Play all audios:
ABSTRACT The aim of our study was to evaluate the association between polymorphisms in the methylenetetrahydrofolate reductase (_MTHFR_) gene and the risk for congenital heart disease (CHD).
Electronic literature databases were searched to identify eligible studies published before _Jun, 2014_. The association was assessed by the odds ratio (OR) with a 95% confidence interval
(CI). The publication bias was explored using Begg's test. Sensitivity analysis was performed to evaluate the stability of the crude results. A total of 35 studies were included in this
meta-analysis. For the _MTHFR_ C677T polymorphism, we detected significant association in all genetic models for Asian children and the maternal population. Significant association was also
detected in T vs. C for a Caucasian paediatric population (OR = 1.163, 95% CI: 1.008–1.342) and in both T vs. C (OR = 1.125, 95% CI: 1.043–1.214) and the dominant model (OR = 1.216, 95%
CI:b1.096–1.348) for a Caucasian maternal population. For the _MTHFR_ A1298C polymorphism, the association was detected in CC vs. AC for the Caucasian paediatric population (OR = 1.484, 95%
CI: 1.035–2.128). Our results support the _MTHFR_ -677T allele as a susceptibility factor for CHD in the Asian maternal population and the -1298C allele as a risk factor in the Caucasian
paediatric population. SIMILAR CONTENT BEING VIEWED BY OTHERS ASSOCIATION OF _MTR_ GENE POLYMORPHISMS WITH THE OCCURRENCE OF NON-SYNDROMIC CONGENITAL HEART DISEASE: A CASE–CONTROL STUDY
Article Open access 09 June 2023 ASSOCIATION OF POLYMORPHISMS OF _FOLR1_ GENE AND _FOLR2_ GENE AND MATERNAL FOLIC ACID SUPPLEMENTATION WITH RISK OF VENTRICULAR SEPTAL DEFECT: A CASE-CONTROL
STUDY Article 10 March 2022 GERMLINE VARIANTS IN _HEY2_ FUNCTIONAL DOMAINS LEAD TO CONGENITAL HEART DEFECTS AND THORACIC AORTIC ANEURYSMS Article 21 August 2020 INTRODUCTION Congenital heart
disease (CHD) is the most frequently occurring congenital disorder in newborns and is the most frequent cause of infant death from birth defects. The aetiology of CHD is largely unknown.
Epidemiological studies reveal a significant environmental contribution to the pathogenesis of CHD1,2. Familial aggregation and twin studies indicate the presence of genetic factors for
susceptibility to this condition3,4,5. Except for a few types of CHD induced by a single gene mutation, the majority of CHDs are polygenic diseases affected by both genetic and environmental
factors. The importance of genetic factors in the development of CHD is also supported by recent data from genome-wide association studies (GWASs). Data from these studies have confirmed
that a region on chromosome 4p16 adjacent to the _MSX1_ and _STX18_ genes was associated with the risk of ostium secundum atrial septal defect (ASD)6 and rs2228638 in _NRP1_ on 10p11
significantly increased the risk of Tetralogy of Fallot (TOF)7. In our studies, we identified _HOMEZ and PLAGL1_ as pathogenic genes in Chinese patients with isolated ventricular septal
defects (VSDs)8,9. In addition, our proteomic study revealed plasma protein changes in CHD patients10. The 5,10-methylenetetrahydrofolate reductase (_MTHFR_) gene is located on chromosome 1
at 1p36.3. MTHFR is the key metabolic enzyme of homocysteine (Hcy). It catalyses 5,10-methylenetetrahydrofolate reduction to 5-methyltetrahydrofolate, which as a methyl donor induces Hcy
remethylation to methionine11. A common C677T mutation (rs1801133) in the _MTHFR_ gene has been described, which results in the conversion of the amino acid alanine to valine at position 226
in the protein. This mutation was associated with a 50% reduction of MTHFR enzyme activity, an increase in plasma Hcy concentration and a decrease in plasma folic acid concentration.
Another polymorphism (A1298C, rs1801131) is located in exon 7, within the presumptive regulatory domain and results in a glutamate-to-alanine change with decreased enzyme activity in
vitro12. It has been reported that _MTHFR_ polymorphisms play important roles in diseases. For example, neural tube defects and pregnancy complications appear to be linked to impaired MTHFR
function13,14. Since Wenstrom first noted an association between _MTHFR_ gene polymorphism and susceptibility to CHD15, other studies have been undertaken to replicate this work. However,
previous case-control reports have yielded inconsistent results. Wang and co-workers carried out a meta-analysis involving 2,554 CHD patients and 3,838 controls by searching the electronic
literature for articles published before _July 22, 2012._ They suggested that the infant and maternal _MTHFR_ C667T polymorphism may be associated with an increased occurrence of CHD16. By
contrast, Mamasoula and co-workers indicated that the _MTHFR_ C677T polymorphism, which directly influences plasma folate levels, is not associated with the risk of CHD17. Therefore, we
performed an up-dated meta-analysis of all published studies (until _Jun, 2014_) to investigate the association between _MTHFR_ polymorphisms (C677T and A1298C) and the risk of CHD. METHODS
SEARCH STRATEGY We conducted a comprehensive search of Embase, Ovid, Web of Science, the Cochrane database, Medline (PubMed), the Chinese Biomedical Literature Database (CBM-disc,
1979–2014), the database of National Knowledge Infrastructure (CNKI, 1979–2014) and the full paper database of Chinese Science and Technology of Chongqing (VIP, 1989–2014) to identify
suitable studies published before _Jun, 2014._ The following keywords were used for searching: (“congenital heart” OR “congenital cardiac” OR “heart defect*” OR “congenital car*”) AND
(“polymorphism*” OR “variant*”) AND (“methylenetetrahydrofolate reductase” OR “MTHFR”). The most complete and recent results were used when there were multiple publications from the same
study group. The references of reviews and retrieved articles were also searched simultaneously to find additional eligible studies. INCLUSION CRITERIA Two investigators reviewed all
identified studies independently to determine whether an individual study was eligible for inclusion. The selection criteria for studies to be considered for this meta-analysis were as
follows: 1) _MTHFR_ polymorphisms in CHD; 2) case-control or case-cohort study; 3) proper CHD diagnosis criteria; 4) original data; 5) human subjects, not animal studies. We expected the
clinical assessment of the patients to include anthropometric measurement and physical examination for dysmorphism and malformation and diagnostic studies to include chest X-ray examination,
electrocardiogram, ultrasonic echocardiogram, etc. Studies would be excluded if the necessary information could not be obtained. DATA EXTRACTION Two investigators extracted the data
independently and a third investigator reviewed the result. The following information was extracted from each study: first author, year of publication, study population (country, ethnicity),
the number of patients and controls in the study, genotype information, genotype methods and main types of CHD. If any data essential to the analysis were not available from a study, best
efforts were made to contact the authors to fill in the missing data. STATISTICAL ANALYSIS Allele frequencies for the _MTHFR_ (C677T and A1298C) polymorphisms from each study were determined
by the allele counting method18. The genotype distributions of controls were used to estimate the frequency of the putative risk allele (-677T and -1298C) using the inverse variance
method19,20. The Hardy-Weinberg Equilibrium (_HWE_) is the most fundamental rule of population genetics. It prescribes the genotype frequencies at a locus in terms of its allele frequencies
in a population. In the most general form, it states that selection, migration and random genetic drift occur with random mating in a population in the absence of mutation21. The deviation
from _HWE_ for the distribution of the allele frequencies was analysed by Fisher's exact test in control groups. We examined the contrast of a vs. A, aa vs. AA, aa vs. Aa and also
examined the recessive genetic model (aa vs. AA+Aa) and the dominant genetic model (Aa+aa vs. AA). The associations between _MTHFR_ polymorphisms and CHD susceptibility were estimated by OR
and its 95% CI. The significance of the pooled OR was determined by the Z-test; _P_ < 0.05 was considered statistically significant. To evaluate the specific effects of ethnicity,
stratified analyses were performed. Heterogeneity across the eligible studies was tested using the Q-test and the results were considered statistically significant when _P_ < 0.122,23.
Heterogeneity was also quantified with the _I__2_ metric (_I__2_ _= (Q - df)/Q × 100%_; _I__2_ < 25%, no heterogeneity; _I__2_ = 25–50%, moderate heterogeneity; _I__2_ = 50–75%, large
heterogeneity; _I__2_ > 75%, extreme heterogeneity). When the effects were assumed to be homogenous (_P_ > 0.1, _I__2_ < 50%), the fixed-effects model was used; otherwise, the
random-effects model was more appropriate24,25,26. Sensitivity analysis was performed to evaluate the stability of the results. If more than seven studies were included, Begg's test was
used to measure publication bias, which was shown as a funnel plot27,28. _P_ < 0.05 was considered representative of statistically significant publication bias. All analyses were
performed using STATA software, version 10.0 (Stata Corporation, College Station, TX, USA), Review Manager (RevMan version 5.1.1, The Nordic Cochrane Centre:
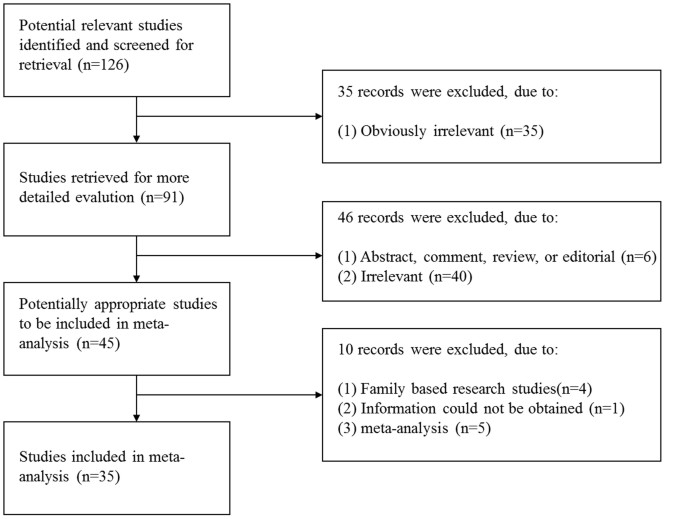
http://ims.cochrane.org/revman/download) and R statistical software (version 2.15.2, http://www.r-project.org). RESULTS STUDIES INCLUDED IN THE META-ANALYSIS A total of 126 abstracts that
met the inclusion criteria were retrieved through the databases. Two reviewers then selected the relevant studies independently. Forty-five relevant studies that described the association
between the _MTHFR_ polymorphism and CHD were identified. However, after reading the full articles and contacting the authors, we excluded five meta-analysis studies29,30,31,32,33, four
family-based studies34,35,36,37 and one study in which information could not be obtained even after the authors were contacted38. Figure 1 shows the process of study selection and exclusion,
with specification of reasons. Finally, 35 studies that met the inclusion criteria, corresponding to 9,329 CHD children and 15,076 normal controls, 3,232 mothers with CHD offspring and
27,174 normal controls for the C677T polymorphism and 1,761 CHD children and 1,868 normal controls/705 mothers with CHD offspring and 15,458 controls for the A1298C polymorphism, were
considered in the meta-analysis15,17,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71. The main characteristics of the included studies are
listed in Table 1–2. POOLED PREVALENCE OF _MTHFR_ -677T AND -1298C IN THE CONTROLS The pooled _MTHFR_ –677T allele frequency determined using the random-effects model was 28.99% (95 CI:
26.14%–32.02%) in the Caucasian paediatric population and was 42.28% (95% CI: 34.17%–50.83%) in the Asian paediatric population. There was no heterogeneity among the Caucasian and Asian
maternal population studies. The _MTHFR_ –677T allele frequency was 31.76% (95 CI: 30.14%–33.43%) in the Caucasian maternal population and was 41.51% (95% CI: 37.50%–45.64%) in the Asian
maternal population. The pooled –1298C allele frequency in the fixed-effects model was 33.12% (95 CI: 29.80%–36.61%) in the Caucasian paediatric population and was 31.09% (95% CI:
25.34%–37.46%) in the Caucasian maternal population using the random-effects model. ASSOCIATION BETWEEN _MTHFR_ C677T POLYMORPHISM AND RISK OF CHD We investigated the association between the
_MTHFR_ C677T polymorphism and the risk of CHD for each study. When all the eligible studies were pooled in the overall population of children with random-effects models, significant
associations were observed in all genetic models: T versus C (OR = 1.248, 95% CI: 1.093–1.426; _P_ = 0.001), TT versus CC (OR = 1.485, 95% CI: 1.140–1.935; _P_ = 0.003) and TT versus CT (OR
= 1.312, 95% CI: 1.100–1.565; _P_ = 0.003), the dominant model (OR = 1.240, 95% CI: 1.053–1.461; _P_ = 0.010) and the recessive model (OR = 1.410, 95% CI: 1.139–1.724; _P_ = 0.001;(Figure
2). In addition, significant associations were observed in the overall maternal population in all genetic models for T versus C (OR = 1.215, 95% CI: 1.085–1.361; _P_ = 0.001), TT versus CC
(OR = 1.488, 95% CI: 1.169–1.859; _P_ = 0.001), TT versus CT (OR = 1.315, 95% CI: 1.042–1.659; _P_ = 0.021), the dominant model (OR = 1.258, 95% CI: 1.144–1.383; _P_ = 2.14e-6) and the
recessive model (OR = 1.408, 95% CI: 1.128–1.757; _P_ = 0.002; (Figure 3). The Z-test indicated that the pooled ORs were statistically significant. In the stratified analysis by ethnicity,
significant associations were found when all studies were pooled with fixed or random-effects models for T versus C (OR = 1.163, 95% CI: 1.008–1.342; _P_ = 0.039) in Caucasian children and
for T versus C (OR = 1.125, 95% CI: 1.043–1.214; _P_ = 0.002), dominant model (OR = 1.216, 95% CI: 1.096–1.348; _P_ = 2.24e-4) in the Caucasian maternal population. In addition, significant
associations were found when all studies were pooled in fixed or random-effects models for all genetic models in Asian children and the maternal population. The main results of meta-analysis
are shown in Table 3. ASSOCIATION BETWEEN MTHFR A1298C POLYMORPHISM AND RISK OF CHD We investigated the association between the _MTHFR_ A1298C polymorphism and the risk of CHD for each
study. Overall, when all the eligible studies were pooled in the fixed-effects model, significant associations were observed for CC vs. AC (OR = 1.354, 95% CI: 1.022–1.793; _P_ = 0.034) and
for the recessive model (OR = 1.322, 95% CI: 1.015–1.732; _P_ = 0.038) in the overall paediatric population. The main results of the meta-analysis are shown in Table 4. In the analysis
stratified by ethnicity, significant associations were found in the Caucasian paediatric population when all studies were pooled in the fixed-effects model for CC versus AC (OR = 1.484, 95%
CI: 1.035–2.128; _P_ = 0.032; Figure 4). The main results of the meta-analysis are shown in Table 4. SENSITIVITY ANALYSES We removed the studies due to the genotype distribution in the
control groups deviating from _HWE_. We found that the corresponding ORs for the C677T polymorphism for the TT vs. CT and recessive models in the overall paediatric population and for all
genetic types in the overall maternal population and the Asian maternal population were not substantially altered (Table 5). This finding supports the reliability of the results. PUBLICATION
BIAS Begg's test and a funnel plot were performed to assess the publication bias of the literature. We detected publication biases for the C677T polymorphism for the T vs. C and
dominant models in the Caucasian paediatric population (Table 3). This might represent a limitation of our analysis because the studies with null findings, especially those with small sample
size, were less likely to be published. By using the trim and fill method, we showed that, if the publication bias was the only source of the funnel plot asymmetry, they needed two and one
more studies, respectively, to balance the funnel plot. The adjusted risk estimate was attenuated. The adjusted OR for T vs. C was 1.142 (95% CI: 0.729–1.786) and for the dominant model was
1.253 (95%CI: 0.738–2.133). The results suggest no evidence of publication biases in other genetic models and populations (Figure 5). DISCUSSION It is estimated that 7.9 million children are
born with a serious birth defect of genetic or partially genetic origin each year in the world. CHDs are the most commonly occurring conditions. However, the aetiology of CHDs is largely
unknown and there are no established strategies for reducing their public health impact. Many studies have demonstrated that genetic factors play important roles in the pathogenesis of CHD.
In our previous studies, we have detected several novel variations of the _PLAGL1_ and _HOMEZ_ genes in Chinese patients with isolated VSD. We believe that these two genes are directly
linked aetiologically with isolated VSD in the population8,9. In addition, the results of recent genome-wide association studies indicated that a region on chromosome 4p16 adjacent to the
_MSX1_ and _STX18_ genes was associated (_P_ = 9.5 × 10−7) with the risk of ostium secundum ASD6. These studies also showed that 1p12 (rs2474937 near _TBX15_; _P_ = 8.44 × 10−10) and 4q31.1
(rs1531070 in _MAML3_; P = 4.99 × 10−12) were associated with congenital heart malformations in Han Chinese populations72. In 1999, Kapusta and associates first reported that maternal
hyperhomocysteinaemia is correlated with an increased risk of CHDs73. More recently, Hobbs and co-workers studied mothers whose pregnancies were affected by congenital heart defects (224
case subjects) or unaffected by any birth defect (90 control subjects) and identified Hcy, S-adenosylhomocysteine and methionine as the most important biomarkers predictive of case or
control status36. The MTHFR protein is a key enzyme in Hcy metabolism. The _MTHFR_ gene is located on chromosome 1 at 1p36.3. The major product of the _MTHFR_ gene is a catalytically active
77 kDa protein that catalyses the conversion of 5,10-methylenetetrahydrofolate into 5-methyltetrahydrofolate, the major circulating form of folate. Two common genetic polymorphisms
associated with reduced MTHFR activity have been identified. The C677T polymorphism is located in exon 4 at the folate-binding site and results in an alanine-to-valine substitution. In
healthy homozygous subjects, the 677TT genotype is associated with higher total Hcy and lower folate plasma level. The other polymorphism (A1298C) is in exon 7 within the presumptive
regulatory domain and results in a glutamate-to-alanine change. Heterozygosity and homozygosity are associated neither with higher total Hcy nor lower folate plasma concentration. The
_MTHFR_ gene polymorphisms are directly linked with many diseases20,74. Our recent meta-analysis demonstrated that the _MTHFR_ C677T polymorphism is associated with the risk of myocardial
infarction in young/middle-aged Caucasians and is associated with susceptibility to preeclampsia20,74. A number of studies have investigated the association between _MTHFR_ genotype and the
risk of CHD. In fact, in the last few years, several case–control studies were performed on this topic. However, the results are inconclusive. The two most recent meta-analyses for
associations between polymorphism and CHD also led to conflicting conclusions. By reviewing all studies published before _April, 2011,_ Yin and co-workers suggested that the foetal and
paternal _MTHFR_ C667T gene may be associated with an increased occurrence of CHD32. By contrast, after analysis of 7,698 cases and 13,159 controls by reviewing studies published before
_2010_, Mamasoula and co-workers indicated that the same polymorphism, which directly influences plasma folate levels, is not associated with CHD risk17. Others also conducted meta-analysis
to evaluate the association between MTHFR polymorphism and CHD29,30,31. It is possible that the relatively small sample size of these studies affected the accuracy of the results. Therefore,
it is essential to re-perform a meta-analysis to evaluate the association. In our present study, we enlarged the sample size to 24,405 participants (9,329 CHD children and 15,076 normal
controls) and performed sensitivity analysis to evaluate the stability of the results. In addition, we are the first to evaluate the association between the _MTHFR_ A1298C polymorphism and
CHD by meta-analysis. We are indebted to Dr. Christensen from McGill University for kindly allowing us access to his previously un-published data for this meta-analysis. Our results indicate
that the frequency of the putative risk allele -677T was 28.99% in Caucasian children and 31.76% in the Caucasian maternal population, whereas the frequency of -677T was 42.28% in Asian
paediatric and 41.51% in the Asian maternal population. In addition, the pooled –1298C allele frequency was 33.12% in Caucasian children and 31.09% in the Caucasian maternal population. The
meta-analysis results showed that associations exist between the _MTHFR_ C677T polymorphism and susceptibility to CHD for all genetic models in all paediatric and maternal populations,
especially in the Asian population. We also detected a significant association in the genetic model for T vs. C in the Caucasian paediatric population and in T vs. C and TT vs. CT for the
Caucasian maternal population (Table 3). In our analysis of the A1298C polymorphism, we detected an association in the genetic model for TT vs. CT in the Caucasian paediatric population
(Table 4). The results showing significant association for all genetic models in the overall maternal population and the Asian maternal population and for the TT vs. CT and recessive models
in the overall paediatric population were found to be stable and reliable by sensitivity analyses (Table 5). Some limitations of this meta-analysis should be discussed. First, significant
heterogeneity was observed in some genetic models when we pooled ORs. Under this condition, we used the random-effects model to pool the data. Sensitivity analysis was performed to evaluate
the stability of the crude results. Second, publication biases appear to substantially contaminate the literature with regard to some genetic associations. The results of the trim and fill
method demonstrated that the publication biases may affect the stability of positive results. In conclusion, our results support the _MTHFR_ –677T allele as a susceptibility factor for CHD
in the Asian maternal population and the -1298C allele as a risk factor in the Caucasian paediatric population. Because of the heterogeneity and publication bias, we believe that other
positive results may not be stable in our meta-analysis. A large number of homogeneous studies should be performed to evaluate these crude results in the future. REFERENCES * Kappagoda, C.
T. & Macartney, F. J. Effect of environmental temperatures on oxygen consumption in infants with congenital disease of the heart. Br Heart J 38, 1–4 (1976). Article CAS PubMed PubMed
Central Google Scholar * Lage, K. et al. Genetic and environmental risk factors in congenital heart disease functionally converge in protein networks driving heart development. Proc Natl
Acad Sci USA 109, 14035–14040 (2012). Article ADS PubMed PubMed Central Google Scholar * Bosi, G. et al. The Italian Multicentric Study on Epidemiology of Congenital Heart Disease:
first step of the analysis. Working Party of the Italian Society of Pediatric Cardiology. Cardiol Young 9, 291–299 (1999). Article CAS PubMed Google Scholar * Caputo, S. et al.
Congenital heart disease in a population of dizygotic twins: an echocardiographic study. Int J Cardiol 102, 293–296 (2005). Article PubMed Google Scholar * Seliem, M. A., Bou-Holaigah, I.
H. & Al-Sannaa, N. Influence of consanguinity on the pattern of familial aggregation of congenital cardiovascular anomalies in an outpatient population: studies from the eastern
province of Saudi Arabia. Community Genet 10, 27–31 (2007). Article PubMed Google Scholar * Cordell, H. J. et al. Genome-wide association study of multiple congenital heart disease
phenotypes identifies a susceptibility locus for atrial septal defect at chromosome 4p16. Nat Genet 45, 822–824 (2003). Article CAS Google Scholar * Xu, J. et al. Genetic variants at
10p11 confer risk of tetralogy of fallot in chinese of nanjing. PLoS One 9, e89636 (2014). Article ADS CAS PubMed PubMed Central Google Scholar * Xuan, C. et al. A novel variation of
PLAGL1 in Chinese patients with isolated ventricular septal defect. Genet Test Mol Biomarkers 16, 984–987 (2012). Article CAS PubMed PubMed Central Google Scholar * Xuan, C. et al.
Identification of two novel mutations of the HOMEZ gene in Chinese patients with isolated ventricular septal defect. Genet Test Mol Biomarkers 17, 390–394 (2013). Article CAS PubMed
PubMed Central Google Scholar * Xuan, C. et al. Proteomic study reveals plasma protein changes in congenital heart diseases. Ann Thorac Surg 97, 1414–1419 (2014). Article PubMed Google
Scholar * Biselli, P. M. et al. Genetic polymorphisms involved in folate metabolism and concentrations of methylmalonic acid and folate on plasma homocysteine and risk of coronary artery
disease. J Thromb Thrombolysis 29, 32–40 (2010). Article CAS PubMed Google Scholar * Hernandez-Diaz, S. et al. Folic acid antagonists during pregnancy and the risk of birth defects. N
Engl J Med 343, 1608–1614 (2000). Article CAS PubMed Google Scholar * Christensen, B. et al. Genetic polymorphisms in methylenetetrahydrofolate reductase and methionine synthase, folate
levels in red blood cells and risk of neural tube defects. Am J Med Genet 84, 151–157 (1999). Article CAS PubMed Google Scholar * Ueland, P. M. et al. Biological and clinical
implications of the MTHFR C677T polymorphism. Trends Pharmacol Sci 22, 195–201(2001). Article CAS PubMed Google Scholar * Wenstrom, K. D. et al. Association of the C677T
methylenetetrahydrofolate reductase mutation and elevated homocysteine levels with congenital cardiac malformations. Am J Obstet Gynecol 184, 806–817 (2001). Article CAS PubMed Google
Scholar * Wang, W. et al. MTHFR C677T polymorphism and risk of congenital heart defects: evidence from 29 case-control and TDT studies. PLoS One 8, e58041 (2013). Article ADS CAS PubMed
PubMed Central Google Scholar * Mamasoula, C. et al. Association between C677T polymorphism of methylene tetrahydrofolate reductase and congenital heart disease: meta-analysis of 7697
cases and 13,125 controls. Circ Cardiovasc Genet 6, 347–353 (2013). Article CAS PubMed Google Scholar * Klug, W. S. Population and evolutionary genetics. Concepts of genetics. Wilbur,
B.(ed.). 700–702 (Pearson Benjamin Cummings, CA, 2011). * Xuan, C. et al. No association between APOE epsilon 4 allele and multiple sclerosis susceptibility: a meta-analysis from 5472 cases
and 4727 controls. J Neurol Sci 308, 110–116 (2011). Article CAS PubMed Google Scholar * Xuan, C. & Lun, L. M. Association between the methylenetetrahydrofolate reductase C677T
polymorphism and susceptibility to preeclampsia: the need for data clarification in a recent meta-analysis. Hypertens Res 36, 463–464 (2013). Article CAS PubMed Google Scholar *
Chakraborty, R. Hardy–Weinberg Equilibrium. Encyclopedia of Biostatistics 4, 1–2 (2005). Google Scholar * Higgins, J. P. & Thompson, S. G. Quantifying heterogeneity in a meta-analysis.
Stat Med 21, 1539–1558 (2002). Article PubMed Google Scholar * Bowden, J. et al. Quantifying, displaying and accounting for heterogeneity in the meta-analysis of RCTs using standard and
generalised Q statistics. BMC Med Res Methodol 11, 41(2011). Article PubMed PubMed Central Google Scholar * Xuan, C. et al. Association between OCTN1/2 gene polymorphisms (1672C-T,
207G-C) and susceptibility of Crohn's disease: a meta-analysis. Int J Colorectal Dis 27, 11–9 (2012). Article PubMed Google Scholar * Xuan, C. et al. PTPN22 gene polymorphism
(C1858T) is associated with susceptibility to type 1 diabetes: a meta-analysis of 19,495 cases and 25,341 controls. Ann Hum Genet 77, 191–203 (2013). Article CAS PubMed Google Scholar *
Zhang, B. B. et al. Genetic 135G/C polymorphism of RAD51 gene and risk of cancer: a meta-analysis of 28,956 cases and 28,372 controls. Fam Cancer 10.1007/s10689-014-9729-0 (2014). [Epub
ahead of print] * Begg, C. B. & Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101 (1994). Article CAS PubMed MATH
Google Scholar * Tian, Q. W. et al. Diagnostic accuracy of glycosylated hemoglobin in chinese patients with gestational diabetes mellitus: a meta-analysis based on 2,812 patients and 5,918
controls. Genet Test Mol Biomarkers 17, 687–695 (2013). Article CAS PubMed Google Scholar * van Beynum, I. M. et al. The MTHFR 677C->T polymorphism and the risk of congenital
heart defects: a literature review and meta-analysis. QJM 100, 743–753 (2007). Article CAS PubMed Google Scholar * Verkleij-Hagoort, A. et al. Hyperhomocysteinemia and MTHFR
polymorphisms in association with orofacial clefts and congenital heart defects: a meta-analysis. Am J Med Genet A 143A, 952–960 (2007). Article CAS PubMed Google Scholar * Nie, Y. et
al. Methylenetetrahydrofolate reductase C677T polymorphism and congenital heart disease: a meta-analysis. Clin Chem Lab Med 49, 2101–2108 (2011). Article CAS PubMed Google Scholar * Yin,
M. et al. Meta analysis of the association between MTHFR C677T polymorphism and the risk of congenital heart defects. Ann Hum Genet 76, 9–16 (2012). Article CAS PubMed Google Scholar *
Chen, K. H. et al. Maternal MTHFR C677T polymorphism and congenital heart defect risk in the Chinese Han population: a meta-analysis. Genet Mol Res 12, 6212–6219 (2013). Article CAS PubMed
Google Scholar * McBride, K. L. et al. A family-based association study of congenital left-sided heart malformations and 5,10 methylenetetrahydrofolate reductase. Birth Defects Res A Clin
Mol Teratol 70, 825–830 (2004). Article CAS PubMed Google Scholar * Pereira, A. C. et al. Lack of evidence of association between MTHFR C677T polymorphism and congenital heart disease
in a TDT study design. Int J Cardiol 105, 15–8 (2005). Article PubMed Google Scholar * Hobbs, C. A. et al. Congenital heart defects and abnormal maternal biomarkers of methionine and
homocysteine metabolism. Am J Clin Nutr 81, 147–153 (2005). Article CAS PubMed Google Scholar * Goldmuntz, E. et al. Variants of folate metabolism genes and the risk of conotruncal
cardiac defects. Circ Cardiovasc Genet 1, 126–132 (2008). Article CAS PubMed PubMed Central Google Scholar * Shaw, G. M. et al. 118 SNPs of folate-related genes and risks of spina
bifida and conotruncal heart defects. BMC Med Genet 10, 49 (2009). Article PubMed PubMed Central Google Scholar * Junker, R. et al. Infant methylenetetrahydrofolate reductase 677TT
genotype is a risk factor for congenital heart disease. Cardiovasc Res 51, 251–254 (2001). Article CAS PubMed Google Scholar * Liu, H. et al. Maternal homocysteine folic acid, MTHFR gene
polymorphism and congenital heart defects in offspring. Chin J Perinatal Med 5, 102–105 (2002). Google Scholar * Storti, S. et al. Association between 5,10-methylenetetrahydrofolate
reductase C677T and A1298C polymorphisms and conotruncal heart defects. Clin Chem Lab Med 41, 276–280 (2003). Article CAS PubMed Google Scholar * Nurk, E. et al. Associations between
maternal methylenetetrahydrofolate reductase polymorphisms and adverse outcomes of pregnancy: the Hordaland Homocysteine Study. Am J Med 117, 26–31 (2004). Article CAS PubMed Google
Scholar * Li, Y. et al. Study of serum Hcy and polymorphisms of Hcy metabolic enzymes in 192families affected by congenital heart disease. J Peking Univ (Health Sci) 37, 75–80 (2005).
Google Scholar * Lee, C. N. et al. Association of the C677T methylenetetrahydrofolate reductase mutation with congenital heart diseases. Acta Obstet Gynecol Scand 84, 1134–1140 (2005).
Article PubMed Google Scholar * Shaw, G. M. et al. Risks of human conotruncal heart defects associated with 32 single nucleotide polymorphisms of selected cardiovascular disease-related
genes. Am J Med Genet A 138, 21–26 (2005). Article PubMed Google Scholar * Hobbs, C. A. et al. Congenital heart defects and genetic variants in the methylenetetrahydroflate reductase
gene. J Med Genet 43, 162–166 (2006). Article CAS PubMed PubMed Central Google Scholar * Zhu, W. L. et al. Maternal and offspring MTHFR gene C677T polymorphism as predictors of
congenital atrial septal defect and patent ductus arteriosus. Mol Hum Reprod 12, 51–54 (2006). Article CAS PubMed Google Scholar * Zhong, Q. A. et al. Association of congenital heart
diseases with MTHFR gene and CBS gene. Guangxi Med J 28, 1140–1142 (2006). CAS Google Scholar * van Beynum, I. M. et al. Maternal MTHFR 677C>T is a risk factor for congenital heart
defects: effect modification by periconceptional folate supplementation. Eur Heart J 27, 981–987 (2006). Article CAS PubMed Google Scholar * Galdieri, L. C. et al. Homocysteine
concentrations and molecular analysis in patients with congenital heart defects. Arch Med Res 38, 212–218 (2007). Article CAS PubMed Google Scholar * Wintner, S. et al. Association of
congenital cardiac defects and the C677T methylenetetrahydrofolate reductase polymorphism. Prenat Diagn 27, 704–708 (2007). Article CAS PubMed Google Scholar * Liu, Y. S. et al. Relation
ship between genetic polymorphism of homocysteine metabolism enzyme and congenital heart disease. Chin J Cardio Rev 15, 210–213 (2007). Google Scholar * van Driel, L. M. et al. Two MTHFR
polymorphisms, maternal B-vitamin intake and CHDs. Birth Defects Res A Clin Mol Teratol 82, 474–481 (2008). Article CAS PubMed Google Scholar * Marinho, C. et al. The
methylenetetrahydrofolate reductase gene variant (C677T) as a susceptibility gene for tetralogy of Fallot. Rev Port Cardiol 28, 809–812 (2009). PubMed Google Scholar * Kuehl, K. et al.
Association of congenital cardiovascular malformations with 33 single nucleotide polymorphisms of selected cardiovascular disease-related genes. Birth Defects Res A Clin Mol Teratol 88,
101–110 (2010). CAS PubMed PubMed Central Google Scholar * Hobbs, C. A., Cleves, M. A., Karim, M. A., Zhao, W. & MacLeod, S. L. Maternal folate-related gene environment interactions
and congenital heart defects. Obstet Gynecol 116, 316–232 (2010). Article PubMed PubMed Central Google Scholar * Xu, J. et al. MTHFR c.1793G>A polymorphism is associated with
congenital cardiac disease in a Chinese population. Cardiol Young 20, 318–326 (2010). Article PubMed Google Scholar * Garcia-Fragoso, L. et al. MTHFR polymorphisms in Puerto Rican
children with isolated congenital heart disease and their mothers. Int J Genet Mol Biol 2, 43–47 (2010). CAS PubMed PubMed Central Google Scholar * Obermann-Borst, S. A. et al.
Congenital heart defects and biomarkers of methylation in children: a case-control study. Eur J Clin Invest 41, 143–150 (2011). Article CAS PubMed Google Scholar * Weiner, A. S. et al.
Polymorphisms in folate-metabolizing genes and risk of having an offspring with congenital anomalies in the West Siberian region of Russia: a case-control study. Prenat Diagn 32, 1041–1048
(2012). Article CAS PubMed Google Scholar * Zhou, S. Y. et al. Study on the association between MTHFR gene polymorphism and tetralogy of fallot. Shandong Med J 52, 1–3 (2012). Google
Scholar * Pishva, S. R. et al. Analysis of MTHFR and MTRR Gene Polymorphisms in Iranian Ventricular Septal Defect Subjects. Int J Mol Sci 14, 2739–2752 (2013). Article CAS PubMed PubMed
Central Google Scholar * Wang, B. et al. Association of SNPs in genes involved in folate metabolism with the risk of congenital heart disease. J Matern Fetal Neonatal Med 26, 1768–1777
(2013). Article CAS PubMed Google Scholar * Jing, X. A. et al. Associations of MTHFR gene polymorphism and environmental factors with congenital heart disease. Chin J Public Health
3,347–349 (2013). Google Scholar * Balderrabano-Saucedo, N. A. et al. Polymorphism 677C > T MTHFR gene in Mexican mothers of children with complex congenital heart disease. Pediatr
Cardiol 34, 46–51 (2013). Article PubMed Google Scholar * Christensen, K. E. et al. Risk of congenital heart defects is influenced by genetic variation in folate metabolism. Cardiol Young
23, 89–98 (2013). Article PubMed Google Scholar * Wang, L. N. et al. Relationship between 5,10-methylenetetrahydrofolate gene polymorphism and congenital heart disease in nuclear family.
Chin J Appl Clin Pediatr 1, 32–35 (2013). CAS Google Scholar * Zidan, H. E., Rezk, N. A. & Mohammed, D. MTHFR C677T and A1298C gene polymorphisms and their relation to homocysteine
level in Egyptian children with congenital heart diseases. Gene 529, 119–124 (2013). Article CAS PubMed Google Scholar * Huang, J. et al. rs1801133 C>T polymorphism is associated
with an increased risk of tetralogy of Fallot. Biomed Rep 2, 172–176 (2014). Article CAS PubMed PubMed Central Google Scholar * Chao, C. S. et al. Correlation Between
Methyltetrahydrofolate Reductase (MTHFR) Polymorphisms and Isolated Patent Ductus Arteriosus in Taiwan. Heart Lung Circ 23, 655–660 (2014). Article PubMed Google Scholar * Sahiner, U. M.
et al. Methylene tetrahydrofolate reductase polymorphisms and homocysteine level in heart defects. Pediatr Int 56, 167–172 (2014). Article CAS PubMed Google Scholar * Hu, Z. et al. A
genome-wide association study identifies two risk loci for congenital heart malformations in Han Chinese populations. Nat Genet 45, 818–821 (2013). Article CAS PubMed Google Scholar *
Kapusta, L. et al. Congenital heart defects and maternal derangement of homocysteine metabolism. J Pediatr 135, 773–774 (1999). Article CAS PubMed Google Scholar * Xuan, C. et al.
Association between polymorphism of methylenetetrahydrofolate reductase (MTHFR) C677T and risk of myocardial infarction: a meta-analysis for 8,140 cases and 10,522 controls. Arch Med Res 42,
677–685 (2011). Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS We thank Karen E. Christensen (Departments of Pediatrics and Human Genetics, McGill
University-Montreal Children's Hospital Research Institute, Quebec, Canada) for providing data from her group's study. The work was fully supported by grants from the National
Natural Science Foundation of China (No. 81301485 & 81170148) and the Shangdong Young Scientists Award Foundation (No. BS2013YY036) AUTHOR INFORMATION AUTHORS AND AFFILIATIONS *
Department of Clinical Laboratory, The Affiliated Hospital of Qingdao University, Qingdao, P.R. China Chao Xuan, Hui Li, Jin-Xia Zhao, Hong-Wei Wang, Yi Wang & Li-Min Lun * Department of
Medical Ultrasonics, The Affiliated Hospital of Qingdao University, Qingdao, P.R. China Chun-Ping Ning * The Key Laboratory of Hypertension, The Affiliated Hospital of Qingdao University,
Qingdao, P.R. China Zhen Liu * Graduate School of Medicine, Mie University, Mie, Japan Bei-Bei Zhang * TEDA International Cardiovascular Hospital, Tianjin & The Affiliated Hospital of
Hangzhou Normal University, Hangzhou, P.R. China Guo-Wei He * Department of Surgery, Oregon Health and Science University, Portland, Oregon Guo-Wei He * Medical College of Qingdao
University, Qingdao, P.R. China Li-Min Lun Authors * Chao Xuan View author publications You can also search for this author inPubMed Google Scholar * Hui Li View author publications You can
also search for this author inPubMed Google Scholar * Jin-Xia Zhao View author publications You can also search for this author inPubMed Google Scholar * Hong-Wei Wang View author
publications You can also search for this author inPubMed Google Scholar * Yi Wang View author publications You can also search for this author inPubMed Google Scholar * Chun-Ping Ning View
author publications You can also search for this author inPubMed Google Scholar * Zhen Liu View author publications You can also search for this author inPubMed Google Scholar * Bei-Bei
Zhang View author publications You can also search for this author inPubMed Google Scholar * Guo-Wei He View author publications You can also search for this author inPubMed Google Scholar *
Li-Min Lun View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Conception and design of the study: C.X. and L.M.L. Acquisition of data: H.L.,
J.X.Z. and H.W.W. Analysis and interpretation of the data: C.X., H.L., J.X.Z., Y.W., C.P.N., Z.L. and B.B.Z. Writing and revision of the manuscript: C.X., L.M.L. G.W.H. All authors reviewed
the manuscript. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons
Attribution 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the
credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a
copy of this license, visit http://creativecommons.org/licenses/by/4.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Xuan, C., Li, H., Zhao, JX. _et al._ Association Between
_MTHFR_ Polymorphisms and Congenital Heart Disease: A Meta-analysis based on 9,329 cases and 15,076 controls. _Sci Rep_ 4, 7311 (2014). https://doi.org/10.1038/srep07311 Download citation *
Received: 30 July 2014 * Accepted: 13 November 2014 * Published: 04 December 2014 * DOI: https://doi.org/10.1038/srep07311 SHARE THIS ARTICLE Anyone you share the following link with will
be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative