Play all audios:
ABSTRACT Influenza A viruses (IAV) pose a constant threat to the human population and therefore a better understanding of their fundamental biology and identification of novel therapeutics
is of upmost importance. Various reporter-encoding IAV were generated to achieve these goals, however, one recurring difficulty was the genetic instability especially of larger reporter
genes. We employed the viral NS segment coding for the non-structural protein 1 (NS1) and nuclear export protein (NEP) for stable expression of diverse reporter proteins. This was achieved
by converting the NS segment into a single open reading frame (ORF) coding for NS1, the respective reporter and NEP. To allow expression of individual proteins, the reporter genes were
flanked by two porcine Teschovirus-1 2A peptide (PTV-1 2A)-coding sequences. The resulting viruses encoding luciferases, fluorescent proteins or a Cre recombinase are characterized by a high
genetic stability _in vitro_ and in mice and can be readily employed for antiviral compound screenings, visualization of infected cells or cells that survived acute infection. SIMILAR
CONTENT BEING VIEWED BY OTHERS A VERSATILE REVERSE GENETICS PLATFORM FOR SARS-COV-2 AND OTHER POSITIVE-STRAND RNA VIRUSES Article Open access 08 June 2021 DIRECTED EVOLUTION OF ENGINEERED
VIRUS-LIKE PARTICLES WITH IMPROVED PRODUCTION AND TRANSDUCTION EFFICIENCIES Article Open access 13 November 2024 THE FIRST VERSATILE HUMAN IPSC-BASED MODEL OF ECTOPIC VIRUS INDUCTION ALLOWS
NEW INSIGHTS IN RNA-VIRUS DISEASE Article Open access 08 October 2020 INTRODUCTION Influenza A viruses (IAV) cause severe respiratory illness in humans and account for 250,000–500,000 annual
deaths worldwide1. Especially zoonotic transmission of IAV from avian reservoirs poses a constant threat to the human population2, as exemplified recently by several fatal human cases upon
H5N1 or H7N9 infections3,4. Albeit infrequently, avian IAV can also establish new, aerosol-transmissible virus lineages in humans, resulting in devastating pandemics with high morbidity and
mortality2. The development of effective countermeasures, such as vaccines or therapeutics has been complicated by the ability of the viruses to rapidly mutate antigenic determinants or
antiviral target structures5,6,7. Improved vaccine approaches, identification of new antivirals and in general a better understanding of the fundamental biology of IAV infection is required
to efficiently antagonize these human pathogens in future8,9. To achieve these tasks, a number of IAV encoding luciferases10,11,12,13,14,15 or fluorescent proteins15,16,17,18 have been
recently generated. The genome of IAV consists of 8 RNA segments with negative polarity19. These segments are numbered according to their size ranging from 2.3 kb (segment 1) to 0.9 kb
(segment 8). Segments 1–3 encoding the polymerase subunits PB2, PB1 and PA respectively, segment 6 coding for the neuraminidase (NA) and segment 8 (NS) encoding both the non-structural
protein 1 (NS1) and the nuclear export protein (NEP) have been shown to tolerate insertion of foreign genes, including that for the green fluorescence protein (GFP) with a length of around
0.7 kb11,12,13,14,15,16,17,18. However, the integration of a reporter gene of this size represents a substantial increase of the overall segment length and is accompanied by substantial
attenuation of viral replication11,14,15,16. As a consequence, especially viruses comprising the GFP gene were shown to lose reporter activity after passage in cell culture or mice15,16,20,
which could render them unfavorable for many experimental approaches, such as multicycle growth experiments, long-term infection of model organisms or transmission studies. The NS-segment
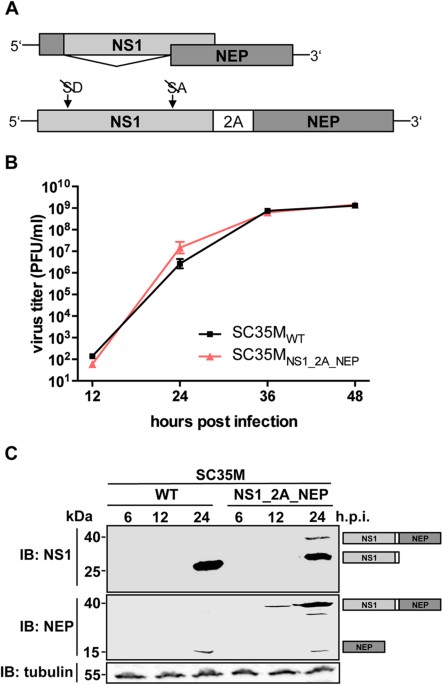
comprises two overlapping ORFs coding for NS1 and NEP. While NS1 is translated from an intron-containing mRNA transcript, the NEP mRNA is generated by exploitation of the cellular splicing
machinery (Fig. 1A)21. Manicassamy _et al._ were the first to utilize the NS-segment as vector for transgene expression in influenza A virus infected cells16. There, an NS1-GFP fusion
protein and NEP are encoded by two non-overlapping genes, which are separated by the porcine Teschovirus-1 2A peptide (PTV-1 2A) coding sequence. PTV-1 2A mediates co-translational
separation of NS1-GFP and NEP by a mechanism termed “stop-carry on” recoding22. The NS1-GFP expressing virus was found to be attenuated in cell culture and mice and it was proposed that this
attenuation is based on an imbalance of NS1 and NEP protein levels, as the coordinated expression of both proteins is no longer regulated by splicing23. In contrast to the
NS1-GFP-expressing virus, however, we could recently show that processing of NS1 and NEP from two non-overlapping genes separated by PTV-1 2A does not result in detectable impairment of
viral replication in cell culture and mice24. Based on this favorable property of PTV-1 2A, we engineered influenza A viruses harboring an NS segment encoding reporter genes flanked by two
genetically distinct PTV-1 2A-encoding sequences. These viruses are genetically stable in cell culture and mice and express a variety of luminescent and fluorescent reporters as well as a
Cre recombinase. RESULTS PTV-1 2A-MEDIATED CO-TRANSLATIONAL SEPARATION OF NS1 AND NEP DOES NOT INTERFERE WITH VIRAL REPLICATION To confirm that the NS-segment of the mouse-adapted IAV
A/SC35M (H7N7)25 allows satisfying co-translational separation of NS1 and NEP, we generated a pHW2000 based26 rescue plasmid (NS1_2A_NEP) that encodes NS1 and NEP in a single ORF (Fig. 1A).
Here, the splice donor and acceptor sites of the NS1 gene were silenced by site-directed mutagenesis without affecting the amino acid sequence. A PTV-1 2A-coding sequence was introduced to
mediate co-translational separation of NS1 and NEP. As expected from our previous work24, a recombinant virus carrying this modified NS-segment (SC35MNS1_2A_NEP) replicates as efficiently as
wild type SC35M (SC35MWT) in mammalian MDCK-II cells (Fig. 1B). To analyze whether this modified NS-segment causes an altered NS1:NEP protein ratio during infection, we determined the
levels of these proteins in the lysates of MDCK-II cells infected with SC35MWT or SC35MNS1_2A_NEP at a multiplicity of infection (MOI) of 0.1. As shown in Fig. 1C, NS1 (26 kDa) and NEP (14.5
kDa) protein levels were comparable between the two viruses 24 hours post infection (h.p.i.). However, we also detected a 40 kDa band corresponding to an NS1-2A-NEP polyprotein with both an
NS1- and an NEP-specific antibody in the cell lysate of SC35MNS1_2A_NEP-infected cells, indicating that the 2A-mediated “stop-carry on” recoding is not completely efficient. FUSION OF A GFP
TO THE NUCLEAR EXPORT PROTEIN (NEP) RESULTS IN COMPROMISED VIRAL GROWTH Based on recent observation that N-terminally GFP-tagged NEP (GFP-NEP) retains its nuclear export and polymerase
co-factor function _in vitro_27,28, we reasoned that reporter genes might be introduced into the viral genome as NEP-fusion constructs. To demonstrate this, we generated a pHW2000-based NS
segment rescue plasmid coding for NS1, 2A and a GFP-NEP fusion protein (Fig. 2A). Using this plasmid, we could indeed successfully generate a recombinant GFP-encoding virus
(SC35MNS1_2A_GFP-NEP). SC35MNS1_2A_GFP-NEP is characterized by severe attenuation in MDCK-II cells, which was most obvious at 24 and 36 h.p.i., when viral titers in the cell supernatant were
reduced by several log10 relative to cells infected with SC35MWT (Fig. 2A). In contrast to SC35MNS1_2A_NEP (Fig. 1C), we observed highly elevated levels of NEP in lysates of cells infected
with SC35MNS1_2A_GFP-NEP (Fig. 2B). This is consistent with earlier observations that fusion of GFP increases the stability of NEP27 and that higher levels of NEP impair viral growth23.
Although there is an increasing body of evidence that NEP is a multifunctional protein crucial for vRNP nuclear export, polymerase activity and viral budding29,30, little is known about the
spatiotemporal regulation of these diverse functions. As we hardly detected any NS1-2A-GFP-NEP polyprotein in the lysate of SC35MNS1_2A_GFP-NEP-infected cells (Fig. 2B), this virus might
serve as a valuable tool for the visualization of changes in the subcellular localization of NEP or its interaction with host factors in the course of an infection. INTRODUCTION OF A SECOND
PTV-1 2A PEPTIDE PERMITS THE GENERATION OF STABLE REPORTER VIRUSES The fusion of GFP to NEP substantially compromised viral growth (Fig. 2A). Since this represents a major drawback for many
experimental approaches, we set out to generate rescue plasmids allowing the expression of a gene of interest without its fusion to NS1 or NEP. To achieve this, we introduced a second PTV-1
2A-coding sequence between the transgene and NEP (Fig. 3A). This sequence was genetically modified to the highest possible extent to prevent homologous recombination with the non-modified
PTV-1 2A nucleotide sequence (see Material and Methods). Using this approach, we were able to rescue viruses encoding three different fluorescent reporter genes: GFP
(SC35MNS1_2A_GFP_2A_NEP), its blue fluorescent derivate Azurite (SC35MNS1_2A_Azurite_2A_NEP)31 and the red fluorescent protein dsRed (SC35MNS1_2A_dsRed_2A_NEP). All reporter viruses
replicated to similar high viral titers of 108 PFU/ml 36 h.p.i., which represents an attenuation of approximately one log10 relative to SC35MWT (Fig. 3A). Replication of
SC35MNS1_2A_dsRed_2A_NEP was particularly delayed, as indicated by the low viral titer 24 hours post infection (Fig. 3A). As expected, infection of A549 cells with the reporter viruses
resulted in well detectable fluorescence signals performing live cell imaging microscopy (Fig. 3B). To analyze the genetic stability of the modified NS segments, viruses were passaged four
times in A549 cells and the proportion of reporter-expressing infectious viral particles was determined by plaque assay and subsequent fluorescent microscopy. As shown in table 1, all
viruses retained their reporter gene after passaging in human cells, indicating a favorable stability of the reporter gene at least over 4 rounds of passaging. To analyze the recoding
efficiency of the two PTV-1 2A peptides, NS1, GFP and NEP protein levels were determined in lysates of cells infected with SC35MNS1_2A_GFP_2A_NEP (Fig. 3C). Besides detection of the three
individual proteins, we observed with both GFP- and NEP-specific antibodies a protein band of approximately 40 kDa corresponding to a GFP-2A-NEP fusion protein (Fig. 3C). A faint signal,
which is visible above this GFP-2A-NEP band in the GFP-immunoblot might indicate the presence of low levels of an NS1-2A-GFP construct. Interestingly, we could not visualize a high molecular
weight band corresponding to an NS1-2A-GFP-2A-NEP polyprotein. Consistently, we observed high levels of unprocessed reporter proteins fused to NEP in the lysates of cells infected with
SC35MNS1_2A_dsRed_2A_NEP and SC35MNS1_2A_Azurite_2A_NEP (Fig. S1). To further characterize the fluorescent reporter viruses _in vivo_, BALB/c mice were infected intranasally with 1,000 PFU
of the respective viruses and viral lung titers were determined 48 h.p.i. All reporter viruses replicated to significant titers between 5 × 105 and 5 × 106 PFU/lung (Fig. 3D). This also
included SC35MNS1_2A_dsRed_2A_NEP, which showed delayed replication properties in cell culture compared to both SC35MNS1_2A_GFP_2A_NEP and SC35MNS1_2A_Azurite_2A_NEP (Fig. 3A). However,
viral lung titers of all reporter viruses were substantially lower than the titers observed after infection of BALB/c mice with SC35MWT. Accordingly, the LD50 of the fluorescent reporter
viruses is increased compared to SC35MWT32 (Table 2). To monitor the genetic stability of the three reporter viruses _in vivo_, plaque assays were performed on the lung homogenates of the
infected BALB/c mice and screened for reporter expression by fluorescent microscopy. This revealed that in all analyzed plaques (100/reporter virus) reporter expression was maintained, again
demonstrating the high genetic stability of the here presented viruses (Table 1). To visualize spread of the reporter viruses in lungs of infected animals, BALB/c mice were infected
intranasally with 10,000 PFU of SC35MNS1_2A_GFP_2A_NEP or SC35MNS1_2A_dsRed_2A_NEP. 48 hours post infection lungs were collected and subjected to microscopic analysis. Virus replication, as
monitored by green or red fluorescence, could be observed in the epithelial layer of the bronchiolar tube (Fig. 3E, filled arrow heads) as well a in the distal lung tissues (Fig. 3E,
transparent arrow heads). Taking together, these data indicate that our fluorescent reporter viruses are genetically stable and allow tracing of different virus-infected lung cells in mice.
GENERATION OF LUCIFERASE-ENCODING VIRUSES FOR SCREENING APPROACHES Luciferases have been proven to be valuable tools in screening approaches for the identification of novel antiviral
substances or host factors33,34,35,36. To generate a stable, luciferase-encoding virus, the gene for the Renilla luciferase (RenLuc) (~0.9 kB) was introduced into the NS-segment. MDCK-II
cells infected with the resulting virus (SC35MNS1_2A_RenLuc_2A_NEP) at an MOI of 0.001 released 108 PFU/ml into the cell supernatant after 48 hours (Fig. 4A). However, compared to
SC35MWT-infected cultures, SC35MNS1_2A_RenLuc_2A_NEP revealed impaired viral growth. To prove stable expression of luciferase, SC35MNS1_2A_RenLuc_2A_NEP was passaged 4 times in human A549
cells. Reporter analysis of ten plaque-purified viruses revealed no loss of luciferase activity (Tab. 1), indicating stable expression of the RenLuc gene. To analyze whether
SC35MNS1_2A_RenLuc_2A_NEP could be employed for antiviral compound screenings, we treated infected MDCK-II cells with increasing concentrations of the viral polymerase inhibitor ribavirin
and determined luciferase activity 24 hours post infection. As shown in Fig. 4B, RenLuc activity decreased in a dose-dependent manner reaching a baseline at approximately 100 μM of
ribavirin. The classic approach to analyze the antiviral activity in cell culture is the determination of viral titers. There, effects at an early stage within the first 6 hours of
infection, prior to efficient particle formation, cannot be visualized. As the expression of virus-encoded luciferase is dependent on viral RNA synthesis and not particle release, we
reasoned that SC35MNS1_2A_RenLuc_2A_NEP would allow quantification of antiviral activity of the viral polymerase inhibitor ribavirin at early time points post infection. To show this,
SC35MNS1_2A_RenLuc_2A_NEP-infected MDCK-II cells were cultured in the presence of 0, 30 or 60 μM of ribavirin and analyzed at different time points post infection for viral titers in the
supernatant and luciferase activity in the cell lysate (Fig. 4C). Already at 3 h.p.i., we observed a substantial dose dependent difference in luciferase activity, whereas a first reduction
of viral titers was detected at 12 hours post infection. Furthermore, we could not resolve differences in the antiviral efficacy between cells treated with 30 μM or 60 μM ribavirin by
determination of viral titers, while this was possible as soon as 6 hours post infection by measuring luciferase activity. The Gaussia luciferase (GLuc) represents an attractive luminescent
reporter for many experimental approaches, not least due to its small size and its secretion from mammalian cells37,38. Indeed, insertion of the GLuc-coding sequence into the viral NS
segment revealed a recombinant virus (SC35MNS1_2A_GLuc_2A_NEP), which was only partially attenuated but highly stable in cell culture (Fig. 4D, Tab.1). As expected, the supernatant of
MDCK-II cells infected with this virus contained significant levels of secreted GLuc (Fig. 4E). To evaluate whether this extracellular reporter activity could be exploited as a measure for
viral replication, we infected MDCK-II cells in the presence of ribavirin and determined luciferase activity and virus titers from the supernatant at different time points post infection. As
anticipated, luciferase activity differed dependent on the concentration of ribavirin (Fig. 4F). However, especially at 24 hours post infection, differences in luciferase activity were not
proportional to differences in virus titers determined from the same supernatants, most probably due to extracellular accumulation of the highly stable GLuc38. As for the fluorescent
reporter viruses, we detected high levels of unprocessed, luciferase-NEP fusion proteins in lysates of cells infected with SC35MNS1_2A_GLuc_2A_NEP and SC35MNS1_2A_RenLuc_2A_NEP (Fig. S1). A
CRE RECOMBINASE-ENCODING VIRUS ALLOWS VISUALIZATION OF CELLS THAT ARE INFECTED OR SURVIVED ACUTE INFECTION The Cre-Lox recombination is a widely used method to control gene expression in
cell culture or animal models39,40. To generate a genetically stable virus that would allow activation or deactivation of genes specifically in infected cells, we introduced a Cre
recombinase gene into the NS segment (SC35MNS1_2A_Cre_2A_NEP). Infection of MDCK-II cells revealed a high viral titer of 108 PFU/ml cell culture supernatant 48 h post infection (Fig. 5A).
However, SC35MNS1_2A_Cre_2A_NEP showed a delayed viral growth especially at 12 and 24 hours post infection compared to SC35MWT (Fig. 5A). To functionally monitor expression of Cre, we
established a human airway derived Calu-3 cell line harboring a loxP-flanked dsRed gene followed by a silenced eGFP gene41 (Fig. 5B). Upon Cre-mediated recombination, the dsRed gene is
eliminated and cells express eGFP, resulting in a switch from red to green fluorescence and consequently constitutive GFP expression is inherited to progeny cells after proliferation. As IAV
suppress host protein synthesis and induce apoptosis42,43,44, we expected that viral infection would complicate identification and analysis of reporter cells upon virus-induced
recombination. Indeed, as judged by live cell imaging, infected Calu-3 cells only showed faint green fluorescence 24 hours post infection and were almost completely eliminated at later time
points (Fig. 5C, infected). To prevent virus-induced cell death and allow limited viral replication, we treated virus-infected cells with ribavirin (0.1 mM) 3 hours post infection and kept
them under treatment to abrogate further replication. We anticipated that this would allow sufficient expression of Cre recombinase and the associated switch to GFP expression as well as
prolonged cell survival (Fig. 5B). Indeed, increasing number of GFP-positive Calu-3 reporter cells were observed in a time dependent manner upon infection at an MOI of 1 with
SC35MNS1_2A_Cre_2A_NEP and subsequent ribavirin treatment (Fig. 5C, infected + ribavirin). As we could show in cell culture that SC35MNS1_2A_Cre_2A_NEP allows the visualization of cells that
survived IAV infection, we hypothesized that we could also trace and identify such cells _in vivo_ using a suitable mouse model. For this reason, rosamT/mG mice45 were infected intranasally
with a sublethal dose of SC35MNS1_2A_Cre_2A_NEP. These transgenic animals comprise a loxP-flanked tdTomato ORF upstream of an eGFP gene integrated into the Rosa26 locus. Analogous to the
earlier described cell culture system, Cre-mediated recombination results in a switch from red to green fluorescence in virtually all cell types. Infected animals were sacrificed at 2, 7 or
21 days post infection to collect their lungs for microscopic examination. Two and 7 days after infection, green fluorescent cells could be detected within the epithelial layer of
bronchioles (Fig. 5D, filled arrow heads) as well as in distal lung tissues (Fig. 5D, transparent arrow heads). A similar distribution of GFP-positive cells was observed 21 days post
infection, a time point where infection was already cleared (data not shown), indicating that diverse populations of epithelial cells survived the virus infection. Interestingly, while 2
days post infection only individual cells in alveolar tissues showed green fluorescence, clusters of adjacent GFP-positive cells could be visualized already 7 days p.i., which might derive
from progenitor cells that survived acute infection. To reveal the identity of the GFP-positive cells found in the distal lung compartment, we subjected lung cells from rosamT/mG mice
infected with SC35MNS1_2A_Cre_2A_NEP to flow cytometric analysis at days 2, 7 and 21 post infection. We could identify recombined, GFP-positive cells within lung epithelial cell populations
(EpCam+), including alveolar epithelial cells type 1 (AEC I, EpCamlowT1α+), alveolar epithelial cells type 2 (AEC II, EpCamlowT1α–), stem and/or progenitor cells (EpCamhighCD24low) and small
airway epithelial cells (EpCamhighCD24high) (Fig. 6 A and B). Of note, all infectious viruses isolated from the lungs of infected animals by plaque purification 2 days post infection
encoded a functional Cre recombinase as judged by GFP expression in the Calu-3 reporter cells, highlighting the genetic stability of SC35MNS1_2A_Cre_2A_NEP in mice (Table 1). Taken together,
this reporter virus in combination with flow cytometric analysis represents a powerful tool to identify and quantify acutely infected lung cells as well as cells that survived acute
infection. STABLE INTRODUCTION OF TRANSGENES INTO THE PB2-SEGMENT OF SC35M IS AT THE COST OF VIRAL REPLICATION CAPACITY As for the NS-segment, it was shown that the PB2-segment tolerates the
integration of foreign genes downstream of the PB2 ORF11,12,46. Since this approach represents an alternative to the NS segment as a vector for transgene expression, we generated
pHW2000-based rescue plasmids encoding GFP or GLuc separated from the PB2 ORF by a PTV-1 2A-coding sequence (Fig. 7A). To guarantee packaging of the modified segment into viral particles, a
terminal stretch of the segment was duplicated and fused downstream to the reporter gene as described by others47. Both viruses, encoding GFP (SC35MPB2_2A_GFP) or GLuc (SC35MPB2_2A_GLuc)
could be successfully generated. Specific fluorescent signals were observed upon infection of A549 cells with SC35MPB2_2A_GFP by live cell imaging microscopy (Fig. 7A) and significant levels
of luciferase could be detected in the supernatant of MDCK-II cells infected with SC35MPB2_2A_GLuc (Fig. 7B). Analysis of the replication efficiency of these viruses in MDCK-II cells,
revealed a substantial attenuation of SC35MPB2_2A_GLuc compared to SC35M (Fig. 7A). In sharp contrast, viral growth of SC35MPB2_2A_GFP was, to our surprise, as efficient as wild type virus
(Fig. 7A). However, analysis of viral plaques obtained with SC35MPB2_2A_GFP revealed that only 1 out of 123 plaques was GFP positive (Tab. 1), indicating that the vast majority of these
viruses lost the intact reporter gene after a single passage on human cells. Indeed, sequencing of the RNA extracted from six GFP-negative plaques revealed that the GFP gene was deleted,
most likely by homologous recombination at the duplicated packaging signals. Interestingly, SC35MPB2_2A_GLuc did not lose its reporter activity after 4 passages (Tab. 1), suggesting that
smaller genes might be tolerated on the expense of viral fitness. To improve the genetic stability of the GFP-encoding PB2 segment, the highest possible number of silent mutations was
introduced into the last 129 nucleotides of the PB2 ORF to prevent homologous recombination at the duplicated packaging signals (Fig. 7C). This modification resulted in a virus, designated
SC35MPB2mod_2A_GFP, which was strongly attenuated in cell culture but genetically stable over four passages in A549 cells (Tab. 1). To rule out the possibility that this attenuation resulted
from mutation of the PB2 ORF rather than from insertion of the transgene, we deleted the GFP-coding sequence from the PB2 segment (Fig. 7D). This virus (SC35MPB2mod) replicated in cell
culture almost as efficiently as SC35MWT. In summary, this suggests that stable integration of a transgene into the PB2-segment of SC35M is possible but associated with severe attenuation.
DISCUSSION Influenza A reporter viruses are important tools to study the biology of IAV and screening approaches. However, these viruses show in general impaired viral replication
efficiencies, probably resulting in an unfavorable selective pressure towards loss of the reporter gene. Especially the latter may cause difficulties in the interpretation of the results
observed with reporter viruses. In this study, we developed a strategy that allows the stable integration of reporter genes into the NS-segment of IAV. We achieved this by converting the NS
segment into a single ORF encoding NS1, the respective gene of interest and NEP. The reporter gene was flanked by two sequences coding for the porcine Teschovirus-1 2A peptide (PTV-1 2A),
thereby allowing co-translational separation of NS1, the reporter protein and NEP. The feasibility of this method was demonstrated by the successful generation of recombinant IAV, encoding a
variety of fluorescent reporter proteins or catalytically active enzymes. Dependent on the gene inserted into the NS segment, we observed various degrees of attenuation. Most importantly,
these genetically modified viruses displayed a high genetic stability over 4 passages in cell culture as well as over a single passage in mice and can be readily used for a broad range of
_in vitro_ and _in vivo_ applications. The levels of viral mRNA transcripts coding for NS1 or NEP in influenza A virus-infected cells is regulated by splicing, resulting in defined protein
ratios of NEP and NS1. This is of special importance with respect to recent findings suggesting that the relative amount of NEP coordinates the intracellular timing of an infection and that
an aberrant NS1 to NEP ratio results in inhibition of viral replication23. Intriguingly, the co-translational processing by PTV-1 2A seems rather inefficient, resulting in a substantial
proportion of uncleaved polyprotein in cells infected with the recombinant viruses (Fig. 1C). As a consequence, the level of free NEP is comparable to that detected in cells infected with
wild type virus preventing significant attenuation as exemplified best with the recombinant virus SC35MNS1_2A_NEP harboring no additional reporter gene (Fig. 1C). Interestingly, cells
infected with SC35MNS1_2A_GFP_2A_NEP expressed high levels of a GFP-2A-NEP fusion protein, while NS1-2A-GFP was almost not detectable (Fig. 3C and Fig. S1). This might be the result of
context-specific differences in 2A-mediated recoding efficiency or could be explained by a higher stability of GFP-2A-NEP compared to NS1-2A-GFP. Integration of foreign genes into the NS
segment using the method presented in this study, can lead to impaired viral fitness. This might be a result of (I) low levels of NEP due to inefficient PTV-1 2A activity (Fig. 3C), (II) an
intrinsic feature of the inserted reporter gene or (III) increased overall length of the NS segment. Indeed, integration of larger genes into the viral genome including firefly luciferase
(~2 kb) or β-galactosidase (~3 kb) failed, suggesting a particular length restriction. We could show for SC35M that insertion of the GFP gene into the PB2-segment results in the rapid loss
of reporter activity due to homologous recombination of the duplicated packaging sequences (Fig. 7A and Tab.1). Loss of the reporter gene could be prevented by extensive modification of
these sequences but was associated with significantly impaired viral growth (Fig. 7C and Tab.1). In contrast to the NS segment, introduction of reporter genes into the PB2 segment of SC35M
did not result in viruses that are suited for further _in vitro_ and _in vivo_ studies. However, we cannot exclude the possibility that this might be different for other influenza A virus
strains. Importantly, the recombinant viruses described in this study harboring reporter genes in the NS segment are genetically stable in cell culture and mice. This includes fluorescent
protein-encoding genes shown to be readily eliminated in cell culture or mice when present as NS1-fusion genes15,16,20. Genetically stable reporter viruses offer several advantages,
including the reliable identification and determination of the relative abundance of virus-infected cells by microscopic tracing of these cells in a model organism. Because of their genetic
stability, luciferase-encoding viruses might be robust tools for high throughput screening approaches. Here, SC35MNS1_2A_RenLuc_2A_NEP might be superior, as the activity of the virus-encoded
RenLuc allows measurements already early upon infection and parallels with viral titers at later time points (Fig. 4C). Sublethal infection of mice with influenza A virus is cleared by day
10–14 and no infectious particles can be isolated from the lung of these animals there upon48. Using the Cre recombinase-encoding virus, we could trace cells that survived viral infection in
the lungs of infected rosamT/mG mice 21 days after viral challenge. GFP-positive cells were found in various lung tissues including the epithelial layers lining the larger and also smaller
airways (alveoli and bronchioles) of the respiratory tract. The fact that we could only observe the formation of GFP-positive cell clusters at later time points post infection (Fig. 5D)
suggests that a proportion of these cells represents progeny of formerly infected and surviving cells, as GFP expression is inherited. Consistently, flow cytometry revealed the presence of
GFP-positive cells among the stem and/or progenitor population 2, 7 and also 21 days post infection (Fig. 6B). Intriguingly, similar experiments were recently performed with an engineered
H1N1 virus (A/Puerto Rico/8/1934) encoding a Cre recombinase within the PB2 segment46. In this case the authors could identify recombined cells exclusively in the larger airways of the
respiratory tract. The reason for this discrepancy is unclear but could be related to differences in the segment used as vector for Cre recombinase-expression or might result from the
different subtype of hemagglutinin (HA) of both mouse-adapted virus strains. While HA of PR/8 (H1N1) possesses a monobasic cleavage site, HA of SC35M (H7N7) harbors a multibasic cleavage
site, which might facilitate infection of a broader spectrum of lung cells. Of note, influenza virus replication is known to be accompanied by the production of defective interfering
particles (DIs) as well as semi-infectious particles (SIs)49,50,51,52. We cannot exclude the possibility that recombination events observed in cells of infected rosamT/mG mice were caused by
the incorporation of such particles, which might not have the capacity to induce cell death. Importantly, the _in vivo_ prevalence of DIs and SIs remains to be elucidated53. Taken together,
we present a strategy that permits the introduction of foreign genes into the NS genome segment of Influenza A viruses. This was achieved by conversion of the intron-containing NS segment
into a segment that allows the expression of reporter genes by two porcine Teschovirus-1 2A peptide (PTV-1 2A)-coding sequences. Such engineered viruses were shown to be genetically stable
over at least 4 passages in cell culture and a single passage in mice and thus represent attractive tools to study influenza A viruses _in vitro_ and _in vivo._ METHODS PLASMID CONSTRUCTION
The Azurite gene was amplified from pGEMHE-X-Azurite, which was kindly provided by Max Ulbrich. The template used to amplify the Cre recombinase gene (pMIG-Cre) was a gift from Hassan Jumaa.
The Gaussia Luciferase gene sequences derived from pT7-NYMVmg-Gluc54 and the Renilla luciferase-coding sequence was obtained from pRL-SV40 (Promega). The eGFP gene was amplified from
pCAGGS-GFP-P27. pHW2000-based26 rescue plasmids to generate SC35M were described by others32. The NS1_2A_NEP plasmid was generated by deletion of the Flag-tag sequence of NS1_2A_Flag-NEP24
by performing overlapping fusion PCR. The same plasmid served as template to create NS1_2A_GFP-NEP where the Flag-tag sequence was replaced by an eGFP gene. NS1_2A_GFP_2A_NEP was obtained by
insertion of a second genetically altered PTV-1 2A encoding sequence (GCCACAAATT TCTCTCTCCT CAAGCAAGCC GGGGACGTCG AGGAGAATCC CGGGCCC) between the genes for eGFP and NEP by overlapping
fusion PCR. Furthermore, a SacII and a KpnI restriction site upstream and downstream of the eGFP gene respectively were introduced. NS1_2A_Renilla_2A_NEP and NS1_2A_Cre_2A_NEP were generated
by overlapping fusion PCR using NS1_2A_GFP_2A_NEP as template. All other transgene-encoding NS-segment rescue plasmids were generated by PCR amplification of the transgene and
SacII/KpnI-digestion-ligation into NS1_2A_GFP_2A_NEP. The PB2_2A_GFP and PB2_2A_GLuc plasmids were generated in two steps. First, a PB2_2A_GFP_2A_NEP and a PB2_2A_GLuc_2A_NEP intermediate
was generated by PCR amplification of PB2 and digestion-ligation into NS1_2A_GFP_2A_NEP and NS1_2A_GLuc_2A_NEP. In a second step, the 2A_NEP sequence was replaced by 166 nucleotides of the
3’ end of the PB2 segment by overlapping fusion PCR. To obtain PB2mod_2A_GFP, silent mutations within the last 129 nucleotides of the 3’ end of the PB2 ORF (GCcAAaGGcG AaAAaGCcAA cGTcCTgATc
GGcCAgGGcG AtGTcGTccT GGTcATGAAa aGaAaaGaGA tagctccATc CTgACcGAtt ccCAaACaGC cACaAAgAGg ATcaGaATGG CtATtAAc) were introduced into PB2_2A_GFP by annealing of two synthetic DNA
oligonucleotides and subsequent overlapping fusion PCR. PB2mod was generated by removal of the 2A_GFP sequence from PB2mod_2A_GFP via PCR amplification and digestion-ligation. CELLS AND
RETROVIRAL TRANSDUCTION HEK293T, A549, Calu-3 and MDCK-II cells were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum, 2 mM L-glutamine and 1%
penicillin/streptomycin. All cells were cultured at 37 °C and 5% CO2. Calu-3 cells harboring the loxp-dsRed-loxp-eGFP expression cassette were generated by retroviral transduction and
subsequent selection in puromycine-containing media as described by the manufacturer (Stratgene). Pseudotyped retroviurses were produced in HEK293T cells upon transfection with
pMSCV-loxp-dsRed-loxp-eGFP-Puro-WPRE (Addgene plasmid 32702, kindly provided by Hans Clevers), pVPack-GP and pVPack-VSV-G as decribed (Stratagene). VIRUS RESCUE To generate recombinant
influenza viruses, HEK293T cells were transfected in a 6 well format with 8 bidirectional pHW2000 rescue plasmids26 encoding the respective viral genome segments (300 ng of plasmid
DNA/segment). 24 hours post transfection, 200 μl of supernatant was transferred to MDCK-II cells (6 well format). Recombinant viruses were plaque-purified upon the observation of cytopathic
effects. DETERMINATION OF VIRAL GROWTH KINETICS MDCK-II cells were infected at an MOI of 0.001 and cultured in infection medium (Dulbecco’s modified Eagle medium supplemented with 0.2%
bovine serum albumin [BSA], 2 mM l-glutamine and 1% penicillin-streptomycin). At 12, 24, 36 and 48 hours post infection 150 μl of supernatant was collected and subjected to plaque assay for
determination of virus titer. PASSAGING AND ANALYSIS OF REPORTER STABILITY For each passage of fluorescent and luminescent reporter viruses, A549 cells were infected at an MOI of 0.01 and
cultured in infection medium (see preceding chapter). 48 hours post infection, supernatant was collected and virus titer was determined by plaque assay. After 4 passages, plaques induced by
fluorescent reporter viruses were analyzed and counted using a fluorescent microscope. To determine the reporter expression of passaged, luciferase-encoding viruses, 10 plaques were randomly
picked and used for infection of MDCK-II cells and 24 hours post infection luciferase activity was measured. SC35MNS1_2A_Cre_2A_NEP was passaged on MDCK-II cells. After 4 passages and
subsequent plaque assay, Calu-3 cells containing the loxp-dsRed-loxp-eGFP expression cassette were infected with plaque-purified viruses. After 3 hours, medium was replaced by
Ribavirin-containing (100 μM) medium. Recombination events resulting in a switch from red to green fluorescence were analyzed with a fluorescent microscope. DETERMINATION OF LUCIFERASE
ACTIVITY Luciferase activity in whole cell lysates or in 5 μl of supernatant of MDCK-cells cultured in 6-well plates was determined using a luciferase assay system (Promega) according to the
manufacturer’s instructions. IMMUNOBLOT ANALYSIS Virus infected cells were incubated with lysis buffer (20 mM Tris, pH 7.5, 100 mM NaCl, 0.5 mM EDTA, 0.5% NP-40, 1% protease inhibitor mix G
[Serva, Heidelberg, Germany], 1 mM dithiothreitol [DTT]) for 15 min on ice. After centrifugation at 13,000 rpm at 4 °C, supernatants were complemented with SDS page sample buffer55 and
incubated at 95 °C. Proteins were separated in SDS-PAGE gels (15%) and transferred to nitrocellulose membranes. Antibodies for detection of NEP and NS1 were a gift from Thorsten Wolff and
Christina Ehrhardt respectively. The commercial antibodies for detection of GFP and tubulin were purchased from Santa Cruz Biotechnology (GFP) and Sigma-Aldrich (tubulin). ANIMAL EXPERIMENTS
All animal experiments were performed in accordance with the relevant guidelines (German animal protection law (TierSchG)) and approved by the welfare committees of the University of
Freiburg, as well as the local authorities. Six-to-eight-week-old mice were anaesthetized with a mixture of ketamin (100 μg per gram body weight) and xylazine (5 μg per gram) administered
intraperitoneally and inoculated intranasally with the indicated doses of viruses in 40 μl PBS containing 0.3% bovine serum albumin (BSA). Animals were sacrificed, if severe symptoms
developed, or body weight loss approached 25% of the initial value. Lung homogenates were prepared using the FastPrep24 system (MP Biomedicals). Briefly, after addition of 800 μl of PBS
containing 0.2% BSA, lungs were subjected to two rounds of mechanical treatment for 10 s each at 6.5 ms−1. Tissue debris was removed by low-speed centrifugation. The LD50 values were
calculated based on the infectious dose (PFU). BALB/c mice were obtained from Janvier (Strasbourg). RosamT/mG mice (_Gt(ROSA)26Sor__tm4__(ACTB-TDTOMATO,-EGFP)LUO_) (Jackson laboratory)
contain the two-color fluorescent rosamT/mG allele from which the cell membrane localized red fluorescent tdTomato is expressed. Upon Cre-Lox recombination directed by lox-P sites flanking
the tdTomato gene, eGFP expression is induced. TISSUE HISTOLOGY At appropriate time points, mice were sacrificed and lungs were transcardially perfused with 0.9% NaCl prior to fixation in 4%
paraformaldehyde (PFA) in 0.1 M phosphate buffer at 4 °C overnight. After washing in ddH2O the lungs were first transferred into a 15% sucrose solution in 0.1 M phosphate buffer (w/v) for 4
h and thereafter in a 30% sucrose solution in 0.1 M phosphate buffer (w/v) overnight (4 °C). For cryosection, lungs were embedded within Tissue-Tek O.C.T. compound, solidified on dry ice
and cut to 15 μm thickness using a cryotome (Leica Microsystems, Germany). The sections were mounted onto gelatine-coated slides and dried at room temperature overnight in the dark. The
slides were washed twice in phosphate buffer, DAPI (Invitrogen) stained at an end concentration of 300 nM in 0.1 M phosphate buffer for 7 min and again washed 3 times in 0.1 M phosphate
buffer. Dried slides were embedded within IMMU-Mount™ (ThermoShandon), coverslipped and stored in the dark at 4 °C until further use. ISOLATION OF MURINE DISTAL LUNG CELLS Mouse lungs were
perfused with HBSS (Gibco) followed by instillation of dispase (BD Biosciences) into the lung through the trachea and incubation in dispase for 40 minutes as previously described56. Trachea
and large airways were dissected and the remaining distal lung tissue was homogenized (GentleMACS, MACS Miltenyi Biotech) in DMEM/2.5% HEPES with 0.01% DNase (Serva) and filtered through 100
μm and 40 μm nylon filters. Cell suspensions were incubated with biotinylated rat anti-mouse CD45, CD16/32 and CD31 mAb (BD Biosciences) for 30 minutes at 37 °C followed by incubation with
biotin-binding magnetic beads and magnetic separation to deplete leukocytes and endothelial cells prior to flow cytometric analysis. FLOW CYTOMETRY The following antibodies were used for
flow cytometric analyses: CD326 (EpCam) APC-Cy7 (clone G8.8), CD24 PE-Cy7 (clone: M1/69), T1α/podoplanin APC (clone: 8.1.1.), CD31 Pacific Blue (clone 390), all Biolegend. CD45 V450 (clone
30-F11, BD Biosciences). Multicolor flow cytometry was performed with an LSR Fortessa® using DIVA software (BD Bioscience). For analytical measurements 0.5–1 × 106 cells were freshly stained
with fluorochrome-labeled antibodies for 15 minutes at 4 °C in MACS buffer. The stained cells were washed and fixed in 4% paraformaldehyde and resuspended in MACS buffer. FLUORESCENCE
MICROSCOPY Fluorescence images of cultured cells seeded in black, clear bottom 96 well microplates (Greiner) were acquired on a Zeiss Observer.Z1 inverted epifluorescence microscope (Carl
Zeiss, Jena) equipped with an AxioCamMR3 camera using a 40x objective. Fluorescence microscopy of lung sections was performed on a Zeiss Axioplan 2 epifluorescence microscope (Carl Zeiss,
Jena) equipped with an ApoTome optical sectioning module using a 10x objective. Images were recorded with an AxioCamMR camera (Carl Zeiss, Jena). ADDITIONAL INFORMATION HOW TO CITE THIS
ARTICLE: Reuther, P. _et al._ Generation of a variety of stable Influenza A reporter viruses by genetic engineering of the NS gene segment. _Sci. Rep._5, 11346; doi: 10.1038/srep11346
(2015). REFERENCES * WHO, _Fact sheet N°211 - Influenza (seasonal)_, <http://www.who.int/mediacentre/factsheets/fs211/en> (2014) (Date of access: 30/12/2014). * Horimoto, T.
& Kawaoka, Y. Influenza: lessons from past pandemics, warnings from current incidents. Nat Rev Micro 3, 591–600; 10.1038/nrmicro1208 (2005). Article CAS Google Scholar * Gao, R. et
al. Human Infection with a Novel Avian-Origin Influenza A (H7N9) Virus. New Engl. J. Med. 368, 1888–1897; 10.1056/NEJMoa1304459 (2013). Article CAS PubMed Google Scholar * Chan, P. K. S.
Outbreak of Avian Influenza A(H5N1) Virus Infection in Hong Kong in 1997. Clin. Infect. Dis. 34, S58–S64; 10.1086/338820 (2002). Article PubMed Google Scholar * Cox, N. J. &
Subbarao, K. Influenza. The Lancet 354, 1277–1282; 10.1016/S0140-6736(99)01241-6 (1999). Article CAS Google Scholar * Moscona, A. Global Transmission of Oseltamivir-Resistant Influenza.
New Engl. J. Med. 360, 953–956; 10.1056/NEJMp0900648 (2009). Article CAS PubMed Google Scholar * Bush, R. M., Bender, C. A., Subbarao, K., Cox, N. J. & Fitch, W. M. Predicting the
Evolution of Human Influenza A. Science 286, 1921–1925; 10.1126/science.286.5446.1921 (1999). Article CAS PubMed Google Scholar * De Clercq, E. Antiviral agents active against influenza
A viruses. Nat. Rev. Drug Discov. 5, 1015–1025; 10.1038/nrd2175 (2006). Article CAS PubMed PubMed Central Google Scholar * Lambert, L. C. & Fauci, A. S. Influenza Vaccines for the
Future. New Engl. J. Med. 363, 2036–2044; 10.1056/NEJMra1002842 (2010). Article CAS PubMed Google Scholar * Sutton, T. C. et al. Genome rearrangement of influenza virus for anti-viral
drug screening. Virus Res. 189, 14–23; 10.1016/j.virusres.2014.05.003 (2014). Article CAS PubMed Google Scholar * Heaton, N. S. et al. _In Vivo_ Bioluminescent Imaging of Influenza A
Virus Infection and Characterization of Novel Cross-Protective Monoclonal Antibodies. J. Virol. 87, 8272 -8281; 10.1128/jvi.00969-13 (2013). Article CAS PubMed PubMed Central Google
Scholar * Munier, S., Rolland, T., Diot, C., Jacob, Y. & Naffakh, N. Exploration of Binary Virus–Host Interactions Using an Infectious Protein Complementation Assay. Mol. Cell.
Proteomics 12, 2845–2855; 10.1074/mcp.M113.028688 (2013). Article CAS PubMed PubMed Central Google Scholar * Tran, V., Moser, L. A., Poole, D. S. & Mehle, A. Highly Sensitive
Real-Time _In Vivo_ Imaging of an Influenza Reporter Virus Reveals Dynamics of Replication and Spread. J. Virol. 87, 13321–13329; 10.1128/jvi.02381-13 (2013). Article CAS PubMed PubMed
Central Google Scholar * Pan, W. et al. Visualizing influenza virus infection in living mice. Nat Commun 4; 10.1038/ncomms3369 (2013). * Eckert, N. et al. Influenza A Virus Encoding
Secreted Gaussia Luciferase as Useful Tool to Analyze Viral Replication and Its Inhibition by Antiviral Compounds and Cellular Proteins. PLoS ONE 9, e97695; 10.1371/journal.pone.0097695
(2014). Article ADS CAS PubMed PubMed Central Google Scholar * Manicassamy, B. et al. Analysis of _in vivo_ dynamics of influenza virus infection in mice using a GFP reporter virus.
Proc. Natl. Acad. Sci. USA 107, 11531–11536; 10.1073/pnas.0914994107 (2010). Article ADS PubMed PubMed Central Google Scholar * Avilov, S. V. et al. Replication-Competent Influenza A
Virus That Encodes a Split-Green Fluorescent Protein-Tagged PB2 Polymerase Subunit Allows Live-Cell Imaging of the Virus Life Cycle. J. Virol. 86, 1433–1448; 10.1128/jvi.05820-11 (2012).
Article CAS PubMed PubMed Central Google Scholar * Lakdawala, S. S. et al. Influenza A Virus Assembly Intermediates Fuse in the Cytoplasm. PLoS Pathog 10, e1003971;
10.1371/journal.ppat.1003971 (2014). Article CAS PubMed PubMed Central Google Scholar * Noda, T. et al. Three-dimensional analysis of ribonucleoprotein complexes in influenza A virus.
Nat Commun 3, 639; 10.1038/ncomms1647 (2012). Article ADS CAS PubMed Google Scholar * Kuznetsova, I. et al. Adaptive mutation in nuclear export protein allows stable transgene
expression in a chimaeric influenza A virus vector. J. Gen. Virol. 95, 337–349; 10.1099/vir.0.056036-0 (2014). Article CAS PubMed Google Scholar * Lamb, R. A. & Lai, C.-J. Sequence
of interrupted and uninterrupted mRNAs and cloned DNA coding for the two overlapping nonstructural proteins of influenza virus. Cell 21, 475–485; 10.1016/0092-8674(80)90484-5 (1980). Article
CAS PubMed Google Scholar * Sharma, P. et al. 2A peptides provide distinct solutions to driving stop-carry on translational recoding. Nucleic Acids Res. ; 10.1093/nar/gkr1176 (2011). *
Chua, M.A., Schmid, S., Perez, J.T., Langlois, R.A. & tenOever, B.R. Influenza A Virus Utilizes Suboptimal Splicing to Coordinate the Timing of Infection. Cell Reports 3, 23–29;
10.1016/j.celrep.2012.12.010. * Reuther, P., Giese, S., Götz, V., Riegger, D. & Schwemmle, M. Phosphorylation of Highly Conserved Serine Residues in the Influenza A Virus Nuclear Export
Protein NEP Plays a Minor Role in Viral Growth in Human Cells and Mice. J. Virol. 88, 7668–7673; 10.1128/jvi.00854-14 (2014). Article PubMed PubMed Central Google Scholar * Li, S. Q.,
Orlich, M. & Rott, R. Generation of seal influenza virus variants pathogenic for chickens, because of hemagglutinin cleavage site changes. J. Virol. 64, 3297–3303 (1990). CAS PubMed
PubMed Central Google Scholar * Hoffmann, E., Neumann, G., Kawaoka, Y., Hobom, G. & Webster, R. G. A DNA transfection system for generation of influenza A virus from eight plasmids.
Proc. Natl. Acad. Sci. USA 97, 6108–6113; 10.1073/pnas.100133697 (2000). Article ADS CAS PubMed PubMed Central Google Scholar * Reuther, P. et al. Adaptive Mutations in the Nuclear
Export Protein of Human-Derived H5N1 Strains Facilitate a Polymerase Activity-Enhancing Conformation. J. Virol. 88, 263–271; 10.1128/jvi.01495-13 (2014). Article PubMed PubMed Central
Google Scholar * Brunotte, L. et al. The Nuclear Export Protein of H5N1 Influenza A Viruses Recruits Matrix 1 (M1) Protein to the Viral Ribonucleoprotein to Mediate Nuclear Export. J. Biol.
Chem. 289, 20067–20077; 10.1074/jbc.M114.569178 (2014). Article CAS PubMed PubMed Central Google Scholar * Paterson, D. & Fodor, E. Emerging Roles for the Influenza A Virus Nuclear
Export Protein (NEP). PLoS Pathog 8, e1003019; 10.1371/journal.ppat.1003019 (2012). Article CAS PubMed PubMed Central Google Scholar * Mänz, B., Schwemmle, M. & Brunotte, L.
Adaptation of Avian Influenza A Virus Polymerase in Mammals To Overcome the Host Species Barrier. J. Virol. 87, 7200–7209; 10.1128/jvi.00980-13 (2013). Article PubMed PubMed Central
Google Scholar * Mena, M. A., Treynor, T. P., Mayo, S. L. & Daugherty, P. S. Blue fluorescent proteins with enhanced brightness and photostability from a structurally targeted library.
Nat Biotech 24, 1569–1571; 10.1038/nbt1264 (2006). Article CAS Google Scholar * Gabriel, G. et al. The viral polymerase mediates adaptation of an avian influenza virus to a mammalian
host. Proc. Natl. Acad. Sci. USA 102, 18590–18595; 10.1073/pnas.0507415102 (2005). Article ADS CAS PubMed PubMed Central Google Scholar * Koutsoudakis, G. et al. Characterization of
the Early Steps of Hepatitis C Virus Infection by Using Luciferase Reporter Viruses. J. Virol. 80, 5308–5320; 10.1128/jvi.02460-05 (2006). Article CAS PubMed PubMed Central Google
Scholar * Blair, W. S. et al. A novel HIV-1 antiviral high throughput screening approach for the discovery of HIV-1 inhibitors. Antiviral Res. 65, 107–116; 10.1016/j.antiviral.2004.11.001
(2005). Article ADS CAS PubMed Google Scholar * Uebelhoer, L. S. et al. High-throughput, luciferase-based reverse genetics systems for identifying inhibitors of Marburg and Ebola
viruses. Antiviral Res. 106, 86–94; 10.1016/j.antiviral.2014.03.018 (2014). Article CAS PubMed Google Scholar * Zou, G., Xu, H. Y., Qing, M., Wang, Q.-Y. & Shi, P.-Y. Development and
characterization of a stable luciferase dengue virus for high-throughput screening. Antiviral Res. 91, 11–19; 10.1016/j.antiviral.2011.05.001 (2011). Article CAS PubMed Google Scholar *
Tannous, B. A. Gaussia luciferase reporter assay for monitoring biological processes in culture and _in vivo_. Nat. Protocols 4, 582–591; 10.1038/nprot.2009.28 (2009). Article CAS PubMed
Google Scholar * Wurdinger, T. et al. A secreted luciferase for ex vivo monitoring of _in vivo_ processes. Nat Meth 5, 171–173; 10.1038/nmeth.1177 (2008). Article CAS Google Scholar *
Turan, S. et al. Recombinase-Mediated Cassette Exchange (RMCE): Traditional Concepts and Current Challenges. J. Mol. Biol. 407, 193–221; 10.1016/j.jmb.2011.01.004 (2011). Article CAS
PubMed Google Scholar * Sauer, B. & Henderson, N. Site-specific DNA recombination in mammalian cells by the Cre recombinase of bacteriophage P1. Proc. Natl. Acad. Sci. USA 85,
5166–5170 (1988). Article ADS CAS PubMed PubMed Central Google Scholar * Koo, B.-K. et al. Controlled gene expression in primary Lgr5 organoid cultures. Nat Meth 9, 81–83;
10.1038/nmeth.1802 (2012). Article CAS Google Scholar * Yatim, N. & Albert Matthew, L. Dying to Replicate: The Orchestration of the Viral Life Cycle, Cell Death Pathways and Immunity.
Immunity 35, 478–490; 10.1016/j.immuni.2011.10.010 (2011). Article CAS PubMed Google Scholar * Sanders, C., Doherty, P. & Thomas, P. Respiratory epithelial cells in innate immunity
to influenza virus infection. Cell Tissue Res. 343, 13–21; 10.1007/s00441-010-1043-z (2011). Article PubMed Google Scholar * Vreede, F. T. & Fodor, E. The role of the influenza virus
RNA polymerase in host shut-off. Virulence 1, 436–439; 10.4161/viru.1.5.12967 (2010). Article PubMed PubMed Central Google Scholar * Muzumdar, M. D., Tasic, B., Miyamichi, K., Li, L.
& Luo, L. A global double-fluorescent Cre reporter mouse. Genesis 45, 593–605; 10.1002/dvg.20335 (2007). Article CAS PubMed Google Scholar * Heaton, N. S. et al. Long-term survival
of influenza virus infected club cells drives immunopathology. J. Exp. Med. 211, 1707–1714; 10.1084/jem.20140488 (2014). Article CAS PubMed PubMed Central Google Scholar * Dos Santos
Afonso, E., Escriou, N., Leclercq, I., van der Werf, S. & Naffakh, N. The generation of recombinant influenza A viruses expressing a PB2 fusion protein requires the conservation of a
packaging signal overlapping the coding and noncoding regions at the 5′ end of the PB2 segment. Virology 341, 34–46; 10.1016/j.virol.2005.06.040 (2005). Article CAS PubMed Google Scholar
* Kumar, Pooja A. et al. Distal Airway Stem Cells Yield Alveoli _In Vitro_ and during Lung Regeneration following H1N1 Influenza Infection. Cell 147, 525–538; 10.1016/j.cell.2011.10.001
(2011). Article CAS PubMed PubMed Central Google Scholar * Janda, J. M., Davis, A. R., Nayak, D. P. & De, B. K. Diversity and generation of defective interfering influenza virus
particles. Virology 95, 48–58; 10.1016/0042-6822(79)90400-8 (1979). Article CAS PubMed Google Scholar * Huang, A. S. & Baltimore, D. Defective Viral Particles and Viral Disease
Processes. Nature 226, 325–327; 10.1038/226325a0 (1970). Article ADS CAS PubMed Google Scholar * Brooke, C. B. et al. Most Influenza A Virions Fail To Express at Least One Essential
Viral Protein. J. Virol. 87, 3155–3162; 10.1128/jvi.02284-12 (2013). Article CAS PubMed PubMed Central Google Scholar * Von Magnus, P. Incomplete forms of influenza virus. Adv. Virus
Res. 2, 59–79 (1954). Article CAS PubMed Google Scholar * Brooke, C. B. Biological activities of ‘noninfectious’ influenza A virus particles. Future virology 9, 41–51; 10.2217/fvl.13.118
(2014). * Herrel, M., Hoefs, N., Staeheli, P. & Schneider, U. Tick-Borne Nyamanini Virus Replicates in the Nucleus and Exhibits Unusual Genome and Matrix Protein Properties. J. Virol.
86, 10739–10747; 10.1128/jvi.00571-12 (2012). Article CAS PubMed PubMed Central Google Scholar * Laemmli, U. K. Cleavage of Structural Proteins during the Assembly of the Head of
Bacteriophage T4. Nature 227, 680–685; 10.1038/227680a0 (1970). Article ADS CAS PubMed Google Scholar * Unkel, B. et al. Alveolar epithelial cells orchestrate DC function in murine
viral pneumonia. J. Clin. Invest. 122, 3652–3664; 10.1172/JCI62139 (2012). Article CAS PubMed PubMed Central Google Scholar * McQualter, J. L., Yuen, K., Williams, B. & Bertoncello,
I. Evidence of an epithelial stem/progenitor cell hierarchy in the adult mouse lung. Proc. Natl. Acad. Sci. USA 107, 1414–1419; 10.1073/pnas.0909207107 (2010). Article ADS PubMed PubMed
Central Google Scholar Download references ACKNOWLEDGEMENTS This study was funded by the Deutsche Forschungsgesellschaft (SCHW 632/11-2) and the Excellence Initiative of the German
Research Foundation (GSC-4, Spemann Graduate School, to A.D.). P.R. was recipient of a Studienstiftung des deutschen Volkes fellowship. We thank Veronika Götz, Sebastian Giese and Linda
Brunotte for critical reading of the manuscript. We are grateful to Max Ulbrich, Hassan Jumaa and Hans Clevers for kindly providing us with plasmid DNA. AUTHOR INFORMATION Author notes *
Reuther Peter and Göpfert Kristina contributed equally to this work AUTHORS AND AFFILIATIONS * Institute for Virology, University of Freiburg, 79104, Freiburg, Germany Peter Reuther,
Kristina Göpfert, Alexandra H. Dudek & Martin Schwemmle * Faculty of Biology, University of Freiburg, 79104, Freiburg, Germany Peter Reuther & Alexandra H. Dudek * Spemann Graduate
School of Biology and Medicine (SGBM), University of Freiburg, 79104, Freiburg, Germany Alexandra H. Dudek * Department of Internal Medicine (Pulmonology), University of Giessen and Marburg
Lung Center, German Center for Lung Research, D-35392, Giessen, Germany Monika Heiner & Susanne Herold Authors * Peter Reuther View author publications You can also search for this
author inPubMed Google Scholar * Kristina Göpfert View author publications You can also search for this author inPubMed Google Scholar * Alexandra H. Dudek View author publications You can
also search for this author inPubMed Google Scholar * Monika Heiner View author publications You can also search for this author inPubMed Google Scholar * Susanne Herold View author
publications You can also search for this author inPubMed Google Scholar * Martin Schwemmle View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS
P.R., S.H. and M.S. conceived and designed the experiments. P.R., K.G., A.D., M.H., S.H. and M.S. performed the experiments and analyzed the data. P.R. and M.S. wrote the manuscript. ETHICS
DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS This work is
licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license,
unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce
the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Reuther, P., Göpfert, K., Dudek,
A. _et al._ Generation of a variety of stable Influenza A reporter viruses by genetic engineering of the NS gene segment. _Sci Rep_ 5, 11346 (2015). https://doi.org/10.1038/srep11346
Download citation * Received: 21 January 2015 * Accepted: 21 May 2015 * Published: 12 June 2015 * DOI: https://doi.org/10.1038/srep11346 SHARE THIS ARTICLE Anyone you share the following
link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature
SharedIt content-sharing initiative