively. The reduction kinetics was monitored by UV-vis absorption spectroscopy of the reaction mixture after
the addition of the catalyst. Only tiny change (from 1.901 to 1.490 at 400 nm) in the absorption was determined even after standing for 24 h with BIF-20, indicating that BIF-20 has no
catalytic activity for the reduction. After addition of a small amount (5.0 mg) of Ag@BIF-20 or Au@BIF-20, the absorption of 4-NP at 400 nm significantly decreases along with a concomitant
increase of the ~300 nm peak of 4-aminophenol (4-AP) (Figure 5a and S10). The UV-vis spectra show an isosbestic point (around 318 nm), suggesting that the catalytic reduction of
4-nitrophenol gives 4-aminophenol only without byproduct33,34. Considering the concentration of BH4− is much higher than that of 4-NP (CKBH4/C4-NP = 1000), the reaction should be of
pseudo-first-order with regard to the reactant. As expected, linear relationships between ln(Ct/C0) and reaction time are obtained in the reduction catalyzed by Ag@BIF-20 or Au@BIF-20
(Figure 5b). The rate constant k was calculated to be 0.37 and 0.22 for the reactions using catalysts Ag@BIF-20 and Au@BIF-20, respectively. Ag@BIF-20 has much higher activity than
Au@BIF-20, which is also superior to that of most Au- and Ag-based catalysts under ambient conditions33,34. To investigate the reusability, four recycles of the activity were examined for
Ag@BIF-20 and Au@BIF-20, respectively. The catalyst exhibits similar catalytic performance without significant reduction in the conversion for the same reaction time (8 min for Ag@BIF-20 and
14 min for Au@BIF-20) (Table S1), revealing the stability of the catalysts. (FE)TEM measurements of the catalysts indicate the size of the Ag and Au NPs almost remained the same after
reaction, further suggesting an excellent stability and long life (Figure 5c–d). In summary, the zeolitic BIF-20 crystal with rich naked B–H bonds in its porous structure successfully used
the reducing function of these B–H bonds to produce small noble-metal (Ag or Au) NPs directly, leading to the formation of NPs-embedding crystalline materials, Ag@BIF-20 and Au@BIF-20. Both
resulting materials have high catalytic activities for the reduction of 4-nitrophenol. The results not only demonstrated the potential activity of the B–H bonds in a BIF structure for
post-synthetic chemical modification, but also opened a new approach toward the fabrication of NPs in zeolitic MOF crystals. METHODS MATERIALS AND INSTRUMENTATION All reagents were purchased
commercially and used without further purification. All Powder X-ray diffraction (PXRD) analyses were recorded on a Rigaku Dmax2500 diffractometer with Cu Kα radiation (λ = 1.54056 Å) with
a step size of 0.05°. TEM measurements were performed by using a JEOL-2010 TEM equipped with the energy dispersive X-ray spectrum operated at 200 kV. ICP analysis was conducted by using
Inductively Coupled Plasma OES spectrometer (Ultima2, JobinYvon). X-ray photoelectron spectra (XPS) were acquired with a PHI Quantum 2000 XPS system with a monochromatic Al Kαsource and a
charge neutralizer. The binding energies (BE) were referred to the C1s peak at 284.6 eV. The electron paramagnetic resonance (EPR) spectra were recorded on a Bruker E 500. The UV-VIS
absorption spectra were measured at room temperature with a Perkin-Elmer Lambda 950 UV/vis spectrophotometer. REFERENCES * Horcajada, P. et al. Metal-organic frameworks in biomedicine. Chem.
Rev. 112, 1232–1268 (2012). Article CAS Google Scholar * Vaidhyanathan, R. et al. Direct observation and quantification of CO2 binding within an amine-functionalized nanoporous solid.
Science 330, 650–653 (2010). Article CAS ADS Google Scholar * Herm, Z. R. et al. Separation of hexane isomers in a metal-organic framework with triangular channels. Science 340, 960–964
(2013). Article CAS ADS Google Scholar * Zhang, J.-P., Zhang, Y.-B., Lin, J.-B. & Chen, X.-M. Metal azolate frameworks: from crystal engineering to functional materials. Chem. Rev.
112, 1001–1033 (2012). Article CAS Google Scholar * Nugent, P. et al. Porous materials with optimal adsorption thermodynamics and kinetics for CO2 separation. Nature 495, 80–84 (2013).
Article CAS ADS Google Scholar * Yoon, M., Srirambalaji, R. & Kim, K. Homochiral metal-organic frameworks for asymmetric heterogeneous catalysis. Chem. Rev. 112, 1196–1231 (2012).
Article CAS Google Scholar * Cooper, E. R. et al. Ionic liquids and eutectic mixtures as solvent and template in synthesis of zeolite analogues. Nature 430, 1012–1016 (2004). Article CAS
ADS Google Scholar * Lin, H.-Y. et al. Crystalline Inorganic Frameworks with 56-Ring, 64-Ring and 72-Ring Channels. Science 339, 811–813(2013). Article CAS ADS Google Scholar * Kang,
Y., Wang, F., Zhang, J. & Bu, X. Luminescent MTN-type cluster−organic framework with 2.6 nm cages. J. Am. Chem. Soc. 134, 17881–17884 (2012). Article CAS Google Scholar * Banerjee,
R. et al. High-throughput synthesis of zeolitic imidazolate frameworks and application to CO2 capture. Science 319, 939–943(2008). Article CAS ADS Google Scholar * Phan, A. et al.
Synthesis, structure and carbon dioxidecapture properties of zeolitic imidazolate frameworks. Acc. Chem. Res. 43, 58–67 (2009). Article Google Scholar * Zhang, J. et al. Zeolitic boron
imidazolate frameworks. Angew. Chem. Int. Ed. 48, 2542–2545 (2009). Article CAS Google Scholar * Wu, T. et al. Zeolite RHO-type net with the lightest elements. J. Am. Chem. Soc. 131,
6111–6113 (2009). Article CAS Google Scholar * Zheng, S. et al. Porous metal carboxylate boron imidazolate frameworks. Angew. Chem., Int. Ed. 49, 5362–5366 (2010). Article CAS Google
Scholar * Zhang, H.-X. et al. Interrupted Zeolite LTA and ATN-Type Boron Imidazolate Frameworks. J. Am. Chem. Soc. 133, 11884–11887 (2011). Article CAS Google Scholar * Tan, J.-C.,
Bennett, T. D. & Cheetham, A. K. Chemical structure, network topology and porosity effects on the mechanical properties of zeolitic imidazolate frameworks. Proc. Nat. Acad. Sci. USA
1107, 9938–9943 (2010). Article ADS Google Scholar * Zhang, J.-P., Zhu, A.-X., Lin, R.-B., Qi, X.-L. & Chen, X.-M. Pore surface tailored SOD-type metal-organic zeolites. Adv. Mater.
23, 1268–1271 (2011). Article CAS Google Scholar * Jiang, H.-L. et al. Au@ZIF-8: CO oxidation over gold nanoparticles deposited to metal-organic framework. J. Am. Chem. Soc. 131,
11302–11303 (2009). Article CAS Google Scholar * Jiang, H.-L. et al. Synergistic catalysis of Au@Ag core-shell nanoparticles stabilized on metal-organic framework. J. Am. Chem. Soc. 133,
1304–1306 (2011). Article CAS Google Scholar * Esken, D. et al. GaN@ZIF-8: Selective formation of gallium nitride quantum dots inside a zinc methylimidazolate framework. J. Am. Chem. Soc.
133, 16370–16373 (2011). Article CAS Google Scholar * Hermes, S. et al. Metal@MOF: Loading of highly porous coordination polymers host lattices by metal organic chemical vapor
deposition. Angew. Chem. Int. Ed. 44, 6237–6241 (2005). Article CAS Google Scholar * Zlotea, C. et al. Pd Nanoparticles embedded into a metal-organic framework: synthesis, structural
characteristics and hydrogen sorption properties. J. Am. Chem. Soc. 132, 2991–2997 (2010). Article CAS Google Scholar * Lim, D.-W., Yoon, J., Ryu, K. Y. & Suh, M. P. Magnesium
nanocrystals embedded in a metal-organic framework: hybrid hydrogen storage with synergistic effect on physi- and chemisorption. Angew. Chem. Int. Ed. 51, 9814–9817 (2012). Article CAS
Google Scholar * Zhu, Q.-L., Li, J. & Xu, Q. Immobilizing metal nanoparticles to metal-organic frameworks with size and location control for optimizing catalytic performance. J. Am.
Chem. Soc. 135, 10210–10213 (2013). Article CAS Google Scholar * Moon, H. R., Kim, J. H. & Suh, M. P. Redox-active porous metal-organic framework producing silver nanoparticles from
AgI ions at room temperature. Angew. Chem. Int. Ed. 44, 1261–1265 (2005). Article CAS Google Scholar * Suh, M. P., Moon, H. R., Lee, E. Y. & Jang, S. Y. A redox-active two-dimensional
coordination polymer: preparation of silver and gold nanoparticles and crystal dynamics on guest removal. J. Am. Chem. Soc. 128, 4710–4718 (2006). Article CAS Google Scholar * Cheon, Y.
E. & Suh, M. P. Enhanced hydrogen storage by palladium nanoparticles fabricated in a redox-active metal-organic framework. Angew. Chem. Int. Ed. 48, 2899–2903 (2009). Article CAS
Google Scholar * Wei, Y., Han, S., Walker, D. A., Fuller, P. E. & Grzybowski, B. A. Nanoparticle core/shell architectures within MOF crystals synthesized by reaction diffusion. Angew.
Chem. Int. Ed. 51, 7435–7439 (2012). Article CAS Google Scholar * Moon, H. R., Limb, D.-W. & Suh, M. P. Fabrication of metal nanoparticles in metal-organic frameworks. Chem. Soc. Rev.
42, 1807–1824 (2013). Article CAS Google Scholar * Yang, H., Wang, Y. & Zheng, N. Stabilizing subnanometer Ag(0) nanoclusters by thiolate and diphosphine ligands and their crystal
structures. Nanoscale 5, 2674–2677 (2013). Article CAS ADS Google Scholar * Yang, H. et al. Ligand-stabilized Au13Cux (x = 2, 4, 8) bimetallic nanoclusters: ligand engineering to control
the exposure of metal sites. J. Am. Chem. Soc. 135, 9568–9571 (2013). Article CAS Google Scholar * Casaletto, M. P. et al. XPS study of supported gold catalysts: the role of Au0 and Au+δ
species as active sites. Surf. Interface Anal. 38, 215–218 (2006). Article CAS Google Scholar * Deng, Y. et al. Multifunctional mesoporous composite microspheres with well-designed
nanostructure: a highly integrated catalyst system. J. Am. Chem. Soc. 132, 8466–8473 (2010). Article CAS Google Scholar * Lee, J., Park, J. C. & Song, H. A nanoreactor framework of a
Au@SiO2 yolk/shell structure for catalytic reduction of p-nitrophenol. Adv. Mater. 20, 1523–1528 (2008). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS This work is
supported by 973 program (2012CB821705 and 2011CB932504), NSFC (91222105, 21203196, 21221001), NSF of Fujian Province (2011J06005), CAS (XDA07070200) and NSF (X.B. DMR-0846958). AUTHOR
INFORMATION AUTHORS AND AFFILIATIONS * State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fujian, Fuzhou,
350002, P.R. China Hai-Xia Zhang, Meng Liu & Jian Zhang * Department of Chemistry and Biochemistry, California State University, Long Beach, 1250 Bellflower Boulevard, Long Beach, CA,
90840, USA Xianhui Bu Authors * Hai-Xia Zhang View author publications You can also search for this author inPubMed Google Scholar * Meng Liu View author publications You can also search for
this author inPubMed Google Scholar * Xianhui Bu View author publications You can also search for this author inPubMed Google Scholar * Jian Zhang View author publications You can also
search for this author inPubMed Google Scholar CONTRIBUTIONS H.Z., J.Z. and M.L. designed and carried out the experiments. H.Z., J.Z. and X.B. analyzed the results and wrote the manuscript.
All authors reviewed the manuscript. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY INFORMATION
Supporting Information RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. To view a copy of this license, visit
http://creativecommons.org/licenses/by-nc-sa/3.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Zhang, HX., Liu, M., Bu, X. _et al._ Zeolitic BIF Crystal Directly Producing
Noble-Metal Nanoparticles in Its Pores for Catalysis. _Sci Rep_ 4, 3923 (2014). https://doi.org/10.1038/srep03923 Download citation * Received: 04 December 2013 * Accepted: 13 January 2014 *
Published: 29 January 2014 * DOI: https://doi.org/10.1038/srep03923 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry,
a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative